Abstract
Flavobacterium johnsoniae, a member of phylum Bacteriodetes, is a gliding bacterium that digests insoluble chitin and many other polysaccharides. A novel protein secretion system, the type IX secretion system (T9SS), is required for gliding motility and for chitin utilization. Five potential chitinases were identified by genome analysis. Fjoh_4555 (ChiA), a 168.9-kDa protein with two glycoside hydrolase family 18 (GH18) domains, was targeted for analysis. Disruption of chiA by insertional mutagenesis resulted in cells that failed to digest chitin, and complementation with wild-type chiA on a plasmid restored chitin utilization. Antiserum raised against recombinant ChiA was used to detect the protein and to characterize its secretion by F. johnsoniae. ChiA was secreted in soluble form by wild-type cells but remained cell associated in strains carrying mutations in any of the T9SS genes, gldK, gldL, gldM, gldNO, sprA, sprE, and sprT. Western blot and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses suggested that ChiA was proteolytically processed into two GH18 domain-containing proteins. Proteins secreted by T9SSs typically have conserved carboxy-terminal domains (CTDs) belonging to the TIGRFAM families TIGR04131 and TIGR04183. ChiA does not exhibit strong similarity to these sequences and instead has a novel CTD. Deletion of this CTD resulted in accumulation of ChiA inside cells. Fusion of the ChiA CTD to recombinant mCherry resulted in secretion of mCherry into the medium. The results indicate that ChiA is a soluble extracellular chitinase required for chitin utilization and that it relies on a novel CTD for secretion by the F. johnsoniae T9SS.
INTRODUCTION
The gliding bacterium Flavobacterium johnsoniae efficiently digests many polysaccharides, including insoluble chitin, a homopolymer of β-1,4-linked N-acetyl-d-glucosamine (GlcNAc) (1). Chitin is one of the most abundant biomolecules on earth, and determination of the mechanisms of its digestion has biotechnological and environmental implications (2). Analysis of the F. johnsoniae genome revealed 10 genes predicted to encode glycohydrolases involved in chitin utilization (3). These included five predicted chitinases to cut the long chitin polymers and five predicted β-N-acetylglucosaminidases to release N-acetylglucosamine from the soluble chitooligosaccharides. The exact functions of each of these enzymes in chitin utilization are not known.
It has been known for many years that mutations that disrupt gliding motility often result in the inability to digest chitin (4). More recently it was recognized that F. johnsoniae has a protein secretion system, originally called the Por secretion system and now referred to as the type IX secretion system (T9SS), that is required for both motility and chitin utilization (5–8). The components of T9SSs are not closely related to those of the well-studied type I to type VI secretion systems of Gram-negative bacteria (5, 9, 10). They are also unrelated to the components of the chaperone-usher pathway that has recently been called the type VII secretion system (11–13), to the components of the extracellular nucleation-precipitation pathway involved in secretion and assembly of curli amyloid fibers, which has been referred to as the type VIII secretion system (13, 14), and to the mycobacterial ESX (ESAT-6) system (12, 15). The T9SS is required for secretion of the cell surface motility proteins SprB and RemA and is thus needed for motility. SprB and RemA are adhesins that move rapidly on the cell surface, apparently propelled by the still poorly defined gliding motor (16–18). Cells with mutations in T9SS genes fail to utilize chitin and lack extracellular chitinase activity (6, 8, 19, 20). One predicted chitinase (Fjoh_4555, which we refer to as ChiA) was identified in the spent culture medium of wild-type cells but not of a T9SS mutant (6). ChiA was thus predicted to be secreted by the T9SS and to have a role in chitin utilization.
T9SSs are common in members of the phylum Bacteroidetes, of which F. johnsoniae is a member (5). They have been studied not only in F. johnsoniae but also in the nonmotile oral pathogen Porphyromonas gingivalis, which uses its T9SS to secrete gingipain protease virulence factors and other proteins (6, 7, 21). Proteins secreted by T9SSs have N-terminal signal peptides, allowing transit across the cytoplasmic membrane via the Sec system, and conserved C-terminal domains (CTDs) that are thought to target the proteins to the T9SS (5, 7, 18, 22–25). These CTDs typically belong to TIGRFAM family TIGR04131 or TIGR04183. F. johnsoniae has 53 proteins with these CTD's, including SprB and RemA (8). One predicted chitinase, Fjoh_4175, has a CTD that belongs to TIGR04183 and thus may be secreted by the T9SS. Surprisingly, the secreted chitinase ChiA does not have a recognizable T9SS CTD, so its relationship to the T9SS or to another secretion system required further study.
Here we demonstrate that chiA encodes the major extracellular chitinase required for chitin utilization and that ChiA is a soluble enzyme that requires the T9SS for secretion. We also show that the C-terminal 105 amino acids of ChiA are necessary for secretion and are sufficient to target a foreign protein for secretion by the T9SS.
MATERIALS AND METHODS
Bacterial and bacteriophage strains, plasmids, and growth conditions.
F. johnsoniae ATCC 17061 strain UW101 was the wild-type strains used in this study (3, 26). F. johnsoniae strains were grown in Casitone-yeast extract (CYE) medium at 30°C (27) or in motility medium (MM) at 25°C (28), as previously described. Escherichia coli strains were grown in Luria-Bertani medium (LB) at 37°C. Strains and plasmids used in this study are listed in Table 1, and primers are listed in Table S1 in the supplemental material. Antibiotics were used at the following concentrations when needed: ampicillin, 100 μg/ml; chloramphenicol, 30 μg/ml; erythromycin, 100 μg/ml; kanamycin, 30 μg/ml; streptomycin, 100 μg/ml; and tetracycline, 20 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and/or descriptiona | Source or reference |
|---|---|---|
| F. johnsoniae strains | ||
| ATCC 17061 strain UW101 | Wild type | 3, 26 |
| UW102-3 | Spontaneous sprA mutant | 19, 62 |
| UW102-57 | Spontaneous gldK mutant | 4, 63 |
| UW102-176 | Nitrosoguanidine-induced gldM mutant | 4, 19 |
| UW102-344 | Spontaneous gldL mutant | 19, 62 |
| CJ1631A | Δ(gldN gldO) | 19 |
| CJ1808 | chiA disruption mutant; (Emr) | This study |
| CJ1827 | Strain used for construction of deletion mutants; rpsL2 (Smr) | 30 |
| CJ2325 | Mutant lacking CTD-encoding region of chiA; rpsL2 (Smr) | This study |
| CJ2355 | Fjoh_4175 deletion mutant; rpsL2 (Smr) | This study |
| FJ149 | sprE disruption mutant; (Emr) | 20 |
| KDF001 | sprT disruption mutant; (Emr) | 6 |
| Plasmids | ||
| pCP23 | E. coli-F. johnsoniae shuttle plasmid; Apr (Tcr) | 64 |
| pET30a | Protein expression vector; Kmr | Novagen |
| pKF002 | pCP23 carrying sprT; Apr (Tcr) | 6 |
| pLYL03 | Suicide vector used for insertional mutagenesis; Apr (Emr) | 65 |
| pME-mCherry | Plasmid expressing fluorescent protein mCherry; Kmr | 66 |
| pNap2 | pCP23 carrying sprE; Apr (Tcr) | 20 |
| pRR51 | rpsL-containing suicide vector for construction of deletions; Apr (Emr) | 30 |
| pSAM1 | 1,299-bp fragment of chiA in pLYL03 for constructing chiA disruption mutant CJ1808; Apr (Emr) | This study |
| pSN48 | pCP23 carrying sprA; Apr (Tcr) | 67 |
| pSSK05 | pCP23 carrying chiA; Apr (Tcr) | This study |
| pSSK07 | 1,833-bp fragment of chiA inserted into pET30a; Kmr | This study |
| pSSK26 | 2,121-bp fragment upstream of chiA CTD-encoding region in pRR51; Apr (Emr) | This study |
| pSSK27 | Construct used to delete CTD-encoding region of chiA; 2,033-bp region downstream of chiA inserted into pSSK26; Apr (Emr) | This study |
| pSSK30 | pCP23 carrying mcherry; Apr (Tcr) | This study |
| pSSK32 | 2,118-bp region downstream of Fjoh_4175 inserted into pRR51; Apr (Emr) | This study |
| pSSK34 | Construct used to delete Fjoh_4175; 1,948-bp region upstream of Fjoh_4175 inserted into pSSK32; Apr (Emr) | This study |
| pSSK45 | mcherry with stop codon amplified with primers 862 and 1443 and cloned into pCP23; Apr (Tcr) | This study |
| pSSK51 | 484-bp fragment spanning the chiA promoter, start codon, and N-terminal signal peptide-encoding region inserted into pSSK30; Apr (Tcr) | This study |
| pSSK52 | 566-bp region encoding 105-amino-acid CTDChiA inserted into pSSK51; Apr (Tcr) | This study |
| pSSK54 | 484-bp fragment spanning the chiA promoter, start codon, and N-terminal signal peptide-encoding region inserted into pSSK45; Apr (Tcr) | This study |
| pTB79 | pCP23 carrying gldN; Apr (Tcr) | 63 |
| pTB81a | pCP23 carrying gldL; Apr (Tcr) | 63 |
| pTB94a | pCP23 carrying gldM; Apr (Tcr) | 63 |
| pTB99 | pCP23 carrying gldK; Apr (Tcr) | 63 |
Antibiotic resistance phenotypes are as follows: ampicillin, Apr; erythromycin, Emr; kanamycin, Kmr; streptomycin, Smr; and tetracycline, Tcr. The antibiotic resistance phenotypes given in parentheses are those expressed in F. johnsoniae but not in E. coli. The antibiotic resistance phenotypes without parentheses are those expressed in E. coli but not in F. johnsoniae.
