ABSTRACT
The development of a vaccine that can induce high titers of functional antibodies against HIV-1 remains a high priority. We have developed an adjuvant based on an oil-in-water emulsion that incorporates Toll-like receptor (TLR) ligands to test whether triggering multiple pathogen-associated molecular pattern receptors could enhance immunogenicity. Compared to single TLR agonists or other pairwise combinations, TLR7/8 and TLR9 agonists combined were able to elicit the highest titers of binding, neutralizing, and antibody-dependent cellular cytotoxicity-mediating antibodies against the protein immunogen, transmitted/founder HIV-1 envelope gp140 (B.63521). We further found that the combination of TLR7/8 and TLR9 agonists was associated with the release of CXCL10 (IP-10), suggesting that this adjuvant formulation may have optimally stimulated innate and adaptive immunity to elicit high titers of antibodies.
IMPORTANCE Combining TLR agonists in an adjuvant formulation resulted in higher antibody levels compared to an adjuvant without TLR agonists. Adjuvants that combine TLR agonists may be useful for enhancing antibody responses to HIV-1 vaccines.
INTRODUCTION
Most effective vaccines induce antibody responses that correlate with protection (1), and for many vaccines, antibody levels remain elevated for decades (2). Vaccines that employ live-attenuated strains of pathogens are often effective by themselves, but many subunit or killed immunogens use adjuvants to provide a delivery formulation to enhance vaccine-induced protective antibody responses. Until recently, the only adjuvant approved for human use in the United States was alum (3), but in 2009 the U.S. Food and Drug Administration (FDA) licensed a human papillomavirus vaccine formulated with a lipid-based adjuvant that contained a Toll-like receptor 4 (TLR4) ligand (4); this was the first TLR ligand-vaccine combination approved by the FDA for use in humans. While adjuvant options for human use in the United States have been limited, adjuvants other than alum have been used for veterinary vaccines in the United States (5), and novel adjuvant formulations for use in humans have been licensed outside the United States (6). Studies have shown that adjuvants could permit antigen sparing (e.g., novel influenza vaccines that would require rapid deployment to combat new pandemics [7]) and could increase the potency and breadth of antibody responses (8, 9). Adjuvants have also been suggested as a means to overcome the problems of inducing broadly neutralizing antibodies against both HIV-1 and influenza virus (10).
Adjuvants can mediate their effects on humoral immunity by multiple mechanisms. These include enhancing uptake of antigen and/or providing a depot of antigen at the site of immunization. Moreover, adjuvants can activate distinct innate immune pathways that profoundly alter both humoral and cellular immunity. Accordingly, the addition of TLR agonists have been used to boost vaccine responses and has been suggested as one means of enhancing the response to HIV-1 immunogens (10). Based on the similarity of TLR expression in rhesus macaques and humans (11), we undertook a systematic comparison of oil-in-water emulsions containing different combinations of TLR agonists formulated with a highly antigenic HIV-1 transmitted/founder envelope B.63521 gp140. We found that a combination of TLR7/8 and TLR9 agonists optimally enhanced humoral responses to HIV-1 envelope protein (Env). This enhanced response was associated with elevated levels of the chemokine CXCL10 (IP-10) in plasma.
MATERIALS AND METHODS
Adjuvant production.
The base adjuvant Span85-Tween 80-squalene (STS) was prepared by mixing Span85, Tween 80, and squalene (Sigma-Aldrich, St. Louis, MO; catalog numbers 85549, P8192, and 53626, respectively) at 0.5, 0.5, and 5% (vol/vol), respectively, in 1× phosphate-buffered saline (PBS; Gibco, Grand Island, NY) (12). For adjuvant combinations containing TLR agonists, 0.2 mg of lipid A (Avanti Polar Lipids, Alabaster, AL; catalog no. 699200P), 6.67 mg of CpG oligodeoxynucleotides (oCpGs; The Midland Certified Reagent Co., Midland, TX; catalog no. ODN10103), and 1 mg of R848 (InvivoGen, San Diego, CA; catalog no. Tlrl-r848-5) were added/ml as shown in Table 1. In all cases, adjuvant mixtures were homogenized for 5 min at room temperature, using an OMNI International homogenizer using plastic soft tissue tips (Kennesaw, GA). After initial homogenization, the adjuvant mixtures were further homogenized using a Microfluidizer model M-110S (Microfluidics Corp., Newton, MA). The cooling coil was kept on ice and the processor was primed three times with 8 ml of homogenized STS mixture, and then each adjuvant mixture was pumped through the instrument at 14,000 lb/in2, making 5 passes prior to collection of the final product. Stable emulsions were stored at room temperature prior to use.
TABLE 1.
Adjuvant compositions
| Adjuvant | TLR agonistsa |
||
|---|---|---|---|
| Lipid A (TLR4) | oCpGs (TLR9) | R848 (TLR7/8) | |
| STS | — | — | — |
| STS+LA | X | — | — |
| STS+oCpG | — | X | — |
| STS+R848 | — | — | X |
| STS+LA+oCpG | X | X | — |
| STS+LA+R848 | X | — | X |
| STS+oCpG+R848 | — | X | X |
TLR agonists were incorporated at 0.2 mg/ml for lipid A, 6.67 mg/ml for oCpGs, and 1 mg/ml for R848. —, Absent from the formulation, X, present in the formulation.
HIV-1 envelope proteins and V1V2 reagents.
Envelope glycoproteins were produced as described for gp140 B.63521 (13), group M consensus gp140 ConS (1, 14), gp120 B.JRFL (2, 13), gp120 E.A244gD+Δ11 (3, 15), and E.A244gDneg (4, 15). HIV-1 Env variable loop 1-variable loop 2 (V1V2) constructs for the detection of V1V2-specific antibodies were produced as described for A.Q23_V1V2, AE.A244_V1V2, and C.1086_V1V2 (5, 16). In addition, constructs using murine leukemia virus (MLV) gp70 as a scaffold were prepared as described previously (6, 17); the gp70 constructs included gp70_B.CaseA2_V1/V2 and MLV gp70 carrier protein without V1V2 sequence as a negative control.
SPR studies.
Surface plasmon resonance (SPR) analysis of the antigenicity of immunogen gp140 B.63521 (Fig. 1) was performed by immobilizing monoclonal antibodies (MAbs) or soluble CD4 (sCD4) to a CM5 sensor chip at about 4,000 response units (RU) using standard amine coupling chemistry as described previously (7, 18–20). Envelope gp140 B.63521 formulated in PBS (pH 7.4) or in 15% STS adjuvant in PBS (equivalent to the final adjuvant concentration used for immunization studies) for 1 h and diluted to 20 μg/ml before being injected for 3 min over the captured MAb or CD4 immobilized surface and binding monitored on a BIAcore 3000 instrument (GE Healthcare Life Sciences, Uppsala, Sweden). Once Env was captured, detection MAbs were injected at 100 μg/ml at a flow rate of 30 μl/min for 2 min over the surface under the same conditions to evaluate antigenicity. Nonspecific binding responses were monitored over a control IgG (palivizumab) surface on the same sensor chip and used for in-line reference subtraction. The data were analyzed using the BIAeval 4.1 software (GE Healthcare).
