Abstract
Streptococcus agalactiae (group B Streptococcus [GBS]) is a leading cause of neonatal sepsis and meningitis, peripartum infections in women, and invasive infections in chronically ill or elderly individuals. GBS can be isolated from the gastrointestinal or genital tracts of up to 30% of healthy adults, and infection is thought to arise from invasion from a colonized mucosal site. Accordingly, bacterial surface components that mediate attachment of GBS to host cells or the extracellular matrix represent key factors in the colonization and infection of the human host. We identified a conserved GBS gene of unknown function that was predicted to encode a cell wall-anchored surface protein. Deletion of the gene and a cotranscribed upstream open reading frame (ORF) in GBS strain 515 reduced bacterial adherence to VK2 vaginal epithelial cells in vitro and reduced GBS binding to fibronectin-coated microtiter wells. Expression of the gene product in Lactococcus lactis conferred the ability to adhere to VK2 cells, to fibronectin and laminin, and to fibronectin-coated ME-180 cervical epithelial cells. Expression of the recombinant protein in L. lactis also markedly increased biofilm formation. The adherence function of the protein, named bacterial surface adhesin of GBS (BsaB), depended both on a central BID1 domain found in bacterial intimin-like proteins and on the C-terminal portion of the BsaB protein. Expression of BsaB in GBS, like that of several other adhesins, was regulated by the CsrRS two-component system. We conclude that BsaB represents a newly identified adhesin that participates in GBS attachment to epithelial cells and the extracellular matrix.
INTRODUCTION
Streptococcus agalactiae (group B Streptococcus [GBS]) can be considered part of the normal human microbiota, as it colonizes the gastrointestinal and/or genital tracts of 15 to 30% of healthy adults (1). However, in certain circumstances, GBS can behave as a life-threatening pathogen that causes infection in neonates, pregnant women, and elderly or immunocompromised persons. Despite the success of prenatal screening and maternal antibiotic prophylaxis in the United States and other countries, GBS remains the leading cause of early-onset neonatal sepsis (2). Neonates acquire GBS from a colonized mother shortly before or during birth by aspiration of infected amniotic fluid or vaginal secretions or by bacterial contamination of the skin or mucosal surfaces (1, 3). Approximately 50% of infants born to a vaginally colonized mother become colonized as a result of peripartum exposure. The ability of GBS to adhere to mucosal and epithelial surfaces is thought to be an essential early step for GBS colonization and for subsequent development of disseminated infection (4).
GBS, like other Gram-positive pathogens, produces surface proteins that mediate bacterium-host receptor interactions (5). Several surface proteins have been characterized as adhesins that are involved in bacterial attachment to host cells and/or the extracellular matrix (ECM) (6–16). Members of one family of surface proteins contain the LPXTG anchor motif at the C terminus followed by a hydrophobic domain and a positively charged tail (17). These proteins, after being synthesized in the cytosol, are exported via the Sec pathway by means of a cleavable N-terminal signal peptide and then anchored to cell wall peptidoglycan through sortase-mediated cleavage within the LPXTG motif and subsequent linkage to a peptidoglycan cross-bridge (18). Analysis of genome sequences of GBS strain 2603V/R (referred to here as 2603) and strain NEM316 revealed 24 or 21 surface proteins, respectively, bearing the LPXTG motif (19, 20). These LPXTG-containing proteins are often involved in (i) bacterial attachment to human cells, such as, for example, the serine-rich repeat family protein Srr-1 and BibA, and/or (ii) binding to ECM components, such as, for example, fibrinogen-binding protein FbsA and fibronectin-binding protein ScpB (6, 10, 11, 13–16).
Searching the genome sequence of GBS strain 515, a clinical isolate from an infected neonate, we identified a predicted cell wall-anchored protein encoded by sal0825 (20, 21). The protein harbors a typical N-terminal signal peptide and C-terminal LPXTG sorting signal, consistent with its being anchored to the GBS cell wall. Sal0825 is one of seven surface proteins that are conserved across GBS strains (22). On the basis of these features and results of the functional studies reported here, we named the protein BsaB (bacterial surface adhesin of GBS). In vitro functional analysis of BsaB revealed that the protein participates in GBS binding to human fibronectin and laminin, in the adhesion of GBS to human epithelial cells, and in biofilm formation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. GBS strains were 515 (21), 2603 (2603V/R) (23), NEM316 (19), A909, H36B, 18RS21 (24), COH1 (25), CJB111 (Carol Baker, Baylor College of Medicine, Houston, TX), and the derivative mutants 515ΔcsrR and 2603ΔcsrR (26). Unless otherwise specified, GBS strains were grown in Todd-Hewitt broth (THB; Difco) or on Trypticase soy agar supplemented with 5% defibrinated sheep blood (PML Microbiologicals). Escherichia coli was grown in Luria-Bertani broth, and Lactococcus lactis was grown in GM17 broth (M17 broth [Oxoid] supplemented with 0.5% glucose) (27). When appropriate, antibiotics were used at the following concentrations: for E. coli, erythromycin (ERM) at 200 μg/ml and ampicillin at 100 μg/ml; for L. lactis, chloramphenicol at 10 μg/ml; and for GBS, ERM at 1 μg/ml and chloramphenicol at 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant property(ies) | Source or reference |
|---|---|---|
| Strains | ||
| GBS strains | ||
| NEM316 | Wild-type strain | 19 |
| 2603V/R | Wild-type strain | 23 |
| A909 | Wild-type strain | 24 |
| H36B | Wild-type strain | 24 |
| CJB111 | Wild-type strain | Carol Baker |
| COH1 | Wild-type strain | 25 |
| 18RS21 | Wild-type strain | 24 |
| 515 | Wild-type strain | 21 |
| 515ΔcsrR | 515 with deletion of csrR gene | 26 |
| 515Δsal0824/5 | 515 with deletion of sal0824-sal0825 genes | This study |
| 515Δsal0825 | 515 with deletion of sal0825 gene | This study |
| 515ΔcsrRΔsal0824/5 | 515 with deletion of csrR and sal0824-sal0825 genes | This study |
| 515ΔcsrRΔsal0825 | 515 with deletion of csrR and sal0825 genes | This study |
| 515pNZ8048 | 515 containing pNZ8048 | This study |
| 515pNZ-sal0825 | 515 expressing Sal0825 from pNZ-sal0825 | This study |
