Abstract
Nitric oxide (NO) is a proposed component of malaria pathogenesis, and the inducible nitric oxide synthase gene (NOS2) has been associated to malaria susceptibility. We analyzed the role of NOS2 polymorphisms on NO bioavailability and on susceptibility to infection, Plasmodium carrier status and clinical malaria. Two distinct West African sample collections were studied: a population-based collection of 1,168 apparently healthy individuals from the Príncipe Island and a hospital-based cohort of 269 Angolan children. We found that two NOS2 promoter single-nucleotide polymorphism (SNP) alleles associated to low NO plasma levels in noninfected individuals were also associated to reduced risk of pre-erythrocytic infection as measured anti-CSP antibody levels (6.25E–04 < P < 7.57E–04). In contrast, three SNP alleles within the NOS2 cistronic region conferring increased NO plasma levels in asymptomatic carriers were strongly associated to risk of parasite carriage (8.00E–05 < P < 7.90E–04). Notwithstanding, three SNP alleles in this region protected from cerebral malaria (7.90E–4 < P < 4.33E–02). Cohesively, the results revealed a dual regimen in the genetic control of NO bioavailability afforded by NOS2 depending on the infection status. NOS2 promoter variants operate in noninfected individuals to decrease both NO bioavailability and susceptibility to pre-erythrocytic infection. Conversely, NOS2 cistronic variants (namely, rs6505469) operate in infected individuals to increase NO bioavailability and confer increased susceptibility to unapparent infection but protect from cerebral malaria. These findings corroborate the hypothesis that NO anti-inflammatory properties impact on different steps of malaria pathogenesis, explicitly by favoring infection susceptibility and deterring severe malaria syndromes.
INTRODUCTION
Malaria is the result of a multistage Plasmodium infection that elicits a multiplicity of host responses. Inflammatory responses are determinants of the clinical course of infection and are influenced by host genetic factors (1). Genetic evidence accumulated in recent years supports a complex role for host genetics in resistance and susceptibility to human malaria (2). Hemoglobin gene variants are well-known malaria resistance factors, but a considerable number of genetic studies focused on clinical malaria syndromes and blood parasite burden also highlighted genes involved in the immune response, inflammation, and cell adhesion (1).
Nevertheless, the exact role of genetic variance in inflammatory responses against Plasmodium infection and in malaria severity remains unclear (1). It is possible that innate immunity genes associated to malaria may play a dual role in the course of infection. Proinflammatory factors would favor an efficacious anti-parasite response leading to parasite clearance and therefore conferring a lower degree of susceptibility to unapparent and mild infections. On the other hand, such factors could increase the risk of developing strong inflammatory responses that trigger severe inflammatory syndromes, namely, cerebral malaria.
Nitric oxide (NO) has been proposed to play a relevant role in malaria pathogenesis, but its mechanisms of action in different stages of infection remain to be elucidated (3). The NOS2 gene codes for the inducible nitric oxide synthase (iNOS) that is responsible for high-level production of NO by activated phagocytes (4). Several studies focused on NOS2 promoter polymorphisms have reported genetic association to different malaria clinical outcomes (5–12), but the role of such variants in malaria infection progression and nitric oxide production appears to be complex (13). Moreover, it is unclear whether NOS2 genetic variants play a role in susceptibility to asymptomatic malaria (14, 15).
Asymptomatic malaria infections have been frequently described in regions where malaria is endemic in both high- and intermediate-transmission areas (16–23). Asymptomatic malaria is suggested to represent an immunological state developed upon repeated exposure that tolerates the parasite in the absence of clinical symptoms (clinical immunity). On the other hand, such unapparent infections are an implicit manifestation of premunition, an immune response that enables control of blood parasite burden at low levels but do not efficiently lead to complete elimination of Plasmodium parasites (24). The mechanisms involved in the acquisition of premunition responses in exposed individuals remain elusive, but some reports have suggested that protection against asymptomatic Plasmodium infection (25) and the malaria reservoir status (23, 26) are influenced by host genetic factors.
To study the involvement of NOS2 gene in controlling NO bioavailability, malaria susceptibility, and severe disease, we analyzed a population-based collection of apparently healthy individuals, conducted in 2005 in the Principe Island on the West Coast of Africa and a hospital-based collection of Angolan children with uncomplicated and cerebral malaria. Using markers of current and past infection in apparently healthy individuals of the Príncipe collection, we analyzed the effect of NOS2 gene variants in susceptibility to acquire infection and their role in controlling NO plasma levels in infected and noninfected individuals. Furthermore, in clinical malaria samples we analyzed the role of NOS2 gene variants in susceptibility to cerebral malaria (CM). We report that Plasmodium infection impacts on the control of NO bioavailability by NOS2 genetic variants and that distinct NOS2 gene regions are associated with infection susceptibility and with the risk of clinical malaria progression.
MATERIALS AND METHODS
Ethics.
Ethical permit to conduct the present study in the Príncipe collection was granted by the Ministry of Health of São Tomé and Principe in the scope of a collaborative protocol on malaria research between the Fundação Calouste Gulbenkian (Portugal) and the government of São Tomé and Principe. Ethical permit for the study in the Angola collection was granted by the Ethical Committee of the Hospital Pediátrico David Bernardino (HPDB) in Luanda, appointed by the Angolan Ministry of Health. All investigations were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from every participant and, in case of children, consent was obtained from their guardians.
