Abstract
Although tractable model organisms are essential to characterize the molecular mechanisms of evolution and adaptation, the ecological relevance of their behavior is not always clear because certain traits are easily lost during long-term laboratory culturing. Here, we demonstrate that despite their long tenure in the laboratory, model organisms retain “ecological memory” of complex environmental changes. We have discovered that Halobacterium salinarum NRC-1, a halophilic archaeon that dominates microbial communities in a dynamically changing hypersaline environment, simultaneously optimizes fitness to total salinity, NaCl concentration, and the [K]/[Mg] ratio. Despite being maintained under controlled conditions over the last 50 years, peaks in the three-dimensional fitness landscape occur in salinity and ionic compositions that are not replicated in laboratory culturing but are routinely observed in the natural hypersaline environment of this organism. Intriguingly, adaptation to variations in ion composition was associated with differential regulation of anaerobic metabolism genes, suggesting an intertwined relationship between responses to oxygen and salinity. Our results suggest that the ecological memory of complex environmental variations is imprinted in the networks for coordinating multiple cellular processes. These coordination networks are also essential for dealing with changes in other physicochemically linked factors present during routine laboratory culturing and, hence, retained in model organisms.
INTRODUCTION
Salinity, or the dissolved ionic content of a body of water, is a ubiquitous property that influences the lifestyle and adaptation of every organism. Diverse prokaryotes have evolved to thrive within limited niches along the entire spectrum of salinity, from trace amounts to saturation, and a variety of strategies for maintaining intracellular salinity at physiologically permissive levels have emerged. One strategy, used by some halophilic prokaryotes, requires the equilibration of extracellular Na+ in excess of 4 M with an equimolar concentration of cytoplasmic K+ via active and passive transport (1, 2). The balance of high intracellular K+ with external Na+ requires the coordination of at least eight different types of membrane channels, including ATP-driven pumps, symporters, antiporters, and light-driven transporters (2). Osmotolerance is thus a complex phenotype dependent on the orchestration of the genetic and regulatory networks that govern osmotic sensory and transport proteins. When environmental salinity conditions overwhelm the system's capacity to balance intra- and extracellular ion concentrations, cells suffer due to membrane depolarization and loss of electrochemical gradients, excessive turgor pressure or loss thereof, and systemic metabolic dysregulation (3, 4).
Environmental salinities vary widely over space and time, even in the hypersaline brines that support extreme halophilic prokaryotes. Salinity is a multifactorial environmental stimulus, consisting of individual ion ratios as well as total salinity. Total salinity varies with precipitation and desiccation, and isosmotic aquatic environments can contain vastly different ion ratios; changes in any or all of these dimensions of salinity carry potential consequences for physiology and fitness. Studies demonstrating the effects of the many dimensions of salinity are uncommon in halophilic prokaryotes, but toxicological data from eukaryotic invertebrates highlight the need for such a higher-dimensional approach to understanding osmotolerance in halophilic bacteria and archaea (5–7). For example, in Ceriodaphnia (water flea), Sphaerium (fingernail clam), Gyraulus (snail), and Tubifex (worm), toxicity from total dissolved solids (TDSs) and chloride is dependent on the ionic composition of the solution. Specifically, increased levels of Ca2+ and Mg2+ reduce the toxicity (increase the 50% lethal dose) of solutions with high levels of TDSs (6), and this relationship has been recapitulated using both field-collected effluent and effluent-mimicking growth media (7).
In contrast, prokaryotic osmotolerance has been examined mainly in terms of single dimensions of salinity using either total salinity or a single ionic salt or nonionic osmolyte (sucrose, polyols), but not both. Studies designed to evaluate the genetic, regulatory, and mechanistic underpinnings of the cellular response to individual salinity factors have laid the foundation for understanding the basic science of prokaryotic osmotolerance (8–12) but are limited in their ability to capture the relative importance of salinity as a multidimensional perturbation for cell fitness and physiology. A few notable exceptions have explored the combinatorial effects of two salts or salt and other environmental effects on halophilic prokaryotes. One study investigated the survival of halophilic bacteria (Bacillus aquimaris, Halomonas salina, Virgibacillus picturae, Salinivibrio costicola) under martian-like conditions with high levels of magnesium and found that growth at a given [MgSO4] is modified by [NaCl] and is further influenced by differences in temperature and pH (13). A 1955 study determined that growth deficiencies in three halophilic prokaryotes induced by a low NaCl concentration could be partially rescued by additional KCl, foreshadowing the later discovery that Na and K gradients in some halophiles are coupled and inversely regulated to maintain osmotic balance (2). Given that halophilic prokaryotes have many potential biotechnological applications, including biofuel synthesis and salinity-dependent crude oil degradation (10, 14, 15), a more nuanced, higher-dimensional investigation of osmotolerance is needed.
Halophiles make attractive models for investigating osmotolerance and complex environmental responses. Halobacterium salinarum in particular is an extreme halophilic archaeon adapted to total salinities upwards of 4 M and has evolved in hypersaline lakes like the Great Salt Lake (GSL), where salinity, temperature, and oxygen frequently fluctuate. In addition, H. salinarum strain NRC-1 has been a model organism for study of the systems biology of complex microbe-environmental interactions, and thus, its culturing regime and molecular biology tools are well established (16, 17). In the natural environment, salinity change often occurs as gradients in time (evaporation, dilution by rainfall) or space (depth gradients, isolated pools with divergent chemistries). Further, these gradients may exist in ion composition, total salinity, or both. Thus, a more comprehensive picture of the salinity response in halophilic archaea is needed, in order to better characterize the niche that these organisms inhabit.
Here, we used a multifactorial approach to assay the contributions of three components of salinity on fitness in H. salinarum NRC-1: total salinity, [NaCl], and [Mg]/[K] ratio. We designed a full-factorial set of 75 growth media around these three factors, and this set included the standard rich Halobacterium CM medium as a control. Although the media are anchored to this synthetic rich medium developed decades ago, the medium compositions were designed to encompass total salinities found in the Great Salt Lake in Utah, a thalassohaline, hypersaline brine that supports dense cultures of haloarchaea. By measuring the maximum growth rate (μ) over gradients in these three environmental factors, we analyzed the contribution of each aspect of salinity to fitness. Upon viewing the range of tolerated environments in three dimensions simultaneously, we gained a measure of the degree to which H. salinarum NRC-1 is specialized to its ionic environment in the laboratory. This measure of specialization in the laboratory has important consequences for the utility of model organisms for investigating molecular mechanisms of adaptation and evolution in the natural environment. Finally, we investigated the similarity between the salinity of the growth medium that enabled the highest fitness and that of Great Salt Lake brine samples. The results of these experiments demonstrate that H. salinarum has “ecological memory” of the complex multifactorial variations in salinity in its natural environment.
MATERIALS AND METHODS
Lab strains and culturing conditions.
H. salinarum NRC-1 Δura3 was cultured in standard CM complex growth medium containing the following: 250 g/liter (4.28 M) NaCl, 20 g/liter (0.081 M MgSO4) MgSO4 · 7H2O, 2 g/liter (0.027 M) KCl, 3 g/liter (0.01 M) sodium citrate dehydrate, 10 g/liter bacteriological peptone (catalog no. LP0034; Oxoid), and 50 mg/liter uracil, without trace metals (18). Solid agar plates were made from the same medium with 20 g/liter bacteriological agar. In all incubations, cultures were grown at 37°C with continuous light and shaking (220 rpm). The Δura3 mutant is the auxotrophic parent strain of the entire H. salinarum NRC-1 mutant library and was selected for use here as an appropriate baseline for future comparisons with mutant strains (19). All experiments here were performed using the Δura3 mutant, here referred to as H. salinarum. All cultures were grown at 37°C with light and shaking, except during growth curve data acquisition, when cultures were grown at 37°C with shaking in the dark.
Growth media and multifactorial design.