| 515pNZ-AB | 515 expressing peptide AB of Sal0825 from pNZ-AB | This study |
| 515pNZ-A | 515 expressing peptide A of Sal0825 from pNZ-A | This study |
| 515pNZ-BC | 515 expressing peptide BC of Sal0825 from pNZ-BC | This study |
| 515pNZ-C | 515 expressing peptide C of Sal0825 from pNZ-C | This study |
| 515pNZ-SEC-sal0825 | 515 expressing Sal0825 from pNZ-SEC-sal0825 | This study |
| 515pNZ-SEC-AB | 515 expressing peptide AB of Sal0825 from pNZ-SEC-AB | This study |
| 515pNZ-SEC-A | 515 expressing peptide A of Sal0825 from pNZ-SEC-A | This study |
| 515pNZ-SEC-BC | 515 expressing peptide BC of Sal0825 from pNZ-SEC-BC | This study |
| 515pNZ-SEC-C | 515 expressing peptide C of Sal0825 from pNZ-SEC-C | This study |
| 2603V/R | Wild-type strain | 23 |
| 2603ΔcsrR | 2603V/R with deletion of csrR gene | 26 |
| 2603Δsal0824/5 | 2603V/R with deletion of sal0824-0825 genes | This study |
| 2603ΔcsrRΔsal0824/5 | 2603V/R with deletion of csrR and sal0824-0825 genes | This study |
| L. lactis strains | ||
| NZ9000 | L. lactis susp. cremoris MG1363 | 27 |
| NZ9700 | Nisin-secreting L. lactis strain | 27 |
| NZ9000::sal0825 | NZ9000 expressing Sal0825 from pNZ-sal0825 | This study |
| NZ9000::AB | NZ9000 expressing peptide AB of Sal0825 from pNZ-AB | This study |
| NZ9000::A | NZ9000 expressing peptide A of Sal0825 from pNZ-A | This study |
| NZ9000::BC | NZ9000 expressing peptide BC of Sal0825 from pNZ-BC | This study |
| NZ9000::C | NZ9000 expressing peptide C of Sal0825 from pNZ-C | This study |
| E. coli DH5α | Chemically competent intermediate host; plasmid free | Zymo Research Corp. |
| Plasmids | ||
| pJRS233 | Temperature-sensitive E. coli-GBS shuttle vector; Err | 57 |
| pNZ8048 | E. coli-L. lactis shuttle vector containing nisin-inducible promoter PnisA and start codon in NcoI site; Cmr | 27 |
| pNZ-sal0825 | pNZ8048 plasmid containing VSV-G-tagged sal0825 gene; Cmr | This study |
| pNZ-A | pNZ8048 plasmid containing VSV-G-tagged fragment A of sal0825 gene; Cmr | This study |
| pNZ-AB | pNZ8048 plasmid containing VSV-G-tagged fragment AB of sal0825 gene; Cmr | This study |
| pNZ-BC | pNZ8048 plasmid containing VSV-G-tagged fragment BC of sal0825 gene; Cmr | This study |
| pNZ-C | pNZ8048 plasmid containing VSV-G-tagged fragment C of sal0825 gene; Cmr | This study |
| pNZ-SEC | Modified pNZ8048, containing P44 promoter from plasmid pNZ44 and SEC signal from the L. lactis MG1363 chromosome; Cmr | 27 |
| pNZ-SEC-sal0825 | pNZ-SEC containing sal0825, Cmr | This study |
| pNZ-SEC-AB | pNZ-SEC plasmid containing VSV-G-tagged fragment AB of sal0825 gene; Cmr | This study |
| pNZ-SEC-A | pNZ-SEC plasmid containing VSV-G-tagged fragment A of sal0825 gene; Cmr | This study |
| pNZ-SEC-BC | pNZ-SEC plasmid containing VSV-G-tagged fragment BC of sal0825 gene; Cmr | This study |
| pNZ-SEC-C | pNZ-SEC plasmid containing VSV-G-tagged fragment C of sal0825 gene; Cmr | This study |
RT-PCR.
GBS RNA was isolated as described previously (28). For reverse transcription-PCR (RT-PCR), 50 ng of total RNA was used with the Invitrogen kit according to the manufacturer's recommendations. The reaction sequence included reverse transcription (30 min at 45°C), denaturation (2 min at 94°C), and 40 cycles of PCR with the following parameters: 94°C for 15 s, 55°C for 30 s, and 68°C for 60 s. PCR primers used in this study are listed in Table 2.
TABLE 2.
Oligonucleotide primers used for PCR
| Category and locus | Primer | Directiona | Sequence (5′–3′) |
|---|---|---|---|
| Primers for analysis of regulation by CsrR | |||
| sal0824 | 1375 | F | CCATTGTCGAATAGGCATGT |
| 1376 | R | TCATTGCCAATACTCCACCT | |
| sal0825 | 1377 | F | ACCTGTGAACGCTAAAGCTG |
| 1378 | R | GCTGACCACTTGTCACCTCT | |
| sal0826 | 1416 | F | AGACGGTGATCAGCTGTTTG |
| 1417 | R | AAGGGCAGAGCCAAAGAATA | |
| sal0827 | 1418 | F | TGCTTCACAAATGGATACCG |
| 1819 | R | GTCACCCCCTGTTTATCGAC | |
| Primers for analysis of transcriptional linkage | |||
| sal0823 | 1404 | F | TCTGTTATGGGCAAGTCTCTCT |
| sal0824 | 1405 | R | ATTAACCAAAGTCAGCCACATC |
| sal0824 | 1443 | F | ACTACATCTGATGACACAGTCCAA |
| sal0825 | 1444 | R | TTGTCACCTCTGACAAAGTTACCA |
| sal0825 | 1406 | F | TACCACCTACTTCGAAACCAAC |
| sal0826 | 1407 | R | CCTGCTTCTCTAATTTCACCAC |
| sal0826 | 1428 | F | TGTGGGTCAACTTCAGTTTGAAGT |
| sal0827 | 1429 | R | CGTGATATAGAAGCATAAGGTATAG |
| sal0827 | 1420 | F | AACAGGTTGATTTAGCTTATACCT |
| sal0828 | 1421 | R | CACTTTCTCCTGCTAACAAATTAT |
| sal0828 | 1422 | F | AACAGGTGAAATGCCATAGTTTGA |
| sal0829 | 1423 | R | TCGATCTACAACAACTTGAGGTAT |
| Primers for deletion of sal0824/5 or sal0825 | |||
| sal0823 | 1381 | F | GCGGGATCCATTAAACAACCACCTCAGGA (BamHI site is underlined) |
| sal0824 | 1383 | R | TCTCTATTAACCAAAGTCAG |
| sal0825 | 1388.4 | F | CTGACTTTGGTTAATAGAGACTGGTGATCAAGCCATTAG (underlined sequence is complementary to that of primer 1383) |
| sal0826 | 1386.3 | R | GCGCGGTACCTGGATTATTGGCAAACAGCT (KpnI site is underlined) |
| sal0824 | 1500 | F | GCGGATCCCAGAGATTCCGCAGATGCCT (BamHI site is underlined) |
| sal0825 | 1501 | R | CTTGCTGCCATTACTGGTG |
| sal0825 | 1502 | F | CACCAGTAATGGCAGCAAGGCTATGACAGCCTTAGCTA (the underlined sequence is complementary to that of primer 1501) |
| sal0826 | 1503 | R | CGGGTACCATTGGCAAACAGCTGATCA (KpnI site is underlined) |
| Primers for expression of Sal0825 and truncated peptides in pNZ8048 | |||
| sal0825 | 1484 | F | CGGCCATGGGTAATAAATCATTCAATACCAAATTAG (NcoI site is underlined) |
| 1485 | R | TTTACCTAAACGATTCATTTCAATATCAGTATAGGCTGTCATAGCTTTTGG (underlined sequence is complementary to VSV-G sequence) | |
| 1486 | F | TATACTGATATTGAAATGAATCGTTTAGGTAAATTAGCTAAAAAATTGCCTAAAACTG (underlined sequence is the VSV-G sequence) | |
| 1483.2 | R | GGCTCTAGATTATTTTGATCGTGATTTTTTAAGGAAGCCTAAC (XbaI site is underlined) | |
| Fragment AB | 1490.1 | R | CGATTCATTTCAATATCAGTATATCGAATAGTATAGCCCTTTGA (underlined sequence is complementary to the VSV-G sequence) |
| Fragment A | 1490.2 | R | CGATTCATTTCAATATCAGTATATGATTCTCCAGGTACAATATC (underlined sequence is complementary to the VSV-G sequence) |
| Fragment BC | 1507 | F | GCCCATGGGTAGAAGTGCATATGTTAATGTTG (NcoI site is underlined) |
| Fragment C | 1508 | F | GCCCATGGGTACTGATGTAGCAGGCTCT (NcoI site is underlined) |
F, forward; R, reverse.
qRT-PCR.
GBS strains were grown to mid-exponential phase, and RNA isolation and quantitative RT-PCR (qRT-PCR) were performed as described previously (28).
Cell culture.
VK2, a human vaginal epithelial cell line, and ME-180, a human cervical carcinoma cell line, were cultured as described previously (28).
