Abstract
Escherichia fergusonii is an emerging pathogen that has been isolated from a wide range of infections in animals and humans. Primers targeting specific genes, including yliE (encoding a conserved hypothetical protein of the cellulose synthase and regulator of cellulose synthase island), EFER_1569 (encoding a hypothetical protein, putative transcriptional activator for multiple antibiotic resistance), and EFER_3126 (encoding a putative triphosphoribosyl-dephospho-coenzyme A [CoA]), were designed for the detection of E. fergusonii by conventional and real-time PCR methods. Primers were screened by in silico PCR against 489 bacterial genomic sequences and by both PCR methods on 55 reference and field strains. Both methods were specific and sensitive for E. fergusonii, showing amplification only for this bacterium. Conventional PCR required a minimum bacterial concentration of approximately 102 CFU/ml, while real-time PCR required a minimum of 0.3 pg of DNA for consistent detection. Standard curves showed an efficiency of 98.5%, with an R2 value of 0.99 for the real-time PCR assay. Cecal and cloacal contents from 580 chickens were sampled from broiler farms located in the Fraser Valley (British Columbia, Canada). Presumptive E. fergusonii isolates were recovered by enrichment and plating on differential and selective media. Of 301 total presumptive isolates, 140 (46.5%) were identified as E. fergusonii by biochemical profiling with the API 20E system and 268 (89.0%) using PCR methods. E. fergusonii detection directly from cecal and cloacal samples without preenrichment was achieved with both PCR methods. Hence, the PCR methods developed in this work significantly improve the detection of E. fergusonii.
INTRODUCTION
Whereas the origin and distribution of Escherichia coli have been the subject of numerous studies, little is known about the ecology of other Escherichia species such as E. fergusonii. This species was established as belonging to Escherichia in 1985 and is genetically most similar to E. coli (1). Since then, E. fergusonii has been recognized as an important emerging opportunistic pathogen of both animals and humans. In animals, E. fergusonii has been isolated from pigs, sheep, cattle, goats, horses, reindeer, ostriches, turkeys, and chickens displaying symptoms of salmonellosis-like infections, including diarrhea as well as mastitis, meningitis, abortion, and septicemia (2–6). This bacterium also has been isolated from human blood, urine, feces, spinal fluid, and, most often, wound exudates from people with conditions such as sepsis, urinary tract infections, enteric diseases, pancreatic carcinoma, and wound infection (2, 3, 6, 7). Several virulence factors, including the presence of a heat-labile toxin on a plasmid, are involved in the pathogenesis of E. fergusonii (2, 5, 8). Interspecies genetic transfer among E. fergusonii, E. coli, and Shigella has already been observed and could lead to more-virulent strains (3, 6). Antimicrobial resistance to several available therapeutic options has also been observed (5), and one recently characterized E. fergusonii isolate was found to be resistant to numerous antibiotics (8).
Poultry production is an important agricultural sector in Canada. Of more than 2,600 poultry farms, over 300 (12%) are located in the province of British Columbia (http://bcchicken.ca/wp-content/uploads/2012/11/BCChickenMarketingBoard-BOOKLETAnnualReport2012.pdf [accessed 4 July 2013]). The majority (over 80%) of British Columbia's poultry farms are located in the Fraser Valley (http://www.chickenfarmers.ca/resources/annual-report-2012/ [accessed 4 July 2013]). Colonization of the poultry gut by potential pathogenic bacteria such as E. fergusonii could result in the contamination of the environment and food chain. Therefore, the potential of E. fergusonii to become an important animal and possible emerging opportunistic zoonotic pathogen (5) raises its importance in food safety and public health.
Current detection methods for E. fergusonii involve the use of differential and selective media as well as biochemical profiling methods such as API 20E. The use of citrate adonitol and sorbitol MacConkey media is an important part of the selection process; however, results can be difficult to interpret, particularly when using complex samples such as chicken cecal and cloacal contents (2, 4). The API 20E identification system is based on 20 different biochemical tests, which greatly increases the probability of an accurate identification. However, the use of biochemical tests is reliant upon having a pure bacterial isolate to start with, which can be a cumbersome process when starting with samples of high bacterial diversity. This identification method is also dependent upon a database of previously documented results which is more limited for species such as E. fergusonii compared to well-investigated bacteria such as E. coli and Salmonella. Molecular methods that employ the PCR assays have been used for the detection of several bacteria (9–11). Thus, the use of PCR primers that are specific to well-conserved genes in E. fergusonii strains may provide increased accuracy as well as speed and simplicity of E. fergusonii detection.
The objectives of this study were to develop a simple and accurate molecular identification model for E. fergusonii from the chicken cecum and cloacae and to investigate the prevalence of this bacterium in broiler chicken farms of the Fraser Valley of British Columbia, Canada.
MATERIALS AND METHODS
Bacterial strains.
The list of the 55 bacterial strains used in this study, including 11 reference strains from the American Type Culture Collection (ATCC; Cedarlane Laboratories, Burlington, Ontario, Canada), the previous characterized E. fergusonii ECD-227 strain (8, 12, 13), and an additional 43 field isolates from our collection, is presented in Table 1.
TABLE 1.