Medium compositions represent a three-factorial design with the following factors: total salinity (5 levels of 100% [8.776 M ionic salts], 90%, 80%, 70%, and 60%, by volume), [NaCl] (3 levels of high [96% of total M], medium [78%], and low [59%]), and the [K]/[Mg] molar ratio (5 levels of 0:100, 25:75, 50:50, 75:25, and 100:0), for a total of 75 media (see Table S1 in the supplemental material). M is defined as the total molarity of dissolved salts; therefore, NaCl levels are defined as [NaCl]/[total salinity] in molar salts. Compositions represent variations on the standard CM medium (labeled medium 2, which has 100% total salinity, 4.28 M NaCl, and a 25:75 [K]/[Mg] ratio), and this medium served as a control for comparison across all H. salinarum experiments. [NaCl] and the [K]/[Mg] ratio were varied by molarity within each level of total salinity. The concentrations of the organic ingredients peptone and citrate were held constant across all media, at 10 g/liter peptone and 3 g/liter citrate. To make the total salinity series, the 100% salinity medium series was diluted in a solution of peptone and citrate to each of the remaining four factor levels (60 to 90%, in 10% increments). The pH of all media was adjusted to ∼7.20 ± 0.03, and all media were autoclave sterilized. Chemical lot numbers were recorded, and all medium batches for all experiments were made using the same lot; only two medium batches were used to complete all experiments, in order to reduce batch effects.
Bioscreen growth assays and growth rate calculations.
Growth assays were performed in two Bioscreen C instruments (Growth Curves USA, Piscataway, NJ) with a maximum throughput of 400 cultures (200 μl each) (see Fig. S1 in the supplemental material). For all strains and replicates in all experiments, fresh colonies were grown in 5-ml cultures of medium 2 to an optical density (OD) at 600 nm (OD600) of 0.4 to 0.6 (mid-exponential phase). These cultures were passaged in medium 2 (standard CM) at an OD of 0.05 to synchronize the cultures and grown to an OD600 of 0.6. All cultures were then diluted in medium 2 to an OD600 of 0.55 to ensure identical starting ODs. Using a Hamilton Microlab Star robot (Hamilton Robotics, Reno, NV) to pair each of 8 cultures with each of up to 16 different media, we inoculated each well of a Bioscreen honeycomb plate with culture and experimental media to a final OD of 0.05. All cultures were maintained in medium 2 until transfer to the Bioscreen plate, when they were added at <1:10 in the experimental media. This method required ∼5% differences between medium salinity and final experimental salinity but was ideal because it did not require a stress-inducing centrifugation or rinse step to remove medium 2. Cultures were incubated in the Bioscreen C instrument for 110 h at 37°C with maximum shaking. The OD600 was measured every 15 min for the duration of the experiment. Experiments designed to test confounding by location on the Bioscreen plate revealed no such bias.
The maximum growth rate was extracted from Bioscreen OD measurements using a custom R package developed in-house, the growth curve analysis function, to automate the analysis (20). We used the maximum growth rate as a surrogate for fitness, and although it is not the only component of fitness, it has been shown to be the most important (21). Briefly, cell optical density measurements, relevant metadata, and plate layout schemes were combined in a single database, which enabled rapid calculation of growth curve parameters (maximum growth rate, maximum OD, area under the curve, etc.). The maximum growth rate was calculated from smooth spline-fitted growth curves as the inflection point where the second derivative transitioned from positive to negative (20).
Statistical analysis.
All analyses were performed in R, versions 2.13 and 2.14. The multifactorial design enabled us to test the hypothesis that the mean maximum growth rate differs significantly as a function of total salinity, [NaCl], and [K]/[Mg]. To test this hypothesis, we used an orthogonal analysis of variance (ANOVA) model of the maximum growth rate on three factors: total salinity, [NaCl], and [K]/[Mg]. Data were means of biological replicates; variance among replicates was low, so replicate was not included as a random effect. No interaction was included because there was no a priori hypothesis for what an interaction would mean biologically. In subsequent exploratory models, total salinity-[NaCl] and total salinity-[K]/[Mg] interactions were included, but models were not originally powered for this analysis, due to the lack of a priori biological reasons to suspect interactions. Models were generated using the R function lm(). Residual-versus-fit plots and additional diagnostics were performed on each model and revealed satisfactory residual normality and homoscedasticity. Outliers included maximum growth rates in media 11 and 12, which produced low growth rates, as expected, due to a low total salinity and a low [K]/[Mg]. These outliers were not omitted.
Great Salt Lake sampling sites.
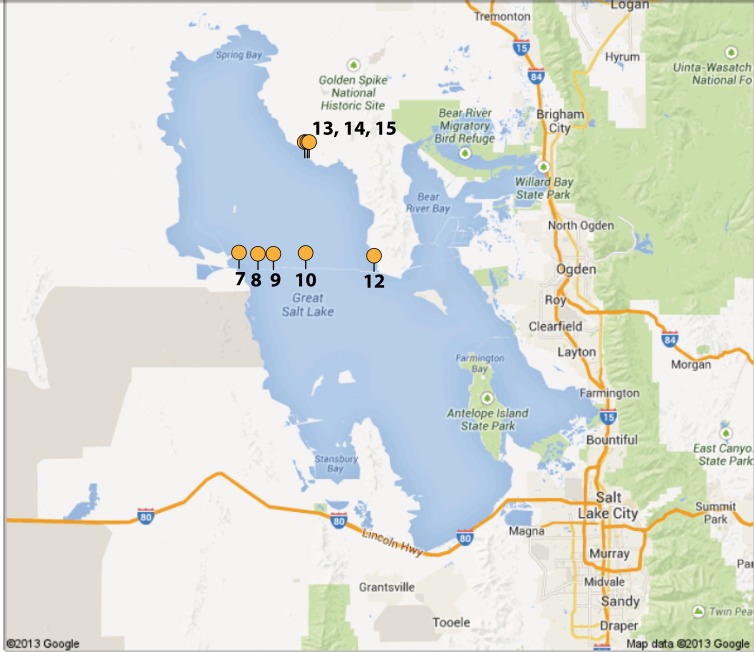
Sites were selected to encompass ecological and chemical diversity. Samples were taken where permission was granted and safety was ensured. Global Positioning System (GPS) coordinates were recorded at each sampling site (Table 1; Fig. 1). No additional permits were required at the time and locations of sampling. Sampling was done from 2 to 6 May 2011, and the samples used for this study represent the subset of samples collected from the north arm of the lake, including along the railroad causeway that bisects the lake into north and south arms. The sites used for this study were GSL2011.7, -8, -9, -10, -12, -13, -14, and -15. Causeway sites were selected to sample the salinity variation near flowthrough sites (culverts and breach) where the north and south arm waters mix.
TABLE 1.
Great Salt Lake north arm sample site locations and water propertiesa
| Sample | Latitude | Longitude | Sampling date (mo/day/yr) | Sampling time (h) | Water depth (m) | Water temp (°C) | pH | Specific conductivity (μS · cm−1 at 25°C) | PSU | DO (mg/liter) | PAR (μE/m2/s1) | UVA (W/m2) | UVB (μW/m−2) | Site description |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSL2011.07 | 41°13.350′ | 112°50.770′ | 5/3/2011 | 13:30 | 0.33 | 13.7 | 8.17 | 148,533 | 118.12 | 8.74 | 683.1 | 15.2 | 7.7 | North causeway at lakeside breach (west side of causeway) |
| GSL2011.08 | 41°13.288′ | 112°47.791′ | 5/3/2011 | 14:45 | 0.24 | 13.6 | 8.13 | 164,200 | 134.46 | 8.98 | NDb | NDb | NDb | North causeway, east of lakeside breach and west of west crack, where the causeway bends slightly |
| GSL2011.09 | 41°13.320′ | 112°45.390′ | 5/3/2011 | 15:10 | 0.25 | 13.0 | 7.90 | 197,600 | 172.18 | 8.77 | NDb | NDb | NDb | North causeway on west crack |
| GSL2011.10 | 41°13.399′ | 112°40.108′ | 5/3/2011 | 15:30 | 0.17 | 12.7 | 7.72 | 201,767 | 177.18 | 8.78 | NDb | NDb | NDb | North causeway, estimated longitudinal center |
| GSL2011.12 | 41°12.980′ | 112°30.385′ | 5/3/2011 | 16:40 | 0.49 | 12.6 | 7.61 | 221,500 | 201.84 | 8.29 | NDb | NDb | NDb | North causeway between east crack and Promontory Point |
| GSL2011.13 | 41°26.284′ | 112°40.100′ | 5/4/2011 | 19:00 | 0.26 | 19.8 | 7.24 | 227,200 | 209.27 | 5.12 | 530 | 4.24 | 0.67 | Spiral Jetty 5 m out from south side of jetty, about halfway between shore and spiral; site used for time course analysis; data here are for time zero |
| GSL2011.14 | 41°26.267′ | 112°40.133′ | 5/5/2011 | 11:30 | 0.265 | 15.12 | 7.35 | 223,800 | 204.82 | 8.12 | NDc | NDc | NDc | Spiral Jetty, center of spiral |
| GSL2011.15 | 41°26.267′ | 112°40.168′ | 5/5/2011 | 12:00 | NDd | NDd | 7.43 | 263,900 | 260.51 | NDd | NDc | NDc | NDc | Spiral Jetty, 50 m west of jetty, out in lake |
Abbreviations: PSU, practical salinity units; DO, dissolved oxygen; PAR, photosynthetically active radiation; UVA, UV A (315 to 400 nm); UVB, UV B (280 to 315 nm).