Disruption and complementation of chiA.
For disruption of chiA, a 1,299-bp region internal to F. johnsoniae chiA was amplified by PCR using primer 937 with an engineered BamHI site and primer 938 with an engineered SalI site. The fragment was inserted into pLYL03 that had been digested with BamHI and SalI to generate pSAM1. pSAM1 was introduced into F. johnsoniae by conjugation (27, 29) and recombined into the chromosome to yield the chiA mutant CJ1808. The insertion was confirmed by PCR using primer 737 and primer 941.
For complementation of chiA, a 4,974-bp fragment was amplified using primer 974 (engineered XbaI site) and 975 (engineered BamHI site). This fragment was introduced into complementation vector pCP23, which had been digested with BamHI and XbaI, to generate pSSK05.
Deletion of the chiA CTD-encoding region.
The previously described strategy to generate unmarked deletions was employed (30) to generate a truncated gene encoding ChiA lacking the C-terminal 106 amino acids. A 2,121-bp fragment upstream of the chiA CTD-encoding region was amplified using Phusion DNA polymerase (New England BioLabs, Ipswich, MA) and primers 1378 and 1379. The amplified fragment was digested with BamHI and SalI and cloned into pRR51 that had been digested with the same enzymes, generating pSSK26. A 2,033-bp fragment downstream of chiA was amplified by PCR using primers 1380 (engineered SalI site) and 1381(engineered SphI site). This fragment was ligated into pSSK26 that had been digested with SalI and SphI, to generate pSSK27. pSSK27 was introduced into the streptomycin-resistant wild-type F. johnsoniae strain CJ1827 by conjugation. The chiA CTD deletion mutant was isolated essentially as previously described (30). The chiA CTD deletion mutant, CJ2325, was confirmed by PCR amplification using primers 1391 and 1392 and by sequencing the product.
Deletion of Fjoh_4175.
A 2,118-bp fragment upstream of Fjoh_4175 was amplified using primers 1229 (engineered SalI site) and 1230 (engineered SphI site). The amplified fragment was digested with SalI and SphI and cloned into pRR51 that had been digested with the same enzymes, generating pSSK32. A 1,948-bp fragment downstream of Fjoh_4175 was amplified using primers 1227 (engineered XbaI site) and 1228 (engineered SalI site). This fragment was introduced into pSSK32 that had been digested with XbaI and SphI, to generate pSSK34. pSSK34 was introduced into F. johnsoniae CJ1827 by conjugation, and the Fjoh_4175 deletion mutant CJ2355 was isolated essentially as previously described (30) and confirmed by PCR amplification and sequencing using primers 1463 and 1464.
Generation of mCherry fusion constructs.
A plasmid expressing the N-terminal region of ChiA (encoding the signal peptide) fused to mCherry and to the C-terminal 105 amino acids of ChiA (CTDChiA) was constructed. A 708-bp region of mCherry was amplified from pME-mCherry using primer 862 (engineered BamHI site) and primer 1266 (engineered XbaI site). This fragment was cloned into the BamHI and XbaI sites of pCP23, generating pSSK30. A 484-bp fragment spanning the chiA promoter, start codon, and N-terminal signal peptide-encoding region was amplified using primer 1593 (engineered KpnI site) and primer 1516 (engineered BamHI site). The fragment was inserted into KpnI- and BamHI-digested pSSK30 to generate pSSK51. To introduce the CTD-encoding region, 566 bp was amplified using primer 1600 (engineered XbaI site) and primer 1404 (engineered SphI site). The product was cloned into pSSK51, to generate pSSK52. A plasmid expressing the ChiA N-terminal signal peptide fused to mCherry without CTDChiA was also constructed as a control. mCherry was amplified using primers 862 and 1443 (engineered XbaI site) and introduced into BamHI- and XbaI-digested pCP23, generating pSSK45. The ChiA N-terminal signal peptide-encoding region was amplified using primers 1593 and 1516 and was cloned into pSSK45 to generate pSSK54.
Determination of chitinase activity.
Chitin utilization on agar was observed as previously described using colloidal chitin prepared from crab shells (19, 26, 31). Chitinase activities in cell-free culture supernatants (spent media), whole cells, and cell extracts were measured as previously described (19) using the synthetic substrates 4-methylumbelliferyl β-d-N,N′-diacetyl-chitobioside [4-MU-(GlcNAc)2] and 4-methylumbelliferyl β-d-N,N′,N″-diacetyl-chitotrioside [4-MU-(GlcNAc)3] (Sigma-Aldrich, St. Louis, MO) except that activities were measured for 30 min. Activities in the spent media (secreted chitinase), whole cells, and cell extracts were indicated as pmol 4-methylumbelliferone released during the 30 min per μg total protein in the original cell suspension. Protein concentrations were determined by the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Waltham, MA).
Protein expression and antibody production.
An 1,833-bp fragment of chiA encoding a region spanning the N-terminal glycoside hydrolase domain was amplified by PCR using primers 1066 (engineered BamHI site) and 1067 (engineered SalI site). This fragment was digested with BamHI and SalI and ligated into pET30a that had been digested with the same enzymes, generating pSSK07. pSSK07 was introduced into E. coli Rosetta 2(DE3) (Novagen, Madison, WI), which expresses seven rare tRNAs required for efficient ChiA expression. Cells were grown to mid-log phase at 37°C in LB, and expression of recombinant ChiA was induced by addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubation for an additional 4 h. Cells were collected by centrifugation and disrupted using a French press, and inclusion bodies containing recombinant ChiA were isolated by centrifugation at 6,415 × g for 30 min. Inclusion bodies were suspended in 20 mM Tris-HCl (pH 7.4) containing 200 mM NaCl, 1 mM EDTA, and 200 μg/ml lysozyme and incubated for 15 min at 25°C. Inclusion bodies were collected by centrifugation at 20,000 × g for 15 min and washed twice by suspension in 20 mM Tris-HCl (pH 7.4) containing 200 mM NaCl, 1 mM EDTA, and 1% Triton X-100 with sonication followed by centrifugation. The inclusion bodies were solubilized in 8 M urea for 1 h at room temperature. Insoluble material was removed by centrifugation at 20,000 × g for 30 min, and the soluble material containing ChiA was boiled in SDS-PAGE loading buffer and separated on 7.5% acrylamide gels by SDS-PAGE. Recombinant ChiA was visualized by CuCl2 staining (32), the band was cut from the gel and destained in 0.25 M Tris (pH 9.0)–0.25 M EDTA, and the protein was electroeluted at 60 mA for 5 h into 25 mM Tris–192 mM glycine–0.1% SDS (pH 8.8) using a model 422 Electro-Eluter (Bio-Rad). Polyclonal antibodies against recombinant ChiA were produced and affinity purified using the recombinant protein by Proteintech Group, Inc. (Chicago, IL).
Western blot analyses.
F. johnsoniae cells were grown to mid-log phase in MM at 25°C with shaking. Cells were pelleted by centrifugation at 22,000 × g for 15 min, and the culture supernatant (spent medium) was filtered using 0.22-μm-pore-size polyvinylidene difluoride filters (Thermo Fisher Scientific). For whole-cell samples, the cells were suspended in the original culture volume of phosphate-buffered saline consisting of 137 mM NaCl, 2.7 mM KCl, 10 mM Na2PO4, and 2 mM KH2PO4 (pH 7.4). Equal amounts of spent media and whole cells were boiled in SDS-PAGE loading buffer for 9 min. Proteins were separated by SDS-PAGE, and Western blot analyses were performed as previously described (19) using affinity-purified antibody against ChiA (1:5,000 dilution). Equal amounts of each sample based on the starting material were loaded in each lane. For cell extracts this corresponded to 15 μg protein, whereas for spent medium this corresponded to the equivalent volume of spent medium that contained 15 μg cell protein before the cells were removed. To determine whether ChiA was associated with membrane vesicles that had been released into the spent medium, the sample was fractionated into soluble and insoluble (membrane) fractions by centrifugation at 352,900 × g for 30 min. For detection of mCherry by Western blotting, commercially available antibodies against mCherry (0.5 mg per ml; BioVision Incorporated, Milpitas, CA) were used at a dilution of 1:5,000.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
F. johnsoniae cells were grown to mid-log phase in MM at 25°C with shaking. Cells were pelleted by centrifugation at 22,000 × g for 15 min, and the spent culture medium was filtered (0.22-μm polyvinylidene difluoride filters) to remove residual cells. The spent medium was concentrated 1,000-fold using Pierce concentrators (Thermo Fisher Scientific), and proteins were separated by SDS-PAGE and detected using the Bio-Rad (Hercules, CA) silver stain kit.