List of bacterial strains used in the present studya
| Organism | Origin |
|---|---|
| Escherichia fergusonii ATCC 35469 | American Type Culture Collection |
| Escherichia fergusonii ECD-227 | Our collection (chicken fecal) |
| Kluyvera ascborbata ATCC 33433 | American Type Culture Collection |
| Escherichia coli ATCC 25922 | American Type Culture Collection |
| Escherichia coli O11:H25 | Retail chicken meat |
| Escherichia coli O?:H7 | Retail chicken meat |
| Escherichia coli O139:NM | Retail chicken meat |
| Escherichia coli O2:H42 | Retail chicken meat |
| Escherichia coli O7:H18 | Retail chicken meat |
| Escherichia coli O157:H7 | Beef fecal material |
| Escherichia coli O82:NM | Human UTI |
| Escherichia coli O2:H7 | Human UTI |
| Escherichia coli O73 | Human blood |
| Escherichia coli Chi7122 | Turkey fecal material |
| Escherichia coli Ec002 | Human |
| Escherichia coli Ec039 | Human |
| Escherichia coli Ec048 | Human |
| Escherichia vulneris | Beef fecal material |
| Salmonella enterica serovars | |
| Enteritidis ABBBS1004 | Chicken fecal material |
| Hadar ABB1048-1 | Chicken fecal material |
| Heidelberg SALB-46 | Chicken fecal material |
| Kentucky SALC-205-3 | Chicken fecal material |
| Typhimurium SALH-394-3 | Chicken fecal material |
| Typhimurium st002 | Human |
| Typhimurium st004 | Human |
| Braenderup H9812 | Colleen Harlton (AAFC, Summerland, BC) |
| Proteus vulgaris ATCC 13351 | American Type Culture Collection |
| Proteus mirabilis ATCC 25933 | American Type Culture Collection |
| Proteus mirabilis PmAgaz | Chicken fecal material |
| Proteus mirabilis Pm001 | Human vagina |
| Proteus mirabilis Pm002 | Human eye |
| Proteus mirabilis Pm003 | Human urine |
| Proteus mirabilis Pm004 | Human hip |
| Proteus mirabilis Pm005 | Human burn |
| Micrococcus luteus ATCC 12698 | American Type Culture Collection |
| Micrococcus luteus ATCC 9341 | American Type Culture Collection |
| Klebsiella oxytoca ATCC 43165 | American Type Culture Collection |
| Klebsiella pneumoniae ATCC 13883 Kp001 | American Type Culture Collection |
| Klebsiella pneumoniae Kp004 | Human |
| Klebsiella pneumoniae Kp005 | Human |
| Klebsiella pneumoniae Kp006 | Human |
| Klebsiella pneumoniae Kp007 | Human |
| Pasteurella multocida Pm016 | Bovine lung |
| Enterobacter cloacae ATCC 13047 ent002 | American Type Culture Collection |
| Enterobacter cloacae ent003 | Human |
| Enterobacter cloacae ent004 | Human |
| Pseudomonas aeruginosa ATCC 27853 | American Type Culture Collection |
| Pseudomonas aeruginosa Pa01 | Human |
| Pseudomonas aeruginosa Pa02 | Retail chicken meat |
| Citrobacter youngae | Beef fecal material |
| Enterobacter spp. | Beef fecal material |
| Providencia stuartii | Beef fecal material |
| Vibrio fluvialis | Beef fecal material |
| Yersinisa pseudotuberculosis | Beef fecal material |
| Shewanella sp. W3-18-1 | Human cellulitis |
The non-ATCC strains were from our collection. BC, British Columbia; UTI, urinary tract infection.
Sample collection and bacterial isolation.
A total of 580 28-to-36-day-old broiler chickens were obtained from 32 farms in the Fraser Valley of British Columbia (Canada), a major area of poultry production. Twenty chickens from each farm were collected in 2 separate growing cycles (10/cycle) except for 6 farms which were sampled once. The 32 sampled farms were assigned to the east (10 farms), north (11 farms), or south (11 farms) regions based on their location within the Fraser Valley. All of the farms used conventional rearing practices approved in Canada. Cloacal and cecal contents were aseptically collected from each bird using a biosafety cabinet and sterile instruments, which were resterilized with hot water and 70% alcohol between birds to avoid cross-contamination. Samples were then transferred to 10 ml of sterile buffered peptone water (EMD Chemicals, Gibbstown, NJ). The cloacal samples were homogenized with a vortex device and the cecal samples with a stomacher for 2 min. A 200-μl volume of each sample was used to inoculate 5 ml of tryptic soy broth (TSB; Benton Dickinson, Mississauga, Ontario, Canada), which was incubated at 37°C overnight with agitation at 175 rpm.
For selection of presumptive E. fergusonii isolates, a loopful of each culture in TSB was first applied to Simmons citrate agar (Oxoid, Nepean, Ontario, Canada) containing 4% adonitol (SCA) as previously described (4). Up to five adonitol-fermenting colonies (dark yellow to orange) were subsequently transferred to sorbitol MacConkey agar (SMA; Oxoid) and incubated overnight at 37°C (2, 7). The non-sorbitol-fermenting colorless colonies on SMA were kept frozen in TSB with 25% glycerol at −80°C for additional analysis. At each sampling time, presumptive E. fergusonii colonies from each positive sample were screened using API 20E strips (bioMérieux, St-Laurent, Quebec, Canada) according to the manufacturer's specifications.
Primer design.
The genome of E. fergusonii ECD-227 has been previously sequenced and investigated (8). Based on this genome, NCBI BLAST (http://www.ncbi.nlm.nih.gov/) was used to select areas of genomic islands that were unique to and ubiquitous in E. fergusonii strains. Areas that had BLAST results of close to 100% coverage and an E value of 0 with respect to the finished genome of E. fergusonii ATCC 35469 (GenBank accession no. CU928158) were selected. The first two primers, Efer13 and EferYP, targeting the yliE gene (encoding a conserved hypothetical protein of the cellulose synthase and regulator of cellulose synthase island) and the EFER_1569 gene (encoding a hypothetical protein, putative transcriptional activator for multiple antibiotic resistance), respectively (Table 2), were selected manually using the melting temperature and GC content specifications provided by iQ SYBR green Supermix manual and using the Tm calculator (Applied Biosystems). Primer3 software (http://bioinfo.ut.ee/primer3/) was used to design the primers for Efer41 targeting the EFER_3126 gene (encoding a putative triphosphoribosyl-dephospho-coenzyme A [CoA]) of the malonate utilization system genomic island. Additionally, PriDimerCheck (http://biocompute.bmi.ac.cn/MPprimer/) was used to select primers that would have less dimer formation. The primers were ordered from IDT Integrated DNA Technologies (Coraville, IA). Primers targeting the β-d-glucuronidase uidA gene present in most E. coli strains (9) were also used as a control to further validate the specificity of the Efer primers.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ to 3′) | Amplicon length (bp) | Positionsa | Gene or producta | Source or reference |
|---|---|---|---|---|---|
| Efer13-F | GGGCATAAATCTGGTTGGC | 233 | 1197158–1197390 | yliE | This study |
| Efer13-R | CGGGCATACCATAACAATCG | Conserved hypothetical protein of the cellulose synthase and regulator of cellulose synthase | |||
| EferYP-F | GCAATATACAGGACACAGTGTCG | 432 | 1602034–1602465 | EFER_1569 | This study |
| EferYP-R | CTATGAAGGGAAGGGTAGGAGC | Hypothetical protein, putative transcriptional activator for multiple antibiotic resistance | |||
| Efer41-F | CCCCTGTTTTACCCTTTG | 635 | 3213423–3214057 | EFER_3126 putative triphosphoribosyl-dephospho-CoA | This study |
| Efer41-R | CGGGGCTTATCCAGTTAT | ||||
| UAL1939b | ATGGAATTTCGCCGATTTTGC | 187 | 11 | ||
| UAL2105b | ATTGTTTGCCTCCCTGCTGC |
Positions and genes correspond to locations and descriptions from E. fergusonii ATCC 35469 (GenBank accession no. CU928158.2).
DNA isolation.