PAR and UV data were not collected due to inclement weather (rainstorm).
PAR and UV data were not collected due to risk of instrument submersion. Collection required wading in deep water.
Depth, temperature, and DO data were not collected at this site due to deep water and an inability to use the instruments properly.
FIG 1.
Map of all GSL sampling sites. Map of the Great Salt Lake in Utah, with orange markers indicating sampling sites. Marker locations were made with GPS coordinates in Table 1. Water data and metadata were collected from each site and recorded, and sites were selected to capture the chemical and ecological diversity within GSL.
Water sampling and processing.
All glassware, bottles, and collection vessels were sterilized with 10% HCl and soaked in a 2% HNO3 solution to remove any residual metal contamination that might confound downstream ion analyses. Water for rinsing filters and diluting samples was treated with a 0.5% Chelex solution and filter sterilized to chelate any remaining ions in the water. At each site, 1 liter of water was collected from the surface. An additional 10 liters of water was collected from each of sites GSL2011.10 and GSL2011.14. Within 6 h of collection, water samples were stored at 4°C before filtering. Five hundred milliliters of the sample from each site was filtered with a 20-μm-pore-size nylon filter in 2 250-ml replicates into metal-free dark polypropylene bottles. Two 50-ml aliquots from each replicate were further filter sterilized using a 0.2-μm-pore-size bottle-top vacuum filter.
Evaporation and rehydration experiments.
The sample taken at site GSL2011.14 was selected for an evaporation time course, in which GSL water chemistry was measured during the course of nearly complete evaporation and crystal rehydration. This site was selected, as it was the most saline of the sites where 10-liter samples were collected. For the experiment, six 1,000-ml beakers were preweighed and then filled with 500 ml each of the GSL2011.14 water sample, which had been prefiltered through a filter with a pore size of 0.2 μm. The beakers were placed in a shaking water bath incubator set at 37°C and shaken at 125 rpm to accelerate the evaporation process. Starting at time zero, the beakers were weighed and two 50-ml samples were removed for later chemical analysis. The beakers were weighed again immediately after sampling. A new beaker was weighed and sampled every 15 to 18 h, until six samples were taken. After a beaker was sampled, it was sacrificed (no other samples were taken from it) and kept until complete evaporation for the rehydration time course experiment. Sacrificing beakers after one sample was taken was important so that changes in salinity could be compared with the overall course of evaporation; removing samples changes the volume dramatically and cannot be corrected post hoc. Samples were taken at 0, 17.25, 33.5, 46.25, 62.5, and 81.75 h. The evaporation experiment was carried out for 82 h (89% evaporation by weight), when the total remaining liquid was just sufficient for a final 100-ml sample.
To measure the changes in salinity and ion ratio as precipitated crystals rehydrated in freshwater (as in a precipitation event), experimental beakers were left to evaporate completely for 7 days until no visible liquid remained. After total evaporation, the mass of lost water was measured from each beaker as follows: mass of lost water = (mass of beaker at time zero with 500 ml GSL water) − (mass of 100 ml sample) − (mass of evaporated beaker with salt crystals). The mass of lost freshwater was quickly added back to each beaker, and two 50-ml samples were immediately taken from one beaker. All six refilled beakers were incubated at 37°C and 125 rpm for 45 h. One sample per beaker was taken at 0, 0.25, 0.75, 1.33, 4.08, and 44.5 h, with each beaker sacrificed after the one sample was taken. Beaker weights were recorded before and after each sample was taken. Samples were collected in technical duplicate, and the entire evaporation/rehydration experiment was pilot studied twice in advance without sampling to ensure the reproducibility of the evaporation course under experimental conditions.
Note that as salt crystals dissolve and rehydrate, evaporation continues to occur. Beakers were not covered to prevent this, in order to recapitulate these competing processes in a hypersaline lake. If the rate of resolvation is fastest just after water is added, then the first few time points (before ∼2 h) should reveal that rehydration has more of an effect than evaporation. Results for samples taken at later time points will be confounded but will nonetheless indicate how salt chemistry changes after a sudden addition of freshwater.
Chemical analysis of GSL and evaporation water samples.
To determine the concentration of the five most abundant ions (Na+, Cl−, Mg2+, SO42−, K+) in GSL, filtered samples from the lake, the evaporation and rehydration time course samples, and controls were sent to Hoh Pak Laboratories, Inc. (New Iberia, LA), for ion analysis (see Tables S2 and S3 in the supplemental material). Hoh Pak was selected, as it specializes in ion analysis of hypersaline samples. Lake samples and samples from time series evaporation and rehydration of sample GSL2011.14, including a deionized water control, were analyzed.
Several additional blind control samples of known concentration were also sent for analysis. This basal salts control is a mixture of the basal salts used in standard CM growth medium for H. salinarum. It contains the following: NaCl (250 g/liter, 4.28 M), MgSO4-7H2O (20 g/liter, 0.081 M MgSO4 alone), and KCl (2 g/liter, 0.027 M). Other components of the CM growth medium were also sent for analysis for the purpose of having reference data for use in additional growth experiments. These included a solution of Oxoid neutralized bacteriological peptone (10 g/liter) and a solution containing both Oxoid peptone (10 g/liter) and sodium citrate (3 g/liter). The peptone and peptone-citrate mixtures were submitted for analysis in triplicate and are labeled a, b, and c in Table S3 in the supplemental material.
The following methods were used at Hoh Pak Laboratories, Inc., to measure the concentrations of each ion: for sodium and potassium, atomic absorption spectroscopy; for chloride, titration with 0.01 N silver nitrate; for magnesium, titration with 0.01 M EDTA; and for sulfate, gravimetric precipitation with barium chloride. Selected samples were submitted in blind duplicate or triplicate to obtain a measure of technical variability. Coefficients of variation ranged from 0 to 5.5%.
Great Salt Lake chemistry and multifactorial growth medium composition comparisons.
We compared pairwise differences between the chemistry of the samples from the north-arm GSL sites and the evaporation/rehydration composition for each growth medium in the multifactorial array (20 GSL samples × 75 media). Pairwise differences were calculated using two independent methods: the average Manhattan distance method and nested sequential comparison of total salinity, followed by Na, Mg, and K concentrations, for each GSL sample-growth medium pair.
(i) Average Manhattan distance.
The Manhattan distance between pairs of GSL sample compositions and growth medium compositions is the sum of the absolute differences in each constituent salinity factor (22). The average distance is this sum divided by 6, because each composition has 6 constituents: total salinity, Na, Cl, Mg, SO4, and K. This distance metric incorporates all ions into a single index, allowing the identification of the growth medium best matched to each GSL sample.
(ii) Nested sequential comparison.