Enzymatic in-gel digestion and mass spectrometric analysis of the peptides were performed at the University of Wisconsin—Madison Mass Spectrometry Facility essentially as previously described (33, 34). Enzymatic digestion and peptide recovery were performed as outlined on the website http://www.biotech.wisc.edu/facilities/massspec/protocols/ingelprotocol. Peptides were analyzed by nanoscale LC-MS/MS (NanoLC-MS/MS) using the Agilent 1100 Nanoflow system (Agilent Technologies, Palo Alto, CA) connected to a hybrid linear ion trap-Orbitrap mass spectrometer (LTQ-Orbitrap XL; Thermo Fisher Scientific) equipped with a nanoscale electrospray ion source. Chromatography of peptides prior to mass spectral analysis was accomplished using a C18 reverse-phase high-pressure liquid chromatography (HPLC) trap column (Zorbax 300SB-C18, 5 μM, 5 by 0.3 mm; Agilent Technologies) and a capillary emitter column (in-house packed with Magic C18, 3 μM, 150 by 0.075 mm; Michrom Bioresources, Inc.) onto which 8 μl of extracted peptides was loaded. NanoHPLC system-delivered solvents were as follows: A, 0.1% (vol/vol) formic acid in water, and B, 95% (vol/vol) acetonitrile–0.1% (vol/vol) formic acid. Sample loading was performed at 10 μl/min, and peptide elution was performed at 0.20 μl/min, using a gradient from 1% (vol/vol) B to 60% (vol/vol) B over 60 min followed by a gradient from 60% (vol/vol) B to 100% (vol/vol) B over 10 min. As peptides eluted, survey MS scans were acquired in the Orbitrap with a resolving power of 100,000 over the mass range m/z 300 to 2000. The 5 most intense peptides detected per scan were fragmented and detected in the ion trap. Raw MS/MS data were converted to mgf file format using the Trans Proteomic Pipeline (Seattle Proteome Center, Seattle, WA). The resulting files were used to search against the F. johnsoniae protein database concatenated with a list of common lab contaminants (5,057 protein entries) with cysteine carbamidomethylation as fixed modification and methionine oxidation and asparagine/glutamine deamidation as variable modifications. Peptide mass tolerance was set at 20 ppm and fragment mass tolerance at 0.8 Da. Matrix Science Mascot version 2.2.07 was used as a search engine, and protein identifications with at least two matched peptides with ion scores of 25 or above were reported.
RESULTS
chiA mutant cells are defective in chitin utilization.
Chitinases have catalytic glycoside hydrolase domains belonging to families 18 (GH18) and 19 (GH19) (35). The F. johnsoniae genome encodes five predicted chitinases with such domains (3). One of these, Fjoh_4555, which we refer to as ChiA (Fig. 1), has previously been implicated in chitin utilization. Cells with a mutation in the T9SS gene sprT failed to accumulate ChiA in the extracellular fluid and failed to utilize chitin (6). ChiA has two GH18 domains, each predicted to have chitinolytic activity (Fig. 1B). We disrupted chiA to determine its role in chitin utilization. Cells of the chiA mutant CJ1808 failed to utilize chitin (Fig. 2A), and the mutant cells were deficient in extracellular chitinase activity (Fig. 3). Complementation with pSSK05, which carries chiA, restored extracellular chitinase activity and the ability to utilize chitin (Fig. 2 and 3). Chitinase activities associated with intact cells and with cell extracts were less affected by disruption of chiA, suggesting that the other predicted chitinases may contribute to these cell-associated activities (Fig. 3). Extracts prepared from cells carrying pSSK05 exhibited elevated levels of activity against 4-MU-(GlcNAc)3, perhaps indicating that ChiA expressed from the plasmid was not efficiently secreted. Deletion of the T9SS genes gldN and gldO also resulted in decreased extracellular chitinase, as previously reported (19), presumably because of a failure to secrete ChiA. Another predicted chitinase, Fjoh_4175, exhibits sequence similarity to the GH18 chitinase domain near the amino terminus of ChiA (GH18N). The CTD of Fjoh_4175 is similar in sequence to the CTDs of members of TIGRFAM family TIGR04183. These CTDs are thought to target the proteins for secretion by the T9SS. Given the importance of the T9SS in chitin utilization, we examined the role of Fjoh_4175 in this process. The Fjoh_4175 deletion mutant CJ2355 digested and grew on chitin (Fig. 2B). Cells of the mutant also had as much extracellular and cell-associated chitinase activities as did wild-type cells (Fig. 3), indicating that Fjoh_4175 does not play a major role in chitin utilization under the conditions examined.
FIG 1.
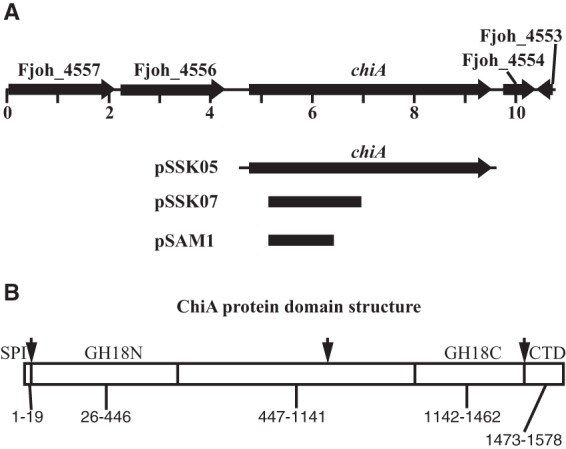
The chiA gene and predicted features of the ChiA protein. (A) Map of the chiA region. Numbers below the map refer to kilobase pairs of sequence. The regions of DNA carried by the plasmids pSSK05 (used for complementation), pSSK07 (used for expression of recombinant ChiA in E. coli), and pSAM1 (used for insertional mutagenesis of chiA) are indicated beneath the map. (B) Predicted features of the ChiA protein. SPI, type I signal peptide. GH18N and GH18C, glycohydrolase 18 family domains located near the amino and carboxy termini, respectively. CTD, C-terminal domain involved in secretion by the type IX secretion system. Arrows denote approximate sites of apparent proteolytic processing, and numbers indicate approximate amino acid ranges for each predicted domain.
FIG 2.
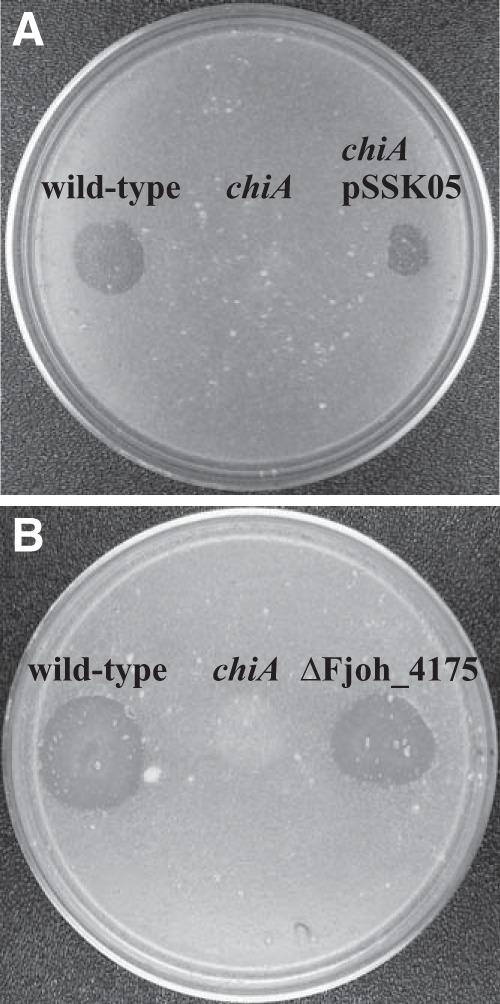
chiA is required for chitin utilization. (A) Approximately 106 cells of wild-type F. johnsoniae UW101, chiA mutant CJ1808, and CJ1808 with pSSK05, which carries chiA, were spotted on MYA-chitin medium and incubated at 25°C for 2.5 days. (B) Wild-type F. johnsoniae CJ1827, chiA mutant CJ1808, and Fjoh_4175 deletion mutant CJ2355 were spotted on MYA-chitin medium and incubated at 25°C for 2.5 days.
