Abstract
Robust CD8+ T cell responses are essential for immune protection against intracellular pathogens. Using parenteral administration of ovalbumin (OVA) protein as a model antigen, the effect of the Toll-like receptor 9 (TLR9) agonist, CpG oligodeoxynucleotide (ODN) 1826, as an adjuvant delivered either topically, subcutaneously, or intramuscularly on antigen-specific CD8+ T cell responses in a mouse model was evaluated. Topical CpG adjuvant increased the frequency of OVA-specific CD8+ T cells in the peripheral blood and in the spleen. The more effective strategy to administer topical CpG adjuvant to enhance CD8+ T cell responses was single-dose administration at the time of antigen injection with a prime-boost regimen. Topical CpG adjuvant conferred both rapid and long-lasting protection against systemic challenge with recombinant Listeria monocytogenes expressing the cytotoxic T lymphocyte (CTL) epitope of OVA257–264 (strain Lm-OVA) in a TLR9-dependent manner. Topical CpG adjuvant induced a higher proportion of CD8+ effector memory T cells than parenteral administration of the adjuvant. Although traditional vaccination strategies involve coformulation of antigen and adjuvant, split administration using topical adjuvant is effective and has advantages of safety and flexibility. Split administration of topical CpG ODN 1826 with parenteral protein antigen is superior to other administration strategies in enhancing both acute and memory protective CD8+ T cell immune responses to subcutaneous protein vaccines. This vaccination strategy induces rapid and persistent protective immune responses against the intracellular organism L. monocytogenes.
INTRODUCTION
Infectious diseases are the leading cause of illness in humans. Immunization is the most cost-effective method to improve population health against infectious diseases. However, current vaccines remain inefficient in part due to a poor ability to elicit cytotoxic T lymphocytes (CTLs), delayed protection, and lack of long-lasting protective effect. An adjuvant is often added to increase the immunogenicity of vaccine antigens. Adjuvants are routinely combined with antigens for efficacy and ease of formulation (1). Split administration, whereby vaccine antigen and adjuvant are delivered separately, has been less commonly studied (2) but has the advantage of flexibility and simplicity. Here, we explore split administration of antigen and adjuvant using the skin as an administration site and a Toll-like receptor 9 (TLR9) agonist as the vaccine adjuvant.
CpG oligodeoxynucleotides (ODNs) bind TLR9, an endosomal pattern recognition receptor that recognizes unmethylated bacterial and viral ODNs with CpG motifs (3). CpG ODNs induce both potent CD8+ T cells and Th1-biased CD4+ T cell immune responses in both mouse and humans (4, 5). The enhanced immune responses induced by CpG ODNs provide protective immunity in several infectious disease models. For example, reduced bacterial burden was detected in mice immunized with CpG ODN and ovalbumin (OVA) coadministered intramuscularly when challenged with Listeria monocytogenes expressing the CTL epitope of OVA257–264 (designated Lm-OVA) 10 days postimmunization (6). Also, intramuscular coinjection of recombinant Toxoplasma gondii protein with CpG ODN as an adjuvant induces a Th1-biased humoral response demonstrated by an increased IgG2a to IgG1 antibody ratio and increased protection against oral T. gondii infection in a mouse model (7). In a human clinical trial, intramuscular delivery of CpG 7909 induced robust specific antibody response to a commercial hepatitis B vaccine (Engerix-B) (8). Although studies have demonstrated the effectiveness of CpG ODNs as adjuvants, concerns remain about the local and systemic side effects observed. In mice, CpG ODNs can induce tumor necrosis factor alpha (TNF-α) release by macrophages, resulting in septic shock (9, 10). Strategies to limit the systemic toxicity of CpG ODNs include conjugation of CpG ODN to antigen (11) and coencapsulation of CpG ODN with antigen (12). These strategies are cumbersome and require validation in humans. Split administration of CpG ODN as an adjuvant and as a means of enhancing vaccine efficacy while limiting toxicity has not been pursued.
The skin is the most accessible organ of our bodies and harbors many immune cells including different subsets of dendritic cells (DCs) (13), mast cells (14), and resident lymphocytes (15–17) that can be harnessed to induce immune responses. We, and others, have explored the skin as a site of vaccination by coadministration of antigen and adjuvant. Topical peptide vaccination with cholera toxin induces robust cellular immune responses in mice (18). However, the toxicity of cholera toxin makes it difficult to handle. Topical CpG ODN coadministered with topical antigen promotes CD8+ T cell production to the antigen (19). Topical administration of CpG ODN also promotes cross-presentation of injected soluble OVA protein antigen with less systemic cytokine production and toxicity than subcutaneous administration (20). When topical CpG ODN is administered epifocally to the antigen administration site, meaning that it is applied to skin that has the same lymphatic drainage as the site to which antigen is given, the CpG adjuvant augments CD8+ T cell responses against melanoma in a mouse model (21). CpG has an adjuvant effect only with epifocal administration and not when it is applied to a site contralateral to the antigen. This demonstrates the ability of splitting antigen and vaccine antigen administration and emphasizes the need for common lymph drainage of antigen and adjuvant for maximum effect (21). The requirement of epifocal administration of adjuvant and antigen suggests that topical CpG ODN instructs antigen-specific T cell generation in the skin draining lymph nodes (SLNs). Here, we sought to examine various strategies to administer CpG ODN 1826 onto the skin to enhance CD8+ T cell responses. We demonstrate that single topical CpG ODN administration at the time of standard (parenteral) immunization is effective, preferentially induces effector memory T cells, and may be used to induce protective immunity against the intracellular pathogen L. monocytogenes.
MATERIALS AND METHODS
Animals.
C57BL/6 female mice 6 to 8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA) and housed in a specific-pathogen-free animal care facility at the Child & Family Research Institute (CFRI) (Vancouver, Canada). TLR9-deficient mice on C57BL/6 background (TLR9 knockout [KO]) were obtained from Oriental Bioservice (Tokyo, Japan) and bred and maintained in the animal care facility at CFRI. Animal experiments were conducted in accordance with protocols approved by the Animal Care Committee of The University of British Columbia and Canadian Council of Animal Care.
Deoxynucleotides and peptide.
Synthetic high-pressure liquid chromatography (HPLC)-purified, single-stranded, phosphorothioated CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was purchased from Sigma-Aldrich Inc. (St. Louis, MO). Control ODN without CpG motifs (5′-TCCAGGACTTCTCTCAGGTT-3′) was purchased from Integrated DNA Technologies, Inc. (San Diego, CA). Lyophilized CpG ODN 1826 and control ODN were reconstituted to 5 mg/ml with phosphate-buffered saline (PBS)–dimethyl sulfoxide (DMSO) (1:1, vol/vol). The immunodominant Kb-restricted OVA8 peptide (OVA257–264, amino acid sequence SIINFEKL) was synthesized by Kinexus (Vancouver, BC).
Immunization.
C57BL/6 or TLR9 KO female mice 6 to 12 weeks of age were anesthetized by intraperitoneal injection of 75 mg ketamine/kg of body weight (Ketalean; Bimeda-MTC Animal Health Inc., Cambridge, ON) and 7.5 mg/kg xylazine (Rompun; Bayer Health Care Inc., Toronto, ON). Mice were shaved on the dorsal back, tape-stripped 15 times using cellophane tape (Staples, Vancouver, BC), and the skin was wiped with acetone (Fisher Scientific, Edmonton, AB) using a cotton swab. One hundred micrograms of chicken ovalbumin protein grade V (OVA) from Sigma-Aldrich Inc. (St. Louis, MO) was injected either subcutaneously or intramuscularly. Next, 250 μg or 50 μg of CpG ODN 1826 was administered topically, subcutaneously, or intramuscularly in an epifocal manner (overlaying the antigen injection site). PBS-DMSO (1:1, vol/vol) or control ODN was applied when no adjuvant was administered. The area was then covered with waterproof tape to prevent oral ingestion of the topical adjuvant or control. When antigen and adjuvant (or control) were administered by the same route, they were given together in a single injection. For a prime-boost regimen, mice were immunized on days 0 and 7, while mice were immunized once on day 0 for a one-time immunization regimen. Multiple applications of the adjuvant CpG ODN 1826 were administered at the time of antigen administration and again at 1 and 2 days (for 3 consecutive days) postimmunization: days 0, 1, and 2 for a one-time immunization regimen and days 0, 1, 2, 7, 8, and 9 for a prime-boost regimen.