We compared medium and GSL samples according to each ion, in order of GSL ion abundance (total salinity, Na, Mg, and K), and preserved the structure of the multifactorial experimental design. To start, we sought the total salinity level that most closely matched the composition of each GSL sample, including evaporation and rehydration time course samples. These total salinity differences had a bimodal distribution, which we used to eliminate GSL brines too dissimilar for comparison. This cutoff point fell at a total salinity difference of 1.6 M. Eleven GSL samples with the closest total salinity matches of less than 1.6 M remained in the analysis, while the remaining nine brines had discrepancies greater than 2.8 M and were omitted. Within this closest-matched group of 15 media with the same total salinity, we then identified the closest matches for each additional ion (Na, followed by Mg and K). The Mg and K comparisons were nested within Na comparisons, which were nested within total salinity comparisons. At each level, the number of the best-matched candidate medium was restricted by the match at the previous level (see Fig. S2 in the supplemental material). Closest matches were defined as the smallest absolute difference between a given GSL brine and the set of growth media. The results of this analysis of salinity differences are presented as heat maps, with red and blue indicating small and large differences between medium and GSL sample compositions, respectively. White asterisks indicate differences in the smallest 5% of all pairwise comparisons, based on empirical cumulative distribution functions. This analysis allowed the identification of a single experimental medium whose composition most closely matched that of each GSL sample and the GSL evaporation/rehydration composition for each major ion. Analyses were performed in R.
Microscopy and cell morphology image analysis.
To evaluate the effects of various salinities on cell physiology, we analyzed images of H. salinarum cultures at ×400 magnification at six time points along the growth curve in media 1, 2, 5, and 12. These media were selected because they represented those with both maximal (medium 2) and minimal (medium 12) growth rates, as well as both extremes in the [K]/[Mg] ratio (media 1 and 5, respectively). Two replicate cultures were grown from colonies in 5 ml CM medium as described above and passaged at an OD of 0.05 after reaching mid-log phase. After reaching mid-log phase once more, cultures were divided into 4 equal aliquots (one per test medium) and pelleted at 8,000 rpm. Pellets were resuspended in 5 ml CM and then diluted further to an OD600 of 0.05 in 50 ml of medium 1, 2, 5, or 12. The value at time zero was recorded after dilution into the test media. At each of six time points (0, 19, 27, 40, 64, and 90 h), ODs were measured, cells were counted in a hemacytometer, and a series of images was taken. Cultures were diluted in their respective experimental media to an OD600 of 0.2 for gel-coated, wet-mount slide preparation. Gel sides were prepared in advance by compressing 2 μl of 2% agarose on slides. Gel slides were dried for 5 min in a 60°C drying oven and allowed to cool just before imaging. These slides immobilize motile cells and allow images to be taken in a single focal plane, making cell area-to-volume extrapolations more meaningful. Images were taken on a Nikon Eclipse E400 bright-field microscope with a ×100 oil immersion objective (phase contrast). Images were captured with an attached Leica camera and analyzed using the Leica Application Suite, version 3.5.0. All images were taken at a resolution of 2,048 by 1,536 pixels at a 118.1-ms exposure time. Image analysis of cell circularity [4π(area/perimeter2); a value of 1.0 indicates a perfect circle], aspect ratio, Feret's distance (the longest distance between any two points), and total area was conducted using the ImageJ program for Macintosh computers and R.
RNA preparation and tiling array analysis.
Genome-wide RNA levels were determined for the Δura3 strain at mid-log phase (OD600 = 0.4 to 0.5) during growth in media 1, 2, 5, 6, 7, 9, 12, 13, 14, and 15 (see Table S1 in the supplemental material) in flasks using standard culturing conditions. These 10 media were selected to accommodate array sample size limitations after omitting media where cultures did not reach an OD600 of 0.4 or growth rates were not significantly different from the others by Tukey's honest significant differences. Cells from 2 ml of culture were pelleted (16,000 × g, 60 s) and flash frozen. Total RNA was isolated using a mirVana RNA kit (Life Technologies) following the manufacturer's instructions and then treated with RNase-free DNase (Promega). RNA from the growth in each medium was compared with a standard RNA reference extracted from H. salinarum NRC-1 grown in batch culture in medium 2 to an OD600 of 0.471. Two biological replicates were performed for each medium, with the exception of one replicate only for M12. Whole-genome tiling arrays were designed using e-Array (Agilent Technologies) for the H. salinarum NRC-1 main chromosome (GenBank accession number NC_002607) and plasmids pNRC100 (GenBank accession number NC_001869) and pNRC200 (GenBank accession number NC_002608) and a 60,000-feature design of 60-mer strand-specific probes spaced at 24 bp. The arrays included the manufacturer's control probes and were printed by Agilent Technologies. RNAs were directly labeled with Alexa 547 and Alexa 647 dyes (Kreatech), followed by hybridization, washing, slide scanning, spot finding, and normalization, as described previously (20), with dye flip experiments performed for each sample. Raw intensity values were median normalized and log10 transformed. Statistical analyses were performed in R and MultiExperiment Viewer (MeV), version 4.8. Gene functional enrichment analysis was performed using DAVID functional enrichment tools (23, 24). Correlations between gene expression and medium composition and growth attributes (μ, [Na], [Cl], [Mg], [K], NaCl/total salt ratio, and [K]/[Mg] ratio) were analyzed using Pearson's r correlation tests.
Microarray data accession number.
Microarray data were deposited in the Gene Expression Omnibus (GEO) database at http://ncbi.nlm.nih.gov (accession number GSE53544).
RESULTS
We generated H. salinarum NRC-1 fitness curves (maximum growth rate [μ]-environment plots) by measuring μ across a set of 75 growth media (5 dilutions of a series of 15 media which varied in [NaCl] and [K]/[Mg]; see Table S1 in the supplemental material). Cellular morphology was observed to vary with ion composition across the growth curve (see Fig. S3 to S5 in the supplemental material), as has been observed in other prokaryotes (25–28). We observed that the timing and intracellular distribution of gas vesicles, cell circularity, and aspect ratio were all associated with the growth medium (see Fig. S3 and S4 in the supplemental material). Nonetheless, measured optical density and manually observed cell counts were proportional throughout the growth curve, indicating that morphology does not cause confounding effects on optical density as a measure of the growth rate (see Fig. S3 in the supplemental material). The series of 75 media were designed to test three independent factors (multifactorial design), testing the individual contributions of total salinity, [NaCl], and [K]/[Mg] to fitness, as measured by μ. The resultant multidimensional salinity landscape (Fig. 2; see Table S1 in the supplemental material) revealed an overall growth optimum (μ = 0.070, doubling time [Td] = 9.9 h) in medium 2 at 7.96 M total ions (90% of that for the control). Among the levels of total salinity, maximum μ occurred at 7.96 M total ions, or 90% of the salinity of standard CM medium (mean μ across 15 media = 0.052, Td = 13.3 h). Higher and lower total salinities were less optimal, with doubling times reaching 31 h in the lowest level of total salinity (2.633 M) (Fig. 3). Among [NaCl] levels, maximum μ occurred at the highest level (97.5% [wt/wt] NaCl; mean μ for high [NaCl] = 0.054, Td = 12.8 h), and in the [K]/[Mg] gradient, maximum μ occurred at a ratio of 75:25 (mean μ for 75:25 [K]/[Mg] = 0.052, Td = 13.3 h).
FIG 2.
Salinity fitness curves. (A) Fitness curves plotting the maximum growth rate over the medium composition show how the salinity niche is shaped by three factors (total salinity, [NaCl], and [K]/[Mg] ratio). Black lines connect the mean maximum growth rate (μ; h−1) from 4 to 8 replicate experiments in each growth medium. Error bars show standard deviations. (B) A three-dimensional landscape representation of the data in panel A. Colors represent growth medium total salinity (purple, 100%; blue, 90%; green, 80%; orange, 70%; red, 60%). Growth media are ordered 1 to 15, as in panel A. The bar height indicates the mean μ. Total salinity is given as the ion concentration, in molar (M ions).