FIG 3.
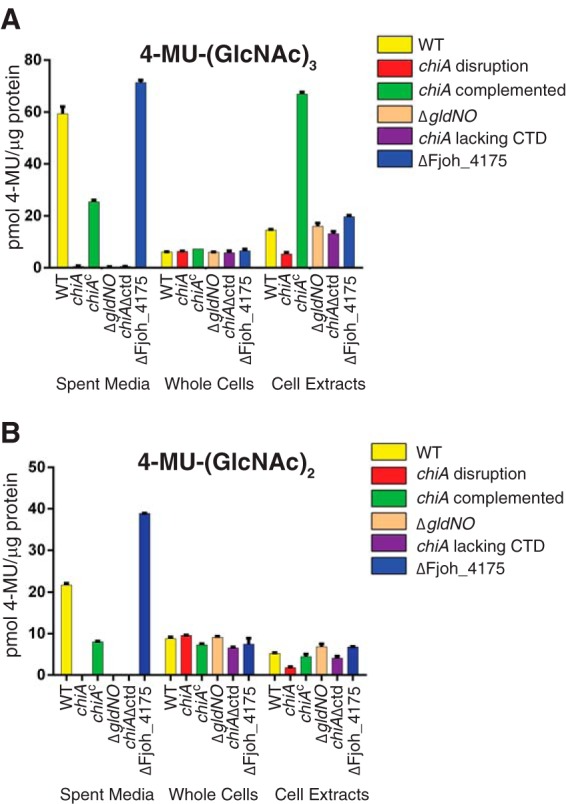
Chitinase activities of wild-type and mutant cells. Chitinase activities of spent media, intact cells, and cell extracts were determined using the synthetic substrates 4-MU-(GlcNAc)2, and 4-MU-(GlcNAc)3. Equal amounts of each sample, based on the protein content of the cell suspension, were incubated with 10 nmol of synthetic substrate for 30 min at 37°C, and the amount of 4-MU released was determined by measuring fluorescence emission at 460 nm following excitation at 360 nm. Yellow, wild-type F. johnsoniae UW101. Red, chiA mutant CJ1808. Green, CJ1808 with pSSK05, which carries chiA. Tan, gldNO deletion mutant CJ1631A. Purple, CJ2325, which produces ChiA lacking the C-terminal 106 amino acids. Blue, Fjoh_4175 deletion mutant CJ2355.
ChiA is a soluble extracellular protein.
A portion of ChiA spanning the N-terminal GH18 domain (GH18N) was overexpressed in E. coli, and polyclonal antiserum was raised against this fragment. The antiserum was used to detect ChiA in cultures of wild-type F. johnsoniae. ChiA was present primarily in the cell-free spent medium, with little if any cell-associated ChiA (Fig. 4A). ChiA was detected in spent medium from wild-type cells but was absent from spent medium of the chiA mutant (Fig. 4B). Introduction of chiA into the mutant on pSSK05 restored production of ChiA. Expression from pSSK05 resulted in large amounts of ChiA, and fragments of ChiA, in the spent medium and in intact cells (Fig. 4B). The extra bands observed for the complemented strain may be the result of failure to efficiently secrete the overexpressed protein, perhaps resulting in degradation. To determine whether ChiA in the spent medium from wild-type cells was present in soluble form or was associated with membrane vesicles or cell debris, particulate material was pelleted by ultracentrifugation twice at 352,900 × g for 30 min. ChiA was found in the soluble fraction (Fig. 4C), indicating that ChiA is a soluble secreted protein.
FIG 4.
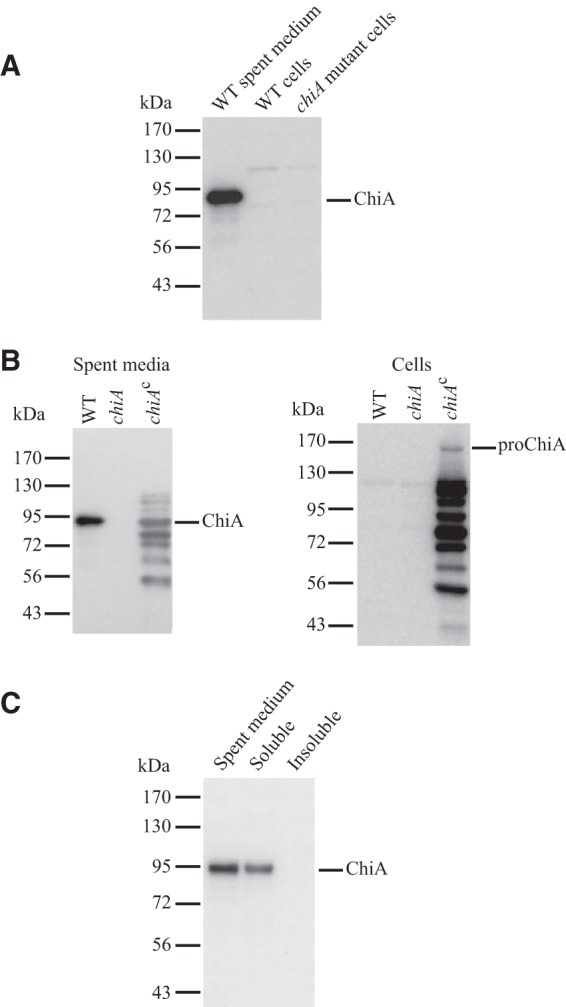
ChiA is a soluble extracellular protein. (A and B) Spent media and whole cells of the wild type (WT), chiA mutant (chiA), and chiA mutant complemented with pSSK05 (chiAc) were examined for ChiA by SDS-PAGE followed by Western blotting. (C) Spent medium from a culture of wild-type F. johnsoniae UW101 was examined for ChiA before (spent medium) and after fractionation into soluble and insoluble (membrane) fractions by centrifugation at 352,900 × g for 30 min. For each panel, equal amounts of each sample based on the starting material were loaded in each lane. For cells this corresponded to 15 μg protein, whereas for spent medium this corresponded to the equivalent volume of spent medium that contained 15 μg cell protein before the cells were removed.
ChiA is predicted to be 166 kDa in size after removal of its N-terminal signal peptide. We refer to the cell-associated 166-kDa protein as proChiA (Fig. 4B). In contrast, the secreted ChiA detected with our antiserum migrated with an apparent molecular mass of approximately 92 kDa (Fig. 4), suggesting that the protein was proteolytically processed. The antiserum used to detect ChiA was raised against the region spanning the N-terminal GH18 domain and thus did not efficiently detect other regions of ChiA released during processing.
Cell-free spent media from wild-type, chiA mutant, and complemented cells were also examined by SDS-PAGE followed by silver staining to identify the prominent bands. Proteins of approximately 92 kDa and 65 kDa were observed in spent media from wild-type and complemented cells but not in spent medium of the chiA mutant (Fig. 5). The 92-kDa and 65-kDa proteins secreted by cells of the chiA mutant CJ1808 complemented with pSSK05, which expresses ChiA, were identified by LC-MS/MS (see Fig. S1 and S2 in the supplemental material). The two bands corresponded to the two GH18 domains of ChiA with flanking sequences, suggesting that proteolytic processing released these in soluble form. The 92-kDa band (ChiAGH18N) corresponded to a fragment containing the amino-proximal GH18 fragment and adjacent regions, and the 65-kDa band (ChiAGH18C) corresponded to a fragment containing primarily the carboxy-proximal GH18 and adjacent regions. LC-MS/MS analysis of the 65-kDa band also revealed two peptides corresponding to regions of the protein closer to the amino terminus. These were apparently low-abundance proteins in the band (see Fig. S2 in the supplemental material) and may have corresponded to breakdown products of the 92-kDa protein. Such breakdown products of approximately 65 kDa were expected, because they were also observed by Western blotting using antiserum against the amino-terminal portion of ChiA (Fig. 4B). LC-MS/MS analysis of the 65-kDa and 92-kDa proteins failed to detect the amino-terminal signal peptide and the C-terminal 91-amino-acid region.
FIG 5.