DNA extraction from isolates was performed according to the method of Bonnet et al. (14). Briefly, 1 ml of a pure culture in TSB incubated overnight at 37°C was spun at 18.8 × g for 3 min. The supernatant was removed, and the pellet was resuspended in 200 μl of sterile water. This suspension was heated in a boiling-water bath for 15 min, cooled on ice for 1 min, and spun again for 3 min. The supernatant was then kept at −20°C until use. Alternatively, DNA was also extracted directly from the cecal and cloacal samples using a QIAamp DNA Stool Minikit (Qiagen, Toronto, Ontario, Canada) according to the manufacturer's recommendations. The purity and concentration of DNA recovered by both methods were determined using a NanoDrop 2000c spectrophotometer (Fisher Scientific, Vancouver, British Columbia, Canada) and/or an Invitrogen Qubit 2.0 Fluorometer (Life Technologies Inc., Carlsbad, CA).
Conventional PCR assay.
A conventional duplex PCR was developed using the primers Efer13 and EferYP on an Applied Biosystems GeneAmp PCR System 9700 thermal cycler (Life Technologies Inc., Burlington, Ontario, Canada). The 25-μl final volume of the PCR mixture contained 12.5 μl of 2× AccuStart PCR Supermix (Quanta Biosciences Inc., Gaithersburg, MD), 0.5 μM each primer, 6.5 μl of molecular analysis-grade water, and 1 μl of bacterial DNA (or half a colony of the bacterial strain tested when a colony PCR was performed). The cycling conditions were 94°C for 3 min followed by 35 cycles of 94°C for 30 s, 56.5°C for 30 s, and 72°C for 30 s and then a hold at 4°C. A range of annealing temperatures (50, 52, 54.4, 55.6, 58, and 60°C) was also investigated. The PCR products were separated on a 2% Tris-acetate-EDTA buffer agarose electrophoresis gel stained with ethidium bromide (1 μl/10 ml) or using Gelred (Biotium Inc., Hayward, CA). The bands were referenced to a GeneRuler 100-bp DNA ladder (Fermentas, Ottawa, Ontario, Canada) and a 1-kb DNA ladder (New England BioLabs Inc., Whitby, Ontario, Canada) to size the amplicons.
Real-time PCR assay.
Real-time PCR was performed with the EferYp and Efer41 primers using a fluorescent intercalating dye in 2× iQ SYBR Green Supermix and a Bio-Rad iQ5 Multicolour real-time PCR detection system (Bio-Rad Laboratories, Inc., Mississauga, Ontario, Canada) according to the manufacturer's protocol. The final 25-μl volume of the real-time duplex PCRs was similar in composition to that of the conventional assay described above. The cycling conditions were 95°C for 3 min, followed by 35 cycles of 95°C for 15 s, 59.5°C for 30 s, and 72°C for 30 s, followed by the default melt curve analysis (an increase of 0.5°C held for 30 s repeated 81 times from 55 to 95°C).
Specificity and efficiency of PCR assays.
In silico PCR amplification (http://insilico.ehu.es/PCR/) was performed using each primer set on 489 (298 Gram-negative and 191 Gram-positive) species, strains, and/or serotypes (see Table S1 in the supplemental material) to validate the specificity of the Efer primers, as well as NCBI BLAST of all primer sequences against the nt nucleotide database using relaxed search parameters (BLASTN with a word size of 7) (accessed November 2013). In addition, a total of 55 reference and field isolates (Table 1) were used to validate the specificity of the conventional and real-time duplex PCR assays for detecting E. fergusonii. To rule out all false negatives, we performed PCRs using universal U165 primers targeting the 16S rRNA gene (15) as a control for the template DNA (U165-F, 5′ AGA GTT TGA TCC TGG CTC AG 3′; U165-R, 5′ AAG GAG GTG ATC CAG CCG CA 3′). Also, the initial DNA concentration was determined using an Invitrogen Qubit 2.0 Fluorometer (Life Technologies Inc.) to ensure that sufficient DNA was present for amplification. The sequencing of PCR products was performed to confirm the identity of the target genes used in this study. The PCR products were purified using a QIAquick PCR purification kit (Qiagen). DNA sequencing was performed by the Nucleic Acid and Protein Sequencing Unit at the University of British Columbia (Vancouver, British Columbia). Sequences of the PCR products were compared with the genome of E. fergusonii ATCC 35469 (GenBank accession no. CU928158) (http://www.ncbi.nlm.nih.gov/) using NCBI BLAST and were aligned with the Clustal Omega multiple-sequence-alignment program at http://www.ebi.ac.uk/Tools/msa/clustalo/.
The minimum concentration for detection by conventional PCR was determined in four replicates of three separate assays using a modified protocol from the Clinical Microbiology Procedures Handbook (16). Briefly, a colony of E. fergusonii ATCC 35469 from a fresh culture on tryptic soy agar (TSA; Becton, Dickinson) was inoculated in 1 ml of TSB and incubated overnight at 37°C. This culture was then diluted 10 times in TSB and incubated for an additional 1 to 1.5 h until a McFarland turbidity of 1.0 was obtained. DNA was extracted as described above from 1 ml of each dilution from 10−1 to 10−7 for PCR. Bacterial numbers (CFU/ml) were determined in each dilution by viable counts on TSA. For real-time PCR, four replicates of three separate assays of 10-fold serial dilutions of E. fergusonii ATCC 35469 DNA ranging from 310 ng to 31 fg were used to construct standard curves and to determine the cycle threshold (CT) and PCR amplification efficiency (E) (11). Again, the DNA concentration was determined using the Invitrogen Qubit 2.0 Fluorometer.
Statistical analysis.
Cochran-Mantel-Haenszel statistics were used to determine the association between isolate and region (east, north, or south), source (cloacae or ceca), collection (first or second visit), and farms using the FREQ procedures of SAS 9.2 (SAS Institute, Inc., Cary, NC). The frequencies of identification obtained by API 20E and PCR were compared by determination of the odds ratio values (95% confidence limits). A P value of 0.05 was used to declare significance.
RESULTS
Conventional and real-time duplex PCR assays.
The conventional duplex PCR using Efer13 and EferYP generated products having similar band intensities on a gel, thereby facilitating visual confirmation. For the real-time PCR assay, EferYP and Efer41 were chosen as they had melting points that were easily distinguishable at 82 to 83.5°C for EferYP and 85.5 to 86.5°C for Efer41. Amplification of expected bands by real-time PCR was confirmed by gel electrophoresis.
Specificity and efficiency.