Bacterial strains, medium, and growth conditions.
Wild-type L. monocytogenes strain 10403s and strain Lm-OVA, which was modified from the wild type to express the CTL epitope of OVA257–264, were provided by H. Shen (University of Pennsylvania, Philadelphia). The construction of the less virulent form, strain ΔactA-Lm-OVA, with a targeted deletion in the virulence determinant ActA has been described elsewhere (22). For immunization and infection experiments, frozen infection aliquots of L. monocytogenes strains were prepared as described previously (23). Briefly, L. monocytogenes strains were grown in brain heart infusion (BHI) broth to mid-logarithmic phase (optical density at 600 nm [OD600], 1.0) at 37°C, washed twice with endotoxin-free, isotonic saline (0.9% NaCl), resuspended in 0.9% NaCl with 20% glycerol (vol/vol), and stored at −80°C until use.
Immunizing infection and animal challenge with L. monocytogenes.
Mice were infected with 5 × 105 CFU of strain Lm-OVA in 100 μl 0.9% NaCl systemically via the tail vein. Three days after infection, mice were euthanized to collect the spleen, which was then homogenized mechanically in 5 ml 0.9% NaCl containing 0.05% Triton X-100. To enumerate the viable bacteria colonizing the spleen postinfection, serial dilutions of the mechanically lysed cell suspensions were plated on BHI agar. For immunizing infection, mice were intraperitoneally injected with 2 × 106 CFU of ΔactA-Lm-OVA.
Flow cytometry antibodies and tetramer.
Antibodies B220-PerCP (clone RA3-6B2), CD8-APC (clone 53-6.7), and gamma interferon (IFN-γ)-APC (clone XMG1.2) were purchased from BD Biosciences Inc. (Mississauga, ON, Canada). Antibodies CD62L-FITC (clone MEL-14) and CD127-APC (clone A7R34) were purchased from eBioscience Inc. (San Diego, CA), whereas CD8-A700 (clone 53.67) was purchased from the AbLab at The University of British Columbia (Vancouver, BC, Canada). Phycoerythrin (PE)-conjugated OVA major histocompatibility complex class I (MHC-I) tetramer specific for the Kb-restricted OVA8 peptide (OVA257–264, amino acid sequence SIINFEKL) was generated by conjugation of biotinylated monomers to streptavidin-PE in R. Tan's laboratory (Child & Family Research Institute, Vancouver, BC, Canada). The anti-Fc receptor monoclonal antibody (clone 2.4G2) was obtained from American Type Culture Collection (Rockville, MD). OVA-specific CD8+ T cells were identified as a B220-negative and CD8 and Kb-OVA MHC-I tetramer double-positive population. Flow cytometry data were acquired using the BD LSRII Flow Cytometer from BD Biosciences Inc. (Mississauga, ON) with the BD FACSDiva software (version 6.0).
Quantification of OVA-specific effector and memory CD8+ T cells.
Spleen and blood were collected from mice. Single-cell suspensions were prepared and were subjected to osmotic lysis to remove erythrocytes. Cells were then incubated with anti-Fc receptor monoclonal antibody to block Fc-binding sites. The B220-negative population was analyzed to detect OVA-specific CTLs ex vivo using Kb-OVA MHC-I tetramers with the B220 and CD8 cell surface marker. OVA-specific memory CD8+ T cells were classified by the cell surface markers CD127 and CD62L. Staining was performed at 4°C for 45 min. Flow cytometry data were analyzed by the FlowJo flow cytometry analysis software for Macintosh (version 8.8.2; Tree Star, Inc., Ashland, OR).
Intracellular cytokine staining.
The ability of the OVA-specific CTL population to produce IFN-γ was determined by restimulating the cells with OVA8 peptide ex vivo at 37°C for 4 h. Cells were then incubated with anti-Fc receptor monoclonal antibody to block Fc-binding sites. Surface marker staining was performed at 4°C for 30 min. Cells were fixed and permeabilized with fixation and permeabilization buffers, respectively, from eBioscience Inc. (San Diego, CA). Intracellular cytokine staining was performed subsequently at room temperature for 30 min.
Statistical analysis.
All quantitative data are presented as means ± standard errors of the means (SEM). Statistical analyses were performed using Prism 4 for Macintosh (version 4.0b or 5.0e). The D'Agostino and Pearson omnibus normality test was used to determine whether the data followed a normal distribution. Data that followed a normal distribution were analyzed by unpaired two-tailed Student's t test (comparison between two groups) or one-way analysis of variance (ANOVA) test followed by Tukey's multiple-comparison posttest (three or more groups). Data that did not follow normal distribution were analyzed by the Mann-Whitney test (two groups) or by the Kruskal-Wallis test followed by Dunn's multiple-comparison posttest (three or more groups). P values of <0.05 were considered to be statistically significant.
RESULTS
Topical CpG ODN 1826 as adjuvant for subcutaneous protein vaccination enhances CD8+ T cell response.
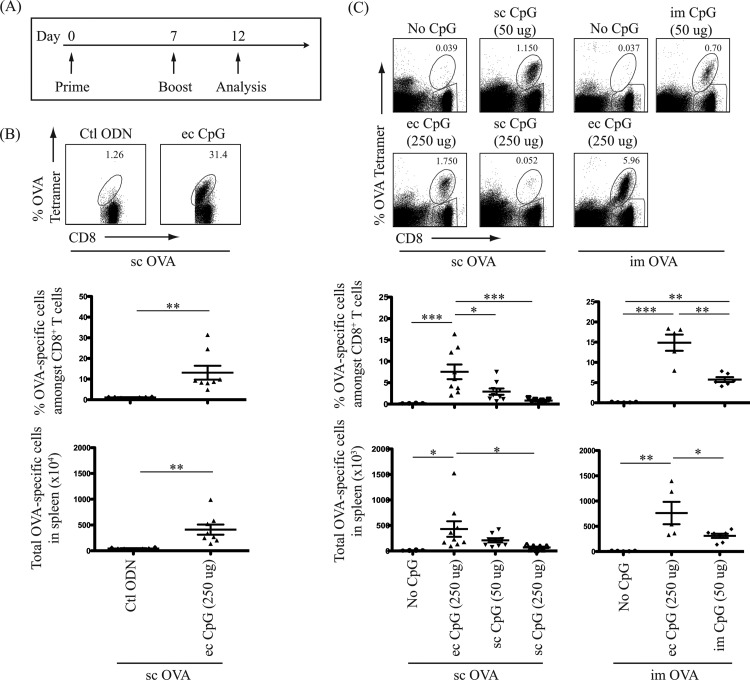
Using CpG ODN 1826 as an adjuvant (CpG adjuvant) to protein-based antigen (OVA), we sought to compare its effects on the generation of antigen-specific CD8+ T cells through different routes of administration. Since a majority of current licensed protein or inactivated-pathogen vaccines require booster doses, three routes of CpG adjuvant administration were tested with a prime-boost immunization regimen (Fig. 1A): topical or subcutaneous route with subcutaneous OVA protein, and topical or intramuscular route with intramuscular OVA protein. Frequencies of OVA-specific CTLs were determined 5 days postimmunization (after boost), a time point that corresponds to a detectable peak in CTL numbers postimmunization (24, 25). Whether the enhanced production of OVA-specific CD8+ T cells with topical CpG adjuvant was CpG motif specific was first determined. Immunization with the control ODN (ODN 1982) without CpG motifs did not enhance OVA-specific CTL generation (Fig. 1B). With subcutaneous OVA protein antigen, we then demonstrated that topical CpG adjuvant increased the proportion of OVA-specific cells among CD8+ T cells. A dose-response curve was performed to determine the optimal subcutaneous and topical doses of CpG adjuvant. A 50 μg subcutaneous dose of CpG adjuvant was optimal in inducing antigen-specific CD8+ T cells (data not shown), while 250 μg was used as a comparative optimal topical CpG adjuvant dose. Topical delivery of CpG adjuvant with subcutaneous OVA protein antigen induced 2.5-fold- and 9-fold-higher proportions of these T cells in the spleen than subcutaneous delivery of 50 μg and 250 μg of CpG adjuvant, respectively (Fig. 1C). When OVA protein was injected intramuscularly, addition of topical CpG adjuvant also increased the proportion of OVA-specific CD8+ T cells 3-fold compared to intramuscular delivery of the adjuvant (Fig. 1C). The same trend was observed when the absolute numbers of OVA-specific CD8+ T cells in the spleen were determined for each immunization group (Fig. 1B and C). These results support the speculation that the topical route is a better route to administer CpG adjuvant to enhance CTL responses than parenteral routes. Data also confirmed that the adjuvant effect of CpG ODN 1826 is CpG motif specific.