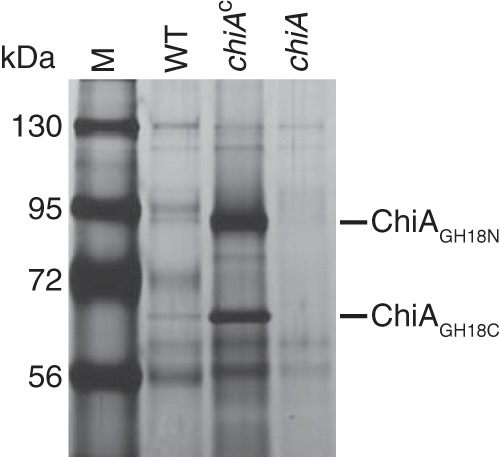
Analysis of secreted ChiA protein by SDS-PAGE. Equal amounts of cell-free spent media from cultures of the wild type (WT), chiA mutant CJ1808 (chiA), and CJ1808 complemented with pSSK05, which carries chiA (chiAc), were separated by SDS-PAGE. Proteins were detected by silver staining. ChiAGH18N and ChiAGH18C refer to amino-proximal and carboxy-proximal GH18-containing fragments, respectively, of ChiA as determined by LC-MS/MS analysis of material from the 92-kDa and 65-kDa bands (see Fig. S1 and S2 in the supplemental material). M, molecular mass markers.
The T9SS is required for secretion of ChiA.
Mutations in the F. johnsoniae T9SS genes gldK, gldL, gldM, gldN, sprA, sprE, and sprT result in defects in chitin utilization (6, 8, 19, 20). The effect of such mutations on secretion of ChiA was examined. ChiA accumulated in the spent culture medium of wild-type cells but not in that of cells of the T9SS mutants (Fig. 6A). Complementation of the T9SS mutants with plasmids carrying the appropriate T9SS genes restored secretion of ChiA into the culture medium. Cells were also examined for ChiA. Wild-type cells accumulated little if any ChiA protein, whereas cells of the T9SS mutants accumulated some proChiA (Fig. 6B). The amount of proChiA that accumulated in cells of the T9SS mutants was less than the amount of processed ChiA found in the culture fluid of wild-type cells. We do not know the reason for this, but likely explanations could include decreased expression of ChiA or degradation of the improperly localized ChiA in the T9SS mutants.
FIG 6.
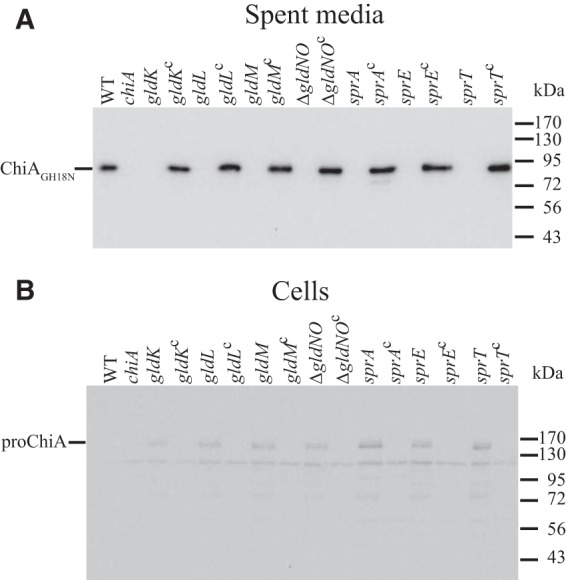
Mutations in T9SS genes disrupt secretion of ChiA. Cell-free spent media (A) and cells (B) were examined for ChiA by SDS-PAGE followed by Western blotting using antiserum against recombinant ChiA. WT, wild-type F. johnsoniae UW101. chiA, chiA mutant CJ1808. gldK, gldK mutant UW102-57. gldKc, UW102-57 complemented with pTB99, which carries gldK. gldL, gldL mutant UW102-344. gldLc, UW102-344 complemented with pTB81a, which carries gldL. gldM, gldM mutant UW102-176. gldMc, UW102-176 complemented with pTB94a, which carries gldM. ΔgldNO, gldNO deletion mutant CJ1631A. ΔgldNOc, CJ1631A complemented with pTB79, which carries gldN. sprA, sprA mutant UW102-3. sprAc, UW102-3 complemented with pSN48, which carries sprA. sprE, sprE mutant FJ149. sprEc, FJ149 complemented with pNap2, which carries sprE. sprT, sprT mutant KDF001. sprTc, KDF001 complemented with pKF002, which carries sprT. Samples loaded in panel B corresponded to 15 μg protein per lane, and samples loaded in panel A corresponded to the volume of spent medium that contained 15 μg cell protein before the cells were removed.
The C-terminal region of ChiA is necessary and sufficient for secretion.
F. johnsoniae proteins known to be secreted by the T9SS have conserved CTDs that belong to TIGRFAM families TIGR04131 (such as SprB) and TIGR04183 (such as RemA). ChiA does not exhibit strong similarity to members of these TIGRFAM families (see Fig. S3 and S4 in the supplemental material). However, ChiA has a C-terminal region of unknown function that might perform a similar role, and this region does exhibit limited similarity to the CTDs of TIGR04183 members (see Fig. S4 in the supplemental material). A mutant, CJ2325, which expresses ChiA lacking the C-terminal 106 amino acids was constructed. Cells of CJ2325 failed to utilize chitin and failed to accumulate ChiA extracellularly (Fig. 3 and 7). Instead, the mutant cells accumulated cell-associated proChiA, suggesting a role for the C-terminal region in secretion.
FIG 7.
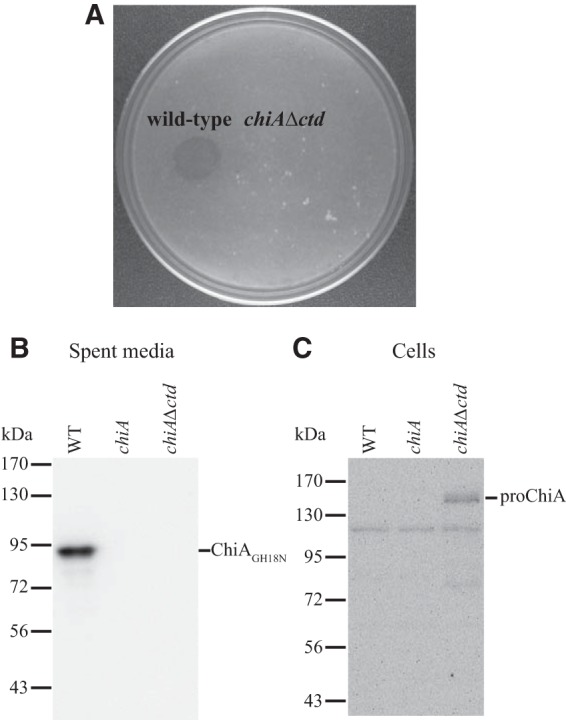
The C-terminal region of ChiA is required for chitin utilization. (A) Deletion of the region of chiA encoding the CTD results in defects in chitin utilization. Approximately 106 cells of wild-type F. johnsoniae CJ1827 and of the chiA Δctd mutant CJ2325 were spotted on MYA-chitin medium and incubated at 25°C for 2.5 days. (B and C) The C-terminal region of ChiA is required for secretion. Cell-free spent media (B) and cells (C) were examined for ChiA by SDS-PAGE followed by Western blotting using antiserum against recombinant ChiA. WT, wild-type F. johnsoniae. chiA, chiA disruption mutant CJ1808. chiA Δctd, chiA mutant CJ2325, which encodes ChiA lacking its CTD. Samples loaded in panel C corresponded to 15 μg protein per lane, and samples loaded in panel B corresponded to the volume of spent medium that contained 15 μg cell protein before the cells were removed.
To determine if the ChiA CTD is sufficient for secretion, we constructed a plasmid that expressed the foreign protein mCherry sandwiched between the ChiA signal peptide (at the amino terminus) and a 105-amino-acid region encompassing the ChiA CTD (at the carboxy terminus). Expression of mCherry with the 105-amino-acid C-terminal region of ChiA resulted in accumulation of mCherry in the spent medium, whereas expression of mCherry without the ChiA C-terminal region did not (Fig. 8). Cells of a strain lacking the T9SS genes gldN and gldO failed to secrete mCherry-CTDChiA. Together these results suggest that the CTD of ChiA targets proteins for secretion by the T9SS.
FIG 8.