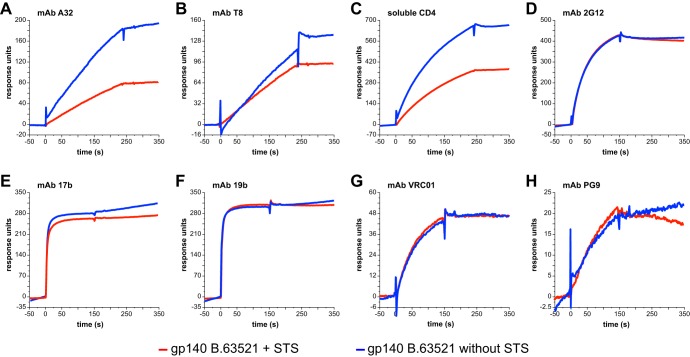
FIG 1.
Envelope protein formulated in an oil-in-water emulsion adjuvant retains antigenicity. Envelope protein gp140 B.63521 formulated in 15% STS adjuvant plus PBS (red curve) or in PBS alone (blue curve) was flowed over captured MAbs or sCD4 and then probed for binding to MAbs. Representative examples of capture by MAbs A32 (A) or T8 (B) or sCD4 (C) are shown. Antigenicity was tested by flowing MAbs over the captured protein. Representative examples are shown, as follows: 2G12 testing sCD4-captured Env (D), 17b testing sCD4-captured Env (E), 19b testing sCD4-captured Env (F), VRC01 testing T8-captured Env (G), and PG9 testing A32-captured Env (H).
Animal studies.
Thirty-three adult rhesus monkeys (Macaca mulatta) were used in the present study. All animals were housed at BioQual (Rockville, MD) and maintained in accordance with the Association for Accreditation of Laboratory Animal Care guidelines at the National Institutes of Health. Twenty-one animals were immunized intramuscularly with gp140 B.63521 at 100 μg/animal/immunization time point; each animal received a 1-ml total injection volume divided into four sites. The final immunization cocktail contained 15% of adjuvant (Table 1), 0.1 mg of gp140 B.63521/ml, with the remaining volume being sterile saline. Three animals per group were immunized for each of the seven adjuvant formulations (Table 1); for this part of the study peripheral blood was obtained prior to study initiation, on each immunization day, and 2 weeks after each immunization.
To assess for adjuvant effect alone, 12 animals were immunized intramuscularly with adjuvant formulations in the absence of immunogen; these animals received the same total injection volume as those in the prior group. Three animals per group were used for this experiment that compared STS, STS+oCpG, STS+R848, and STS+oCpG+R848. For this part of the study, peripheral blood was obtained immediately prior to immunization and at 6 h, 24 h, 7 days, and 14 days after adjuvant administration.
To assess for the durability of the immune response, six rhesus macaques were assessed in a separate study. These animals received a heterologous prime-boost regimen that included gp120 E.A244gDneg given at weeks 0, 18, 24, and 84. The animals received no immunizations between weeks 24 and 84. Peripheral blood samples taken at weeks 0, 24, 26, 30, 32, 36, 38, 40, 84, 86, and 88 were available for analysis.
Isolation of plasma and PBMCs.
EDTA anticoagulated blood from immunized monkeys was centrifuged over Ficoll (Ficoll-Paque) and plasma and peripheral blood mononuclear cell (PBMC) layers were collected in separate tubes. PBMCs were washed in 1× PBS containing 2% fetal bovine serum. Prior to use, plasma was divided into aliquots and stored at −80°C; PBMCs were cryopreserved in freezing media (10% dimethyl sulfoxide–90% fetal bovine serum) and stored in the vapor phase of liquid nitrogen.
Antibody characterization by ELISA.
Plasma samples were studied for reactivity to HIV-1 Env protein antigens and V1V2 constructs by enzyme-linked immunosorbent assay (ELISA) as described previously (8, 9, 21). Blocking assays were performed as described previously (10, 13) modified to use rhesus detection reagents (10, 21). Plasma titers were determined using an initial 1:25 dilution (for Env reagents) or 1:30 (for V1V2 reagents), followed by a 3-fold dilution series; the background for each analyte was determined based on nonimmune plasma. Endpoint titers were calculated by applying four-parameter logistic regression to the binding data using the drc package in R (12, 22) and using the resulting parameters to calculate the dilution value that would be equivalent to optical density (OD) = 3 × the background for Env reagents and OD = 4 × the background for V1V2 reagents.
Neutralization assays.
Neutralization of Env-pseudotyped viruses was measured in 96-well culture plates by using Tat-regulated firefly luciferase (Luc) reporter gene expression to quantify reductions in virus infection in TZM-bl cells (23). Neutralization of Env.IMC.LucR viruses was similarly measured by using Tat-regulated Renilla Luc reporter gene expression A3R5 cells (24). Both assays have been formally optimized and validated (25, 26). Heat-inactivated (56°C, 1 h) serum samples were assayed at 3-fold dilutions starting at 1:20. Neutralization titers (50% inhibitory dose [ID50]) are the serum dilutions at which relative luminescence units (RLU) were reduced by 50% compared to RLU in virus control wells after subtraction of background RLU in cell control wells. Assay stocks of Env-pseudotyped viruses and Env.IMC.LucR viruses were produced by transfection in 293T cells and titrated in either TZM-bl or A3R5 cells as described previously (23, 27).
ADCC assay.
Antibody-dependent cell-mediated cytotoxicity (ADCC) activity was detected according to our previously described ADCC-GranToxiLux (GTL) procedure (28). We used HIV-1 subtype B Bal gp120 (Immune Technology Corp., New York, NY) to coat the CEM.NKRCCR5 (29) target cell line; in a second set of experiments, the chronically B.HXB2c-infected cell line A1953 was used (28). All PBMC samples from seronegative donors used as effector cells were obtained according to the appropriate Institutional Review Board protocol. An effector to target (E:T) ratio of 30:1 for whole PBMC effector cells was used. A minimum of 2.5 × 103 events representing viable gp120-coated target cells was acquired for each well. Data analysis was performed using FlowJo 9.3.2 software (TreeStar, Ashland, OR). The results are expressed as the percent granzyme B (GzB) activity, defined as the percentage of cells positive for proteolytically active GzB out of the total viable target cell population. The final results are expressed after subtracting the background represented by %GzB activity observed in wells containing effector and target cell populations in the absence of plasma. The results were considered positive if %GzB activity after background subtraction was >8% for the gp120-coated target cells.
Cytokine and chemokine assays.