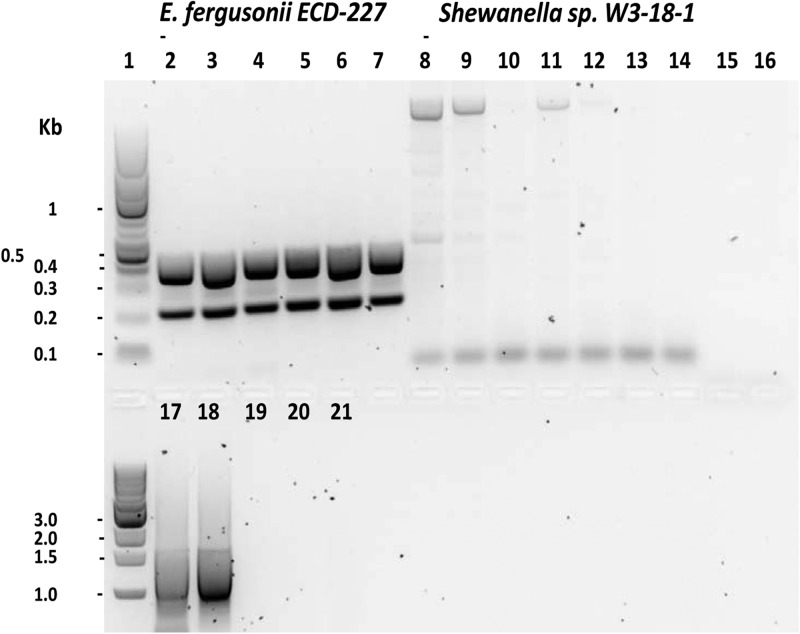
Of the 489 species, strains, and/or serotypes tested by in silico PCR (see Table S1 in the supplemental material), only E. fergusonii showed the predicted positive results and no amplification was obtained in other bacteria. An NCBI BLAST search of the nt nucleotide database (accessed November 2013) identified partial matches of our primer sequences to those of Shewanella (aquatic Gram-negative and facultative anaerobic Proteobacteria) and Pelobacter (strictly anaerobic, Gram-negative bacteria that use only a very limited number of substrates and are unable to ferment sugars); however, no results were generated via in silico PCR using their genomic sequences. Moreover, inspection of the NCBI BLAST for each primer pair revealed that the partial exact matches to Shewanella or Pelobacter were not within proper orientation or distance to produce viable PCR products. Of 55 isolates, including a Shewanella isolate (Table 1 and Fig. 1; see also Fig. S1 in the supplemental material), tested by the duplex PCR assays, amplifications were obtained only with E. fergusonii ATCC 35469 and ECD-227. The DNA concentrations extracted from isolates presented in Table 1 as well as any isolates negative by PCR were within the range of 0.3 to 44.6 ng/μl (300 to 4,460 pg/μl). A threshold of bacterial concentration of approximately 102 CFU/ml was needed for consistent detection by conventional PCR, and an amount of 0.3 pg of DNA, which corresponds to approximately 60 genomic equivalents, was determined to be required for consistent detection by real-time PCR, indicating the sensitivity of our PCRs. The CT value for this quantity of DNA was found to be 30.6 ± 1.0 and the number of endpoint relative fluorescence units (RFU) to be 653.3 ± 201.2 (Table 3). The PCR amplification efficiency “E” was 98.5%, with an R2 value of 0.99.
FIG 1.
Conventional duplex PCR using the Efer13 and EferYP primers, showing amplification in Escherichia fergusonii ECD-227 but not in Shewanella sp. W3-18-1. A variety of annealing temperatures were used in an attempt to generate amplicons in Shewanella. Universal U165 primers (targeting the 16S rRNA gene) were used as a control for the template DNA. Lanes: 1, 1-kb ladder; 2, Escherichia fergusonii ECD-227, 50°C; 3, 52°C; 4, 54.4°C; 5, 55.6°C; 6, 58°C; 7, 60°C; 8, Shewanella sp. W3-18-1, 50°C; 9, 52°C; 10, 54.4°C; 11, 55.6°C; 12, 58°C; 13, 60°C; 14, Efer13 and EferYP primers, no DNA, 55.6°C; 15, no Taq, E. fergusonii DNA, 55.6°C; 16, no Taq, Shewanella DNA, 55.6°C; 17, universal U165 primers, E. fergusonii DNA; 18, universal U165 primers, Shewanella DNA, 55.6°C; 19, universal U165 primers, no DNA; 20, no Taq, E. fergusonii DNA; 21, no Taq, Shewanella DNA.
TABLE 3.
Means of the cycle threshold values and endpoint relative fluorescence units of 10-fold serially diluted DNA from E. fergusonii ATCC 35469a
| Amount of DNA | Mean (± SD) CT valuec | Endpoint RFU |
|---|---|---|
| NTC | NA | 130.00 ± 67.3 |
| 310 ng | 10.7 ± 1.3 | 3,949.0 ± 850.1 |
| 31 ng | 12.7 ± 0.7 | 3,657.5 ± 195.3 |
| 3.1 ng | 16.1 ± 0.8 | 3,947.0 ± 164.2 |
| 310 pg | 19.8 ± 0.6 | 3,809.8 ± 189.9 |
| 31 pg | 23.6 ± 0.5 | 3,119.1 ± 138.1 |
| 3.1 pg | 27.2 ± 0.7 | 1,893.3 ± 119.9 |
| 0.31 pgb | 30.6 ± 1.0 | 653.3 ± 201.2 |
| 0.031 pg | 3.0 ± 0.5 | 216.7 ± 62.1 |
CT, cycle threshold; NA, not available; NTC, no-template control; RFU, relative fluorescence units.
Minimum amount of DNA needed for reliable detection in real-time PCR; approximately 60 genomic equivalents.
Data represent results from three separated and four replicated experiments.
Some of the isolates positive by PCR were identified as Kluyvera spp. by API 20E (99.3% accuracy). The sequences of Efer13 and EferYP PCR products from one such Kluyvera isolate and E. fergusonii ATCC 35469 were compared. A 100% similarity alignment of the Efer13 product (231 bp) was obtained between E. fergusonii ATCC 35469 and the putative Kluyvera spp. Within a 419-bp stretch for the PCR product of EferYP, there were four mismatches between E. fergusonii ATCC 35469 and the putative Kluyvera spp. Compared to the expected products of both E. fergusonii ATCC 35469 and ECD-227, two of the mismatches in this putative Kluyvera spp. were unique and the other two positions matched E. fergusonii ECD-227. A BLAST analysis of the Efer13 and EferYP products yielded E values of 4 × 10−117 for Efer13 and 0 for EferYP from E. fergusonii ATCC 35469. The BLAST analysis using the full sequence of PCR products did not generate similarities with Shewanella or Pelobacter.
Direct detection by PCR.
DNA was extracted directly from 12 (6 positive by culture and 6 negative by culture) cecal and cloacal samples and analyzed by both conventional and real-time PCR methods. The six samples that were previously found to have presumptive E. fergusonii using cultural methods were again positive by both PCR methods. Two samples for which presumptive isolates were recovered by cultural methods but subsequently found to not contain E. fergusonii yielded positive results by PCR detection from DNA extracted directly from cecal and cloacal samples. Four additional samples were tested from a farm that had produced no presumptive isolates, and one of these samples was also found to be positive for E. fergusonii. These data showed that the PCR methods could be developed to shorten the detection time while increasing the chance to avoid false-negative samples.