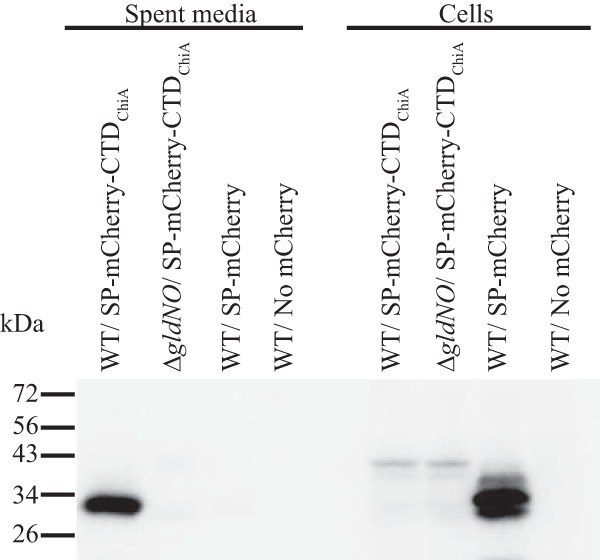
The ChiA CTD is sufficient for secretion of the heterologous protein mCherry. Cell-free spent media and whole cells were analyzed for cultures of wild-type (WT) cells carrying pSSK54, which expresses mCherry with the N-terminal signal peptide from ChiA (SP-mCherry), or carrying pSSK52, which expresses mCherry with the N-terminal signal peptide from ChiA and the 105-amino-acid C-terminal region of ChiA (SP-mCherry-CTDChiA). Cells and spent media from cultures of the T9SS mutant CJ1631A (ΔgldNO) were also analyzed. “No mCherry” indicates spent media or cells from F. johnsoniae carrying the control vector pCP23. Cell samples corresponded to 15 μg protein per lane, and samples from spent media corresponded to the volume of spent medium that contained 15 μg cell protein before the cells were removed. Samples were separated by SDS-PAGE, and mCherry was detected using antiserum against mCherry.
Cells of the chiA mutant exhibit wild-type gliding motility.
Many mutants of F. johnsoniae that have defects in chitin utilization have been studied, and each of these also had defects in gliding motility (6, 8, 19, 20, 26, 36, 37). The connection between chitin utilization and gliding motility was unclear until it was recognized that assembly of the gliding motility apparatus and secretion of ChiA relied on the same T9SS. Unlike the T9SS mutants, cells of the chiA mutant CJ1808 formed spreading colonies on agar (see Fig. S5 in the supplemental material), and cells moved rapidly over surfaces similarly to wild-type cells, demonstrating that the ability to utilize chitin was not required for gliding motility.
DISCUSSION
F. johnsoniae rapidly digests insoluble chitin, and its genome encodes five predicted chitinases that may have roles in this process (3). Here we demonstrate that one of these, ChiA, is essential for chitin utilization. ChiA is a soluble extracellular enzyme. Disruption of chiA eliminates soluble extracellular chitinase activity and results in an inability of cells to digest insoluble chitin. Cell-associated chitinase activities were still present, presumably contributed by some of the remaining four predicted chitinases. Some of these may reside on the cell surface, whereas others may be periplasmic, allowing the digestion of oligomers of chitin that have been imported across the outer membrane. At least one of the predicted chitinases, Fjoh_4175, was not essential for chitin digestion under the conditions employed. Deletion of Fjoh_4175 failed to decrease the levels of secreted or cell-associated chitinase activities. Fjoh_4175 may be a minor chitinase, may not be expressed under the conditions of our experiments, or may not assist in digestion of the form of chitin (insoluble colloidal chitin prepared from crab shells) used in this study.
ChiA appears to be secreted by the T9SS. Mutations in any of the T9SS genes resulted in failure to secrete soluble ChiA and accumulation of unprocessed proChiA inside cells. Sequence analysis did not predict that ChiA would be secreted by the T9SS. Most other proteins known to be secreted by T9SSs have CTDs that belong to TIGRFAM families TIGR04131 (which includes SprB) and TIGR04183 (which includes RemA), but ChiA was not recognized by algorithms used to detect members of these families. ChiA does, however, have a region C-terminal to the predicted catalytic domains that appears to perform a similar function. Deletion of this region resulted in failure to secrete ChiA, and attachment of this region to a foreign protein, mCherry, resulted in secretion in soluble form. BLASTP analysis of the C-terminal 106-amino-acid sequence against the nonredundant protein sequences in GenBank identified only three proteins that exhibit significant similarity to the F. johnsoniae ChiA CTD. Each of these is a predicted chitinase from Flavobacterium species, and each is similar to ChiA not only over the CTD but also over the entire protein. The ChiA CTD thus does not seem to represent a large family of previously unrecognized T9SS CTDs. The results reported here and those previously published (21–25, 38–40) indicate that CTDs are involved in secretion by the T9SS but that there is considerable variation in the CTD sequences. Some features are apparently common to all, including the presence of multiple positively charged residues near the carboxy terminus. The sequence variations suggest that the structures of the CTDs may be more important than the exact sequences in targeting proteins to the T9SS.
The T9SS probably secretes many proteins besides those involved in motility and chitin utilization. F. johnsoniae is predicted to encode 53 proteins that have CTDs that belong to TIGRFAM families TIGR04131 and TIGR04183, which are thought to target proteins for secretion by the T9SS (8). This list includes nine predicted glycoside hydrolases, one polysaccharide lyase, and four peptidases in addition to proteins such as SprB and RemA that were previously known to be secreted by this system. Mutations that disrupt the T9SS are thus likely to have pleiotropic effects in addition to the known defects in motility and chitin utilization.
Proteins secreted by T9SSs often localize to the outer surface of the outer membrane. The F. johnsoniae motility proteins SprB and RemA and the P. gingivalis gingipains and adhesins are examples of such proteins (8, 18, 23, 24, 39–41). Some of these surface-associated proteins have been shown to be modified by attachment of a glycolipid that may anchor them to the cell surface (41). This modification has been proposed as a general property of proteins secreted by T9SSs (39). Our results with ChiA indicate that it is secreted in soluble form by the T9SS, suggesting that this type of modification is not a requirement for secretion by the system. Many members of the phylum Bacteroidetes have dozens or even hundreds of genes predicted to encode CTD-containing proteins secreted by T9SSs (5, 39). It is perhaps not surprising that among this large number of proteins, some are cell-surface associated proteins and others are soluble extracellular proteins.
ChiA may undergo multiple processing events during or after secretion from the cell. ChiA has a predicted cleavable N-terminal signal peptide that is thought to target it to the Sec system for transit across the cytoplasmic membrane. Mutations in secDF result in decreased digestion of chitin (42), which is consistent with the involvement of the Sec system in export of ChiA. T9SS mediated secretion across the outer membrane may involve cleavage of the CTD from ChiA. This C-terminal region was not detected by LC-MS/MS analysis of secreted ChiA, suggesting that it may have been removed from the major secreted products. Evidence of removal of T9SS CTDs by proteolytic processing during secretion was recently reported for proteins of P. gingivalis, Tannerella forsythia, Parabacteroides distasonis, Prevotella intermedia, and Cytophaga hutchinsonii (7, 22, 24, 39, 40). PG0026, also referred to as PorU, was required for removal of the CTDs from secreted proteins of P. gingivalis (22). F. johnsoniae has an ortholog of PorU that may perform a similar function. In addition to removal of the amino- and carboxy-terminal regions, F. johnsoniae ChiA may have had another processing event involving proteolysis between the two GH18 domains, resulting in two major soluble products, each predicted to have chitinase activity. We do not know whether this processing event is important for the functioning of ChiA or whether it is the result of nonspecific digestion by one of the many proteases produced by F. johnsoniae (3). ChiAGH18N is similar in sequence to Bacillus circulans ChiA1 (43), and ChiAGH18C is similar in sequence to B. circulans ChiD (44) (see Fig. S6 and S7 in the supplemental material). The two GH18 domains of F. johnsoniae ChiA exhibit little similarity to each other, but each has the signature active-site sequence (DXXDXDXE) that is characteristic of GH18 chitinases (35). B. circulans chiA1 and chiD are adjacent on the genome, and the protein products presumably work together to digest chitin (44). Additional experiments are needed to determine the exact functions of F. johnsoniae ChiAGH18N and ChiAGH18C and the synergy, if any, that they exhibit.
In addition to their catalytic domains, many bacterial chitinases have carbohydrate-binding modules (CBMs) belonging to family 5 or 12 (35). Examination of proteins encoded by the F. johnsoniae genome revealed the complete absence of such domains, as presented in the Carbohydrate Active enZYmes (CAZY) database (http://www.cazy.org/) (45, 46). ChiA itself does not harbor a recognizable CBM of any family. ChiA may have novel CBMs or may rely on its catalytic domains to interact with chitin.
ChiA is required for F. johnsoniae chitin digestion, but further experiments are needed to determine if the four other predicted chitinases (3) have roles in this process. Synergistic interactions between multiple chitinases may be needed to efficiently digest crystalline chitin in nature. Such synergy has been demonstrated for the chitinases of other bacteria (47). Variations in organization of the polymer strands in the α, β, and γ forms of crystalline chitin, variation in the degree of acetylation, and variations regarding the components complexed with chitin (proteins, polysaccharides, or inorganic materials) (35, 48) may mean that no single enzyme or set of enzymes is ideally suited to efficiently digest all forms of chitin. Additional experiments will be needed to determine the entire complement of chitinolytic enzymes that allows optimal digestion and utilization of different forms of chitin by F. johnsoniae cells.