Cell wall protein extracts.
For identification of recombinant protein on the bacterial cell surface, cell wall-anchored proteins were released by the following procedure. Bacterial cells were collected from 20 ml overnight culture, washed twice with phosphate-buffered saline (PBS), pH 7.4, resuspended in 0.5 ml of protoplast buffer (50 mM HEPES, 0.01 M MgCl2, 0.5 M sucrose, pH 7.0), and then treated with lysozyme (2 mg/ml) and mutanolysin (60 U/ml) at 37°C for 1 h. The supernatant was collected after centrifugation of protoplasts at 5,000 × g for 45 min at 4°C, and supernatant proteins were analyzed by SDS-PAGE and Western immunoblotting.
Construction of mutagenesis plasmid for deletion of the sal0824/5 locus.
For construction of a plasmid to delete the sal0824-sal0825 (sal0824/5) locus, primers 1381 and 1383 were used to PCR amplify the first 114 bp of sag0824 and 799 bp of adjacent upstream flanking sequence using GBS strain 515 chromosomal DNA as the template. Primers 1388.4 and 1386.3 were used to amplify the last 99 bp of sal0825 and 806 bp of downstream flanking DNA. Primer 1388.4 contains 20 bp of DNA that is complementary to primer 1383. The two gel-purified PCR products containing complementary ends were mixed and amplified with primers 1381 and 1386.3 to create a 2,190-bp internal deletion of the sal0824/5 gene locus by overlap PCR. The 1,817-bp overlap PCR product was digested with BamHI and KpnI and ligated into BamHI/KpnI-digested pJRS233. We used a similar strategy to construct a plasmid to introduce a large internal deletion in sal0825 using primer pairs 1500/1501 and 1502/1503 to amplify the sal0825 5′ and 3′ termini and flanking regions, followed by overlap PCR with primer pair 1500/1503 to fuse the 5′ and 3′ amplicons.
Construction of GBS mutants 515Δsal0824/5, 2603Δsal0824/5, 515ΔcsrRΔsal0824/5, 2603ΔcsrRΔsal0824/5, 515Δsal0825, and 515ΔcsrRΔsal0825.
The deletion construct in plasmid pJRS233 was introduced into GBS candidate strains 515, 515ΔcsrR, 2603, and 2603ΔcsrR by electroporation. Exchange of the internally deleted sal0824/5 or sal0825 locus for the native alleles on the GBS chromosome and identification of mutants was accomplished as described previously (28).
Expression of Sal0825 and truncated peptides in L. lactis NZ9000 or GBS 515.
To express Sal0825 in L. lactis NZ9000 or GBS 515, primers 1484 and 1485 were used to PCR amplify the first 1,419 bp of sal0825. Primers 1486 and 1483.2 were used to amplify the last 120 bp of sal0825, which encodes the C terminus sorting signal. Primers 1486 and 1485 included a 33-bp vesicular stomatitis virus G protein (VSV-G) epitope tag sequence. The two gel-purified PCR products containing complementary ends were mixed and amplified with primers 1484 and 1383.2 to create a sal0825--VSV-G fusion by overlap PCR. The overlap PCR product was digested with NcoI and XbaI and ligated into a similarly digested pNZ8048 vector.
To express the truncated Sal0825 peptides, four primer pairs were used to amplify the specific regions AB, A, BC, and C (see Fig. 6). Primers 1484 and 1490.1 were used to PCR amplify the first 873 bp of sal0825 (fragment AB), primers 1484 and 1490.2 were used to PCR amplify the first 627 bp of sal0825 (fragment A), primers 1507 and 1485 were used to PCR amplify the region from bp 628 to 1419 of sal0825 (fragment BC), and primers 1508 and 1485 were used to PCR amplify the region from bp 874 to 1419 (fragment C) of sal0825. As described for the construction of pNZ-sal0825, the primers 1486 and 1483.2 were used to amplify the last 120 bp of sal0825. The resultant amplicon was fused with fragment AB, A, BC, or C by overlap PCR using primer pairs 1484/1490.1, 1484/1490.2, 1507/1483.2, and 1508/1483.2 to create fusion fragments AB–VSV-G, A–VSV-G, BC–VSV-G, and C–VSV-G, respectively. The overlap PCR product was digested with NcoI and XbaI and ligated onto the pNZ8048 vector digested with the same enzymes. To make pNZ-SEC constructs, the digested PCR product was ligated to the pNZ-SEC vector, which harbors the P44 promoter from plasmid pNZ44 and the SEC signal sequence from the L. lactis MG1363 chromosome (27).
FIG 6.
Analysis of the functional domains of Sal0825 in GBS. (A) Diagram of the fragments of Sal0825 expressed from the pNZ8048 vector in GBS 515. (B) Proteins anchored to the bacterial cell wall were isolated and then separated by SDS-PAGE. (C) Western blot analysis with specific antiserum against the epitope tag VSV-G. (D) Relative levels of adherence to human fibronectin among different GBS strains. (B and C) Lanes 1, molecular weight standards (numbers beside the lanes are in thousands); lanes 2, proteins isolated from GBS 515 containing the vector alone (pNZ-SEC); lanes 3 to 7, proteins isolated from 515pNZ-SEC-sal0825 (pNZ-SEC-sal0825), 515pNZ-SEC-AB (pNZ-SEC-AB), 515pNZ-SEC-A (pNZ-SEC-A), 515pNZ-SEC-BC (pNZ-SEC-BC), and 515pNZ-SEC-C (pNZ-SEC-C), respectively. ***, P < 0.001; **, P < 0.01.
The recombinant plasmids were transformed into E. coli DH5α chemically competent cells (Zymo Research). After verification of DNA sequences, the plasmid construct was subsequently transformed into electrocompetent L. lactis NZ9000 or GBS cells. Colonies were screened by PCR after 24 h of incubation.
Western immunoblotting.
For immunoblotting, protein preparations were fractionated by SDS-PAGE under reducing conditions using a NuPAGE 12% bis-Tris gel (Invitrogen) and transferred onto a nitrocellulose membrane. The membrane was incubated in TBS (PBS with 0.005% Tween 20) containing 5% milk to block nonspecific binding, followed by washing three times in TBS. Primary antibody (anti-VSV-G; Sigma) was added at 1:5,000 in TBS for 1 h at room temperature. After being washed three times in TBS, membranes were incubated for 1 h with goat anti-rabbit IgG conjugated to horseradish peroxidase, diluted 1:10,000. Membranes were washed three times in substrate buffer. Positive bands were visualized with the addition of peroxidase substrate (Pierce).
GBS adherence to human epithelial cells.
Adherence assays were performed as described previously using cell lines VK2 and ME-180 (28). Assays were repeated at least three times in triplicate. The percentage of adherent GBS was calculated as follows: (number of CFU of adherent GBS/number of CFU in initial inoculum) × 100%.
GBS adherence to human extracellular matrix proteins.
To investigate the adhesion of GBS to immobilized ECM components, adherence assays were performed in 24-well polystyrene plates coated with individual ECM proteins. Plates coated with fibronectin or laminin were purchased from BD Biosciences. Adherence assays were performed as described previously (8). Assays were repeated at least three times in triplicate. The percentage of adherent GBS was calculated as follow: (number of CFU of adherent GBS/number of CFU in initial inoculum) × 100%.
Biofilm formation assay.
Biofilm assays were performed in 96-well polystyrene flat-bottom microtiter plates (Costar) as described previously (8, 29, 30). Each assay was performed in triplicate and repeated at least three times.
Statistical analysis.
Data are reported as means ± standard deviations (SD) unless otherwise stated. Statistical analysis was performed using Prism 5.0 (Graphpad Software Inc.). Differences between groups were analyzed using a two-tailed t test. Differences with P values of <0.05 were considered statistically significant. Asterisks in the figures represent ranges of P values for differences between groups (not significantly different [NS], P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001).