Plasma from the second monkey group was assayed for the presence of cytokines/chemokines using a cytokine monkey magnetic 29-plex panel (Life Technologies, Frederick, MD) and was performed according to the manufacturer's instructions. Biomarker profiling was performed in the Duke Human Vaccine Institute Immune Reconstitution & Biomarker Analysis Shared Resource Facility (Durham, NC) under the direction of Gregory D. Sempowski. Plasma samples were also tested for alpha interferon (IFN-α) by capture ELISA according to the manufacturer's instructions (Mabtech, Mariemont, OH).
Statistical analysis.
Statistical tests were performed in SAS v9.2 (SAS Institute, Cary, NC). Comparisons of preplanned contrasts for multiple groups were performed using multiple-degree-of-freedom F tests using PROC GLM in SAS with subsequent pairwise comparisons. When multiple comparisons were performed, P values were corrected using the false discovery rate method (30). The statistical test used is noted when P values are presented. Estimation of antibody half-life was performed using NLIN in SAS. Graphs of the data were created using GraphPad Prism (GraphPad Software, La Jolla, CA) with layout in Illustrator CS5 (Adobe, San Jose, CA).
RESULTS
Oil-in-water emulsion adjuvant formulation retains antigenicity of HIV-1 Env immunogens.
In order to determine whether an oil-in-water emulsion adjuvant would disrupt the antigenicity of a recombinant HIV-1 envelope protein, we formulated gp140 B.63521 in 15% STS adjuvant in PBS and tested for antigenicity using SPR. Using immobilized reagents A32, T8, and sCD4, we captured the envelope protein and then probed for binding to a panel of MAbs. In all cases, antigenicity was retained in the adjuvant formulation (Fig. 1). We did observe cross blocking of binding for the combinations VRC01 testing of sCD4-bound protein and 17b testing of T8 bound protein; both of these were expected based on the known binding epitopes for each reagent.
Oil-in-water emulsion adjuvants combined with Env immunogens elicit HIV-1 Env-reactive antibodies.
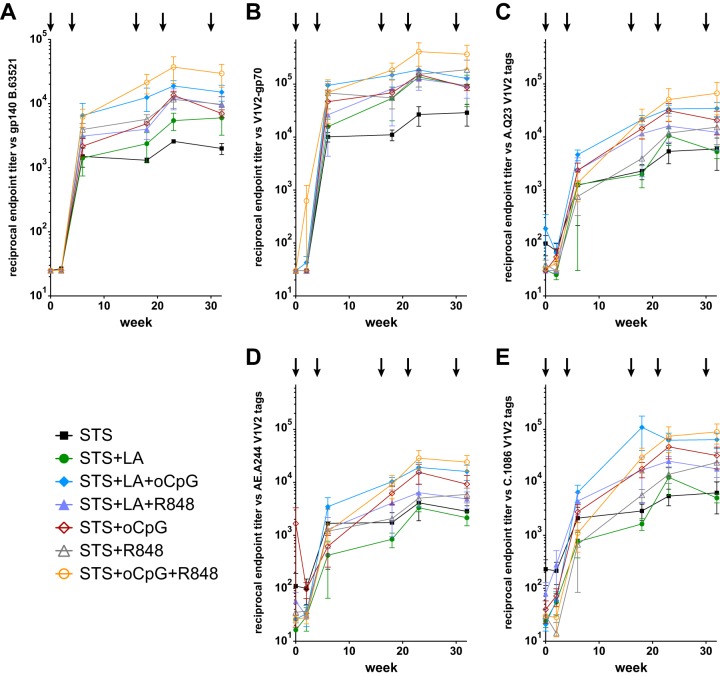
We then assessed the ability of the different squalene-based adjuvant formulations (Table 1) to induce antibodies reactive with the transmitted/founder Env immunogen, gp140 B.63521. Env gp140 B.63521 is a highly antigenic protein that expresses sites for broadly neutralizing monoclonal antibodies (MAbs) directed against glycans, variable loop 1-variable loop 2 (V1V2), the CD4 binding site (CD4bs), and the membrane-proximal external region (MPER). After two immunizations, all animals developed robust titers against gp140 B.63521 that remained elevated for the remainder of the study (Fig. 2A; see also Fig. S1 in the supplemental material). After the fifth immunization, animals immunized with adjuvant STS had the lowest endpoint titer (1:1,905; 95% confidence interval [CI] 1:728 to 1:4,989), while animals immunized with STS+oCpG+R848 had the highest endpoint titer (1:25,704; 95% CI = 1:5,420 to 1:121,899; P = 0.004 [t test]).
FIG 2.
Oil-in-water emulsion adjuvants combined with Env immunogens elicit HIV-1 Env-reactive and V1V2-directed antibodies. (A) All animals developed antibodies against gp140 B.63521. After five immunizations, STS elicited the lowest endpoint titer (1:1,905; 95% CI = 1:728 to 1:4,989), and STS+oCpG+R848 elicited the highest endpoint titer (1:25,704; 95% CI = 1:5,420 to 1:121,899; P = 0.004 [t test]). (B) Binding to case A2 V1V2-gp70. STS elicited the lowest endpoint titer (1:19,890; 95% CI = 1:912 to 1:434,011), and STS+oCpG+R848 elicited the highest titer (1:298,498; 95% CI = 1:44,722 to 1:1,992,000). Similar binding patters were observed against V1V2 tags representing clades A, CRF01_AE, and C (C, D, and E, respectively).
HIV-1 Env-reactive antibodies elicited by STS+oCpG+R848 are durable.
In order to assess the durability of HIV-1 Env-reactive antibodies, we performed a second study wherein rhesus macaques were immunized with gp120 E.A244gDneg envelope and where the animals were rested without immunization for 60 weeks. Plasma antibody binding was assessed against the immunogen, and the data were evaluated for a decline in antibody titers. The estimated half-life of antibodies during this interval was 8.5 weeks (Fig. 3), a value similar to that observed in large-scale human clinical trials (31).
FIG 3.
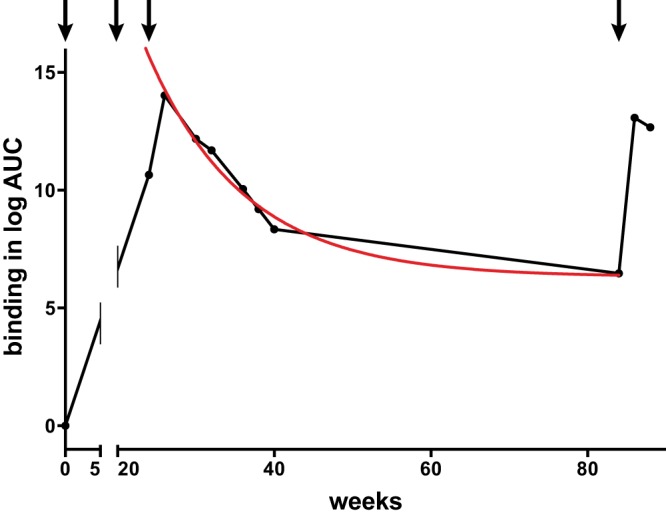
STS+oCpG+R848 adjuvant elicits a durable antibody response. Animals were immunized with gp120 E.A244gDneg (arrows) and tested between weeks 24 and 84 (60-week rest without immunization). ELISA binding data plotted as the log area under the curve (log AUC) were fitted to a decay model (red curve), and the half-life of the antibody response was found to be 8.5 weeks. Antibody levels remained above background at week 84 and were boosted by a subsequent immunization.