Chitin is one of the most abundant biopolymers produced on earth and is a common component of organisms in soil, freshwater, and marine environments (2, 49–51). Bacteria of the phylum Bacteroidetes are important and sometimes dominant members of the chitinolytic communities in these environments (52). Members of the phylum Bacteroidetes are known to use novel strategies to utilize polysaccharides (53), and an improved understanding of the mechanisms used by F. johnsoniae and related bacteria to digest chitin may enhance our understanding of the turnover of this important biopolymer in nature. Such studies may also have more targeted practical value. For example, F. johnsoniae and closely related bacteria are common in the rhizosphere (54–59) and have been linked to enhanced disease resistance of plants (57, 60). Chitinases released by these bacteria may contribute to this resistance because of their activities against fungal or insect pests. The chitin-modifying enzymes may also be useful for the production of chitooligosaccharides and other pharmaceutical products (61).
The results presented in this paper identify the major extracellular chitinase, ChiA, of F. johnsoniae and characterize its secretion by the T9SS. The motility adhesins SprB and RemA are also known to be secreted by the F. johnsoniae T9SS. Unlike SprB and RemA, ChiA is not attached to the cell surface after secretion but instead is released in soluble form. Further study is needed to determine what features of the proteins result in anchoring on the cell surface or release in soluble form. The results of such studies could have broad implications. Analysis of the F. johnsoniae genome suggests that many proteins are secreted by the T9SS, and these are likely to undergo similar CTD recognition and processing events. Moreover, T9SSs appear to be common in the large and diverse phylum Bacteroidetes (5, 39), and an understanding of the events occurring during secretion of cell surface and extracellular proteins of these bacteria will likely be of both practical and fundamental significance.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by MCB-1021721 from the National Science Foundation and by a University of Wisconsin—Milwaukee Research Growth Initiative Grant.
We thank Greg Sabat and Greg Barrett-Wilt at the University of Wisconsin—Madison Mass Spectrometry Facility for LC-MS/MS analyses and Ryan Rhodes and Yongtao Zhu for helpful suggestions.
Footnotes
Published ahead of print 20 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01170-13.
REFERENCES
- 1.Stanier RY. 1947. Studies on non-fruiting myxobacteria. I. Cytophaga johnsonae, n. sp., a chitin-decomposing myxobacterium. J. Bacteriol. 53:297–315 [PMC free article] [PubMed] [Google Scholar]
- 2.Muzzarelli RAA. 1999. Native, industrial and fossil chitins, p 1–6 In Jolles P, Muzzarelli RAA. (ed), Chitin and chitinases. Birkhauser Verlag, Basel, Switzerland: [DOI] [PubMed] [Google Scholar]
- 3.McBride MJ, Xie G, Martens EC, Lapidus A, Henrissat B, Rhodes RG, Goltsman E, Wang W, Xu J, Hunnicutt DW, Staroscik AM, Hoover TR, Cheng YQ, Stein JL. 2009. Novel features of the polysaccharide-digesting gliding bacterium Flavobacterium johnsoniae as revealed by genome sequence analysis. Appl. Environ. Microbiol. 75:6864–6875. 10.1128/AEM.01495-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang LYE, Pate JL, Betzig RJ. 1984. Isolation and characterization of nonspreading mutants of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 159:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride MJ, Zhu Y. 2013. Gliding motility and Por secretion system genes are widespread among members of the phylum Bacteroidetes. J. Bacteriol. 195:270–278. 10.1128/JB.01962-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato K, Naito M, Yukitake H, Hirakawa H, Shoji M, McBride MJ, Rhodes RG, Nakayama K. 2010. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 107:276–281. 10.1073/pnas.0912010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K, Yukitake H, Narita Y, Shoji M, Naito M, Nakayama K. 2013. Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol. Lett. 338:68–76. 10.1111/1574-6968.12028 [DOI] [PubMed] [Google Scholar]
- 8.Shrivastava A, Johnston JJ, van Baaren JM, McBride MJ. 2013. Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell-surface gliding motility adhesins SprB and RemA. J. Bacteriol. 195:3201–3212. 10.1128/JB.00333-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Economou A, Christie PJ, Fernandez RC, Palmer T, Plano GV, Pugsley AP. 2006. Secretion by numbers: protein traffic in prokaryotes. Mol. Microbiol. 62:308–319. 10.1111/j.1365-2958.2006.05377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland IB. 2010. The extaordinary diversity of bacterial protein secretion mechanisms. Methods Mol. Biol. 619:1–20. 10.1007/978-1-60327-412-8_1 [DOI] [PubMed] [Google Scholar]
- 11.Busch A, Waksman G. 2012. Chaperone-usher pathways: diversity and pilus assembly mechanism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367:1112–1122. 10.1098/rstb.2011.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chagnot C, Zorgani MA, Astruc T, Desvaux M. 2013. Proteinaceous determinants of surface colonization in bacteria: bacterial adhesion and biofilm formation from a protein secretion perspective. Front. Microbiol. 4:303. 10.3389/fmicb.2013.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desvaux M, Hebraud M, Talon R, Henderson IR. 2009. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 17:139–145. 10.1016/j.tim.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60:131–147. 10.1146/annurev.micro.60.080805.142106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5:883–891. 10.1038/nrmicro1773 [DOI] [PubMed] [Google Scholar]
- 16.Nakane D, Sato K, Wada H, McBride MJ, Nakayama K. 2013. Helical flow of surface protein required for bacterial gliding motility. Proc. Natl. Acad. Sci. U. S. A. 110:11145–11150. 10.1073/pnas.1219753110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson SS, Bollampalli S, McBride MJ. 2008. SprB is a cell surface component of the Flavobacterium johnsoniae gliding motility machinery. J. Bacteriol. 190:2851–2857. 10.1128/JB.01904-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrivastava A, Rhodes RG, Pochiraju S, Nakane D, McBride MJ. 2012. Flavobacterium johnsoniae RemA is a mobile cell surface lectin involved in gliding. J. Bacteriol. 194:3678–3688. 10.1128/JB.00588-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes RG, Samarasam MN, Shrivastava A, van Baaren JM, Pochiraju S, Bollampalli S, McBride MJ. 2010. Flavobacterium johnsoniae gldN and gldO are partially redundant genes required for gliding motility and surface localization of SprB. J. Bacteriol. 192:1201–1211. 10.1128/JB.01495-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes RG, Samarasam MN, Van Groll EJ, McBride MJ. 2011. Mutations in Flavobacterium johnsoniae sprE result in defects in gliding motility and protein secretion. J. Bacteriol. 193:5322–5327. 10.1128/JB.05480-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Sakai E, Veith PD, Shoji M, Kikuchi Y, Yukitake H, Ohara N, Naito M, Okamoto K, Reynolds EC, Nakayama K. 2005. Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J. Biol. Chem. 280:8668–8677. 10.1074/jbc.M413544200 [DOI] [PubMed] [Google Scholar]
- 22.Glew MD, Veith PD, Peng B, Chen YY, Gorasia DG, Yang Q, Slakeski N, Chen D, Moore C, Crawford S, Reynolds E. 2012. PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J. Biol. Chem. 287:24605–24617. 10.1074/jbc.M112.369223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, Reynolds EC. 2006. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J. Bacteriol. 188:6376–6386. 10.1128/JB.00731-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoji M, Sato K, Yukitake H, Kondo Y, Narita Y, Kadowaki T, Naito M, Nakayama K. 2011. Por secretion system-dependent secretion and glycosylation of Porphyromonas gingivalis hemin-binding protein 35. PLoS One 6:e21372. 10.1371/journal.pone.0021372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slakeski N, Seers CA, Ng K, Moore C, Cleal SM, Veith PD, Lo AW, Reynolds EC. 2011. C-terminal domain residues important for secretion and attachment of RgpB in Porphyromonas gingivalis. J. Bacteriol. 193:132–142. 10.1128/JB.00773-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride MJ, Braun TF. 2004. GldI is a lipoprotein that is required for Flavobacterium johnsoniae gliding motility and chitin utilization. J. Bacteriol. 186:2295–2302. 10.1128/JB.186.8.2295-2302.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride MJ, Kempf MJ. 1996. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 178:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, McBride MJ, Subramaniam S. 2007. Cell-surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J. Bacteriol. 189:7503–7506. 10.1128/JB.00957-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunnicutt DW, McBride MJ. 2000. Cloning and characterization of the Flavobacterium johnsoniae gliding motility genes, gldB and gldC. J. Bacteriol. 182:911–918. 10.1128/JB.182.4.911-918.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodes RG, Pucker HG, McBride MJ. 2011. Development and use of a gene deletion strategy for Flavobacterium johnsoniae to identify the redundant motility genes remF, remG, remH, and remI. J. Bacteriol. 193:2418–2428. 10.1128/JB.00117-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichenbach H. 1992. The genus Lysobacter, p 3256–3275 In Balows A, Truper H, Dworkin M, Harder W, Schleifer K. (ed), The prokaryotes, 2nd ed. Springer-Verlag, Berlin, Germany [Google Scholar]