FIG 1.
Topical administration of CpG adjuvant is more effective in antigen-specific effector CD8+ T cell generation. One hundred micrograms of OVA protein antigen was injected subcutaneously (sc) or intramuscularly (im) in combination with CpG adjuvant administered topically (ec), sc, or im as indicated. (A) Schematic diagram of prime-boost immunization regimen and T cell analysis timeline. (B, C) Top panels show representative flow cytometric dot plots in log axis scale identifying OVA-specific CD8+ T cells in the spleen. Cells were gated on a B220-negative population, and OVA-specific CD8+ T cells were identified as CD8 and Kb-OVA class I tetramer double-positive population. Bottom panels show scatter plots of the percentage of OVA-specific cells among CD8+ T cells and total OVA-specific cells detected in the spleen 5 days postimmunization (after boost). Animals labeled “No CpG” or “Ctl ODN” received antigen and had skin treatment of tape-stripping and acetone (topical placebo treatment) without CpG or with control deoxynucleotide (ODN 1982), respectively. Animals treated with “sc CpG” did not receive any skin treatment. In parallel, naive mice that did not receive antigen or skin treatment were analyzed. Data summarize at least two independent experiments (n = 6 to 9) and are expressed in means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
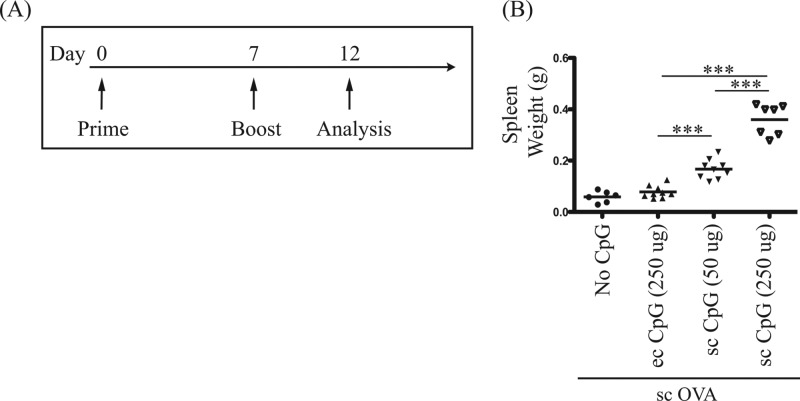
Toxicities caused by parenteral (intraperitoneal) CpG ODNs include follicle microarchitecture disruption as well as splenomegaly due to erythroid and myeloid expansion in the red pulp of the spleen (26, 27). Thus, the spleens of immunized and untreated (naive) mice were weighed as an indirect method to evaluate potential toxicity of CpG adjuvant administered via the three routes mentioned above. In contrast to subcutaneous injection of 50 μg and 250 μg of CpG adjuvant, which increased spleen weight 3- and 7-fold, respectively, no significant increase of spleen weight was detected in mice treated with topical CpG adjuvant (Fig. 2). Overall, topical delivery of CpG ODN 1826 as adjuvant was more effective and less toxic than parenteral administration when given at the same time as parenteral protein-based vaccine, by either the subcutaneous or the intramuscular route.
FIG 2.
Topical administration is less toxic than subcutaneous administration of CpG adjuvant. One hundred micrograms of OVA protein antigen was injected subcutaneously (sc) with CpG adjuvant administered topically (ec) or subcutaneously (sc) as indicated. (A) Schematic diagram of prime-boost immunization regimen and T cell analysis timeline. (B) Toxicity analysis by spleen weight. Scatter plot of spleen weights measured 5 days postimmunization (after boost). Data summarize at least two independent experiments (n = 6 to 9) and are expressed in means ± SEM. ***, P < 0.001.
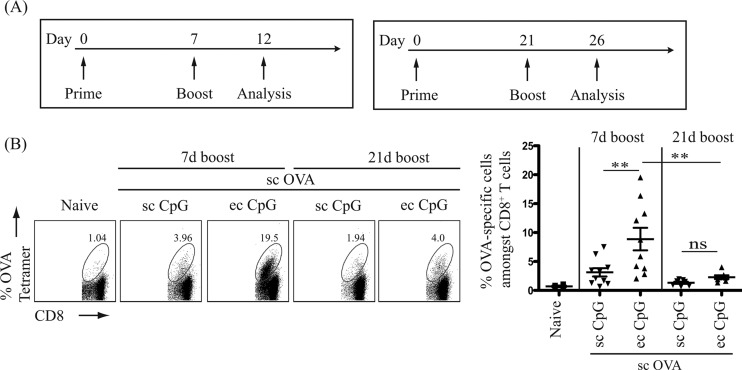
A rapid (7-day) prime-boost immunization regimen can be used to enhance CTL responses with topical CpG ODN 1826.
Proper spacing between doses of a given vaccine series is essential for optimal immune responses. A shorter interval between vaccine booster doses would be more practical. We thus tested a 7-day (common in murine cancer vaccine protocols) and a 21-day (standard timing for antibody production) prime-boost immunization schedule for the optimal induction of antigen-specific CD8+ T cells (Fig. 3A). Percentages of antigen-specific cells among CD8+ T cells were determined by tetramer staining 5 days postimmunization (after boost). In the spleen, a 3-fold-higher proportion of OVA-specific CD8+ T cells was detected when CpG adjuvant was administered topically than subcutaneously (Fig. 3B) using the 7-day-interval prime-boost immunization regimen. In addition, the prime-boost immunization regimen with a 7-day interval between the initial and the booster dose induced a higher level of OVA-specific CD8+ T cells than with a 21-day interval (Fig. 3B). Hence, the short-interval (7 days apart) prime-boost immunization regimen was more effective than the long-interval (21 days apart) regimen in inducing antigen-specific CD8+ CTLs.
FIG 3.
Optimal intervals between doses of a vaccine series for the rapid generation of antigen-specific CD8+ T cells. One hundred micrograms of OVA protein antigen was injected subcutaneously (sc) with CpG adjuvant administered topically (ec, 250 μg) or subcutaneously (sc, 50 μg). (A) Schematic diagrams of prime-boost immunization with interval of 7 days or 21 days and T cell analysis timeline. (B) Left panels show representative flow cytometric dot plots in log axis scale identifying OVA-specific cells CD8+ T cells in the spleen. Cells were gated as described for Fig. 1. Right panel shows a scatter plot of the percentage of OVA-specific cells among CD8+ T cells detected in the spleen. Data summarize at least two independent experiments (n = 4 to 10) and are expressed in means ± SEM. **, P < 0.01; ns, no significance.
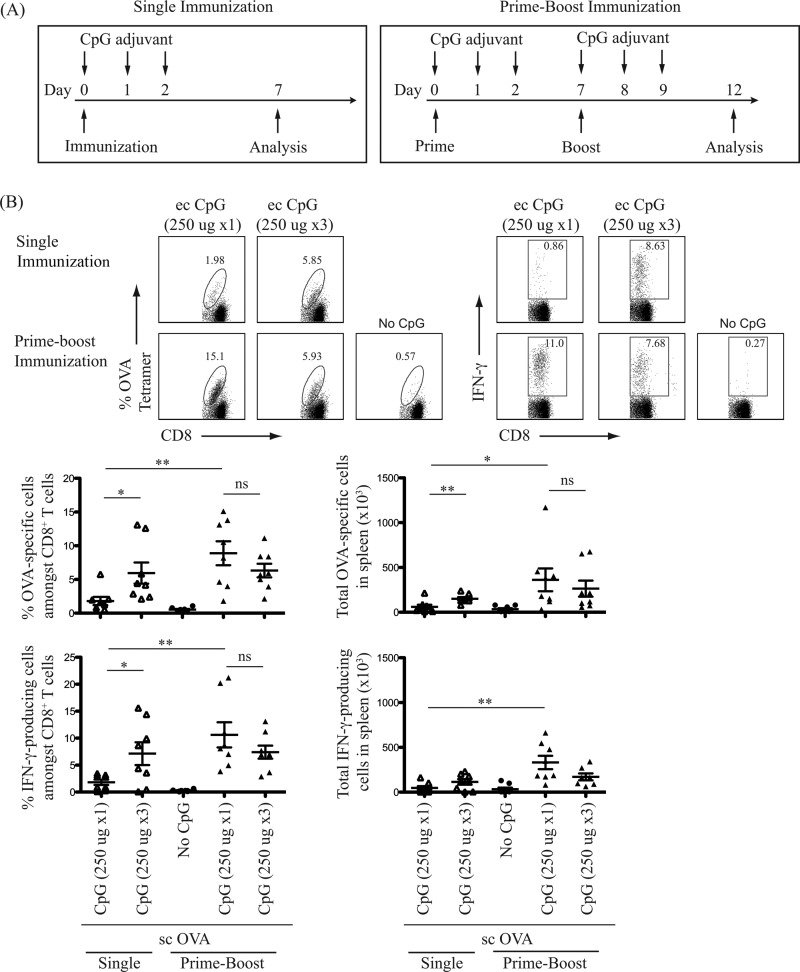
A single application of topical CpG ODN 1826 at the time of protein-based vaccine administration is sufficient to enhance antigen-specific CTL response.