Prevalence using the API 20E microbial identification kit.
A total of 398 presumptive isolates were collected, and 301 were screened with the API 20E kit. An identification accuracy of 70% or more (API 20E identification [ID] ≥ 70% accuracy) was assumed to be confirmative for E. fergusonii. Using this criterion, 46.5% (140) of the isolates from 22 of the 32 (68.8%) farms sampled were confirmed to be E. fergusonii. The isolates were recovered from cloacal or cecal samples at both sampling times (collection 1 or 2). Significant differences were observed between the east, north, and south regions of the Fraser Valley for the numbers of E. fergusonii isolates (P < 0.05). This species was detected in 70% (7/10), 73% (8/11), and 64% (7/11) of farms located in the east, north, and south, respectively (Table 4). Significant intrafarm variations in prevalence were also noted within given regions (data not shown).
TABLE 4.
Prevalence of E. fergusonii in broiler chickens of the Fraser Valley by API 20E identification and both conventional and real-time PCR detection
| Parameter | No. (%) of isolates by indicated identification method or P value |
|
|---|---|---|
| API 20E | PCR | |
| Regiona | ||
| East (n = 96) | 32 (33.3) | 85 (88.5) |
| North (n = 99) | 64 (64.5) | 94 (94.5) |
| South (n = 106) | 44 (41.5) | 89 (84.0) |
| P value | <0.01d | 0.06 |
| Location in chickenb | ||
| Ceca (n = 166) | 82 (49.4) | 147 (88.6) |
| Cloaca (n = 135) | 58 (43.0) | 121 (89.6) |
| P value | 0.26 | 0.89 |
| Collectionc | ||
| 1 (n = 137) | 58 (42.34) | 119 (86.7) |
| 2 (n = 164) | 82 (50.0) | 149 (90.9) |
| P value | 0.18 | 0.36 |
Data represent comparisons of the prevalences of E. fergusonii in the indicated regions of the Fraser Valley using either API 20E or PCR detection.
Data represent comparisons of the prevalences of E. fergusonii in the ceca versus the cloaca using either API20E or PCR detection.
Data represent comparisons of the prevalences of E. fergusonii in all samples from collection 1 versus collection 2 using either API20E or PCR detection.
P values ≤ 0.05 were considered to represent a significant difference in the levels of prevalence.
Prevalence by PCR identification.
Of the 301 presumptive isolates screened by both PCR assays, 89.0% (268) were identified as E. fergusonii from 31 of 32 farms (96.9%). Isolates were recovered from 90% (9/10), 100% (11/11), and 100% (11/11) of the farms located in the east, north, and south regions, respectively. No significant differences were noted between the regions for the prevalence of E. fergusonii by PCR (P = 0.06). Intrafarm variations in E. fergusonii for a given region were also confirmed by PCR.
Comparison of API 20E and PCR results.
Analysis of the 301 isolates by API 20E (at API 20E ID ≥ 70% accuracy as mentioned above) and our PCR methods detected E. fergusonii at similar rates regardless of sample source (cecal and cloacal) and time (collection 1 and 2). However, the two PCR detection methods proved more accurate than API 20E when identification accuracy was 68% or less (API 20 ID ≤ 68% accuracy). Both the PCR and API (at ID ≥ 98.4% accuracy) methods could effectively discriminate between E. fergusonii and E. coli; below that accuracy level, PCR-positive isolates were obtained. Using the API 20E kit, 23 different profiles with various 2-nitrophenyl-ß-d-galactopyranoside (ONPG), l-arginine (ADH), l-lysine (LDC), l-ornithine (ODC), sodium pyruvate (VP), d-sorbitol (SOR), L-rhamnose (RHA), d-sucrose (SAC), d-melibiose (MEL), and amygdaline (AMY) results were observed (Table 5), 16 of which were associated with isolates identified as E. fergusonii by PCR. Interestingly, 18 of the 301 isolates were identified by API 20E as Kluyvera spp. (ID = 99.3% accuracy); all were also identified as E. fergusonii using the PCR primers designed for this work. However, no amplification products were obtained with Kluyvera ascorbata ATCC 33433. Furthermore, all the putative Kluyvera isolates generated the same colony characteristics as E. fergusonii ATCC and ECD-227 strains (beige colonies on a beige plate) on MacConkey agar and were clearly unlike the reference Kluyvera ATCC strains (pink colonies on pink plates).
TABLE 5.
Biochemical profiles of presumptive E. fergusonii isolates determined by API 20E
| Type of isolate | No. of isolates | API 20E ID % | Biochemical profilec |
Total no. of E. fergusonii isolates by PCRa,b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ONPG | ADH | LDC | ODC | VP | SOR | RHA | SAC | MEL | AMY | ||||
| Control | 1 | E. fergusonii (ATCC) | + | − | + | + | − | − | + | − | − | + | 1 |
| Control | 1 | E. coli 1 (ATCC) | + | − | + | + | − | + | + | − | + | − | 0 |
| Control | 1 | Kluyvera spp. (ATCC)d | + | − | − | + | − | − | + | + | + | + | 0 |
| A | 7 | E. fergusonii 99.8% | + | − | + | + | − | − | − | − | − | + | 7 |
| B | 23 | E. fergusonii 99.6% | − | − | + | + | − | − | + | − | − | + | 23 |
| C | 102 | E. fergusonii 98.9% | + | − | + | + | − | − | + | − | − | + | 102 |
| D | 3 | E. fergusonii 97.4% | − | − | + | + | − | − | − | − | − | + | 3 |
| E | 2 | E. fergusonii 89.2%, Kluyvera spp. 5.8% | + | + | + | + | − | − | + | − | + | + | 2 |
| F | 3 | E. fergusonii 70.8%, E. coli 1 22.5% | + | − | + | + | − | − | + | − | − | − | 3 |
| G | 7 | E. fergusonii 68.2%, Kluyvera spp. 26.4% | + | − | + | + | − | − | − | − | + | + | 7 |
| H | 18 | E. fergusonii 56.2%, Kluyvera spp. 40.6% | + | − | + | + | − | − | + | − | + | + | 18 |
| I | 5 | E. fergusonii 55.9%, Kluyvera spp. 40.4% | + | − | + | + | + | − | + | − | + | + | 5 |
| J | 8 | Kluyvera spp. 42.7%, E. fergusonii 29% | − | − | + | + | − | − | + | + | − | + | 8 |
| K | 61 | E. hermannii 59.5%, E. fergusonii 17.3% | + | − | + | + | − | − | + | + | − | + | 61 |
| L | 2 | E. coli 1 99.9% | + | − | + | − | − | + | + | + | + | − | 0 |
| M | 1 | E. coli 1 99.8% | + | + | + | + | − | + | + | + | + | − | 0 |
| N | 5 | E. coli 1 99.8% | + | − | + | − | − | + | + | − | + | − | 0 |
| O | 3 | E. coli 1 98.9% | + | + | + | + | − | − | + | + | + | − | 0 |
| P | 2 | E. coli 1 98.4% | + | − | + | + | − | + | + | + | + | − | 0 |
| Q | 1 | E. coli 1 98.1% | + | + | + | + | − | − | + | − | + | − | 1 |
| R | 9 | E. coli 1 97.7% | + | − | + | + | − | − | + | − | + | − | 3 |
| S | 3 | E. coli 1 97.2% | + | − | + | − | − | − | + | − | + | − | 0 |
| T | 6 | E. coli 1 96.3% | + | − | + | + | − | − | − | − | + | − | 6 |
| U | 1 | E. coli 1 93.3% | − | − | + | + | − | − | + | − | + | − | 1 |
| V | 11 | Leclercia adecarboxylata 64.2%, E. coli 22.7% | + | − | − | − | − | − | + | − | + | − | 0 |
| W | 18 | Kluyvera spp. 99.3% | + | − | + | + | − | − | + | + | + | + | 18 |
| Total | 304e | 269f | |||||||||||
A total of 301 presumptive isolates were tested by both API 20E and PCR; 140 were identified as E. fergusonii by API 20E at an identification accuracy of ≥70%, and 268 were identified as E. fergusonii by PCR.