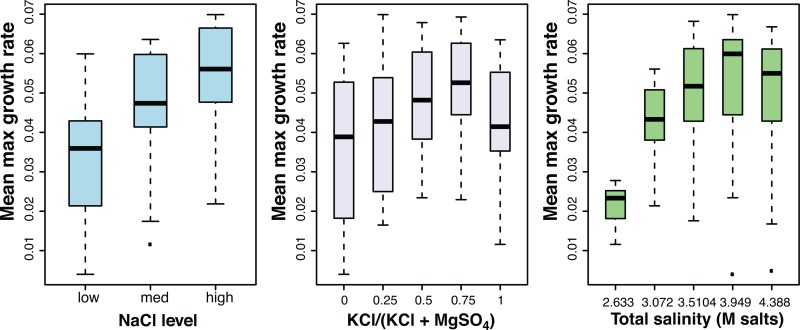
FIG 3.
The mean maximum growth rate varies by total salinity, [NaCl] level, and [K]/[Mg] ratio. Box plots indicate the median (black bar in each box) and 1st and 3rd quartiles of μ (bottom and top edges of each box, respectively) within each level of each factor. Whiskers extend to 1.5 times the interquartile range, and individual points lie outside this range. [K]/[Mg] is represented as the potassium fraction of total potassium plus magnesium.
Within the five levels of total salinity, the [NaCl] fraction was positively associated with μ, except in samples with the most dilute total salinity level (60% of that for the control), where this association disappeared. Holding total salinity and [NaCl] constant, distinct μ maxima (peaks) emerged across gradients in [K]/[Mg]. These peaks emerged at different [K]/[Mg] levels, depending on the encompassing total salinity and [NaCl] levels. For example, a [K]/[Mg] of 25:75 produced peaks in most total salinity groups when [NaCl] was high. Peaks shifted to a [K]/[Mg] of 100:0 as [NaCl] decreased, and this effect was modified by total salinity. While the peak μ in medium 2 at 90% total salinity was the largest, local peaks in other compositions were defined as being significantly greater than those in medium sharing the same total salinity and [NaCl].
We then tested the hypothesis that total salinity, [NaCl], and [K]/[Mg] each has an independent effect on μ. Mixed ANOVA models revealed that all three salinity dimensions were significantly associated with μ and, thus, fitness (Table 2), demonstrating that H. salinarum perceives salinity to be a complex perturbation comprising total salinity and ion ratio components. Biological replicate was included as a random effect. From the fitness curves, we made the a priori decision to evaluate interactions between total salinity and [NaCl] fraction and between [NaCl] and [K]/[Mg]. Both interaction terms were highly significant, reflecting the underlying coordination of many ion-specific transport mechanisms.
TABLE 2.
Mixed ANOVA modela of associations between growth medium components and maximum growth rate
| Parameter | F | P |
|---|---|---|
| Total salinity | 461.2 | <0.001 |
| [NaCl] | 622.97 | <0.001 |
| [K]/[Mg] | 140.04 | <0.001 |
| Total salinity · [NaCl] | 36.71 | <0.001 |
| [NaCl] · ([K]/[Mg]) | 81.06 | <0.001 |
The ANOVA model describes the contributions of each medium component to the mean maximum growth rate (µ). Salinity components were included as fixed effects; biological replicate was included as a random effect.
Upon the unexpected observation that H. salinarum NRC-1 attains peak maximum growth rates in noncontrol medium, we asked whether any of the noncontrol, peak fitness-producing media shared saline properties with natural hypersaline environments that have supported the evolution of H. salinarum. If a laboratory model organism reaches peak fitness under salinity conditions that it has never experienced, perhaps it is successful under these conditions because they resemble ancestral environments experienced prior to laboratory culturing. To answer this question, we identified which of the 75 growth media had compositions that best matched each of the 20 brine compositions measured from the Great Salt Lake north arm. These 20 compositions were found in eight water samples taken from the north arm of GSL at sites selected for the diversity of saline chemistry and microbial ecology (Fig. 1). The remaining 12 compositions were found in samples taken from evaporation and rehydration time courses, using sample GSL2011.14 as a starting point. These time course samples recapitulated the temporal variation in brine chemistry due to evaporation and rehydration (see Tables S2 and S3 in the supplemental material).
To identify the closest matches among the GSL sample-growth medium pairs of multivariate brine compositions, we used two methods to measure the similarity of each pair of brine samples: average Manhattan distance and a nested comparison that preserves the multifactorial medium design. Before applying either method, we identified the total salinity level among the five total salinity factor levels (60 to 100%, or 5.33 to 8.94 M) in the growth media that most closely matched the level in each of 20 GSL brine samples. These total salinity differences had a bimodal distribution, which we used to eliminate GSL brines too dissimilar for comparison. This cutoff point fell at a total salinity difference of 1.6 M. Eleven GSL samples with the closest total salinity matches if they were less than 1.6 M remained in the analysis, while the remaining nine brine samples had a discrepancy of greater than 2.8 M and were omitted.
For the GSL sample-medium comparisons, we first used a simple average Manhattan distance metric to generate a distance matrix that incorporates all ion (Na, Cl, Mg, SO4, and K) concentrations and total salinity into pairwise distances (Fig. 4; see Table S4 in the supplemental material). The Manhattan distance between any GSL sample-growth medium pair was the sum of the absolute differences in each ion, and each ion contributed equally to the difference between brines. The distance metric was then divided by 6, to combine the salinity-specific differences and obtain the average distance for each GSL sample-growth medium pair. Using this method, the growth media whose compositions matched those of 9 of the 11 GSL samples were those that produced peaks in the maximum growth rate (hypergeometric P < 0.0005).
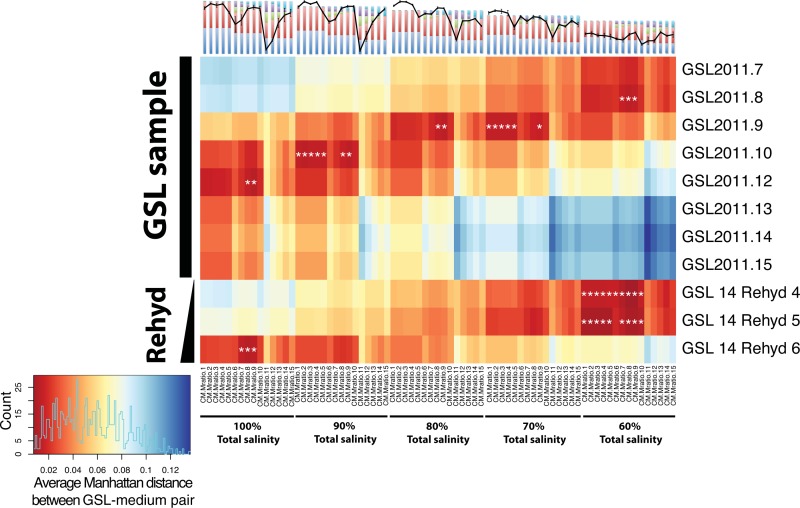
FIG 4.
Pairwise Manhattan distances between GSL sample and growth medium salinities. The salinity compositions of GSL samples (y axis) and 75 growth media (x axis) were compared by calculating the average Manhattan distance. The average Manhattan distance is the sum of the absolute differences in each salinity attribute (total salinity, Na, Cl, Mg, SO4, and K, in molar) divided by the total number of attributes (six). Colors indicate the magnitude of the distance, with red indicating the smallest differences and blue indicating the largest differences. Differences in the smallest 5% by cumulative distribution function are indicated with white asterisks. GSL samples are distinguished from sample GSL2011.14 rehydration (Rehyd) time course samples on the left-hand y axis. GSL samples shown are those with differences in total salinity from the total salinity of the most closely matching growth medium of no greater than 1.6 M. The key at the lower left indicates the scale and distribution of distances among sample pairs. The colors denoting the ion compositions of the growth media (top x axis) are defined in Fig. 2A. The CM.Mratio labels distinguish each of the 75 media. The final number in the CM.Mratio name indicates the ion composition (1 to 15). For media from series of less than 100% salinity, the number following “Mratio” represents the dilution (90, 80, 70, or 60%). See Table S1 in the supplemental material.