TLR stimulation commonly results in short-term stimulation of innate immunity. Consistent with this concept, repeated daily topical administration of TLR7 agonist has been shown to further improve CD8+ T cell induction following immunization with protein antigen (28). Thus, whether daily multiple doses of topical CpG ODN further enhance the adjuvant effect with subcutaneous protein-based vaccines was investigated. Whether a regimen of single immunization induces frequencies of antigen-specific CD8+ T cells comparable to those of a prime-boost regimen was also investigated. OVA protein was injected subcutaneously with a one-time application of topical CpG adjuvant or a three-time application on 3 consecutive days (Fig. 4A). Using flow cytometry, the frequencies of OVA-specific CD8+ T cells and IFN-γ-producing CTLs were determined. A booster dose at 7 days increased the pool of OVA-specific and IFN-γ-producing CD8+ T cells as detected in the spleen 5 days postimmunization compared to the single immunization (Fig. 4B). Multiple doses of CpG adjuvant administered over 3 consecutive days did not further enhance the generation of OVA-specific or IFN-γ-producing CTLs over single dose (Fig. 4B). The frequency of IFN-γ-producing CD8+ T cells paralleled that of OVA-specific CTLs. Thus, a single topical application of CpG adjuvant at the time of subcutaneous protein antigen administration with a prime-boost regimen, 7 days apart, is effective for the induction of functional antigen-specific CTLs.
FIG 4.
Better immunization strategy using topical CpG ODN as vaccine adjuvant to elicit acute CD8+ T cell responses. (A) Schematic diagrams of single and prime-boost immunization regimens and T cell analysis timelines. One hundred micrograms of OVA protein antigen was injected subcutaneously (sc) with or without topical (ec) CpG adjuvant. Arrows indicate three consecutive days of topical CpG adjuvant administration (250 μg per administration). (B) Top panels show representative flow cytometric dot plots identifying OVA-specific CD8+ T cells and IFN-γ-producing cells in the spleen after restimulation with OVA8 peptide ex vivo. Bottom panels show scatter plots of the percentage and the total number of OVA-specific CD8+ T cells and IFN-γ-producing cells detected in the spleen on day 7 or day 12 post-initial immunization (after prime) using a single or a prime-boost immunization regimen, respectively. Data summarize two independent experiments (n = 8) and are expressed as means ± SEM. *, P < 0.05; **, P < 0.01; ns, no significance.
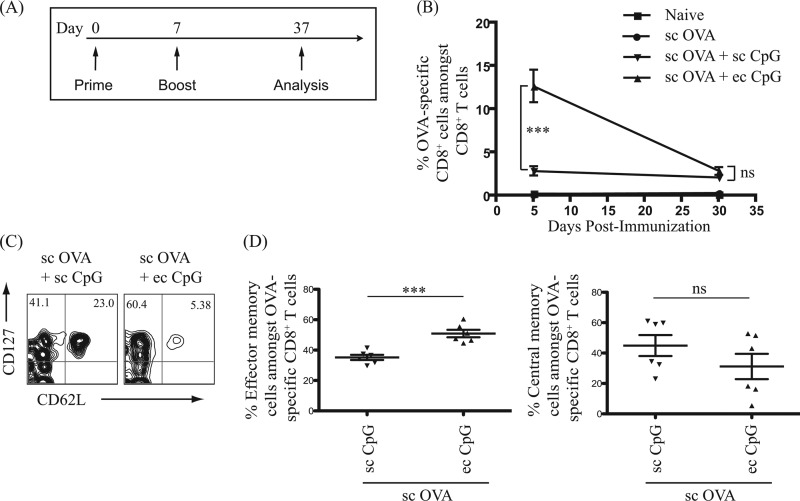
Topical administration of CpG ODN 1826 induces a higher proportion of antigen-specific CD8+ effector memory T cells than the subcutaneous route.
Effector T cells undergo contraction and differentiate into memory T cells. Memory T cells provide sustained protection against invading pathogens in a faster and more effective manner than primary effector T cells (29). The ability of topical and subcutaneous CpG adjuvant to augment the generation of antigen-specific CD8+ memory T cells was thus determined. Mice were immunized using the prime-boost immunization regimen with OVA protein administered subcutaneously with CpG adjuvant administered either topically or subcutaneously. The levels of circulating OVA-specific CD8+ effector T cells (5 days after boost) and long-term memory T cells (30 days after boost) in peripheral blood were determined postimmunization (Fig. 5A). Topical administration of CpG adjuvant robustly increased the frequency of OVA-specific CD8+ effector T cells detected in the peripheral blood up to 6-fold, compared to subcutaneous administration, at 5 days postimmunization (after boost) (Fig. 5B). At 30 days postimmunization (after boost), no significant difference in the frequency of CD8+ memory T cells in the blood of mice immunized with topical and subcutaneous adjuvant was observed (Fig. 5B).
FIG 5.
Topical administration of CpG adjuvant induced a higher proportion of antigen-specific CD8+ T effector memory cells than did subcutaneous administration. One hundred micrograms of OVA protein antigen was injected subcutaneously (sc) with CpG adjuvant administered subcutaneously (sc, 50 μg) or topically (ec, 250 μg). (A) Schematic diagram of immunization regimen and T cell analysis timeline. (B) Point graph of the percentage of OVA-specific cells among CD8+ T cells detected in peripheral blood 5 days and 30 days postimmunization (after boost). Data summarize two independent experiments (n = 3 to 6). (C) Representative flow cytometric density plots in log axis scale of CD8+ T effector memory and central-memory cells in the peripheral blood 30 days postimmunization (after boost). (D) Left panel and right panel show a scatter plot of the percentage of CD127+CD62L− effector memory cells and CD127+CD62L+ central-memory cells among OVA-specific CD8+ T cells detected in the peripheral blood 30 days postimmunization (after boost), respectively. Data represent two independent experiments (n = 6) and are expressed in means ± SEM. ***, P < 0.001; ns, no significance.
Despite similar proportions of the memory T cells, does topical CpG adjuvant change the phenotype of the memory T cells? There are two major subsets of memory T cells: central memory and effector memory T cells (30), (31). Central memory T cells are CD127+CD62L+, have a higher proliferative potential than naive cells, and preferentially reside in lymphoid organs. Effector memory T cells are CD127+CD62L−, have a faster effector function, and preferentially reside in nonlymphoid (stromal) tissues. To further characterize the OVA-specific memory CD8+ T cells induced by immunization with CpG adjuvant administered topically or subcutaneously, the surface markers CD127 and CD62L were used to differentiate effector memory from central memory T cells (Fig. 5C). Interestingly, the population of CD127+CD62L− OVA-specific effector memory T cells in peripheral blood was 1.5-fold higher upon immunization in the presence of CpG adjuvant administered topically than subcutaneously (Fig. 5D). Also, a trend of a lower proportion of CD127+CD62L+ OVA-specific central memory T cells was detected with topical CpG adjuvant than with subcutaneous administration (Fig. 5D). Thus, immunization in the presence of CpG adjuvant (either subcutaneously or topically) induces long-lived antigen-specific memory CD8+ T cells. In addition, topical delivery of CpG adjuvant induces a higher proportion of antigen-specific effector memory CD8+ T cells than does subcutaneous delivery.