TLR agonists enhance epitope-specific HIV-1 Env reactive antibody levels.
We further assessed the plasma samples for the presence of epitope-specific antibodies through direct binding assays. The RV144 ALVAC HIV-1/AIDSVAX B/E vaccine trial demonstrated 31.2% vaccine efficacy (32), and the immune correlates analysis showed a direct correlation between antibodies directed against V1V2 and a decreased risk of infection (33). All rhesus macaque groups in the present study developed antibodies that bound to B.CaseA2 V1V2-gp70, the same protein used in the immune correlates case-control study (33) (Fig. 2B). After the fifth immunization, adjuvant STS again elicited the lowest endpoint titer (1:19,890; 95% CI = 1:912 to 1:434,011) while STS+oCpG+R848 elicited the highest titer (1:298,498; 95% CI = 1:44,722 to 1:1,992,000). We analyzed for the presence of V1V2 cross-clade reactivity and found a similar trend but with lower titers against clade A, CRF01_AE, and C V1V2 protein constructs (Fig. 2C, D, and E, respectively).
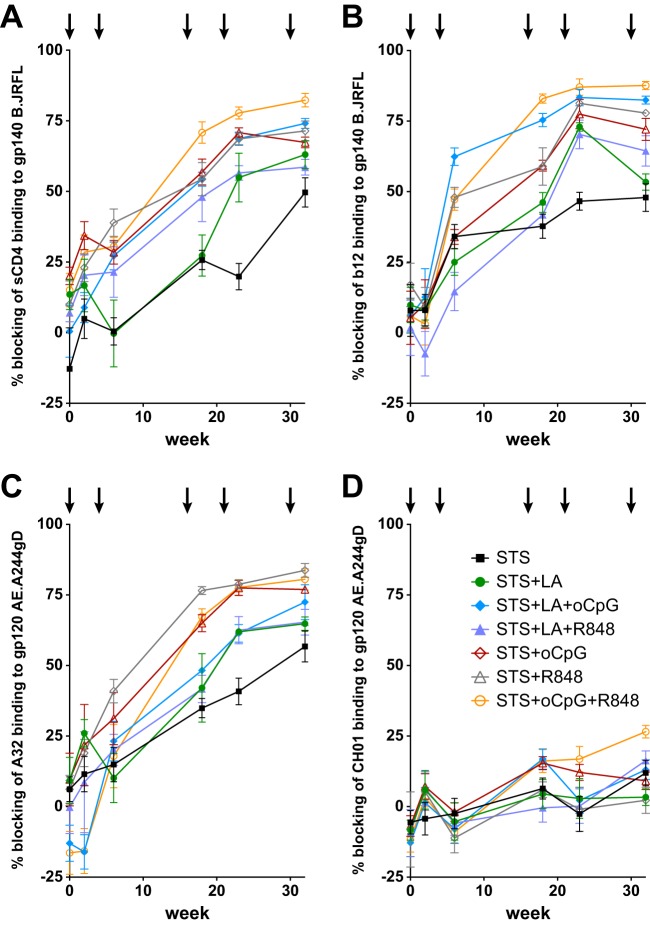
We next looked for the presence of antibodies against other known specificities through the use of assays of plasma competition with MAbs of known specificity or soluble CD4 (sCD4). All adjuvant combinations were able to elicit antibodies that blocked the binding of sCD4 and MAb b12 to gp140 B.JRFL (Fig. 4A and 4B, respectively). Similar to the pattern observed with overall Env binding, after five immunizations antibodies were lowest for STS and highest for STS+oCpG+R848, both for those that blocked sCD4 binding (blocking of 50 and 82%, respectively; Fig. 4A) and those that blocked CD4bs MAb b12 binding (blocking of 48 and 88%, respectively; Fig. 4B). Blocking of ADCC-mediating MAb A32 showed a different pattern; after five immunizations, STS elicited 57% blocking while STS+R848 was slightly higher than STS+oCpG+R848 (84% versus 81%, respectively; Fig. 4C). No adjuvant combination elicited high level blocking of V1V2-binding broadly neutralizing MAb CH01; low-level blocking was seen in some samples after five immunizations (e.g., STS+oCpG+R848 at 27%; Fig. 4D).
FIG 4.
Plasma antibodies block the binding of MAbs and sCD4. Plasma antibodies blocked binding of labeled ligands to Env proteins. Binding of sCD4 (A) and MAb b12 (B) to gp140 B.JRFL was inhibited by immune plasma. Titers were lowest for STS and highest for STS+oCpG+R848. (C) Blocking of ADCC-mediating MAb A32 was lowest for STS and highest for STS+R848. (D) Low-level blocking of broadly neutralizing MAb CH01 was found in the STS+oCpG+R848-immunized group.
Combined TLR agonists elicit higher titers of neutralizing and ADCC-mediating antibodies.
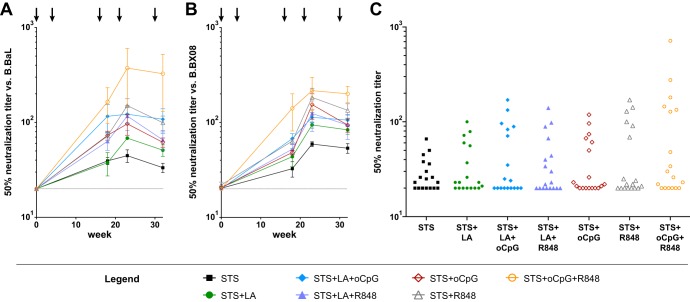
We tested for the ability of vaccine-elicited antibodies to neutralize HIV-1 in the TZM-bl pseudovirus neutralization assay. Similar to what was observed for binding antibody titers, the 50% neutralization titers against B.BaL and B.BX08 were lowest for STS alone and highest for STS+oCpG+R848 (Fig. 5A and B). The largest difference in neutralization activity was observed after the fourth immunization, and titers were found to be slightly lower after the fifth immunization (Fig. 5AB). The neutralization titer against B.BaL elicited by STS was 1:45, while that elicited by STS+oCpG+R848 was 1:374 (t test P < 0.05); similarly, the titers against B.BX08 elicited by these two adjuvant combinations after four immunizations were 1:59 and 1:216, respectively (t test P < 0.05).
FIG 5.
Plasma neutralization. Neutralization titers with 50% activity against B.BaL (A) and B.BX08 (B) were determined. After four immunizations, the titer elicited against B.BaL by STS was 1:45 versus STS+oCpG+R848 at 1:374 (P < 0.05 [t test]). Titers against B.BX08 were 1:59 and 1:216, respectively, at the same time points (P < 0.05 [t test]). (C) Aggregate neutralization data for six isolates tested in the TZM-bl assay (B.BaL.26, B.Bx08.16, B.PVO.4, C.DU123.6, C.DU172.17, and CRF01_AE.93TH976.1) show that STS+oCpG+R848 demonstrated a trend toward higher titers compared to the other adjuvant groups. The data underlying this figure appear in Table S1 in the supplemental material.