RESULTS
Identification of a GBS gene predicted to encode a surface protein of unknown function.
Surface components play an important role for tissue colonization and infection by mediating interactions between the pathogen and the host cells and in evasion of immune defense. Analysis of the completed GBS genomes identified a gene, designated sal0825 in strain 515, which is present in all reported sequenced strains. Because the genome sequences of several strains indicated disruption of the homologous sequence by frameshift mutations, we amplified by PCR the chromosomal region in strains 2603, NEM316, CJB111, H36B, A909, 18RS21, and COH1 and sequenced the amplicons. The results showed an uninterrupted open reading frame (ORF) for all strains except COH1, with 98 to 99% identity in its predicted amino acid (aa) sequence to that of sal0825 in strain 515. Strain COH1 appears to have a single nucleotide deletion that results in a frameshift and premature termination at aa 229. Sal0825 harbors a bacterial immunoglobulin-like domain (BID1) spanning aa 210 to 291. BID1 domains are found in bacterial surface proteins such as intimin-like proteins and cell adhesion molecules of Gram-negative pathogens that mediate bacterial adhesion and/or invasion into host cells (31, 32).
The upstream ORF, sal0824, is transcribed in the same orientation as sal0825 (Fig. 1). Sal0824 contains 6 membrane-spanning domains, which suggests that it is located on the cell surface. Homologs of sal0824 and sal0825 were found in GBS strains 2603, NEM316, CJB111, H3bB, A909, 18RS21, and COH1. BLAST analysis performed with sal0824 did not reveal any homologous proteins of known function in the database. The downstream ORF, sal0826, was predicted to encode a protein with homology to peptide chain release factor 3 of E. coli, which is involved in the release of newly synthesized polypeptide chains from the ribosome (33, 34). The function of the hypothetical protein encoded by sal0827 is unknown. We performed RT-PCR to define the transcriptional linkage between sal0825 and flanking ORFs. The results demonstrated cotranscription of four consecutive ORFs, sal0824, sal0825, sal0826, and sal0827 (see Fig. S1 in the supplemental material). The functional relationship of proteins encoded by the four cotranscribed genes remains to be investigated.
FIG 1.
Schematic of the chromosomal region of GBS strain 515 that contains the sal0824/5 locus and flanking genes.
Expression of Sal0824/5 is repressed by CsrR.
The CsrRS (or CovRS) two-component regulatory system controls the expression of multiple virulence factors in GBS (26, 35–40). Inactivation of CsrRS in GBS strains was associated with increased adherence to epithelial cells and increased expression of several adhesins (8, 38). To test whether CsrR regulates the expression of sal0825, we compared the expression of sal0825 in the 515 wild type to that in the 515ΔcsrR mutant. The result demonstrated a marked increase in the expression of sal0825 in strain 515ΔcsrR relative to that in wild-type strain 515 (Fig. 2). As discussed above, three other genes, sal0824, sal0826, and sal0827, were cotranscribed with sal0825, so we also tested whether their expression was regulated by CsrR. We found that the expression of sal0824 was increased to an extent similar to that of sal0825 in strain 515ΔcsrR. In contrast, we found no increase in the expression of sal0826 and sal0827 (Fig. 2). In summary, the data suggested that the expression of sal0824 and sal0825 is under the negative control of CsrR. Despite the apparent transcriptional linkage of the two downstream ORFs to sal0824 and sal0825, their expression may be controlled predominantly by a separate, CsrR-independent, promoter.
FIG 2.
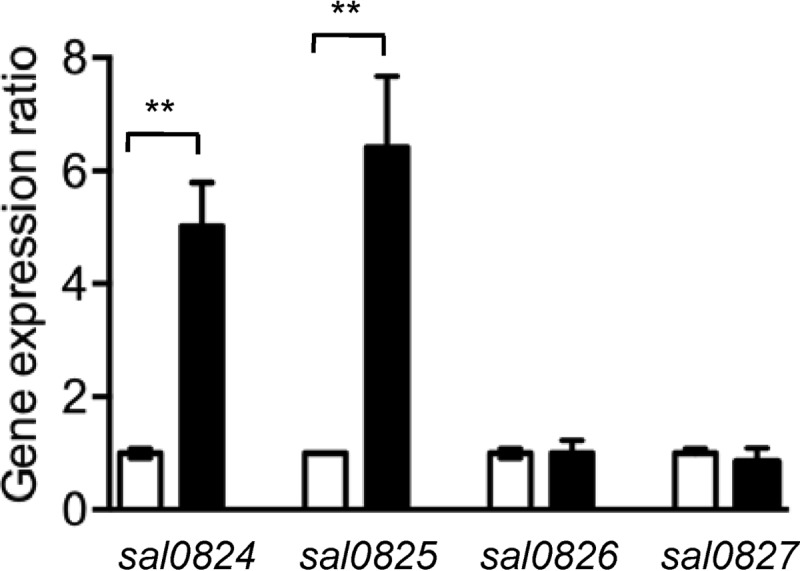
CsrR regulation of expression of sal0824/5 in GBS strain 515. Relative transcript abundances for the indicated genes were compared by RT-PCR between strains 515 (open bars) and 515ΔcsrR (filled bars). Values represent gene expression relative to that in 515 (mean ± SD). Each assay was performed at least three times in triplicate. **, P < 0.01.
Effect of Sal0824/5 inactivation on GBS adherence.
We hypothesized that Sal0825 participates in bacterium-host cell interactions based on its surface localization and the presence of a BID1 domain. To investigate the biological function of the protein, we first tested whether it contributes to the adherence of GBS to human epithelial cells. A deletion mutant was developed by allelic exchange in which both sal0824 and sal0825 were deleted from GBS strain 515. We chose to include sal0824 in this deletion with the consideration that it may form a functional unit with sal0825 based on their transcriptional linkage, similar regulation by CsrR, and surface localization. We then examined the relative association of the GBS wild-type and mutant strains with two types of human epithelial cells, VK2 (vaginal epithelial cell line) and ME-180 (cervical carcinoma cell line). After 1 h of exposure to GBS, cell monolayers were washed to remove the unbound bacteria, detached from wells, and lysed, and quantitative cultures of the lysates were performed to enumerate the cell-bound bacteria. We observed 31% less adherence to VK2 cells of 515Δsal0824/5 than to those of wild-type strain 515 (Fig. 3). Similarly, adherence of strain 515Δsal0824/5 to ME-180 cells was reduced by 29% compared to that of the wild type, although overall adherence to this cell line was quite low. Since expression of sal0824/5 is repressed by CsrR in strain 515 (Fig. 2), the relatively modest effect on adherence from inactivation of this locus might reflect the low level of sal0824/5 expression. To explore this possibility, we constructed a double mutant, 515ΔcsrRΔsal0824/5, and compared its adherence with that of 515ΔcsrR. While inactivation of csrR resulted in increased adherence to VK2 cells, adherence of the double mutant strain to both cell lines was not significantly different from that of 515ΔcsrR (Fig. 3).
FIG 3.
Effect of inactivation of the sal0824/5 locus on GBS adherence to human epithelial cells or immobilized ECM proteins. Levels of adherence were compared among GBS strains 515, 515Δsal0824/5 (Δsal0824/5), 515ΔcsrR (ΔcsrR), 515ΔcsrR/Δsal0824/5 (ΔcsrR/Δsal0824/5). Adherence is shown as a percentage of the initial inoculum (mean ± SD). Each assay was performed at least three times in triplicate. **, P < 0.01; *, P < 0.05.