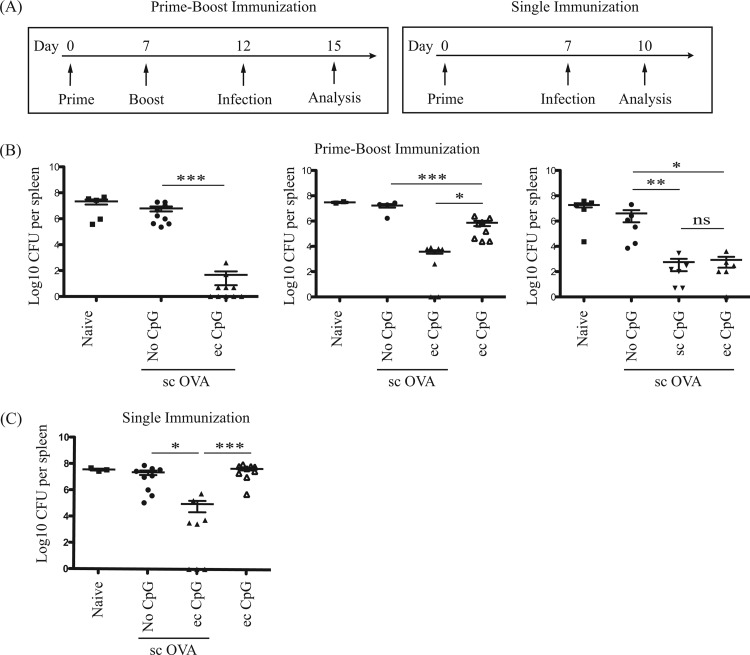
Topical CpG ODN 1826 confers rapid TLR9-dependent protection against systemic L. monocytogenes infection.
Topical CpG adjuvant induced rapid and lasting CD8+ T cell responses to subcutaneous antigen. To determine whether these responses were adequate to protect against infection, we tested the ability of this vaccination method to protect against Listeria monocytogenes, an intracellular bacterium predominantly controlled by host CD8+ T cell responses (32, 33). The prime-boost and the one-time immunization regimens were tested for their ability to protect animals from systemic L. monocytogenes challenge. Mice were immunized with OVA antigen subcutaneously with or without topical CpG adjuvant and then challenged with mutant strain Lm-OVA 5 or 7 days postimmunization (Fig. 6A). A 5-log decrease of bacterial load was noted in the spleen following a prime-boost immunization regimen with topical CpG adjuvant compared to immunization without adjuvant (Fig. 6B). Immunization with CpG adjuvant alone (i.e., without antigen) reduced bacterial burden. However, this was not as effective as immunization with antigen and adjuvant (Fig. 6B). Thus, repeated innate stimulation provides a modicum of rapid protection that is improved upon with antigen vaccination. Unexpectedly, topical administration of CpG adjuvant did not further decrease bacterial load in the spleen compared to subcutaneous administration after acute systemic Lm-OVA challenge (Fig. 6B). In addition, a 3-log decrease of bacterial burden in the spleen was detected in mice immunized with topical CpG adjuvant compared to those immunized without adjuvant in the one-time immunization regimen (Fig. 6C). Thus, immunization using topical or subcutaneous CpG adjuvant with subcutaneous protein antigen can provide protection against L. monocytogenes infections, which is not observed with immunization with antigen alone. Although a prime-boost immunization regimen provides optimal protection against L. monocytogenes, a one-time immunization strategy can confer protection as well.
FIG 6.
Acute protection against systemic L. monocytogenes infection induced by immunization with topical or subcutaneous CpG adjuvant. Naive mice, mice treated with topical (ec, 250 μg) CpG adjuvant, and mice immunized with 100 μg OVA protein antigen subcutaneously (sc, 50 μg) with or without topical (ec, 250 μg) CpG adjuvant were challenged with 5 × 105 CFU Lm-OVA intravenously. (A) Schematic diagram of immunization, infection, and bacterial burden analysis timelines. (B, C) Mice were immunized with a prime-boost or a single-immunization regimen. Mice were then challenged with Lm-OVA at the time points indicated. Scatter dot plots in log axis scale of bacterial counts in the spleen 3 days postinfection. Data summarize at least two independent experiments (n = 2 to 10) and are expressed in means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significance.
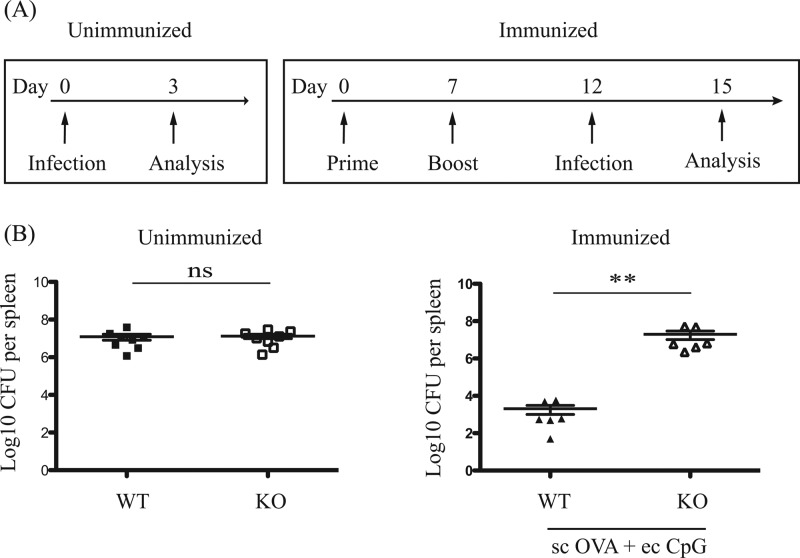
The uptake of CpG ODNs by cells is CpG motif independent, and CpG ODNs have been reported to induce TLR9-independent immune effects (34, 35). To confirm that the adjuvant effect of CpG ODN 1826 on L. monocytogenes bacterial burden was TLR9 dependent, we compared the bacterial load 3 days postinfection by Lm-OVA between immunized wild-type (WT) and TLR9-deficient (KO) mice (Fig. 7A). Bacterial loads in WT and KO mice following Lm-OVA infection were identical (Fig. 7B, left panel), suggesting similar susceptibility to infection. Unlike WT animals with a 4-log decrease in bacterial load in the spleen, bacterial load was not reduced in the KO mice (Fig. 7B, right panel), demonstrating TLR9 dependency of the topical CpG adjuvant effect.
FIG 7.
Acute protection against systemic L. monocytogenes infection induced by immunization with topical or subcutaneous CpG adjuvant. Wild-type (WT) and TLR9-deficient (KO) mice were unimmunized or immunized with 100 μg OVA protein subcutaneously (sc) with topical (ec, 250 μg) CpG adjuvant. All mice were challenged with 5 × 105 CFU Lm-OVA intravenously. (A) Schematic diagram of immunization, infection, and bacterial burden analysis timelines. (B) Scatter dot plots in log axis scale of bacterial counts in the spleen 3 days postinfection. Data summarize two independent experiments (n = 6 to 8) and are expressed as means ± SEM. **, P < 0.01; ns, no significance.
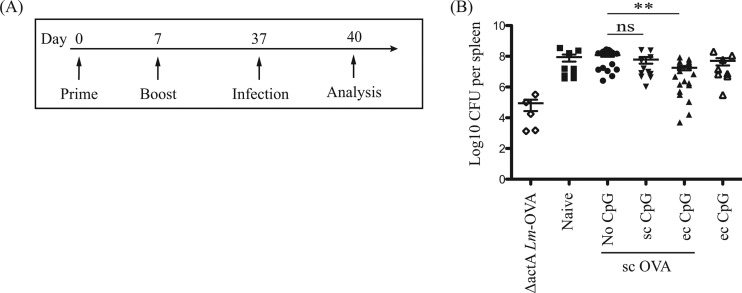
Topical CpG ODN 1826 improves long-term protection against systemic L. monocytogenes infection.
Topical CpG adjuvant induced persistent CTL responses to subcutaneously administered protein vaccine. Hence, we assessed the ability of our proposed prime-boost immunization strategy with protein antigen injected subcutaneously and topical CpG ODN 1826 as adjuvant to provide long-term protective immunity against intracellular bacterial infection (Fig. 8A). Mice immunized in the presence of CpG adjuvant administered topically but not subcutaneously showed a modest but significant decrease in bacterial burden (1-log reduction) (Fig. 8B). An attenuated strain, ΔactA-Lm-OVA, was used as a live vaccine to serve as a positive control (22). Thus, our proposed prime-boost immunization strategy with soluble protein antigen and topical CpG adjuvant but not subcutaneous adjuvant is able to confer long-term protection against systemic L. monocytogenes infection. In our experiments, topical adjuvant use at the time of protein immunization approximated the protective effect of a live vaccine.