- 32.Lee C, Levin A, Branton D. 1987. Copper staining: a five minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal. Biochem. 166:308–312. 10.1016/0003-2697(87)90579-3 [DOI] [PubMed] [Google Scholar]
- 33.Crosby HA, Heiniger EK, Harwood CS, Escalante-Semerena JC. 2010. Reversible N epsilon-lysine acetylation regulates the activity of acyl-CoA synthetases involved in anaerobic benzoate catabolism in Rhodopseudomonas palustris. Mol. Microbiol. 76:874–888. 10.1111/j.1365-2958.2010.07127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saveliev SV, Woodroofe CC, Sabat G, Adams CM, Klaubert D, Wood K, Urh M. 2013. Mass spectrometry compatible surfactant for optimized in-gel protein digestion. Anal. Chem. 85:907–914. 10.1021/ac302423t [DOI] [PubMed] [Google Scholar]
- 35.Hoell IA, Vaaje-Kolstad G, Eijsink VGH. 2010. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. 27:331–366. 10.1080/02648725.2010.10648156 [DOI] [PubMed] [Google Scholar]
- 36.Braun TF, McBride MJ. 2005. Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility. J. Bacteriol. 187:2628–2637. 10.1128/JB.187.8.2628-2637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBride MJ, Braun TF, Brust JL. 2003. Flavobacterium johnsoniae GldH is a lipoprotein that is required for gliding motility and chitin utilization. J. Bacteriol. 185:6648–6657. 10.1128/JB.185.22.6648-6657.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen KA, Travis J, Potempa J. 2007. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-negative bacteria? J. Bacteriol. 189:833–843. 10.1128/JB.01530-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veith PD, Nor Muhammad NA, Dashper SG, Likic VA, Gorasia DG, Chen D, Byrne SJ, Catmull DV, Reynolds EC. 2013. Protein substrates of a novel secretion system are numerous in the Bacteroidetes phylum and have in common a cleavable C-terminal secretion signal, extensive post-translational modification and cell surface attachment. J. Proteome Res. 12:4449–4461. 10.1021/pr400487b [DOI] [PubMed] [Google Scholar]
- 40.Zhou XY, Gao JL, Hunter N, Potempa J, Nguyen KA. 2013. Sequence-independent processing site of the C-terminal domain (CTD) influences maturation of the RgpB protease from Porphyromonas gingivalis. Mol. Microbiol. 89:903–917. 10.1111/mmi.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YY, Peng B, Yang Q, Glew MD, Veith PD, Cross KJ, Goldie KN, Chen D, O'Brien-Simpson N, Dashper SG, Reynolds EC. 2011. The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol. Microbiol. 79:1380–1401. 10.1111/j.1365-2958.2010.07530.x [DOI] [PubMed] [Google Scholar]
- 42.Nelson SS, McBride MJ. 2006. Mutations in Flavobacterium johnsoniae secDF result in defects in gliding motility and chitin utilization. J. Bacteriol. 188:348–351. 10.1128/JB.188.1.348-351.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Suzuki K, Oyanagi W, Ohnishi K, Tanaka H. 1990. Gene cloning of chitinase A1 from Bacillus circulans WL-12 revealed its evolutionary relationship to Serratia chitinase and to the type III homology units of fibronectin. J. Biol. Chem. 265:15659–15665 [PubMed] [Google Scholar]
- 44.Watanabe T, Oyanagi W, Suzuki K, Ohnishi K, Tanaka H. 1992. Structure of the gene encoding chitinase D of Bacillus circulans WL-12 and possible homology of the enzyme to other prokaryotic chitinases and class III plant chitinases. J. Bacteriol. 174:408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. 2004. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem. J. 382:769–781. 10.1042/BJ20040892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. 2009. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37:D233–238. 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brurberg MB, Nes IF, Eijsink VG. 1996. Comparative studies of chitinases A and B from Serratia marcescens. Microbiology 142:1581–1589. 10.1099/13500872-142-7-1581 [DOI] [PubMed] [Google Scholar]
- 48.Beier S, Bertilsson S. 2013. Bacterial chitin degradation—mechanisms and ecophysiological strategies. Front. Microbiol. 4:149. 10.3389/fmicb.2013.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeuniaux C, Vossfoucart MF. 1991. Chitin biomass and production in the marine-environment. Biochem. Syst. Ecol. 19:347–356. 10.1016/0305-1978(91)90051-Z [DOI] [Google Scholar]
- 50.Kirchner M. 1995. Microbial colonization of copepod body surfaces and chitin degradation in the sea. Helgolander Meeresun. 49:201–212. 10.1007/BF02368350 [DOI] [Google Scholar]
- 51.Poulicek M, Jeuniaux C. 1991. Chitin biodegradation in marine environments—an experimental approach. Biochem. Syst. Ecol. 19:385–394. 10.1016/0305-1978(91)90055-5 [DOI] [Google Scholar]
- 52.Kirchman DL. 2002. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 39:91–100. 10.1111/j.1574-6941.2002.tb00910.x [DOI] [PubMed] [Google Scholar]
- 53.Salyers AA, Reeves A, D'Elia J. 1996. Solving the problem of how to eat something as big as yourself: diverse bacterial strategies for degrading polysaccharides. J. Ind. Microbiol. 17:470–476 [Google Scholar]
- 54.Johansen JE, Binnerup SJ. 2002. Contribution of Cytophaga-like bacteria to the potential of turnover of carbon, nitrogen, and phosphorus by bacteria in the rhizosphere of barley (Hordeum vulgare L.). Microb. Ecol. 43:298–306. 10.1007/s00248-002-2006-z [DOI] [PubMed] [Google Scholar]
- 55.Johansen JE, Nielsen P, Binnerup SJ. 2009. Identification and potential enzyme capacity of flavobacteria isolated from the rhizosphere of barley (Hordeum vulgare L.). Can. J. Microbiol. 55:234–241. 10.1139/W08-116 [DOI] [PubMed] [Google Scholar]
- 56.Kolton M, Green SJ, Harel YM, Sela N, Elad Y, Cytryn E. 2012. Draft genome sequence of Flavobacterium sp strain F52, isolated from the rhizosphere of bell pepper (Capsicum annuum L. cv. Maccabi). J. Bacteriol. 194:5462–5463. 10.1128/JB.01249-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolton M, Harel YM, Pasternak Z, Graber ER, Elad Y, Cytryn E. 2011. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 77:4924–4930. 10.1128/AEM.00148-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manter DK, Delgado JA, Holm DG, Stong RA. 2010. Pyrosequencing reveals a highly diverse and cultivar-specific bacterial endophyte community in potato roots. Microb. Ecol. 60:157–166. 10.1007/s00248-010-9658-x [DOI] [PubMed] [Google Scholar]
- 59.Peterson SB, Dunn AK, Klimowicz AK, Handelsman J. 2006. Peptidoglycan from Bacillus cereus mediates commensalism with rhizosphere bacteria from the Cytophaga-Flavobacterium group. Appl. Environ. Microbiol. 72:5421–5427. 10.1128/AEM.02928-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sang MK, Kim KD. 2012. The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J. Appl. Microbiol. 113:383–398. 10.1111/j.1365-2672.2012.05330.x [DOI] [PubMed] [Google Scholar]
- 61.Dahiya N, Tewari R, Hoondal GS. 2006. Biotechnological aspects of chitinolytic enzymes: a review. Appl. Microbiol. Biotechnol. 71:773–782. 10.1007/s00253-005-0183-7 [DOI] [PubMed] [Google Scholar]
- 62.Wolkin RH, Pate JL. 1985. Selection for nonadherent or nonhydrophobic mutants co-selects for nonspreading mutants of Cytophaga johnsonae and other gliding bacteria. J. Gen. Microbiol. 131:737–750 [Google Scholar]
- 63.Braun TF, Khubbar MK, Saffarini DA, McBride MJ. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J. Bacteriol. 187:6943–6952. 10.1128/JB.187.20.6943-6952.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal S, Hunnicutt DW, McBride MJ. 1997. Cloning and characterization of the Flavobacterium johnsoniae (Cytophaga johnsonae) gliding motility gene, gldA. Proc. Natl. Acad. Sci. U. S. A. 94:12139–12144. 10.1073/pnas.94.22.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L-Y, Shoemaker NB, Salyers AA. 1995. Location and characterization of the transfer region of a Bacteroides conjugative transposon and regulation of the transfer genes. J. Bacteriol. 177:4992–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. 2007. The Tol2kit: a multisite Gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dynam. 236:3088–3099. 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- 67.Nelson SS, Glocka PP, Agarwal S, Grimm DP, McBride MJ. 2007. Flavobacterium johnsoniae SprA is a cell-surface protein involved in gliding motility. J. Bacteriol. 189:7145–7150. 10.1128/JB.00892-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