In order to further assess the ability of this group of adjuvants to elicit neutralizing antibodies, we tested for neutralizing activity against additional virus isolates in the TZM-bl assay. Aggregate neutralization data showed a trend for STS+oCpG+R848 to elicit higher neutralization titers compared to the other adjuvant formulations tested (Fig. 5C; see Table S1 in the supplemental material). From all groups tested, one animal in the STS+oCpG+R848 group developed activity against a tier 2 virus (C.DU123.6) in the TZM-bl assay at week 32 (Table 2; see also Table S1 in the supplemental material). In order to further assess the breadth of activity elicited by STS+oCpG+R848, we tested samples from that group in the A3R5.7 assay and found that the animals developed substantial breadth, with only two isolates being resistant to all samples (Table 2). Activity was observed against viruses in all HIV-1 subtypes tested with the exception of subtype G.
TABLE 2.
Neutralization assays for the STS+oCpG+R848 group
| Assay | Neutralization (serum dilution)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal 4841 |
Animal 4842 |
Animal 4843 |
|||||||
| Wk 0 | Wk 23 | Wk 32 | Wk 0 | Wk 23 | Wk 32 | Wk 0 | Wk 23 | Wk 32 | |
| TZM-bl assay | |||||||||
| B.BaL.26 | <20 | 827 | 716 | <20 | 173 | 134 | <20 | 121 | 128 |
| B.Bx08.16 | <20 | 380 | 276 | <20 | 114 | 181 | <20 | 155 | 145 |
| B.PVO.4 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| C.DU123.6 | <20 | 26 | 48 | 24 | 27 | 34 | <20 | <20 | 26 |
| C.DU172.17 | 21 | 26 | 30 | <20 | 27 | 22 | <20 | <20 | <20 |
| CRF01_AE.93TH976.17 | <20 | 31 | 23 | <20 | 24 | <20 | <20 | 22 | <20 |
| A3R5.7 assay | |||||||||
| A.398-F1-F6_20.LucR | 33 | >43,740 | 26,396 | 34 | NT | 2,494 | 26 | NT | 5,621 |
| AC.246-F3_C10_2.LucR | 26 | 193 | 247 | 10 | 149 | 256 | 23 | 72 | 283 |
| AC.BJOX002000.03.2.LucR | 32 | 219 | 152 | 45 | 114 | 146 | 24 | 65 | 103 |
| B.TRO.11.LucR | 28 | 78 | 171 | 35 | 157 | 184 | <20 | 49 | 74 |
| B. X2278_C2_B6.LucR | <20 | 4,925 | 3,159 | 21 | 250 | 373 | <20 | 129 | 62 |
| C.25710-2.43.LucR | 20 | 55 | 109 | <20 | 55 | 70 | <20 | 32 | 40 |
| C.Ce703010217_B6.LucR | 59 | 43 | 50 | 49 | 76 | 69 | 40 | 37 | 58 |
| G.X1635_S2_B10.LucR | 36 | 72 | 98 | 55 | 57 | 78 | 41 | 55 | 66 |
| CRF01_AE.CNE8.LucR | <20 | 2,557 | 1,317 | <20 | 508 | 407 | <20 | 327 | 804 |
| CRF01_AE.CNE55.LucR | <20 | 57 | 92 | <20 | 48 | 75 | <20 | <20 | 22 |
| CRF07_BC.Ce1176_A3.LucR | 22 | 37 | 42 | 30 | 48 | 77 | <20 | 40 | 28 |
| CRF07_BC.CH119.10.LucR | 29 | 184 | 184 | 37 | 108 | 157 | <20 | 55 | 92 |
The serum dilutions for three animals at three different time points postinfection at which the relative luminescence units (RLUs) were reduced 50% compared to virus control wells (no test sample) are presented. NT, not tested. Boldfacing indicates responses above the background observed at week 0.
We then tested for the ability of vaccine-elicited antibodies to mediate ADCC against B.BaL-coated target cells (Fig. 6; see also Fig. S2 in the supplemental material). After five intramuscular immunizations, STS elicited the lowest endpoint ADCC titer (1:2,317, 95% CI = 1:579 to 1:9,268), while STS+oCpG+R848 elicited the highest titer (1:47,753, 95% CI = 1:27,227 to 1:83,946; P = 0.001 [t test]; Fig. 6A). The peak activity in the ADCC assay displayed a similar pattern, with the peak activity elicited by STS at 14.9% ± 0.9% versus that elicited by STS+oCpG+R848 at 31.5% ± 0.9% (P = 0.0002 [t test]; Fig. 6B). The ADCC activity elicited by STS+oCpG+R848 was markedly higher than that elicited by any other adjuvant tested; the next highest group after five immunizations was STS+LA+oCpG, which elicited an endpoint titer of 1:18,290 and peak activity of 22.0% (Fig. 6). We also observed ADCC activity against the chronically B.HXB2c-infected cell line A1953 (see Fig. S2 in the supplemental material), confirming that the antibodies elicited by this vaccination regimen can recognize infected cell targets.
FIG 6.
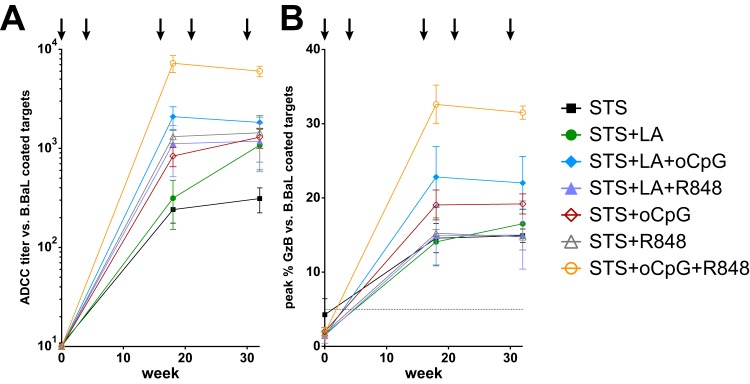
Plasma ADCC activity. (A) The ADCC plasma titer against B.BaL-coated target cells after five immunizations was lowest for STS (1:2,317, 95% CI = 1:579 to 1:9,268) and was highest for STS+oCpG+R848 (1:47,753, 95% CI = 1:27,227 to 1:83,946; P = 0.001 [t test]). (B) ADCC peak activity was lowest for STS at 14.9% ± 0.9% and highest for STS+oCpG+R848 at 31.5% ± 0.9% (P = 0.0002 [t test]).
Formulation of TLR7/8 and TLR9 selectively results in elevation of plasma CXCL10 (IP-10).