Bacterium-host tissue interaction often involves the attachment of the bacterium to human ECM components, which in turn bind host cell surface integrins (41). To investigate whether the inactivation of sal0824/5 affects GBS adherence to ECM proteins, we compared the levels of adherence of GBS strains using 24-well plates coated with either fibronectin or laminin. Coated wells were each inoculated with approximately 6 × 106 CFU of GBS, and the adherence rate was calculated as the ratio of the number of CFU of GBS bound to the number of CFU in the initial inoculum. We found 32% and 29% decreases, respectively, in adherence to fibronectin when sal0824/5 was deleted from strain 515 and 515ΔcsrR (Fig. 3). Deletion of sal0824/5 resulted in a more modest reduction in binding to laminin, a difference that reached statistical significance only in the 515ΔcsrR background.
In summary, inactivation of sal0824/5 led to a moderate reduction in adherence of GBS 515 to human epithelial VK2 cells and immobilized fibronectin. This limited effect on adherence may be explained by the presence of other adhesins on the surface of GBS. Indeed, several surface components of GBS are involved in the adherence to host cells and ECM and may be responsible for the residual adherence of the sal0824/5 deletion mutant (6–16).
sal0824 is predicted to encode a protein closely associated with the cell membrane; therefore, we thought it unlikely that the reduced-adherence phenotype observed in the sal0824/5 mutant was attributable to loss of Sal0824. Rather, we focused our subsequent studies on sal0825, which is predicted to encode a cell wall-anchored protein with an exposed extracellular domain that is more likely to mediate adherence. To test the role of Sal0825, we constructed a mutant in which only sal0825 was deleted. We found that loss of Sal0825 had an impact on adherence similar to that of deletion of both Sal0824 and Sal0825 (see Fig. S2 in the supplemental material). Thus, it appears that Sal0825, and not Sal0824, is the major component that contributes to the adherence phenotype.
Effect of Sal0825 expression on the adherence of L. lactis.
Because of overlapping functions of multiple GBS adhesins, deletion of one or two proteins may not have a major impact on overall bacterial adhesion. To further investigate the potential role of Sal0825, we expressed the protein on the surface of L. lactis. Nonpathogenic L. lactis has been a useful tool to express and decipher the function of heterologous proteins. It supports expression of surface proteins from Gram-positive bacteria and has the further advantage of relatively low intrinsic adherence to cells and ECM proteins (42, 43). The sal0825 sequence was fused with a VSV-G epitope tag and cloned into pNZ8048. The recombinant plasmid was introduced into L. lactis (see Materials and Methods). The NICE (nisin-induced controlled expression) system of plasmid pNZ8048 enabled expression of Sal0825 protein in L. lactis after a 1-h induction with nisin (27). As detected by Western blotting, Sal0825 is anchored to the lactococcal cell wall (see Fig. S3 in the supplemental material). The adherence of L. lactis pNZ-sal0825 to fibronectin was markedly increased (46-fold) relative to that of L. lactis pNZ, and its adherence to laminin was modestly increased (2-fold) (Fig. 4). The Sal0825-expressing strain also showed 8-fold-greater adherence to VK2 cells. All these data suggested that Sal0825 functions as an adhesin in bacterial interactions with host cells and/or ECM. The expression of Sal0825 did not change lactococcal adherence to ME-180 cells in initial experiments (Fig. 4). Since ECM proteins can act as bridging molecules between bacterial adhesins and cell surface receptors, such as integrins, we tested whether fibronectin might function in this role for Sal0825-mediated adherence. When ME-180 monolayers were coated with fibronectin (10 μg/ml), we observed a 2-fold increase in the binding of L. lactis pNZ-sal0825 compared to that on the uncoated cell monolayer (Fig. 4). In summary, using L. lactis as a heterologous expression host, we identified Sal0825 as a novel surface adhesin. The expression of Sal0825 significantly increased the association of L. lactis with the human ECM components fibronectin and laminin as well as human epithelial VK2 cells and ME-180 cells. The data suggest that Sal0825 adherence to epithelial cells is mediated by binding to fibronectin (and perhaps other ECM proteins), which acts as a bridging molecule linking the bacteria to the host cell surface.
FIG 4.
The expression of Sal0825 protein increases the adherence of L. lactis to human epithelial cells or immobilized ECM proteins. Levels of adherence were compared among strains L. lactis(pNZ) and L. lactis(pNZ-sal0825). ME-180 cells were exposed to L. lactis strains before or after the cell monolayer was coated with human fibronectin (Fne). Adherence is shown as a percentage of the initial inoculum (mean ± SD). Each assay was performed at least three times in triplicate. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01.
Effect of Sal0825 expression on biofilm formation in GBS and L. lactis.
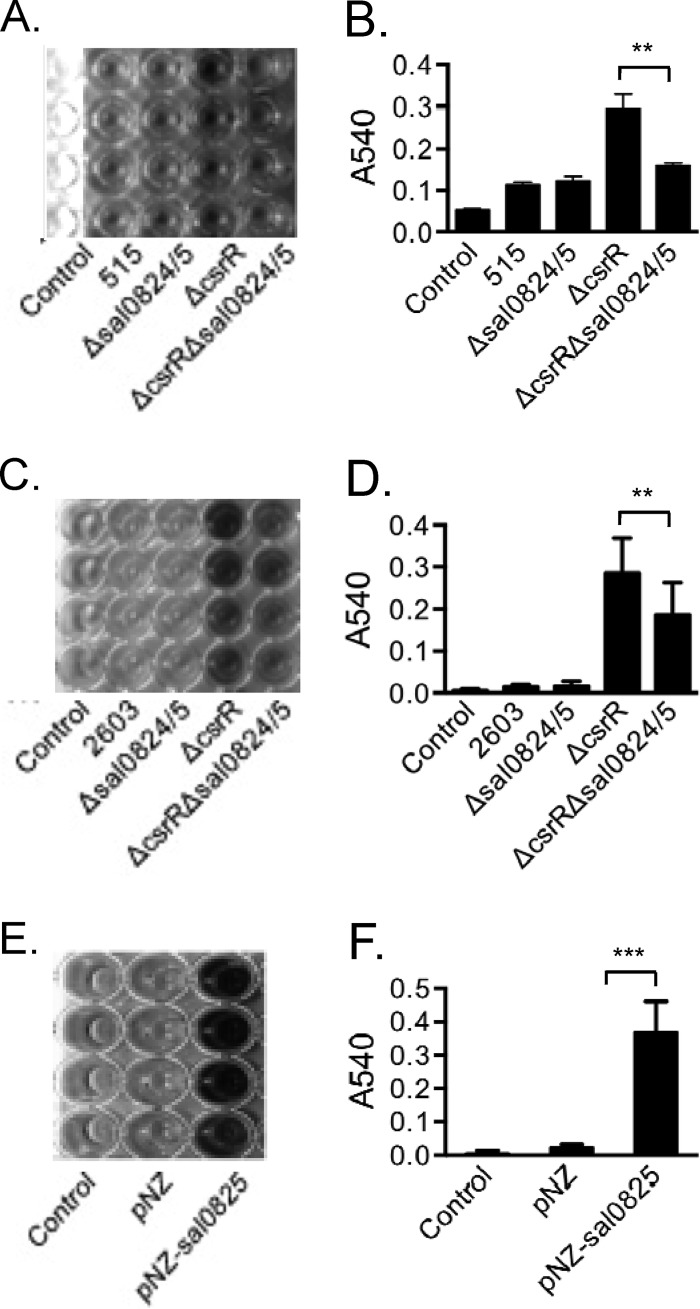
Biofilm formation may have an important role in the pathogenesis of GBS infection (29, 30, 44). GBS has been isolated from the population of biofilm-forming bacteria on intrauterine devices and has been shown to form biofilm on abiotic and cell surfaces in vitro. Two research groups found that pilus PI-2a participates in biofilm formation (29, 30). However, it was also noted that GBS strains that do not produce pilus PI-2a can form biofilms, indicating that additional factors may contribute (30). To investigate whether Sal0825 is involved in biofilm formation, GBS strains were grown in LB medium supplemented with 1% glucose in polystyrene plates, and biofilm formation was detected by crystal violet staining followed by dye solubilization with acetic acid and measurement of absorbance at 540 nm. As shown in Fig. 5A and B, strain 515 produced very little biofilm. Similar results were observed in the mutant strain 515Δsal0824/5. As biofilm formation is relatively low in wild-type strain 515, we also assessed the importance of sal0824/5 in biofilm formation in the background of 515ΔcsrR, in which expression of sal0824/5 is upregulated. Our results showed that inactivation of sal0824/5 in 515ΔcsrR resulted in a 47% reduction in biofilm formation (Fig. 5A and B). We also investigated the role of sal0824/5 in biofilm formation by GBS strain 2603, as this strain exhibits a greater increase in biofilm formation upon deletion of csrR than does strain 515. Biofilm formation was decreased 34% in strain 2603ΔcsrRΔsal0824/5 relative to that in strain 2603ΔcsrR. As shown in Fig. 5, there is still some production of biofilm in the double mutant 2603ΔcsrRΔsal0824/5, indicating that Sal0824/5 is not the only factor contributing to biofilm formation. Together, these data suggested that Sal0824/5 is one of the factors involved in biofilm formation in GBS 515 and 2603 and that CsrR regulates biofilm formation, at least partially, through regulation of Sal0824/5 expression.