In the second method, we performed nested, sequential comparisons of total salinity, sodium, and magnesium or potassium in each GSL sample-medium pair in order to preserve the factor structure of the multifactorial medium design (see Fig. S2 in the supplemental material). The first comparison that we made was at the level of total salinity (see Fig. S6 in the supplemental material). Conditioning on the set of 15 media that comprised the best-matched total salinity level for a given GSL sample, we then asked which of the three sodium factor levels best matched the [Na] of the GSL samples (see Fig. S6 in the supplemental material). Again, conditioning on the best-matched sodium level within the best-matched total salinity level, we performed the same analysis of differences on both [Mg] and [K] (see Fig. S6 and S7 in the supplemental material). Because the media were designed to evaluate the effect of various [K]/[Mg] ratios, the GSL sample-medium comparison of potassium could not be nested with magnesium. Therefore, they were compared separately, each nested within the best-matched sodium level. Using the nested comparison method (total salinity → [Na] → [Mg]) for finding the growth medium whose composition most closely matched that of each GSL sample, 8 of 11 matching growth media produced peak maximum growth rates (hypergeometric P < 0.005). Due to the anticorrelation between [Mg] and [K] in the medium design, a larger proportion of Mg-matched GSL samples than K-matched samples corresponded to peak fitness-producing media. The Mg-based comparison is likely more relevant due to the higher relative abundance of Mg versus K in both the GSL sample and standard growth medium. Both the Manhattan distance and nested comparison methods were highly concordant in their identification of the growth medium that was the most similar in composition to that of each GSL sample and of peak fitness-producing media whose compositions did not correspond to that of any GSL sample. H. salinarum NRC-1 reached a peak μ in 8 media across the set of 75 growth media whose compositions were not matched to those of any of the GSL samples. More striking, the compositions of a majority of GSL samples mapped to those of peak fitness-producing media (Fig. 5).
FIG 5.
Great Salt Lake sample compositions map to those of the growth media that produce peak fitness. GSL samples (blue and yellow dots numbered with GSL sample numbers) are superimposed onto a three-dimensional landscape of H. salinarum NRC-1 fitness, where each colored bar represents the maximum growth rate in 1 of 75 growth media. Dots are located on the individual medium compositions that were best matched to GSL sample salinity by Manhattan distance (yellow) or nested pairwise comparison (blue). The colors in the three-dimensional bar plot indicate growth medium total salinity (purple, 100%; blue, 90%; green, 80%; orange, 70%; red, 60%), and media within a single total salinity (TS) level are numbered 1 to 15 from left to right. Bar height measures indicate the maximum growth rate (in hours−1). See Fig. 2A for standard deviations around the mean. Samples with an “R” prefix in the sample number were rehydrated.
To understand the salinity response at the level of gene regulation and cellular physiology, we asked whether the changes in fitness due to ion composition are accompanied by changes in gene expression. Using whole-genome tiling microarrays, we measured genome-wide transcript levels for cells grown to mid-log phase in 10 media with various ion compositions in the 100% total salinity series, chosen to cover the range of growth phenotypes observed in media 1 to 15. From these data, we identified genes with expression responsive to growth rate and genes with expression responsive to salinity. H. salinarum is known to display several growth phase-associated transitions in cell physiology and gene expression (29). In the transition from active growth in log phase to stationary phase, H. salinarum is known to switch from aerobic to anaerobic metabolism in anticipation of nutrient limitation, generating gas vesicles to vertically relocate in the water column (29). In the current experiments, genes involved in these processes were significantly correlated with μ, including the phototrophy genes for light-driven energy production under anaerobic conditions (specifically, the bacteriorhodopsin pump components bacterioopsin [bop] and retinal chromophore synthesizing phytoene synthase [crtB1]) and gas vesicle genes for flotation (gvpA1, gvpC1, gvpA2, and gvpV1).
To identify genes correlated with medium ion composition alone, we removed the μ-correlated genes from the analysis and found that 37 genes were significantly correlated with ion composition (see Table S5 and Fig. S8 in the supplemental material). These 37 genes were correlated at a P value of <0.10 with both [Cl] and [Mg], but in opposite directions, as the medium series design caused an anticorrelation between [Cl] and [Mg] as a result of maintaining constant salinity across compositions. The observed growth rate-independent expression patterns indicate that changes in ion composition may signal the need for switching between metabolic physiologies: a transcriptional regulator involved in anaerobic metabolism on dimethyl sulfoxide (dmsR) was significantly correlated with [Mg], while a transporter involved in a second anaerobic metabolic strategy that allows growth on arginine (VNG1238C) was significantly anticorrelated with [Mg]. The biosynthesis of several enzyme cofactors that are critical to metabolism and DNA synthesis was also affected by environmental ion composition: [Cl]- and [Mg]-correlated genes included genes functioning in the biosynthesis or binding of the redox cofactors coenzyme pyrroloquinoline quinone (a cofactor in glucose dehydrogenase), flavin mononucleotide (a cofactor in NADH dehydrogenase), and cobalamin (vitamin B12; a cofactor in ribonucleotide reductase). These effects may stem from the use of Mg as a cofactor in cofactor biosynthesis enzymes, such as in the synthesis of the cobalamin precursor porphyrin (30). We also observed that variations in ion composition induced up to an ∼10-fold upregulation of a globally acting transcription factor IIB ortholog, TFBa (tfbA), in media 1, 5, 7, and 13. The tfbA gene was previously shown to be differentially regulated under transition metal perturbations (31, 32) and to contribute to fitness at high pH and low temperature (20). Taken together, it appears that this global regulator is sensitive to ion composition, in addition to its role in the pH and temperature response. Thus, adaptation to complex variations across the salinity landscape is accompanied by both growth rate-dependent and growth rate-independent changes in gene expression.
DISCUSSION
The major aim of this study was to systematically query the osmotolerance repertoire of an extreme halophilic archaeon. H. salinarum is simultaneously a seasoned laboratory model and an extremophile, requiring salinities that preclude most other life. Despite the known complex network of membrane-bound sensors, channels, and pumps with functional annotations for ion transport, the H. salinarum NRC-1 response to the multiple ionic constituents of its unique hypersaline environment has hardly been explored from an integrated, systems perspective. We grew H. salinarum in a series of media that encompass multifactorial gradients in total salinity and two dimensions of ion composition. The strength of this design lies in its power to distinguish which of these factors contributes significantly to growth and fitness and in our ability to detect interactions that provide clues for exploring the underlying genetic, regulatory, and physiological networks that govern osmotolerance. By expanding the traditional experimental definition of salinity to include three dimensions, we observed that H. salinarum perceives and responds to all three tested dimensions of salinity independently, and we corroborated results from an early paper by quantifying the observed interaction between sodium and potassium responses (33).
The fitness curve plots produced by this experimental design revealed unexpected patterns. First, the growth medium that produced the highest value of fitness was not the standard CM control medium (medium 2) but, rather, the 90% dilution of medium 2. This is curious, because the salt content of this growth medium was first described in 1960 (34) and it became a standard rich medium for culturing H. salinarum by many research groups (18, 35). It is unclear how this medium was developed and whether or not it was formulated to produce the highest value of μ. Regardless of the medium formulation, our surprise is borne from the numerous examples of model organisms adapting to their culturing environments (36–39).
A second unexpected result was the intermediate peaks (local maxima) in μ along the ion composition gradients within each level of total salinity. Within each level of total salinity, three peaks in μ emerged, one within each level of [NaCl]. The highest of these peaks in μ generally occurred in medium 2 or 3 across all dilutions except 60%, where several patterns broke down due to extremely low growth rates. This is consistent with the conclusion that the ion ratio of CM enables the highest fitness independently of total salinity. However, additional peaks occurred in media with ion ratios and total salinities that do not resemble those in CM at all. For example, medium 9 and all of its dilutions produced fitness peaks nearly as high as those produced by media 2 and 3 (but still significantly different by Tukey's honest significant difference). Close in composition to medium 9, media 8 and 10 also produced μ peaks in other total salinity levels. Similarly, media 14 and 15 also produced local peaks in μ, but at the lowest level of [NaCl].