FIG 8.
Long-term protection against systemic L. monocytogenes infection induced by immunization with topical or subcutaneous CpG adjuvant. Naive mice, mice treated with topical (ec, 250 μg) CpG adjuvant, and mice immunized with 100 μg OVA protein antigen subcutaneously (sc) without or with topical (ec, 250 μg) or subcutaneous (sc, 50 μg) CpG adjuvant were challenged with 5 × 105 CFU Lm-OVA intravenously 30 days postimmunization (after boost). Mice immunized with ΔactA-Lm-OVA intraperitoneally were analyzed in parallel as positive controls. (A) Schematic diagram of immunization, infection, and bacterial burden analysis timeline. (B) Scatter plots in log axis scale of bacterial counts in the spleen 3 days postinfection. Data summarize at least two independent experiments (n = 5 to 19) and are expressed in means ± SEM. **, P < 0.01; ns, no significance.
DISCUSSION
Using the skin as a site of adjuvant administration, we demonstrate that a TLR9 agonist may be applied onto the skin to enhance CTL responses to locally administered protein antigen and to provide protective immunity to the intracellular pathogen L. monocytogenes. The structural and immune properties of the skin enable a split adjuvant and antigen vaccination strategy: Guebre-Xabier et al. first demonstrated that topical administration of an enterotoxin improved the amplitude of the antibody response to intramuscular or subcutaneous influenza virus antigen in mice (36). This group also showed that an enterotoxin-containing patch could induce a trend toward higher antibody responses to influenza vaccination in the elderly (37), demonstrating the relevance of this approach in humans. Other groups showed that topical TLR7 agonists induce CTL responses to protein antigen in mice (2), improve immunity to Leishmania in mice (38), and are able to induce immune responses to tumor antigens in humans (39).
Separate administration of vaccine and adjuvant at immunization has several advantages over standard coadministration. Such advantages include potentially more efficacious sites of adjuvant administration, increased safety, and the ability to tailor adjuvant use to specific populations without a need for vaccine reformulation. However, bacterial toxins, such as enterotoxins, have persistent safety concerns. Further, even limited amounts of topical TLR7 agonist may induce systemic cytokine release and splenomegaly in mice (28) and may induce systemic symptoms and side effects in humans (40). Synthetic ODNs with CpG motifs are larger molecules that have less penetration after topical application than imiquimod and similar TLR7 agonists. We thus explored split skin administration of adjuvant and antigen using synthetic CpG ODN 1826 as a vaccine adjuvant.
Synthetic ODNs with CpG motifs have been explored for immunotherapeutic uses, including the reduction of allergic responses and the enhancement of cancer therapy and innate immune responses (4). CpG ODNs have also been extensively studied for their ability to improve vaccine efficacy. These studies have always used coadministration of antigen and adjuvant. Studies in nonhuman primates indicate that CpG ODNs are as effective as TLR7 adjuvants in their ability to induce long-lasting protective immunity but without the induction of associated skin inflammation (1). Topical administration of CpG ODNs coadministered with parenteral antigen enhances CTL responses to antigen (20) and induces CTL response to tumors in a murine model (21). The adjuvant must be administered within the same lymph node draining area as the antigen and results in movement of Langerin+ (epidermally and dermally derived) dendritic cells (DCs) and antigen-bearing DCs into the draining lymph nodes. This results in an effective adjuvant effect allowing CTL priming without the cytokine toxicity and splenomegaly noted with parenteral administration of CpG adjuvant (20): we have previously shown that subcutaneous administration of CpG adjuvant results in rapid elevation of TNF-α, interleukin-12p70 (IL-12p70), IL-6, and monocyte chemoattractant protein-1 (MCP-1), as well as IFN-γ release in the serum (20). None of these occurred with topical administration of CpG adjuvant, supporting the contention that topical administration induces less systemic inflammation. Here, we expanded on our previous observations and explored the use of synthetic CpG ODN 1826 as topical adjuvant in the context of intracellular infection. Our results indicate that topical CpG ODN administration is superior to coadministration with antigen via the subcutaneous or intramuscular route in terms of CTL induction, has fewer systemic effects, and is better than parenteral CpG in terms of long-term protection against systemic L. monocytogenes infection.
Current protein vaccines require booster doses to achieve effective immunity. A protective CD8+ T cell threshold is often not reached with a single immunization (41). Instead, prime-boost regimens are required for long-term protection (42). Proper spacing between doses of a given vaccine series is essential for optimal immune responses. In contrast, rapid induction of immunity is desired as a response to a pandemic infection or bioterrorist attack. We find that an effective strategy of CpG ODN 1826 administration for antigen-specific CTL induction is to administer a single dose of topical CpG ODN 1826 as an adjuvant with subcutaneous protein antigen followed by a booster dose 7 days later. Unlike what is observed with the TLR7 agonist R848, this strategy does not result in splenomegaly and multiple applications are not required to increase efficacy (28). In addition, a single immunization with topical CpG adjuvant also enhanced protective immunity. High levels of inflammatory mediators, such as IFN-γ, which may be induced by CpG adjuvant, inhibit durable memory T cell responses (43, 44). We show in this paper, and have noted previously, that topical administration of CpG adjuvant, which does not induce splenomegaly or systemic cytokine release (20) (and thus induces less systemic inflammation than subcutaneous CpG), nevertheless induced memory CD8+ T cell responses. We propose that the local adjuvant administration induces the activation of antigen-presenting cells (20) without the widespread inflammation and possibly direct cytokine stimulation of T cells that may occur with parenteral administration. This may simulate the much more technically demanding DC vaccination, which has been shown to induce rapid memory CD8+ T cell responses (44).
Topical CpG adjuvant induces a rapid increase of antigen-specific CTL and a higher proportion of effector memory CD8+ T cells. We next asked whether these changes were sufficient to protect against intracellular bacterial infection. CD8+ T cells are required for protection against L. monocytogenes infection (32, 33). Further, intraperitoneal CpG ODN injection alone offers non-antigen-specific short-term protection against L. monocytogenes infection, possibly due to IL-12 and IFN-γ release (45). We thus used Lm-OVA to assess whether antigen-specific CTLs induced by our immunization strategy confers protection against systemic L. monocytogenes infection. Topical administration of CpG adjuvant at the time of OVA protein immunization induced rapid protection against intravenously administered Lm-OVA, as indicated by the reduction of the bacterial load when mice were challenged shortly after immunization. This protection was similar in extent to that provided by subcutaneous CpG adjuvant (despite higher CTL frequencies), possibly due to a saturation in the efficacy of clearance of intravenously administered organisms by CTL. Repeated administration of topical CpG without antigen also decreased the bacterial load slightly, suggesting that topical application can have a broad, antigen-nonspecific effect, as noted following intraperitoneal administration (45). Phosphorothioate modification of CpG ODNs to render them more resistant to nuclease degradation may result in phosphorothioate CpG but TLR9-independent immune activation (46, 47, 48, 49). We confirmed that the protective effect of topical ODN 1826 as adjuvant was TLR9 dependent using TLR9 KO mice.
Memory T cells have higher affinity for antigen and a decreased threshold for activation (50). Memory CD8+ T cells are crucial in providing protective immunity against intracellular pathogens such as Plasmodium (41) and HIV (51). Topical CpG adjuvant administration at the time of subcutaneous protein immunization induces antigen-specific memory T cells to form a stable pool of circulating CD8+ T cells for up to 4 months (20). However, topical administration of CpG adjuvant did not result in a further increase in the frequency of blood-circulating antigen-specific memory CD8+ T cells compared to subcutaneous administration.