There was a significant difference in the detection of E. fergusonii by API 20E versus detection by PCR (P < 0.0001), with a risk factor of 56.57 when using PCR as the detection method.
All samples were negative for sodium thiosulfate (H2S), urea (URE), l-tryptophane (TDA), gelatin (GEL), and inositol (INO); all samples were positive for l-tryptophane (IND), d-glucose (GLU), d-mannitol (MAN), and l-arabinose (ARA).
K. ascorbata ATCC 33433 was the only sample positive for trisodium citrate (CIT) fermentation; all other profiles were negative.
301 presumptive + 3 controls.
268 identified + 1 control.
A total of 33 isolates were identified by API 20E as E. coli (ID ≥ 93% accuracy), among which 5 isolates were positive with the tested primers. All 301 isolates were also screened using the previously characterized UAL1939b and UAL2105b primer set targeting the uidA gene. All 5 of the isolates that were positive using the Efer duplex PCR assays were negative using the uidA primer set regardless of the API 20E profile and accuracy level. All isolates that were identified as E. coli by API 20E but negative by Efer duplex PCR were positive using the uidA primer set (Table 5).
There was agreement between the API 20E kit and PCR assays for 140 E. fergusonii isolates. Therefore, a discrepancy was observed for 128 isolates that were detected as E. fergusonii by PCR but not by API 20E (P < 0.05). The logit estimators of Cochran-Mantel-Haenszel statistics (based on table scores) showed that PCR was approximately 0.8 times more likely to detect E. fergusonii than API 20E (95% confidence limits, 0.7 to 0.9).
DISCUSSION
Although culture-based methods in bacteriology are widely used, they are time-consuming and laborious. Alternative molecular methods targeting nucleic acids have shown great potential in food safety, agricultural, regulatory, public health, and industrial settings (10, 17). Reagents for conventional PCR are less expensive than for real-time PCR; however, the real-time PCR assay provides convenience, as no gel electrophoresis is required, thereby reducing the risk of false positives and contaminations (18). The availability of a duplex assay also provides additional specificity as only one of two genes needs to be available for PCR amplification in order to obtain a positive result. In the duplex conventional PCR assay described in this work, two isolates showed a product (band) for the Efer13 primer alone (EferYP product missing) whereas the EferYP product alone was amplified in four isolates by the real-time PCR assay (Efer41 product missing). The absence of these products was confirmed in a single PCR with either EferYP or Efer41, suggesting a lack of these genes in these isolates or the presence of some unknown inhibitory factors affecting their proper amplification (19). Hence, using duplex assays permitted a higher level of specificity for the detection of E. fergusonii, which is a potential emerging multidrug-resistant pathogen.
To our knowledge, there are currently no molecular methods available for the detection of E. fergusonii. Both the conventional and real-time PCR assays described here are simple methods that could reduce the detection time of E. fergusonii from 6 days to 1. The proposed PCR methods could also allow the simultaneous processing of multiple samples, as up to 96 reactions can be run in one assay, which could be repeated more than once a day. Selective culture on Simmons citrate adonitol media followed by PCR detection can be achieved in 5 days, representing a 1-day reduction of the process compared to methods that rely on the API 20E kit, which require 6 days. In the present study, direct detection of E. fergusonii by PCR in cecal and cloacal DNA yielded some encouraging results. Three of six samples that did not produce positive results by cultivation were positive by PCR. It is possible that E. fergusonii was present in these samples but was unable to compete with other gut bacteria during the enrichment step. E. fergusonii may also be present at nonculturable levels that are detectable only by PCR with DNA extracted directly from cecal and cloacal samples. Consequently, direct DNA extraction provides a method with increased detectability and speed in comparison to cultivation methods. Optimization of this method is under way in our laboratory and will be the subject of a further manuscript.
The present work showed that PCR assays provide improved discrimination over biochemical methods to distinguish E. fergusonii from closely related E. coli and Kluyvera species. Multiple selective and differential medium types were used to first select presumptive isolates. On SCA agar, E. fergusonii is able to utilize adonitol for growth, leading to dark yellow to orange colonies (4). In contrast, E. coli is typically not able to use adonitol or citrate as a primary carbon source and does not grow on this medium (4). K. ascorbata is able to utilize citrate but not adonitol, leading to the formation of white colonies and the development of a blue color in the medium (20). As an additional purification step before testing by API 20E, SMA was used. E. fergusonii does not utilize the sorbitol as a carbon source in SMA, resulting in white/beige colonies and the development of a beige color in the medium. Growth of E. coli on SMA leads to pink colonies (20). Since some K. ascorbata can use sorbitol, traditional MacConkey agar was also used for differentiation from Kluyvera on the basis of lactose fermentation capability, since E. fergusonii does not ferment lactose, in contrast to Kluyvera spp. and most E. coli strains (95% of active and 25% of inactive E. coli) (20). The isolates that were identified as Kluyvera spp. by API 20E (99.3% accuracy) were applied to MacConkey agar along with the E. fergusonii, K. ascorbata, and E. coli ATCC control strains (Table 1). All Kluyvera isolates generated the same colony characteristics as E. fergusonii ATCC and ECD-227 strains (beige colonies on a beige plate) that were obviously different from those of the reference ATCC strains of Kluyvera and E. coli (pink colonies on pink plates). Although the API 20E “W” profile of Table 5 provides a strong result for Kluyvera spp., these samples were found to ferment adonitol and not lactose, which contradicts the previously documented findings for Kluyvera spp. (98%, 95%, and 83%) (20). These observations could partially explain the limited ability to discriminate between E. fergusonii, E. coli, and Kluyvera spp. using biochemical tests such as API 20E.