FIG 5.
Role of Sal0825 in biofilm formation. Biofilms were compared among GBS strain 515 and isogenic mutants strains 515Δsal0824/5 (Δsal0824/5), 515ΔcsrR (ΔcsrR), and 515ΔcsrR/sal0824/5 (ΔcsrR/sal0824/5) (A and B), among GBS strain 2603 and isogenic mutant strains 2603Δsal0824/5 (Δsal0824/5), 2603ΔcsrR (ΔcsrR), and 2603ΔcsrR/sal0824/5 (ΔcsrR/sal0824/5) (C and D), and between L. lactis strains containing the plasmid construct pNZ-sal0825 and the pNZ vector alone (E and F). (A, C, E) Adherent bacteria stained with crystal violet; (B, D, F) quantification of biofilm by measurement of absorbance at 540 nm after the release of bound dye from each well using glacial acetic acid. ***, P < 0.001; **, P < 0.01.
To test whether Sal0825 alone is sufficient to confer the ability to form biofilm, we compared the biofilm formation of L. lactis pNZ-sal0825 with that of L. lactis harboring the pNZ8048 vector alone. As shown in Fig. 5E and F, a striking increase in biofilm production was observed when Sal0825 was expressed in L. lactis. Together, these data provide evidence that Sal0825 contributes to GBS biofilm formation.
Analysis of the function of the BID1 domain of Sal0825.
As mentioned above, Sal0825 harbors a BID1 domain at aa 210 to 291, which is also present in other adhesion proteins (31, 32). To understand the role of the BID1 domain and other regions of the protein in adherence, we constructed recombinant pNZ plasmids corresponding to various regions of Sal0825 and expressed the recombinant peptides in L. lactis (Fig. 6A). Peptide AB corresponds to the first 291 aa, including the amino-terminal portion of the protein and the BID1 domain. Peptide BC corresponds to aa 210 to 512 and includes the BID1 domain and the adjacent C-terminal region. As indicated in Fig. 6A, we also expressed peptides A and C individually, which correspond, respectively, to the N-terminal region and C-terminal region, excluding the central BID1 domain. The N-terminal signal peptide and C-terminal sorting signal were included in each construct to allow correct localization and display of peptides on the bacterial surface. Each recombinant plasmid was introduced into L. lactis, and protein expression was induced with nisin. However, while trying to compare the levels of adherence to fibronectin of L. lactis strains, we found that our work was hampered by the poor growth of L. lactis pNZ-BC and L. lactis pNZ-C. Although strains L. lactis pNZ-AB and pNZ-A grew normally, neither of them showed any difference in adherence to fibronectin from that of L. lactis with the pNZ8048 vector alone (data not shown).
In order to further characterize the functional domain, we also expressed the same fragments of Sal0825 in GBS strain 515. Because nisin induction slowed GBS growth, we cloned and expressed full-length Sal0825 and derivative fragments under the control of the constitutive P44 promoter in pNZ-SEC in GBS strain 515 with the following plasmids: pNZ-SEC-sal0825, pNZ-SEC-A, pNZ-SEC-AB, pNZ-SEC-BC, pNZ-SEC-C, and the pNZ-SEC vector alone. The expression and localization of the entire or partial Sal0825 protein was confirmed by SDS-PAGE and Western blotting (Fig. 6B and C). We evaluated the relative adherence of each GBS strain to fibronectin-coated plates. We found that overexpression of full-length Sal0825 increased the binding to fibronectin of GBS 515pNZ-SEC-sal0825 by approximately 10-fold compared to that of 515pNZ-SEC (Fig. 6D), which is in agreement with the result in L. lactis (Fig. 4). We then compared the adherence capacities of GBS strains expressing peptides corresponding to various regions of Sal0825. We found that the overexpression of peptide BC (strain 515pNZ-SEC-BC) increased binding by 4.5-fold relative to that of 515pNZ-SEC. For peptide C, we detected only a very small amount on the GBS surface by Western blotting. However, even the small amount of peptide enhanced binding of 515pNZ-SEC-C by 3.2-fold relative to that of 515pNZ-SEC. On the other hand, the surface expression of peptides A and AB in strains 515pNZ-SEC-A and 515pNZ-SEC-AB was very low (Fig. 6B and C), and we did not observe any increase in GBS adherence related to the expression of these two peptides (Fig. 6D). In summary, our data suggest that the C-terminal region of Sal0825 corresponding to peptide C as well as the BID1 domain itself contributes to the adherence function of Sal0825. In addition, region C appears to play an important role in the expression, surface display, or stability of Sal0825, since constructs lacking this domain were poorly expressed on the bacterial surface in both L. lactis and GBS.
DISCUSSION
The present study investigated the importance of a previously uncharacterized surface protein encoded by sal0825 in GBS adherence and biofilm formation. Based on the results of these experiments, we propose to name the protein bacterial surface adhesin of GBS (BsaB). Our data demonstrated that BsaB is localized on the surface of GBS and is able to interact directly with immobilized fibronectin and laminin. BsaB promoted the adherence of GBS to VK2 cells and ME-180 cells, representing human vaginal and cervical epithelial cells. In addition, BsaB enhanced GBS adherence to abiotic surfaces in strains 515 and 2603. In an attempt to identify the functional adherence domain of the protein, we found that binding to immobilized fibronectin involved both a central BID1 domain and the C-terminal region from aa 292 to 512. The latter domain is critical not only for binding but also for expression and proper display of BsaB on the bacterial surface.
Deletion of sal0824/5 resulted in a moderate decrease in GBS adherence to human vaginal epithelial VK2 cells and immobilized human fibronectin. However, a loss-of-function approach is likely limited by the presence of multiple adherence factors in GBS, so the effect of sal0824/5 inactivation might be largely compensated for by the function of other adhesins (6–16). A complementary gain-of-function approach provided evidence that overexpression of BsaB resulted in a substantial increase in the adherence of both L. lactis and GBS.