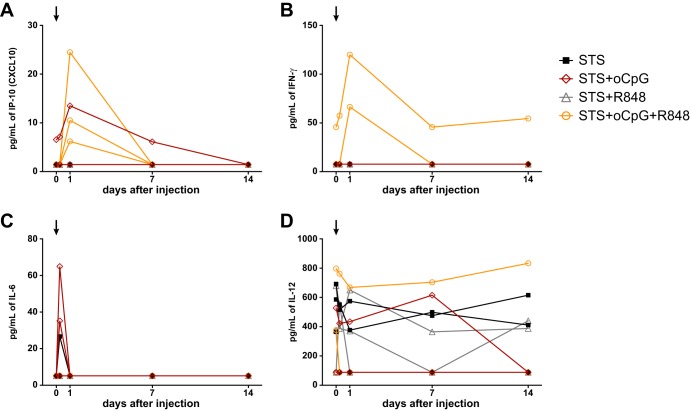
We sought to determine whether TLR agonist combinations could elicit cytokines and chemokines that correlate with the observed differences in induced antibody levels. Using a separate group of naive rhesus macaques, we immunized with oil-in-water emulsions containing TLR agonists. We obtained plasma samples after 6 h, 24 h, 1 week, and 2 weeks and tested for the presence of 30 cytokines/chemokines. Across all five time points, we found no detectable changes for 11 markers (IFN-α, interleukin-4 [IL-4], IL-5, IL-10, IL-15, IL-17, granulocyte-monocyte colony-stimulating factor, granulocyte colony-stimulating factor, macrophage inflammatory protein 1α [MIP-1α], MIP-1β, and vascular endothelial growth factor [data not shown]). For 16 markers, we observed detectable changes across different time points, but without a discernible pattern related to immunization; representative data are shown for IL-12 (Fig. 7D), and similar nonspecific patterns were observed for 15 other markers (IL-1 receptor α, IL-1β, IL-2, IL-8, fibroblast growth factor basic, monocyte chemotactic protein 1[MCP-1], eotaxin, RANTES, epidermal growth factor, hepatocyte growth factor, chemokine [C-C motif] ligand [CCL]-22, chemokine [C-X-C motif] ligand 9 [CXCL-9], CXCL-11, macrophage migration inhibitory factor, and tumor necrosis factor alpha [data not shown]).
FIG 7.
Cytokine/chemokine stimulation by TLR agonists in oil-in-water emulsion. (A) CXCL10 (IP-10) was elevated in 3/3 animals immunized with STS+oCpG+R848, peaking at 24 h and returning to baseline by 1 week. One of three animals immunized with STS+oCpG had an elevation at baseline, peaked at 24 h, and waned at later points. (B) IFN-γ was transiently elevated in two of three animals immunized with STS+oCpG+R848, peaking at 24 h. (C) IL-6 was elevated in two of three animals immunized with STS+oCpG and in one of three animals immunized with STS alone. The peak occurred at 6 h and returned to baseline by 24 h. (D) IL-12 showed a nonspecific pattern over the course of the study. This lack of a pattern was observed for 15 other chemokines/cytokines (data not shown).
A transient elevation of IFN-γ was observed in two of three animals immunized with STS+oCpG+R848; the elevation peaked at 24 h and had returned to baseline by the 1 week time point (Fig. 7B). Similarly, a transient elevation of IL-6 was observed in two of three animals immunized with STS+oCpG and in one of three animals immunized with STS alone, with the peak occurring at 6 h and returning to baseline by 24 h (Fig. 7C). These elevations were not observed in any of the other immunized animals (Fig. 7B and C).
When we measured CXCL10 (IFN-γ-induced protein 10 [IP-10]), we found that three of three animals immunized with STS+oCpG+R848 had elevated levels that peaked 24 h after immunization and that had returned to baseline by the 1-week time point (Fig. 7A). Only one other animal, immunized with STS+oCpG, had elevated levels of CXCL10; this animal had higher levels at baseline that then returned to baseline by the 1-week time point (Fig. 7A).
DISCUSSION
In this study, we have demonstrated that a combination of a TLR9 agonist (type B oCpG [ODN10103]) with a TLR7/8 agonist (R848) formulated in an oil-in-water emulsion with transmitted/founder Env gp140 B.63521 resulted in significantly higher levels of ADCC and tier 1 neutralizing antibodies compared to other TLR agonist combinations. Adjuvants stimulate immune responses through triggering of host defense pathways designed to recognize damage or threats. By combining agonists for different molecular pattern recognition pathways, an adjuvant can trigger signaling events that activate both immediate inflammatory responses and later adaptive T and B cell antipathogen responses (34, 35). Using a combination of stimuli to selectively trigger the immune system with an adjuvant formulation will be critical for enhancing vaccine responses against HIV-1 Env immunogens.
There is a global need for an effective vaccine against HIV-1 (36), but, to date, only one of the four HIV-1 vaccine efficacy trials in humans has shown any degree of protection from infection (32, 37–40). Although the estimated vaccine efficacy afforded by the RV144 ALVAC HIV-1/AIDSVAX B/E vaccine regimen was modest and short-lived (32), a correlates of risk analysis showed that higher levels of IgG antibodies against V1V2 directly correlated with decreased risk of infection (33). Moreover, it has recently been shown that RV144 vaccine-elicited antibodies directed against specific epitopes in the V1V2 loops can mediate ADCC (41) and neutralize some isolates of HIV-1 (16, 42). A major problem with the alum-based vaccine used in RV144 was that antibody responses declined over the first year following completion of the vaccine regimen, such that the estimated vaccine efficacy at 1 year was 60.5% (43) and at year 3 was 31.2% (32). Similar declines in antibody levels were observed in the VAX003 and VAX004 trials (31, 39, 44), highlighting the need for immunogen-adjuvant combinations that can elicit durable responses. Although much work remains to develop novel immunogens that can extend these results, the parallel development of adjuvants that enhance the magnitude and durability of desirable responses is critically important. The benchmark for vaccine-elicited immune responses are currently licensed vaccines that have been shown to elicit protective antibodies lasting many years (2). However, these analyses have depended on the availability of samples collected over many years and exclude data of initial rapid decline seen following immunization (45, 46). Although we do not have data extending over multiple years, our demonstration of sustained antibody levels lasting for 1 year (Fig. 3) suggests that adjuvant preparations such as those reported here might be useful in enhancing the durability of HIV-1 vaccine responses.
One desirable feature in an adjuvant formulation is that it not perturb the antigenicity of the vaccine insert. For this reason it was important that the protein immunogen, transmitted/founder Env gp140 B.63521, retained antigenicity to a panel of MAbs representing targets of HIV-1 vaccine development. As shown in Fig. 1, the immunogen formulated in an oil-in-water emulsion retained binding to broad neutralizing antibodies VRC01, PG9, and 2G12, as well as MAbs 17b, 19b, A32, and T8, in addition to sCD4. These data show that this vaccine formulation is capable of preserving critical antigenic targets and that the immunogen can be presented to the immune system in an optimal manner.