Common to these intermediate peaks (media 8, 9, 14, and 15) was the [K]/[Mg] ratio of 75:25 or greater. The [K]/[Mg] ratio of medium 2 (25:75) did not produce peaks within other levels of [NaCl], indicating that increased [K]/[Mg] compensates for a lowered [NaCl]. While this study design cannot distinguish between the effect of increased potassium versus that of decreased magnesium, we can make inferences based on previous potassium-specific studies in haloarchaea. In H. salinarum, intracellular potassium levels are maintained to balance extracellular sodium, and this balance is regulated by a combination of passive transport and active, ATP-driven pumps. At K concentrations below 5 mM, growth defects were observed in a study of H. salinarum potassium transport (12). At K concentrations below 200 μM, the Kdp active transport system is upregulated and requires ATP to pump potassium into the cell and maintain the cationic balance across the membrane (12). In our data, the rescue of low-sodium defects by potassium suggests that monovalent cations, whether they be sodium or potassium, are needed to restore fitness when [NaCl] falls below 78% of the total salt concentration. If a simple Na-K balance across the membrane were the most critical, we would not expect to see a defect in any dilution of medium 12 (274 to 456 mM potassium), because these concentrations fall above the known threshold for growth defects caused by potassium depletion.
We observed that peaks in μ occur in media with diverse compositions that depart from the composition for the control, and this reinforces the need to approach osmotolerance as a complex phenotype and salinity as a multidimensional perturbation. These local μ maxima also led us to ask whether the peak fitness-producing media shared salinity characteristics with natural hypersaline brines, where H. salinarum has evolved in the environment. Samples collected from the Great Salt Lake, a prototypical ecosystem dominated by haloarchaea, were analyzed for the presence of the five most dominant ions, and their compositions were compared with the composition of each of the 75 growth media. Of GSL samples with comparable total salinity, a significant majority had ion compositions that closely matched those of the peak fitness-producing growth media. This result emerged from two different analysis strategies and suggests that evolutionary growth history is preserved in the osmotolerance repertoire of laboratory model organisms. Most reassuring is the fact that peaks in μ outside control medium 2 and the preservation of osmotolerance under GSL-like conditions indicate a lack of laboratory specialization in H. salinarum NRC-1.
Insight into the physiological response to salinity was provided by morphology studies and gene expression analyses. Time series images showed that changes in ion composition produce significant changes to cell morphology and gas vesicle content: the timing and distribution of gas vesicles as well as cell circularity and aspect ratio were associated with the growth medium. A preliminary gene expression analysis offered clues to the interrelationships among responses to salinity-coupled environmental stimuli. Transcriptome analysis identified ion composition-induced changes in the expression of genes involved in anaerobic metabolism and transcriptional regulation. These results suggested that adaptation to salinity might require metabolic and regulatory changes to simultaneously deal with factors that covary with ion composition in the natural environment (e.g., oxygen). A physicochemical relationship exists between salinity, temperature, and oxygen concentration: higher salinity and water activity (as influenced by the ratios of mono- and divalent ions) support lower dissolved oxygen, and this relationship is modified by temperature. In H. salinarum, substantial gene expression changes occur in response to oxygen perturbations as cells switch between aerobic and anaerobic metabolic strategies (40), and expression of several of the same genes changes in a manner correlated with variations in ion composition. Thus, the cellular responses to ion composition and oxygen may be coupled, reflecting the coincident variation of these parameters in the natural environment. Together, analyses of growth dynamics and gene expression offer insight into the complexity of the salinity response in this halophilic model organism, which involves changes in growth rate, cell morphology, and gene regulation.
The utility of a model organism for investigating the cellular response to environmental stimuli is high, only as long as the model can recapitulate the phenomena of interest within its controlled and invariant laboratory culturing regime. Saccharomyces cerevisiae (36), Escherichia coli (21), and several pathogens (37–39) have diverged in their growth phenotypes or become specialists during their tenure in the laboratory. Such laboratory specialization has major implications for the environmental or clinical relevance of these model organisms. Here, we show that H. salinarum retains its utility as a model for investigating the complex networks that govern osmoregulation in extreme halophilic archaea. Nevertheless, the question that remains is why osmotolerance phenotypes appear to be so deeply ingrained at the level of growth and fitness. One explanation is the underlying genetic, regulatory, and mechanistic complexity inherent in maintaining osmotic balance in an environment where salinity, temperature, and oxygen are physicochemically linked. The oxygen concentration depends on temperature and salinity, and regulatory networks governing oxic and anoxic physiologies involve ∼10% of the H. salinarum genome (40). The overlap in oxygen, temperature, and salinity networks is likely vast, making the genome robust to changes with pleiotropic consequences for a multiple-stress response. Our data support this concept: in comparing GSL sample and growth medium compositions, H. salinarum cultures achieved peaks in μ in media whose compositions were not either of the same composition as the control medium or at all similar to the compositions of samples from GSL. Even though H. salinarum has likely never experienced these conditions, it thrives there due to the evolution of biological networks wired to couple osmotolerance with other environmental responses critical for survival in the laboratory. In the trade-off between flexibility and robustness, we have demonstrated robustness, or memory, in a model halophilic archaeon, thus preserving its utility for systems-level investigations of the cellular response to complex salinity perturbations.
Supplementary Material
ACKNOWLEDGMENTS
For the Great Salt Lake sampling, we relied on a collaboration with Bonnie Baxter and Jaimi Butler at the Great Salt Lake Institute (GLSI), Westminster College (Salt Lake City, UT), whose group has the experience, expertise, and permits to regularly sample the lake. We are grateful for the hospitality at GSLI during our sampling trip. In addition, the USGS Utah Water Science Center (David Naftz and Michael Freeman) enabled access to additional sites within GSL and along the railroad causeway that bisects the lake from east to west. We thank these collaborators for their continued assistance and advice. We also thank Monica Orellana and Ben Kerr for helpful discussion and insight.
Funding was provided by grants from the U.S. National Science Foundation (NSF-1262637 and NSF-1330912 to N.S.B.) and the U.S. National Institutes of Health (2P50GM076547 to N.S.B. and 5F32GM097931 to E.J.W.). Work was also conducted under the ENIGMA (Ecosystems and Networks Integrated with Genes and Molecular Assemblies) program, supported by the Office of Science, Office of Biological and Environmental Research, of the U.S. Department of Energy under contract no. DE-AC02-05CH11231.
Footnotes
Published ahead of print 10 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03280-13.