The most prominent function of BsaB is fibronectin binding (Fig. 4). Fibronectin is a large glycoprotein constituent of the ECM and blood plasma that has been shown to be a binding substrate for a variety of pathogenic bacteria, such as Staphylococcus aureus and Streptococcus pyogenes (41, 45–54). At least one of two genes coding for closely related fibronectin-binding proteins is found in almost all clinical isolates of S. aureus (55). Streptococcus pyogenes can express at least five different cell wall-anchored proteins with fibronectin-binding activity (52). In GBS, ScpB (C5a-peptidase) was identified as a bifunctional protein, working as a peptidase that inactivates human C5a and also mediating bacterial binding to fibronectin (6, 56). Fibronectin forms a molecular bridge between the bacterial surface and host cell integrins and is important for bacterium-host interactions (52). In this study, we found that fibronectin enhanced the attachment of GBS to ME-180 epithelial cells, presumably by a bridging mechanism (Fig. 4).
Our previous study showed that CsrR negatively regulates the expression of multiple GBS adhesins (8). The current work demonstrates that CsrR modulates the expression of BsaB in the same manner, consistent with the previously described central regulatory role of CsrR in GBS adherence (8, 38). sal0825, encoding BsaB, is cotranscribed with the upstream ORF sal0824, and expression of both is regulated by CsrR, but BsaB alone is sufficient to confer increased adherence on GBS and L. lactis.
Previous studies have shown that pilus type 2a is involved in biofilm formation by GBS. In this report, BsaB was found to contribute to biofilm formation in both strain 515, which produces type 2a pili, and strain 2603, which does not. In both strain backgrounds, BsaB-dependent biofilm formation was strongly regulated by CsrR.
Adherence of GBS to epithelial cells and/or ECM is a key step in bacterial colonization of host mucosal surfaces and in subsequent infection. The current work adds BsaB to a growing number of adhesins reported to be involved in GBS-host interaction. Expression of multiple surface proteins with adherence functions enables efficient interactions of GBS with different host components and likely enhances the organism's adaptability in occupying different niches of the host. While redundancy in function makes it difficult to establish a definitive role in pathogenesis for any single GBS adhesin, the multiplicity of adhesins produced by GBS underlines the importance of bacterial attachment in GBS colonization and invasion.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AI59502 and AI29952 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 16 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01014-13.
REFERENCES
- 1.Edwards MS, Baker CJ. 2001. Group B streptococcal infections, p 1091–1156 In Remington JS, Klein JO. (ed), Infectious diseases of the fetus and newborn, 5th ed. W. B. Saunders, Philadelphia, PA [Google Scholar]
- 2.Verani JR, McGee L, Schrag SJ. 2010. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm. Rep. 59:1–36 [PubMed] [Google Scholar]
- 3.Spellerberg B, Martin S, Brandt C, Lutticken R. 2000. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol. Lett. 188:125–128. 10.1111/j.1574-6968.2000.tb09182.x [DOI] [PubMed] [Google Scholar]
- 4.Tamura GS, Kuypers JM, Smith S, Raff H, Rubens CE. 1994. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect. Immun. 62:2450–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindahl G, Stalhammar-Carlemalm M, Areschoug T. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin. Microbiol. Rev. 18:102–127. 10.1128/CMR.18.1.102-127.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Q, Stafslien D, Purushothaman SS, Cleary P. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 70:2408–2413. 10.1128/IAI.70.5.2408-2413.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutekunst H, Eikmanns BJ, Reinscheid DJ. 2004. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 72:3495–3504. 10.1128/IAI.72.6.3495-3504.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SE, Jiang S, Wessels MR. 2012. CsrRS and environmental pH regulate group B streptococcus adherence to human epithelial cells and extracellular matrix. Infect. Immun. 80:3975–3984. 10.1128/IAI.00699-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenau A, Martins K, Amor S, Gannier F, Lanotte P, van der Mee-Marquet N, Mereghetti L, Quentin R. 2007. Evaluation of the ability of Streptococcus agalactiae strains isolated from genital and neonatal specimens to bind to human fibrinogen and correlation with characteristics of the fbsA and fbsB genes. Infect. Immun. 75:1310–1317. 10.1128/IAI.00996-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samen U, Eikmanns BJ, Reinscheid DJ, Borges F. 2007. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect. Immun. 75:5405–5414. 10.1128/IAI.00717-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santi I, Scarselli M, Mariani M, Pezzicoli A, Masignani V, Taddei A, Grandi G, Telford JL, Soriani M. 2007. BibA: a novel immunogenic bacterial adhesin contributing to group B Streptococcus survival in human blood. Mol. Microbiol. 63:754–767. 10.1111/j.1365-2958.2006.05555.x [DOI] [PubMed] [Google Scholar]
- 12.Schubert A, Zakikhany K, Pietrocola G, Meinke A, Speziale P, Eikmanns BJ, Reinscheid DJ. 2004. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect. Immun. 72:6197–6205. 10.1128/IAI.72.11.6197-6205.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert A, Zakikhany K, Schreiner M, Frank R, Spellerberg B, Eikmanns BJ, Reinscheid DJ. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46:557–569. 10.1046/j.1365-2958.2002.03177.x [DOI] [PubMed] [Google Scholar]
- 14.Spellerberg B, Rozdzinski E, Martin S, Weber-Heynemann J, Schnitzler N, Lutticken R, Podbielski A. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenenbaum T, Bloier C, Adam R, Reinscheid DJ, Schroten H. 2005. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect. Immun. 73:4404–4409. 10.1128/IAI.73.7.4404-4409.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, Doran KS. 2009. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J. Infect. Dis. 199:1479–1487. 10.1086/598217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarre WW, Schneewind O. 1994. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol. Microbiol. 14:115–121. 10.1111/j.1365-2958.1994.tb01271.x [DOI] [PubMed] [Google Scholar]
- 18.Mazmanian SK, Ton-That H, Schneewind O. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049–1057. 10.1046/j.1365-2958.2001.02411.x [DOI] [PubMed] [Google Scholar]
- 19.Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couve E, Lalioui L, Poyart C, Trieu-Cuot P, Kunst F. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499–1513. 10.1046/j.1365-2958.2002.03126.x [DOI] [PubMed] [Google Scholar]
- 20.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O'Connor KJ, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. U. S. A. 102:13950–13955. 10.1073/pnas.0506758102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wessels MR, Paoletti LC, Rodewald AK, Michon F, DiFabio J, Jennings HJ, Kasper DL. 1993. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect. Immun. 61:4760–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosinski-Chupin I, Sauvage E, Mairey B, Mangenot S, Ma L, Da Cunha V, Rusniok C, Bouchier C, Barbe V, Glaser P. 2013. Reductive evolution in Streptococcus agalactiae and the emergence of a host adapted lineage. BMC Genomics 14:252. 10.1186/1471-2164-14-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tettelin H, Masignani V, Cieslewicz MJ, Eisen JA, Peterson S, Wessels MR, Paulsen IT, Nelson KE, Margarit I, Read TD, Madoff LC, Wolf AM, Beanan MJ, Brinkac LM, Daugherty SC, DeBoy RT, Durkin AS, Kolonay JF, Madupu R, Lewis MR, Radune D, Fedorova NB, Scanlan D, Khouri H, Mulligan S, Carty HA, Cline RT, Van Aken SE, Gill J, Scarselli M, Mora M, Iacobini ET, Brettoni C, Galli G, Mariani M, Vegni F, Maione D, Rinaudo D, Rappuoli R, Telford JL, Kasper DL, Grandi G, Fraser CM. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. U. S. A. 99:12391–12396. 10.1073/pnas.182380799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancefield RC, McCarty M, Everly WN. 1975. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J. Exp. Med. 142:165–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin TR, Rubens CE, Wilson CB. 1988. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in neonatal lung. J. Infect. Dis. 157:91–100. 10.1093/infdis/157.1.91 [DOI] [PubMed] [Google Scholar]