To date, regulatory authorities in the United States have only licensed two adjuvants for human use: alum, which is used in a number of vaccines (3), and a lipid-based adjuvant system formulated with a human papillomavirus vaccine (4). However, even though they were not added to the vaccine formulation, it has been shown that the presence of “hidden” TLR agonists enhances the immunogenicity of FDA-approved vaccines directed against Streptococcus pneumoniae (47). In addition, live attenuated vaccines trigger TLR pathways during the time of abortive infection that induces long-lasting immunity (48). Thus, there is precedent for the use of TLR agonists in vaccines, and the FDA has issued guidance on what would be needed to license new adjuvants in the context of influenza vaccination (49).
Thus, TLR agonists would appear to be excellent choices for enhancing vaccine responses in humans. It is critically important to select animal models that mimic human responses when evaluating TLR agonists in adjuvant formulations that may ultimately lead to a licensed product. Although mice and other small-animal models are attractive for cost and ease-of-use concerns, substantial differences between human and mouse response to TLR agonists have been described (50). In contrast, TLR7/8 and TLR9 agonists have been shown to be similarly active in both human and rhesus plasmacytoid dendritic cells and B cells and to enhance antibody responses (51). TLR4 expression in cells of the immune system has also been shown to be similar between humans and rhesus macaques (11). Based on these data, we expect that the adjuvant formulations in the present study would also work in humans.
Although both TLR7 and TLR9 appear to converge on the same signaling pathway, we observed enhancement of vaccine response using a combination of ligands for these two receptors. TLR7 (52) and TLR9 (53) both act through MyD88, and so the increase in activity that we found through the use of this combination was not expected. The pathogen ligands for these two TLRs differ (single-stranded RNA for TLR7/8 and CpG DNA for TLR9 [54]); thus, differences in their downstream effects might be expected, and our data suggest that combined triggering can lead to desirable responses. It is possible that the result observed is due to an increased dose of TLR agonist and that similar results might be obtained through an increased dose of TLR7/8 or TLR9 agonist alone; further investigation will be required to determine whether the effect observed in the present study is a dose effect or due to differential TLR triggering. There is evidence that other combinations of TLR agonists known to signal through different pathways can combine to enhance vaccine response; for example, combinations of TLR3 and TLR4 that signal through TRIF and combinations of TLR7, TLR8, and TLR9 that signal through MyD88 can combine to enhance dendritic cell function (55). In addition, the yellow fever vaccine has been shown to trigger TLR2, which uses adapter proteins TIRAP and MyD88, and through TLR7, TLR8, and TLR9, all of which act through MyD88 (56). Our finding of a combined effect using TLR7/8 and TLR9 triggering suggests that this combination in addition to other known synergistic combinations could be exploited to enhance vaccine responses for novel vaccine candidates. The examples of yellow fever vaccine and our oil-in-water emulsion demonstrate that different delivery vehicles can be harnessed to administer combinations of TLR agonists that can enhance vaccine responses.
We found that there was a transient elevation of CXCL10 (IP-10) following vaccination with combined TLR7/8 and TLR9 agonists. These agonists have been shown to stimulate IP-10 secretion in rhesus macaques when administered individually (57). Furthermore, secretion of IP-10 triggered by TLR agonists has been shown to cause regulatory dendritic cells to recruit Th1 cells and to then inhibit their proliferation (58). Given the role of Th1 cells in promoting cellular immunity over humoral immunity (59), inhibition of this helper T cell subset may explain why IP-10 elevation correlated with enhanced antibody responses.
In summary, we show that the inclusion of TLR7/8 and TLR9 agonists in a squalene-based oil-in-water emulsion improves the induction of HIV-1 antibodies. Such an adjuvant regimen does not perturb the antigenicity of recombinant HIV-1 Envs and should be a powerful adjuvant formulation to use with highly antigenic Envs that can induce high titers of potentially protective antibodies.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Duke Human Vaccine Institute Immune Reconstitution and Biomarker Analysis Shared Resource Facility (Durham, NC) directed by Gregory D. Sempowski for assistance with biomarker profiling. We also thank Kara Anasti for expert technical assistance with SPR experiments.
Support for this work was provided by a Collaboration for AIDS Vaccine Discovery grant to B.F. Haynes from the Bill and Melinda Gates Foundation, by the Center For HIV/AIDS Vaccine Immunology (CHAVI; grant U19 AI067854), and by the Center For HIV/AIDS Vaccine Immunology-Immunogen Discovery grant (CHAVI-ID; grant UM1 AI100645). This study was further supported by CAVD funding for the CTVIMC, grant OPP1032325, and also by the Duke University Center for AIDS Research Flow Cytometry and Virology cores (CFAR; P30-AI-64518).
M.A.M., H.-X.L., D.C.M., and B.F.H. have applied for patents related to this work.
Footnotes
Published ahead of print 3 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03309-13.
REFERENCES
- 1.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915. 10.1056/NEJMoa066092 [DOI] [PubMed] [Google Scholar]
- 3.Baylor NW, Egan W, Richman P. 2002. Aluminum salts in vaccines–US perspective. Vaccine 20(Suppl 3):S18–S23. 10.1016/S0264-410X(02)00166-4 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2010. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 59:626–629 [PubMed] [Google Scholar]
- 5.Meeusen ENT, Walker J, Peters A, Pastoret P-P, Jungersen G. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489–510. 10.1128/CMR.00005-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, Medina M-J, Nguyen-Van-Tam JS, Read RC, Warren FC, Zambon M. 2011. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect. Dis. 11:91–101. 10.1016/S1473-3099(10)70296-6 [DOI] [PubMed] [Google Scholar]
- 7.Caillet C, Piras F, Bernard M-C, de Montfort A, Boudet F, Vogel FR, Hoffenbach A, Moste C, Kusters I. 2010. AF03-adjuvanted and non-adjuvanted pandemic influenza A (H1N1) 2009 vaccines induce strong antibody responses in seasonal influenza vaccine-primed and unprimed mice. Vaccine 28:3076–3079. 10.1016/j.vaccine.2010.02.050 [DOI] [PubMed] [Google Scholar]
- 8.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3:85ra48. 10.1126/scitranslmed.3002336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, Golding H. 2010. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2:15ra5. 10.1126/scitranslmed.3000624 [DOI] [PubMed] [Google Scholar]
- 10.Karlsson Hedestam GB, Fouchier RAM, Phogat SK, Burton DR, Sodroski J, Wyatt RT. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6:143–155. 10.1038/nrmicro1819 [DOI] [PubMed] [Google Scholar]
- 11.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. 2008. Expression and function of Toll-like receptors on dendritic cells and other antigen-presenting cells from non-human primates. Vet. Immunol. Immunopathol. 125:18–30. 10.1016/j.vetimm.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 12.Ott G, Barchfeld GL, van Nest G. 1995. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine 13:1557–1562. 10.1016/0264-410X(95)00089-J [DOI] [PubMed] [Google Scholar]