Most of the variability in the API 20E results occurred with the fermentation profiles of d-melibiose (MEL), amygdaline (AMY), and d-sucrose (SAC). According to the API 20E guidelines, only very small percentages (1%) of E. fergusonii ferment MEL, as shown by the two isolates of API 20E profile E (Table 5). High percentages (99%) of E. fergusonii utilize AMY, for which 98% of the isolates were positive except for the three isolates with API 20E profile F (Table 5). Although it is clearly possible that E. fergusonii can ferment MEL, none of the isolates with API 20E profiles G, H, and I provided positive results. It appears as though a positive MEL result is also coupled to a positive ADH result for which the profiles G, H and I are negative, thereby causing the positive identification of E. fergusonii to be less definitive in these cases. It is well documented that E. fergusonii does not ferment SAC (20). However, SAC fermentation is less informative, as many strains of E. coli and some Salmonella also are not able to ferment this sugar. In the present study, all isolates represented by API 20E profiles J and K were positive for SAC, resulting in split identification (J, 42.7% Kluyvera and 29.0% E. fergusonii; K, 59.0% E. hermannii and 17.3% E. fergusonii). Genetic determinants for SAC fermentation have been found on a plasmid or a mobile genetic element inserted into the chromosomes of E. coli and Salmonella (21–23). The ability to ferment sucrose has also been found to be transferable through conjugation from Salmonella to E. coli and then between other strains of E. coli (24). Thus, it is possible that these genes were transferred to E. fergusonii from Salmonella or E. coli, the species most closely genetically related to this bacterium (1), as reported for other genetic determinants (8). Additionally, it has been suggested that distinct evolutionary rates occur among Escherichia lineages and that E. fergusonii has been subjected to more changes likely due to more frequent genome rearrangements during its evolution than in E. coli (25). In the present study, a negative reaction for sucrose in profiles J and K would lead to E. fergusonii results at 99.6% accuracy (profile B) and 99.8% accuracy (profile A), respectively (Table 5). Interestingly, profile K is the largest category next to profile C (98.9% E. fergusonii). With profiles J and K being considered positive, this would increase the total number of isolates identified by API 20E as E. fergusonii by 23% (to 209 of 301).
Molecular biology methods also support the idea that it may be challenging to provide reliable identifications using biochemical methods. Targeting the uidA gene was suggested to discriminate between E. coli and E. fergusonii (9). In the present study, all isolates were screened for uidA. API 20E profiles Q, R, S, T, and U resulted in high-percentage identifications for E. coli; however, molecular analysis of isolates with these profiles for which amplification was obtained with the Efer primers did not yield any amplification with the uidA primers. The sequencing results supported the medium selection findings in regard to Kluyvera identification. The BLAST analysis of all products from the putative Kluyvera isolate resulted in E. fergusonii as the only result with 100% coverage and an E value equivalent to zero. In addition, the sequences aligned with those from E. fergusonii ATCC 35469 and ECD-227 at 100% and 99.5%. It seems unlikely that Kluyvera species are exactly aligned with E. fergusonii within two different regions of genomic islands previously found to be unique to E. fergusonii (8). It has been reported that confirmation of adonitol and cellulose fermentation is critical for E. fergusonii identification (26). One of our primer sets specifically targeted yliE related to cellulose utilization.
Determining the prevalence of food-borne pathogens such as Salmonella and Campylobacter in poultry production has been a topic of scientific concern for many years. However, attention must be paid to potential emerging pathogens such as E. fergusonii. In a previous study, a virulent multidrug-resistant strain of E. fergusonii ECD-227 was isolated from broiler chickens (8). This finding was important, but a more detailed survey was required to determine the prevalence of this bacterium in broiler production. Both the API 20E and PCR methods developed in the present study showed that E. fergusonii is widespread in broilers from the Fraser Valley. This suggests that E. fergusonii may be a normal member of the broiler microflora, like its closest genetic relative, E. coli. As for E. coli, some strains of E. fergusonii can be pathogenic due to the presence of virulence factors (2, 5, 8). Thus, it would be interesting to establish the pathogenicity potential of E. fergusonii isolates to assess the risk to chicken health and food safety. Such knowledge will provide insight into proactively managing risks associated with the presence of E. fergusonii in chicken production. It will also be important to determine the role of E. fergusonii in the overall dynamic of the broiler gut microflora. The importance of having specific and reliable molecular detection methods for E. fergusonii is evident, and the conventional and real-time PCR assays described in this work could be highly useful in this regard.
In conclusion, the present study revealed that E. fergusonii is widely distributed throughout poultry farms in the Fraser Valley. The role of this bacterium in the chicken gut remains to be established. Due to the potential for opportunistic veterinary and human-pathogenic E. fergusonii strains to emerge as significant pathogens, accurate identification is imperative to facilitate ecological investigation of this species. Rapid and efficient molecular detection is one of the critical strategies deployed in the control of potential pathogens. Detection by PCR can increase specificity while reducing the identification time. The PCR detection methodologies described in this study allowed a clear differentiation between E. fergusonii and genetically related species. These detection methods could be adapted to use in a clinical setting to direct subsequent patient treatment as well as in food safety and environmental assessment studies.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Agriculture and Agri-Food Canada through the Risk Mitigation Strategies and the Sustainable Agriculture Environmental Systems (SAGES) programs. We acknowledge funding support from grant 89758-2010 of the Natural Sciences and Engineering Research Council of Canada to F.M.
We are grateful to the British Columbia Chicken Marketing Board (Abbotsford, British Columbia) and the participating broiler chicken farmers in the Fraser Valley. We also thank N. Obradovic for excellent technical assistance.
Footnotes
Published ahead of print 17 January 2014
This article is contribution 821 from the Pacific Agri-Food Research Centre.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04169-13.