Two types of CD8+ memory T cells with differential proliferative and protective potential have been identified based on surface expression of CD62L: CD62L+ central memory T cells, with low proliferative potential, and CD62L− effector memory T cells, with high proliferative potential (52). Both central and effector memory T cells may be crucial to combat pathogens, acting at different times following infection. Cui et al. showed that parenteral CpG-B administration at the time of vaccination increases CD127low KLRG1hi short-lived effector cells (SLEC) formation but not CD127hi KLRG1low CD8+ memory T cells (MPEC) (53). Obar et al. showed that intraperitoneal injection of CpG ODN 1826 at the time of vaccination increases central memory CD62Lhi CD8+ T cells (54). We note that topical CpG initially induces a larger burst of blood-resident antigen-specific CD8+ T cells in an acute response compared to parenteral CpG, but we did not detect a subsequent difference in the frequency of memory T cells within the blood compartment 1 month later. Although we did not directly phenotype the memory T cells into the CD127hi KLRG1low MPEC, we found that topical administration of CpG adjuvant, unlike the intraperitoneal administration of CpG by Obar et al., resulted in a preferential induction of a pool of CD127+CD62L− effector memory cells. Preferential tissue distribution and effector cell population contraction within the blood compartment may explain why protective immunity following subcutaneous or topical CpG adjuvant vaccination resulted in a weaker protective response to delayed systemic L. monocytogenes challenge (30 days postimmunization) than to acute challenge (5 days postimmunization).
Taken together, our work indicates that a rapid (7-day) prime-boost regimen with a single dose of topical CpG adjuvant at the time of parenteral antigen administration is a good strategy to induce protective CD8+ T cell response for protein-based vaccines. We extend these observations to an in vivo mouse infection model using Lm-OVA and show rapid protection when mice are immunized with subcutaneous antigen with topical CpG ODN 1826. Our work thus supports the use of topical CpG adjuvant to increase the cell-mediated immune response to protein vaccines. The rapid protection induced makes this strategy attractive for protection against novel intracellular pathogens, as in pandemic viral outbreaks. This strategy has the added advantage of not requiring novel vaccine formulations for developed vaccine. Further, it may be applied selectively to at-risk populations such as the elderly or the very young.
CpG ODNs are excellent adjuvants in nonhuman primates when given subcutaneously (1). It is important to note that TLR9 expression pattern differs between humans and mice. Could topical CpG ODN application have adjuvant effects in humans? TLR9 is constitutively expressed or can be induced to express on murine myeloid DCs, plasmacytoid DCs, B cells, monocytes, and mast cells (55, 56). In normal mouse skin, TLR9 mRNA expression is induced following intradermal injection of CpG 1668 (57). In humans, TLR9 expression is more restricted than in mice to plasmacytoid DCs and B cells but can also be induced in monocytes and keratinocytes (58, 59, 60), suggesting that topical application may also have an additional adjuvant effect in humans. Topical administration of adjuvant is a superior strategy to parenteral administration because topical application of adjuvant limits the distribution of the adjuvant (39). We speculate that topical CpG adjuvant induces keratinocyte as well as DC activation, multiplying immunogenic stimuli without promoting systemic inflammation.
ACKNOWLEDGMENTS
W.K.C. was supported by the CIHR Canada Graduate Scholarship Master's Award and a CIHR Skin Research Training Centre Training Scholarship. K.W. is supported by the NSERC industrial postgraduate scholarship award. J.P.D. is a Senior Scientist of the Michael Smith Foundation for Research and the Child and Family Research Institute (CFRI). The work was supported by a CIHR operating grant (FRN 89878).
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 3 January 2014
REFERENCES
- 1.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 203:1249–1258. 10.1084/jem.20052433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston D, Bystryn J-C. 2006. Topical imiquimod is a potent adjuvant to a weakly-immunogenic protein prototype vaccine. Vaccine 24:1958–1965. 10.1016/j.vaccine.2005.10.045 [DOI] [PubMed] [Google Scholar]
- 3.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745. 10.1038/35047123 [DOI] [PubMed] [Google Scholar]
- 4.Klinman DM. 2004. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 4:249–259. 10.1038/nri1329 [DOI] [PubMed] [Google Scholar]
- 5.Rothenfusser S, Hornung V, Ayyoub M, Britsch S, Towarowski A, Krug A, Sarris A, Lubenow N, Speiser D, Endres S, Hartmann G. 2004. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood 103:2162–2169. 10.1182/blood-2003-04-1091 [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Luo X, Yang C, Yu S, Xu H. 2011. Three CpG oligodeoxynucleotide classes differentially enhance antigen-specific humoral and cellular immune responses in mice. Vaccine 29:5778–5784. 10.1016/j.vaccine.2011.05.087 [DOI] [PubMed] [Google Scholar]
- 7.Sánchez VR, Pitkowski MN, Fernández Cuppari AV, Rodríguez FM, Fenoy IM, Frank FM, Goldman A, Corral RS, Martin V. 2011. Combination of CpG-oligodeoxynucleotides with recombinant ROP2 or GRA4 proteins induces protective immunity against Toxoplasma gondii infection. Exp. Parasitol. 128:448–453. 10.1016/j.exppara.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 8.Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, Cameron DW. 2005. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS 19:1473–1479. 10.1097/01.aids.0000183514.37513.d2 [DOI] [PubMed] [Google Scholar]
- 9.Wagner H, Lipford GB, Häcker H. 2000. The role of immunostimulatory CpG-DNA in septic shock. Springer Semin. Immunopathol. 22:167–171. 10.1007/s002810000023 [DOI] [PubMed] [Google Scholar]
- 10.Sparwasser T, Miethke T, Lipford G, Erdmann A, Häcker H, Heeg K, Wagner H. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur. J. Immunol. 27:1671–1679. 10.1002/eji.1830270712 [DOI] [PubMed] [Google Scholar]
- 11.Heit A, Schmitz F, O'Keeffe M, Staib C, Busch DH, Wagner H, Huster KM. 2005. Protective CD8 T cell immunity triggered by CpG-protein conjugates competes with the efficacy of live vaccines. J. Immunol. 174:4373–4380 http://www.jimmunol.org/content/174/7/4373 [DOI] [PubMed] [Google Scholar]
- 12.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. 2007. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur. J. Immunol. 37:2063–2074. 10.1002/eji.200737169 [DOI] [PubMed] [Google Scholar]
- 13.Romani N, Thurnher M, Idoyaga J, Steinman RM, Flacher V. 2010. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol. Cell Biol. 88:424–430. 10.1038/icb.2010.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawicki W, Marshall JS. 2007. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 19:31–38. 10.1016/j.coi.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Macleod AS, Havran WL. 2011. Functions of skin-resident γδ T cells. Cell. Mol. Life Sci. 68:2399–2408. 10.1007/s00018-011-0702-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RA. 2010. Skin-resident T cells: the ups and downs of on site immunity. J. Investig. Dermatol. 130:362–370. 10.1038/jid.2009.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nestle FO, Di Meglio P, Qin J-Z, Nickoloff BJ. 2009. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9:679–691. 10.1038/nri2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahlon R, Hu Y, Orteu CH, Kifayet A, Trudeau JD, Tan R, Dutz JP. 2003. Optimization of epicutaneous immunization for the induction of CTL. Vaccine 21:2890–2899. 10.1016/S0264-410X(03)00141-5 [DOI] [PubMed] [Google Scholar]
- 19.Klimuk SK, Najar HM, Semple SC, Aslanian S, Dutz JP. 2004. Epicutaneous application of CpG oligodeoxynucleotides with peptide or protein antigen promotes the generation of CTL. J. Investig. Dermatol. 122:1042–1049. 10.1111/j.0022-202X.2004.22411.x [DOI] [PubMed] [Google Scholar]
- 20.Najar HM, Dutz JP. 2007. Topical TLR9 agonists induce more efficient cross-presentation of injected protein antigen than parenteral TLR9 agonists do. Eur. J. Immunol. 37:2242–2256. 10.1002/eji.200636212 [DOI] [PubMed] [Google Scholar]
- 21.Najar HM, Dutz JP. 2008. Topical CpG enhances the response of murine malignant melanoma to dacarbazine. J. Investig. Dermatol. 128:2204–2210. 10.1038/jid.2008.59 [DOI] [PubMed] [Google Scholar]