- 13.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao H-X, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SSA, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463. 10.1128/JVI.01708-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao H-X, Sutherland LL, Xia S-M, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma B-J, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268–282. 10.1016/j.virol.2006.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam SM, Liao H-X, Tomaras GD, Bonsignori M, Tsao C-Y, Hwang K-K, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. 2013. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J. Virol. 87:1554–1568. 10.1128/JVI.00718-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao H-X, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang K-K, Chen X, Tsao C-Y, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang Z-Y, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. 10.1016/j.immuni.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinter A, Honnen WJ, Kayman SC, Trochev O, Wu Z. 1998. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine 16:1803–1811. 10.1016/S0264-410X(98)00182-0 [DOI] [PubMed] [Google Scholar]
- 18.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. 2009. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113:3716–3725. 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 19.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. 1996. T-cell-receptor affinity and thymocyte positive selection. Nature 381:616–620. 10.1038/381616a0 [DOI] [PubMed] [Google Scholar]
- 20.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 178:4424–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma B-J, Alam SM, Go EP, Lu X, Desaire H, Tomaras GD, Bowman C, Sutherland LL, Scearce RM, Santra S, Letvin NL, Kepler TB, Liao H-X, Haynes BF. 2011. Envelope deglycosylation enhances antigenicity of HIV-1 gp41 epitopes for both broad neutralizing antibodies and their unmutated ancestor antibodies. PLoS Pathog. 7:e1002200. 10.1371/journal.ppat.1002200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritz C, Streibig JC. 2005. Bioassay analysis using R. J. Statist. Software 15:1–22 [Google Scholar]
- 23.Montefiori DC. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. Chapter 12:Unit 12.11. 10.1002/0471142735.im1211s64 [DOI] [PubMed] [Google Scholar]
- 24.McLinden RJ, LaBranche CC, Chenine A-L, Polonis VR, Eller M, Ochsenbauer C, Kappes JC, Perfetto SP, Montefiori DC, Michael NL, Kim JH. 2013. Detection of HIV-1 neutralizing antibodies in a human CD4+/CCR5+/CXCR4+ T-lymphoblastoid cell assay system. PLoS One 8:e77756. 10.1371/journal.pone.0077756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarzotti-Kelsoe M, Daniell X, Todd CA, Bilska M, Martelli A, LaBranche CC, Perez LG, Ochsenbauer C, Kappes JC, Rountree W, Denny TN, Montefiori DC. Optimization and validation of a neutralizing antibody assay for HIV-1 in A3R5 cells. J. Immunol. Methods, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Mascola JR, Montefiori DC. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. 10.1016/j.virol.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J. Virol. 73:8966–8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R Statist. Soc. (B) 57:289–300 [Google Scholar]
- 31.Mascola JR, Montefiori DC. 2010. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28:413–444. 10.1146/annurev-immunol-030409-101256 [DOI] [PubMed] [Google Scholar]
- 32.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, Investigators MOPH-TAVEG 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 33.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H-X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, de Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenten D, Medzhitov R. 2011. The control of adaptive immune responses by the innate immune system. Adv. Immunol. 109:87–124. 10.1016/B978-0-12-387664-5.00003-0 [DOI] [PubMed] [Google Scholar]
- 35.Olive C. 2012. Pattern recognition receptors: sentinels in innate immunity and targets of new vaccine adjuvants. Expert Rev. Vaccines 11:237–256. 10.1586/erv.11.189 [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Rerks-Ngarm S, Excler J-L, Michael NL. 2010. HIV vaccines: lessons learned and the way forward. Curr. Opin. HIV AIDS 5:428–434. 10.1097/COH.0b013e32833d17ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald DW, Janes H, Robertson M, Coombs R, Frank I, Gilbert P, Loufty M, Mehrotra D, Duerr A, Step Study Protocol Team 2011. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step study). J. Infect. Dis. 203:765–772. 10.1093/infdis/jiq114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol Team 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K, Bangkok Vaccine Evaluation Group 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661–1671. 10.1086/508748 [DOI] [PubMed] [Google Scholar]
- 40.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, rgp120 HIV Vaccine Study Group 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654–665. 10.1086/428404 [DOI] [PubMed] [Google Scholar]
- 41.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang K-K, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao C-Y, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao H-X, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 86:11521–11532. 10.1128/JVI.01023-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441. 10.1093/infdis/jis367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, Khamboonruang C, Thongcharoen P, Morgan P, Benenson M, Paris RM, Chiu J, Adams E, Francis D, Gurunathan S, Tartaglia J, Gilbert P, Stablein D, Michael NL, Kim JH. 2012. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 12:531–537. 10.1016/S1473-3099(12)70088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, Sinangil F, Burke D, Berman PW. 2005. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 191:666–677. 10.1086/428405 [DOI] [PubMed] [Google Scholar]
- 45.Herrmann KL, Halstead SB, Wiebenga NH. 1982. Rubella antibody persistence after immunization. JAMA 247:193–196. 10.1001/jama.247.2.193 [DOI] [PubMed] [Google Scholar]
- 46.Simonsen O, Bentzon MW, Kjeldsen K, Venborg HA, Heron I. 1987. Evaluation of vaccination requirements to secure continuous antitoxin immunity to tetanus. Vaccine 5:115–122. 10.1016/0264-410X(87)90057-0 [DOI] [PubMed] [Google Scholar]
- 47.Sen G, Khan AQ, Chen Q, Snapper CM. 2005. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J. Immunol. 175:3084–3091 [DOI] [PubMed] [Google Scholar]
- 48.Pulendran B. 2009. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat. Rev. Immunol. 9:741–747 [DOI] [PubMed] [Google Scholar]
- 49.Food and Drug Administration. 2007. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 50.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503. 10.1016/j.immuni.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gujer C, Sundling C, Seder RA, Karlsson Hedestam GB, Loré K. 2011. Human and rhesus plasmacytoid dendritic cell and B-cell responses to Toll-like receptor stimulation. Immunology 134:257–269. 10.1111/j.1365-2567.2011.03484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. 2002. Small antiviral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 53.Hemmi H, Kaisho T, Takeda K, Akira S. 2003. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J. Immunol. 170:3059–3064 [DOI] [PubMed] [Google Scholar]
- 54.Wickelgren I. 2006. Immunology: targeting the tolls. Science 312:184–187. 10.1126/science.312.5771.184 [DOI] [PubMed] [Google Scholar]
- 55.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769–776. 10.1038/ni1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203:413–424. 10.1084/jem.20051720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwissa M, Nakaya HI, Oluoch H, Pulendran B. 2012. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood 119:2044–2055. 10.1182/blood-2011-10-388579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian C, An H, Yu Y, Liu S, Cao X. 2007. TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood 109:3308–3315. 10.1182/blood-2006-08-040337 [DOI] [PubMed] [Google Scholar]
- 59.Zygmunt B, Veldhoen M. 2011. T helper cell differentiation more than just cytokines. Adv. Immunol. 109:159–196. 10.1016/B978-0-12-387664-5.00005-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.