REFERENCES
- 1.Oren A. 2001. The bioenergetic basis for the decrease in metabolic diversity at increasing salt concentrations: implications for the functioning of salt lake ecosystems. Hydrobiologia 466:61–72. 10.1023/A:1014557116838 [DOI] [Google Scholar]
- 2.Oren A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csonka LN, Hanson AD. 1991. Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol. 45:569–606. 10.1146/annurev.mi.45.100191.003033 [DOI] [PubMed] [Google Scholar]
- 4.Csonka LN. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tietge JE, Hockett JR, Evans JM. 1997. Major ion toxicity of six produced waters to three freshwater species: application of ion toxicity models and TIE procedures. Environ. Toxicol. Chem. 16:2002–2008. 10.1002/etc.5620161004 [DOI] [Google Scholar]
- 6.Soucek DJ, Linton TK, Tarr CD, Dickinson A, Wickramanayake N, Delos CG, Cruz LA. 2011. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 30:930–938. 10.1002/etc.454 [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AJ, Cherry DS, Zipper CE. 2005. Evaluation of ionic contribution to the toxicity of a coal-mine effluent using Ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 49:155–162. 10.1007/s00244-004-0034-z [DOI] [PubMed] [Google Scholar]
- 8.Coker JA, Dassarma P, Kumar J, Müller JA, Dassarma S. 2007. Transcriptional profiling of the model archaeon Halobacterium sp. NRC-1: responses to changes in salinity and temperature. Saline Syst. 3:6. 10.1186/1746-1448-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z, Zhou A, Baidoo E, He Q, Joachimiak MP, Benke P, Phan R, Mukhopadhyay A, Hemme CL, Huang K, Alm EJ, Fields MW, Wall J, Stahl D, Hazen TC, Keasling JD, Arkin AP, Zhou J. 2010. Global transcriptional, physiological, and metabolite analyses of the responses of Desulfovibrio vulgaris Hildenborough to salt adaptation. Appl. Environ. Microbiol. 76:1574–1586. 10.1128/AEM.02141-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Mailem DM, Sorkhoh NA, Al-Awadhi H, Eliyas M, Radwan SS. 2010. Biodegradation of crude oil and pure hydrocarbons by extreme halophilic archaea from hypersaline coasts of the Arabian Gulf. Extremophiles 14:321–328. 10.1007/s00792-010-0312-9 [DOI] [PubMed] [Google Scholar]
- 11.Browne RA, Wanigasekera G. 2000. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. J. Exp. Mar. Biol. Ecol. 244:29–44. 10.1016/S0022-0981(99)00125-2 [DOI] [Google Scholar]
- 12.Strahl H, Greie J-C. 2008. The extremely halophilic archaeon Halobacterium salinarum R1 responds to potassium limitation by expression of the K+-transporting KdpFABC P-type ATPase and by a decrease in intracellular K. Extremophiles 12:741–752. 10.1007/s00792-008-0177-3 [DOI] [PubMed] [Google Scholar]
- 13.Crisler JD, Newville TM, Chen F, Clark BC, Schneegurt MA. 2012. Bacterial growth at the high concentrations of magnesium sulfate found in martian soils. Astrobiology 12:98–106. 10.1089/ast.2011.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapilatu YH, Grossi V, Acquaviva M, Militon C, Bertrand J-C, Cuny P. 2010. Isolation of hydrocarbon-degrading extremely halophilic archaea from an uncontaminated hypersaline pond (Camargue, France). Extremophiles 14:225–231. 10.1007/s00792-010-0301-z [DOI] [PubMed] [Google Scholar]
- 15.Minai-Tehrani D, Minoui S, Herfatmanesh A. 2009. Effect of salinity on biodegradation of polycyclic aromatic hydrocarbons (PAHs) of heavy crude oil in soil. Bull. Environ. Contam. Toxicol. 82:179–184. 10.1007/s00128-008-9548-9 [DOI] [PubMed] [Google Scholar]
- 16.Baliga NS. 2008. Systems biology. The scale of prediction. Science 320:1297–1298. 10.1126/science.1159485 [DOI] [PubMed] [Google Scholar]
- 17.Bonneau R, Facciotti MT, Reiss DJ, Schmid AK, Pan M, Kaur A, Thorsson V, Shannon P, Johnson MH, Bare JC, Longabaugh W, Vuthoori M, Whitehead K, Madar A, Suzuki L, Mori T, Chang D-E, Diruggiero J, Johnson CH, Hood L, Baliga NS. 2007. A predictive model for transcriptional control of physiology in a free living cell. Cell 131:1354–1365. 10.1016/j.cell.2007.10.053 [DOI] [PubMed] [Google Scholar]
- 18.DasSarma S, Fleischmann EM, Rodriguez-Valera F. 1995. Archaea: a laboratory manual—halophiles. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 19.Peck RF, DasSarma S, Krebs MP. 2000. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol. Microbiol. 35:667–676. 10.1046/j.1365-2958.2000.01739.x [DOI] [PubMed] [Google Scholar]
- 20.Turkarslan S, Reiss DJ, Gibbins G, Su WL, Pan M, Bare JC, Plaisier CL, Baliga NS. 2011. Niche adaptation by expansion and reprogramming of general transcription factors. Mol. Syst. Biol. 7:554. 10.1038/msb.2011.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper VS, Bennett AF, Lenski RE. 2001. Evolution of thermal dependence of growth rate of Escherichia coli populations during 20,000 generations in a constant environment. Evolution 55:889–896. 10.1554/0014-3820(2001)055[0889:EOTDOG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Paul S, Bag SK, Das S, Harvill ET, Dutta C. 2008. Molecular signature of hypersaline adaptation: insights from genome and proteome composition of halophilic prokaryotes. Genome Biol. 9:R70. 10.1186/gb-2008-9-4-r70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang DW, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37:1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4:44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 25.Doudoroff M. 1940. Experiments on the adaptation of Escherichia coli to sodium chloride. J. Gen. Physiol. 23:585–611. 10.1085/jgp.23.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. 2012. Hyperosmotic stress response of Campylobacter jejuni. J. Bacteriol. 194:6116–6130. 10.1128/JB.01409-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen F, Stephens PJ, Knøchel S. 1995. The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J. Appl. Bacteriol. 79:274–281. 10.1111/j.1365-2672.1995.tb03137.x [DOI] [Google Scholar]
- 28.Singh SP, Montgomery BL. 2013. Salinity impacts photosynthetic pigmentation and cellular morphology changes by distinct mechanisms in Fremyella diplosiphon. Biochem. Biophys. Res. Commun. 433:84–89. 10.1016/j.bbrc.2013.02.060 [DOI] [PubMed] [Google Scholar]
- 29.Facciotti MT, Pang WL, Lo F-Y, Whitehead K, Koide T, Masumura K-I, Pan M, Kaur A, Larsen DJ, Reiss DJ, Hoang L, Kalisiak E, Northen T, Trauger SA, Siuzdak G, Baliga NS. 2010. Large scale physiological readjustment during growth enables rapid, comprehensive and inexpensive systems analysis. BMC Syst. Biol. 4:64. 10.1186/1752-0509-4-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storbeck S, Rolfes S, Raux-Deery E, Warren MJ. 2010. A novel pathway for the biosynthesis of heme in Archaea: genome-based bioinformatic predictions and experimental evidence. Archaea 2010:175050. 10.1155/2010/175050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corratgé-Faillie C, Jabnoune M, Zimmermann S, Véry A-A, Fizames C, Sentenac H. 2010. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell. Mol. Life Sci. 67:2511–2532. 10.1007/s00018-010-0317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur A, Pan M, Meislin M, Facciotti MT, El-Gewely R, Baliga NS. 2006. A systems view of haloarchaeal strategies to withstand stress from transition metals. Genome Res. 16:841–854. 10.1101/gr.5189606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown H, Gibbons NE. 1955. The effect of magnesium, potassium, and iron on the growth and morphology of red halophilic bacteria. Can. J. Microbiol. 1:486–494. 10.1139/m55-062 [DOI] [PubMed] [Google Scholar]
- 34.Sehgal SN, Gibbons NE. 1960. Effect of some metal ions on the growth of Halobacterium cutirubrum. Can. J. Microbiol. 6:165–169. 10.1139/m60-018 [DOI] [PubMed] [Google Scholar]
- 35.Baliga NS, Kennedy SP, Ng WV, Hood L, DasSarma S. 2001. Genomic and genetic dissection of an archaeal regulon. Proc. Natl. Acad. Sci. U. S. A. 98:2521–2525. 10.1073/pnas.051632498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kvitek DJ, Will JL, Gasch AP. 2008. Variations in stress sensitivity and genomic expression in diverse S. cerevisiae isolates. PLoS Genet. 4:e1000223. 10.1371/journal.pgen.1000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassat J, Dunman PM, Murphy E, Projan SJ, Beenken KE, Palm KJ, Yang S-J, Rice KC, Bayles KW, Smeltzer MS. 2006. Transcriptional profiling of a Staphylococcus aureus clinical isolate and its isogenic agr and sarA mutants reveals global differences in comparison to the laboratory strain RN6390. Microbiology 152:3075–3090. 10.1099/mic.0.29033-0 [DOI] [PubMed] [Google Scholar]
- 38.Fine DH, Furgang D, Schreiner HC, Goncharoff P, Charlesworth J, Ghazwan G, Fitzgerald-Bocarsly P, Figurski DH. 1999. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology 145:1335–1347. 10.1099/13500872-145-6-1335 [DOI] [PubMed] [Google Scholar]
- 39.Gaynor EC, Cawthraw S, Manning G, MacKichan JK, Falkow S, Newell DG. 2004. The genome-sequenced variant of Campylobacter jejuni NCTC 11168 and the original clonal clinical isolate differ markedly in colonization, gene expression, and virulence-associated phenotypes. J. Bacteriol. 186:503–517. 10.1128/JB.186.2.503-517.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid AK, Reiss DJ, Kaur A, Pan M, King N, Van PT, Hohmann L, Martin DB, Baliga NS. 2007. The anatomy of microbial cell state transitions in response to oxygen. Genome Res. 17:1399–1413. 10.1101/gr.6728007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.