- 22.Kollmann TR, Reikie B, Blimkie D, Way SS, Hajjar AM, Arispe K, Shaulov A, Wilson CB. 2007. Induction of protective immunity to Listeria monocytogenes in neonates. J. Immunol. 178:3695–3701 http://www.jimmunol.org/content/178/6/3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loeffler DIM, Smolen K, Aplin L, Cai B, Kollmann TR. 2009. Fine-tuning the safety and immunogenicity of Listeria monocytogenes-based neonatal vaccine platforms. Vaccine 27:919–927. 10.1016/j.vaccine.2008.11.047 [DOI] [PubMed] [Google Scholar]
- 24.Kearney ER, Pape KA, Loh DY, Jenkins MK. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1:327–339. 10.1016/1074-7613(94)90084-1 [DOI] [PubMed] [Google Scholar]
- 25.Wirth TC, Harty JT, Badovinac VP. 2010. Modulating numbers and phenotype of CD8(+) T cells in secondary immune responses. Eur. J. Immunol. 40:1916–1926. 10.1002/eji.201040310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparwasser T, Hültner L, Koch ES, Luz A, Lipford GB, Wagner H. 1999. Immunostimulatory CpG-oligodeoxynucleotides cause extramedullary murine hemopoiesis. J. Immunol. 162:2368–2374 [PubMed] [Google Scholar]
- 27.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. 2004. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 10:187–192. 10.1038/nm987 [DOI] [PubMed] [Google Scholar]
- 28.Chang BA, Cross JL, Najar HM, Dutz JP. 2009. Topical resiquimod promotes priming of CTL to parenteral antigens. Vaccine 27:5791–5799. 10.1016/j.vaccine.2009.07.062 [DOI] [PubMed] [Google Scholar]
- 29.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim S-K, Clute SC, Welsh RM. 2006. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 211:164–181. 10.1111/j.0105-2896.2006.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, Andrian Von UH, Ahmed R. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. 10.1038/ni889 [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 32.Pamer EG. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812–823. 10.1038/nri1461 [DOI] [PubMed] [Google Scholar]
- 33.Lara-Tejero M, Pamer EG. 2004. T cell responses to Listeria monocytogenes. Curr. Opin. Microbiol. 7:45–50. 10.1016/j.mib.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Saxena M, Busca A, Pandey S, Kryworuchko M, Kumar A. 2011. CpG protects human monocytic cells against HIV-Vpr-induced apoptosis by cellular inhibitor of apoptosis-2 through the calcium-activated JNK pathway in a TLR9-independent manner. J. Immunol. 187:5865–5878. 10.4049/jimmunol.1100115 [DOI] [PubMed] [Google Scholar]
- 35.Sanjuan MA, Rao N, Lai K-TA, Gu Y, Sun S, Fuchs A, Fung-Leung W-P, Colonna M, Karlsson L. 2006. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol. 172:1057–1068. 10.1083/jcb.200508058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guebre-Xabier M, Hammond SA, Ellingsworth LR, Glenn GM. 2004. Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice. J. Virol. 78:7610–7618. 10.1128/JVI.78.14.7610-7618.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frech SA, Kenney RT, Spyr CA, Lazar H, Viret J-F, Herzog C, Glück R, Glenn GM. 2005. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine 23:946–950. 10.1016/j.vaccine.2004.06.036 [DOI] [PubMed] [Google Scholar]
- 38.Zhang W-W, Matlashewski G. 2008. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect. Immun. 76:3777–3783. 10.1128/IAI.01527-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams S, O'Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, Cruz CM, Angiulli A, Angiulli F, Ritter E, Holman RM, Shapiro RL, Berman RS, Berner N, Shao Y, Manches O, Pan L, Venhaus RR, Hoffman EW, Jungbluth A, Gnjatic S, Old L, Pavlick AC, Bhardwaj N. 2008. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J. Immunol. 181:776–784 http://jimmunol.org/content/181/1/776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantisani C, Lazic T, Richetta AG, Clerico R, Mattozzi C, Calvieri S. 2012. Imiquimod 5% cream use in dermatology, side effects and recent patents. Recent Pat. Inflamm. Allergy Drug Discov. 6:65–69. 10.2174/187221312798889301 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. 2008. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. U. S. A. 105:14017–14022. 10.1073/pnas.0805452105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabbari A, Harty JT. 2006. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 203:919–932. 10.1084/jem.20052237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badovinac VP, Porter BB, Harty JT. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5:809–817. 10.1038/ni1098 [DOI] [PubMed] [Google Scholar]
- 44.Badovinac VP, Messingham KAN, Jabbari A, Haring JS, Harty JT. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 11:748–756. 10.1038/nm1257 [DOI] [PubMed] [Google Scholar]
- 45.Krieg AM, Love-Homan L, Yi AK, Harty JT. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428–2434 http://www.jimmunol.org/content/161/5/2428 [PubMed] [Google Scholar]
- 46.Vollmer J, Weeratna RD, Jurk M, Samulowitz U, McCluskie MJ, Payette P, Davis HL, Schetter C, Krieg AM. 2004. Oligodeoxynucleotides lacking CpG dinucleotides mediate Toll-like receptor 9 dependent T helper type 2 biased immune stimulation. Immunology 113:212–223. 10.1111/j.1365-2567.2004.01962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts TL, Sweet MJ, Hume DA, Stacey KJ. 2005. Cutting edge: species-specific TLR9-mediated recognition of CpG and non-CpG phosphorothioate-modified oligonucleotides. J. Immunol. 174:605–608 http://www.jimmunol.org/content/174/2/605 [DOI] [PubMed] [Google Scholar]
- 48.Sester DP, Brion K, Trieu A, Goodridge HS, Roberts TL, Dunn J, Hume DA, Stacey KJ, Sweet MJ. 2006. CpG DNA activates survival in murine macrophages through TLR9 and the phosphatidylinositol 3-kinase-Akt pathway. J. Immunol. 177:4473–4480 http://www.jimmunol.org/content/177/7/4473 [DOI] [PubMed] [Google Scholar]
- 49.Luganini A, Caposio P, Landolfo S, Gribaudo G. 2008. Phosphorothioate-modified oligodeoxynucleotides inhibit human cytomegalovirus replication by blocking virus entry. Antimicrob. Agents Chemother. 52:1111–1120. 10.1128/AAC.00987-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. 2007. Memory CD8+ T cells require CD28 costimulation. J. Immunol. 179:6494–6503 http://www.jimmunol.org/content/179/10/6494 [DOI] [PubMed] [Google Scholar]
- 51.Belyakov IM, Ahlers JD. 2008. Functional CD8+ CTLs in mucosal sites and HIV infection: moving forward toward a mucosal AIDS vaccine. Trends Immunol. 29:574–585. 10.1016/j.it.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 52.Usherwood EJ, Hogan RJ, Crowther G, Surman SL, Hogg TL, Altman JD, Woodland DL. 1999. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J. Virol. 73:7278–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui W, Joshi NS, Jiang A, Kaech SM. 2009. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 27:2177–2187. 10.1016/j.vaccine.2009.01.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham Q-M, Zickovich JM, Lefrançois L. 2011. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 187:4967–4978. 10.4049/jimmunol.1102335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran N, Koch A, Berkels R, Boehm O, Zacharowski PA, Baumgarten G, Knuefermann P, Schott M, Kanczkowski W, Bornstein SR, Lightman SL, Zacharowski K. 2007. Toll-like receptor 9 expression in murine and human adrenal glands and possible implications during inflammation. J. Clin. Endocrinol. Metab. 92:2773–2783. 10.1210/jc.2006-2697 [DOI] [PubMed] [Google Scholar]
- 56.Krieg AM. 2007. Antiinfective applications of Toll-like receptor 9 agonists. Proc. Am. Thorac. Soc. 4:289–294. 10.1513/pats.200701-021AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L, Zhou X, Shi J, Xie X, Yuan Z. 2003. Toll-like receptor-9 induced by physical trauma mediates release of cytokines following exposure to CpG motif in mouse skin. Immunology 110:341–347. 10.1046/j.1365-2567.2003.01739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saikh KU, Kissner TL, Sultana A, Ruthel G, Ulrich RG. 2004. Human monocytes infected with Yersinia pestis express cell surface TLR9 and differentiate into dendritic cells. J. Immunol. 173:7426–7434 http://www.jimmunol.org/content/173/12/7426 [DOI] [PubMed] [Google Scholar]
- 59.Tripp CH, Ebner S, Ratzinger G, Romani N, Stoitzner P. 2010. Conditioning of the injection site with CpG enhances the migration of adoptively transferred dendritic cells and endogenous CD8+ T-cell responses. J. Immunother. 33:115–125. 10.1097/CJI.0b013e3181b8ef5f [DOI] [PubMed] [Google Scholar]
- 60.Lebre MC, der Aar van AMG, van Baarsen L, van Capel TMM, Schuitemaker JHN, Kapsenberg ML, de Jong EC. 2007. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 127:331–341 [DOI] [PubMed] [Google Scholar]