- 26.Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR. 2005. Regulation of virulence by a two-component system in group B Streptococcus. J. Bacteriol. 187:1105–1113. 10.1128/JB.187.3.1105-1113.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahey-El-Din M, Griffin BT, Gahan CG. 2008. Nisin inducible production of listeriolysin O in Lactococcus lactis NZ9000. Microb. Cell Fact. 7:24. 10.1186/1475-2859-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang S, Park SE, Yadav P, Paoletti LC, Wessels MR. 2012. Regulation and function of pilus island 1 in group B Streptococcus. J. Bacteriol. 194:2479–2490. 10.1128/JB.00202-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konto-Ghiorghi Y, Mairey E, Mallet A, Dumenil G, Caliot E, Trieu-Cuot P, Dramsi S. 2009. Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PLoS Pathog. 5:e1000422. 10.1371/journal.ppat.1000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaudo CD, Rosini R, Galeotti CL, Berti F, Necchi F, Reguzzi V, Ghezzo C, Telford JL, Grandi G, Maione D. 2010. Specific involvement of pilus type 2a in biofilm formation in group B Streptococcus. PLoS One 5:e9216. 10.1371/journal.pone.0009216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40:D302–305. 10.1093/nar/gkr931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo KS, Kim JW, Park JY, Viall AK, Minnich SS, Rohde HN, Schnider DR, Lim SY, Hong JB, Hinnebusch BJ, O'Loughlin JL, Deobald CF, Bohach GA, Hovde CJ, Minnich SA. 2012. Role of a new intimin/invasin-like protein in Yersinia pestis virulence. Infect. Immun. 80:3559–3569. 10.1128/IAI.00294-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grentzmann G, Brechemier-Baey D, Heurgue-Hamard V, Buckingham RH. 1995. Function of polypeptide chain release factor RF-3 in Escherichia coli. RF-3 action in termination is predominantly at UGA-containing stop signals. J. Biol. Chem. 270:10595–10600 [DOI] [PubMed] [Google Scholar]
- 34.Mikuni O, Ito K, Moffat J, Matsumura K, McCaughan K, Nobukuni T, Tate W, Nakamura Y. 1994. Identification of the prfC gene, which encodes peptide-chain-release factor 3 of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 91:5798–5802. 10.1073/pnas.91.13.5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cumley NJ, Smith LM, Anthony M, May RC. 2012. The CovS/CovR acid response regulator is required for intracellular survival of group B Streptococcus in macrophages. Infect. Immun. 80:1650–1661. 10.1128/IAI.05443-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Palo B, Rippa V, Santi I, Brettoni C, Muzzi A, Metruccio MM, Grifantini R, Telford JL, Paccani SR, Soriani M. 2013. Adaptive response of group B streptococcus to high glucose conditions: new insights on the CovRS regulation network. PLoS One 8:e61294. 10.1371/journal.pone.0061294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang SM, Ishmael N, Hotopp JD, Puliti M, Tissi L, Kumar N, Cieslewicz MJ, Tettelin H, Wessels MR. 2008. Variation in the group B Streptococcus CsrRS regulon and effects on pathogenicity. J. Bacteriol. 190:1956–1965. 10.1128/JB.01677-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, Pellegrini E, Glaser P, Kunst F, Msadek T, Trieu-Cuot P, Poyart C. 2004. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol. Microbiol. 54:1250–1268. 10.1111/j.1365-2958.2004.04365.x [DOI] [PubMed] [Google Scholar]
- 39.Lembo A, Gurney MA, Burnside K, Banerjee A, Mde los Reyes Connelly JE, Lin WJ, Jewell KA, Vo A, Renken CW, Doran KS, Rajagopal L. 2010. Regulation of CovR expression in group B Streptococcus impacts blood-brain barrier penetration. Mol. Microbiol. 77:431–443. 10.1111/j.1365-2958.2010.07215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patras KA, Wang NY, Fletcher EM, Cavaco CK, Jimenez A, Garg M, Fierer J, Sheen TR, Rajagopal L, Doran KS. 2013. Group B Streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell. Microbiol. 15:1154–1167. 10.1111/cmi.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauck CR, Ohlsen K. 2006. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr. Opin. Microbiol. 9:5–11. 10.1016/j.mib.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 42.Kunji ER, Slotboom DJ, Poolman B. 2003. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta 1610:97–108. 10.1016/S0005-2736(02)00712-5 [DOI] [PubMed] [Google Scholar]
- 43.Mierau I, Kleerebezem M. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 68:705–717. 10.1007/s00253-005-0107-6 [DOI] [PubMed] [Google Scholar]
- 44.Kaur H, Kumar P, Ray P, Kaur J, Chakraborti A. 2009. Biofilm formation in clinical isolates of group B streptococci from north India. Microb. Pathog. 46:321–327. 10.1016/j.micpath.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 45.Amelung S, Nerlich A, Rohde M, Spellerberg B, Cole JN, Nizet V, Chhatwal GS, Talay SR. 2011. The FbaB-type fibronectin-binding protein of Streptococcus pyogenes promotes specific invasion into endothelial cells. Cell. Microbiol. 13:1200–1211. 10.1111/j.1462-5822.2011.01610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldassarri L, Creti R, Imperi M, Recchia S, Pataracchia M, Orefici G. 2007. Detection of genes encoding internalization-associated proteins in Streptococcus pyogenes isolates from patients with invasive diseases and asymptomatic carriers. J. Clin. Microbiol. 45:1284–1287. 10.1128/JCM.02119-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dziewanowska K, Carson AR, Patti JM, Deobald CF, Bayles KW, Bohach GA. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68:6321–6328. 10.1128/IAI.68.11.6321-6328.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher M, Huang YS, Li X, McIver KS, Toukoki C, Eichenbaum Z. 2008. Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A streptococcus. Infect. Immun. 76:5006–5015. 10.1128/IAI.00300-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fowler T, Wann ER, Joh D, Johansson S, Foster TJ, Hook M. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 79:672–679. 10.1078/0171-9335-00104 [DOI] [PubMed] [Google Scholar]
- 50.Lammers A, Nuijten PJ, Smith HE. 1999. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol. Lett 180:103–109. 10.1111/j.1574-6968.1999.tb08783.x [DOI] [PubMed] [Google Scholar]
- 51.Marjenberg ZR, Ellis IR, Hagan RM, Prabhakaran S, Hook M, Talay SR, Potts JR, Staunton D, Schwarz-Linek U. 2011. Cooperative binding and activation of fibronectin by a bacterial surface protein. J. Biol. Chem. 286:1884–1894. 10.1074/jbc.M110.183053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz-Linek U, Hook M, Potts JR. 2006. Fibronectin-binding proteins of gram-positive cocci. Microbes Infect. 8:2291–2298. 10.1016/j.micinf.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 53.Sinha B, Francois PP, Nusse O, Foti M, Hartford OM, Vaudaux P, Foster TJ, Lew DP, Herrmann M, Krause KH. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell. Microbiol. 1:101–117. 10.1046/j.1462-5822.1999.00011.x [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi M, Terao Y, Kawabata S. 29 November 2012. Pleiotropic virulence factor—Streptococcus pyogenes fibronectin-binding proteins. Cell. Microbiol. 10.1111/cmi.12083 [DOI] [PubMed] [Google Scholar]
- 55.Peacock SJ, Day NP, Thomas MG, Berendt AR, Foster TJ. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23–31. 10.1053/jinf.2000.0657 [DOI] [PubMed] [Google Scholar]
- 56.Beckmann C, Waggoner JD, Harris TO, Tamura GS, Rubens CE. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 70:2869–2876. 10.1128/IAI.70.6.2869-2876.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Casal J, Price JA, Maguin E, Scott JR. 1993. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol. Microbiol. 8:809–819. 10.1111/j.1365-2958.1993.tb01628.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.