REFERENCES
- 1.Farmer JJ, III, Fanning GR, Davis BR, O'Hara CM, Riddle C, Hickman-Brenner FW, Asbury MA, Lowery MA, III, Brenner DJ. 1985. Escherichia fergusonii and Enterobacter taylorae, two new species of Enterobacteriaceae isolated from clinical specimens. J. Clin. Microbiol. 21:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh J-Y, Kang M-S, An B-K, Shin E-G, Kin M-J, Kwon J-H, Kwon Y-K. 2012. Isolation and epidemiological characterization of heat-labile enterotoxin-producing Escherichia fergusonii from healthy chickens. Vet. Microbiol. 160:170–175. 10.1016/j.vetmic.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 3.Weiss ATA, Lübke-Becker A, Krenz M, van der Grinten E. 2011. Enteritis and septicemia in a horse associated with infection by Escherichia fergusonii. J. Equine Vet. Sci. 31:361–364. 10.1016/j.jevs.2011.01.005 [DOI] [Google Scholar]
- 4.Foster G, Evans J, Tryland M, Hollamby S, MacArthur I, Gordon E, Harley J, Voigt K. 2010. Use of citrate adonitol agar as a selective medium for the isolation of Escherichia fergusonii from a captive reindeer herd. Vet. Microbiol. 144:484–486. 10.1016/j.vetmic.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 5.Rayamajhi N, Cha SB, Shin SW, Jung BY, Lim SK, Yoo HS. 2011. Plasmid typing and resistance profiling of Escherichia fergusonii and other Enterobacteriaceae isolates from South Korean farm animals. Appl. Environ. Microbiol. 77:3163–3166. 10.1128/AEM.02188-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azmuda N, Rahman MZ, Sultana M, Jenssen EL, Khan SI, Birkeland NK. 2012. Evidence of interspecies O antigen gene cluster transfer between Shigella boydii 15 and Escherichia fergusonii. APMIS 120:959–966. 10.1111/j.1600-0463.2012.02926.x [DOI] [PubMed] [Google Scholar]
- 7.Wragg P, La Ragione RM, Best A, Reichel R, Anjum MF, Majura M, Woodwar MJ. 2009. Characterisation of Escherichia fergusonii isolates from farm animals using an Escherichia coli virulence gene array and tissue culture adherence assays. Res. Vet. Sci. 86:27–35. 10.1016/j.rvsc.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 8.Forgetta V, Rempel H, Malouin R, Vaillancourt R, Jr, Topp E, Dewar K, Diarra MS. 2012. Pathogenic and multidrug-resistant Escherichia fergusonii from broiler chicken. Poult. Sci. 91:512–525. 10.3382/ps.2011-01738 [DOI] [PubMed] [Google Scholar]
- 9.Maheux AF, Picard FJ, Boissinot M, Bissonnette L, Paradis S, Bergeron MG. 2009. Analytical comparison of nine PCR primer sets designed to detect the presence of Escherichia coli/Shigella in water samples. Water Res. 43:3019–3028. 10.1016/j.watres.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 10.Wallace PS, MacKay WG. 2013. Quality in the molecular microbiology laboratory. Methods Mol. Biol. 943:49–79. 10.1007/978-1-60327-353-4_3 [DOI] [PubMed] [Google Scholar]
- 11.Fricker M, Messelhäuβer U, Busch U, Scherer S, Ehling-Schulz M. 2007. Diagnostic real-time PCR assays for the detection of emetic Bacillus cereus strains in foods and recent food-borne outbreaks. Appl. Environ. Microbiol. 73:1892–1898. 10.1128/AEM.02219-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diarrassouba F, Diarra MS, Bach S, Delaquis P, Pritchard J, Topp E, Skura BJ. 2007. Antibiotic resistance and virulence genes in commensal Escherichia coli and Salmonella isolates from commercial broiler chicken farms. J. Food Prot. 70:1316–1327 [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre B, Gattuso M, Moisan H, Malouin F, Diarra MS. 2009. Genotype comparison of sorbitol-negative Escherichia coli isolates from healthy broiler chickens from different commercial farms. Poult. Sci. 88:1474–1484. 10.3382/ps.2008-00426 [DOI] [PubMed] [Google Scholar]
- 14.Bonnet C, Diarrassouba F, Brousseau R, Masson L, Diarra MS. 2009. Pathotype and antibiotic resistance gene distribution of Escherichia coli isolates from broiler chickens raised on antimicrobial supplemented diets. Appl. Environ. Microbiol. 75:6955–6962. 10.1128/AEM.00375-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall V, O'Neill GL, Magree JT, Duerden BI. 1999. Development of amplified 16S ribosomal DNA restriction analysis for identification of Actinomyces species and comparison with pyrolysis-mass spectrometry and conventional biochemical tests. J. Clin. Microbiol. 37:2255–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapp C, Moody JA. 1992. Tests to assess bactericidal activity, p. 5.16.1–5.16.20 In Isenberg HD. (ed), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, DC [Google Scholar]
- 17.Rasooly A, Herold KE. 2008. Food microbial pathogen detection and analysis using DNA microarray technologies. Foodborne Pathog. Dis. 5:531–550. 10.1089/fpd.2008.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heid AC, Stevens J, Livak KJ, Williams PM. 1996. Real time quantitative PCR. Genome Res. 6:986–994. 10.1101/gr.6.10.986 [DOI] [PubMed] [Google Scholar]
- 19.Gallup JM. 2011. qPCR inhibition and amplification of difficult templates, p 23–65 In Kennedy S, Oswald N. (ed), PCR troubleshooting and optimization: the essential guide. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 20.Farmer JJ., III 2003. Enterobacteriaceae: introduction and identification, p 636–653 In Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. (ed), Manual of clinical microbiology, 8th ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 21.Palchaudhuri S, Rahn S, Santos DS, Maas WK. 1977. Characterization of plasmids in a sucrose-fermenting strain of Escherichia coli. J. Bacteriol. 130:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archer CT, Kim JF, Jeong H, Park JH, Vickers CE, Lee SY, Nielsen LK. 2011. The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome-scale reconstruction of E. coli. BMC Genomics 12:9. 10.1186/1471-2164-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahreis K, Bentler L, Bockmann J, Hans S, Meyer A, Siepelmeyer J, Lengeler JW. 2002. Adaptation of sucrose metabolism in the Escherichia coli wild-type strain EC3132. J. Bacteriol. 184:5307–5316. 10.1128/JB.184.19.5307-5316.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohlhieter JA, Lazere JR, Snellings NJ, Johnson EM, Synenki RM, Baron LS. 1975. Characterization of transmissible genetic elements from sucrose-fermenting Salmonella strains. J. Bacteriol. 122:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walk ST, Alm EW, Gordon DM, Ram JL, Toranzos GA, Tiedje JM, Whittam TS. 2009. Cryptic lineages of the genus Escherichia. Appl. Environ. Microbiol. 75:6534–6544. 10.1128/AEM.01262-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford-Miksza LK, Himathongkham S, Dodd ML, Badoiu AS, Badoiu OM, Guthertz LS. 2009. Misidentification of a variant biotype of Escherichia coli O157:H7 as Escherichia fergusonii by Vitek 2 compact. J. Clin. Microbiol. 47:872-873. 10.1128/JCM.02425-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.