Sample collection.
The Island of Principe covers ∼55 square miles (142 square kilometers) with about 6,000 inhabitants representing a well-circumscribed, nonmigratory community. The Príncipe collection resulted from a cross-sectional study conducted in 2005 that covered 10 villages in the Northern Principe Island (within a 50-km2 region) and enrolled 1,387 apparently healthy donors within a period of 3 weeks. Blood samples were collected from participants without any prior selection or restrictions on participation. The age distribution of the sampled individuals represented the whole population, ranging from 1 month to 85 years of age. Individuals with tracked relatedness up to the second ancestor generation to a participant were excluded from the study.
The details of the Angola collection have been described previously (27). Patients were selected from attendees to the HPDB from February 2005 to May 2007. Malaria was diagnosed on the basis of a positive asexual parasitemia detected on a Giemsa-stained thick smear (28). CM was defined according to the World Health Organization criteria: either a coma score of <3 on the Blantyre scale for children <60 months or a coma score <7 on the Glasgow scale for children ≥60 months. Meningitis and encephalitis were ruled out by cerebrospinal fluid analysis after lumbar puncture. Cases with known causes of encephalopathy and hypoglycemia (glycemia < 40 mg/dl) were excluded. The UM group represents patients with malaria diagnosis by microscopic examination and febrile illness without any clinical finding suggestive of other causes of infection and with no manifestations of severe malaria.
Plasmodium detection.
Parasite DNA was extracted from frozen packed red blood cells using a 96 QIAmp blood kit (Qiagen, USA). A nested PCR amplification assay was used for the detection of the main Plasmodium species of human malaria (P. falciparum, P. vivax, P. malariae, and P. ovale) as described previously (29). In the Príncipe collection, asymptomatic individuals were considered Plasmodium carriers if they tested positive for any Plasmodium species. In the Angola samples, malaria laboratory diagnosis was confirmed using this method.
ELISA.
In the Principe collection, IgG antibodies against P. falciparum circumsporozoite protein (CSP) were detected by enzyme-linked immunosorbent assay (ELISA) in the 1,209 plasma samples using PpCSP-M2 antigen (30). Microtiter plates were coated with antigen (5 μg/ml) in coating buffer was added, followed by incubation overnight at 4°C. The antigen solution was then removed, and the plate was washed three times with phosphate-buffered saline (PBS). After blocking with PBS-gelatin (1%), a 100-μl aliquot of 1/100 diluted plasma in PBS-gelatin-Tween was incubated overnight at 4°C, followed by five washes with PBS. As a secondary antibody, 100 μl of 1/600 dilution of goat anti-human IgG coupled to alkaline phosphatase (Sigma-Aldrich, USA) in PBS-Gelatin-Tween was incubated for 2 h at 37°C. The supernatant was discarded and the plates were washed five times with PBS and incubated with 100 μl of substrate solution (10 mg of pNPP in 1 ml of 1 M Tris [pH 9.8] plus 9 ml of 1.5 M NaCl). Known positive and negative control plasma samples were included in each plate. The absorbance at 405 nm was determined by using a micro-ELISA plate reader, and results were expressed in arbitrary units (AU) calculated as follows: log [(A405-test plasma − A405-blank)/(A405-positive control − A405-blank) × 10,000)]. ELISA specificity was evaluated in 44 samples of unexposed European individuals that showed very low or undetectable levels of anti-CSP antibodies. To ensure specificity, the positivity cutoff value (>0.90 AU) was set at the AU average + 4× standard deviation obtained in 44 samples of unexposed European individuals.
Nitric oxide quantification.
Plasma NO levels (μM) were assessed by chemiluminescence-based measurement of nitrate (NO3−) and nitrite (NO2−) concentrations, as previously described (31). This method is based on the vanadium III-induced reduction of NO2− and NO3− to NO, at a high temperature (90°C), using a Sievers 280 NO analyzer (Sievers Instruments, USA).
Genotyping.
Genomic DNA was extracted from whole blood using Chemagen magnetic beads technology. DNA preparations were quantified using PicoGreen reagents (Invitrogen, Portugal) according to the supplier's instructions. A total of 25 single-nucleotide polymorphisms (SNPs) covering the NOS2 region were initially genotyped by using the Sequenom iPlex assay (Sequenom, San Diego, CA) and the Sequenom MassArray K2 platform at the Genomics Unit of the Instituto Gulbenkian de Ciência. Extensive quality control was performed using eight HapMap (http://hapmap.ncbi.nlm.nih.gov/) controls of diverse ethnicity, a Hardy-Weinberg equilibrium (HWE) with P > 0.01, and a minimum of 90% call rate for each SNP. Genotype determinations were performed blinded to affection status. One SNP (rs2297514) was excluded due to a genotyping failure. Furthermore, four SNPs did not meet the quality control criteria—three in both sample populations (rs28973255, rs9895831, and rs9906835) and one (rs28998790) in the Angola collection—and were also excluded. Samples with <75% call rate and duplicates were excluded from analysis, such that 45 samples from Príncipe and 3 from Angola were removed from the analysis.
Genetic analysis.
The final data set used in the analysis consisted of 1,168 subjects that had both genetic and plasma data for the Principe population and 269 individuals for the Angolan collection comprising 127 CM children and 142 UM patients. Association analysis of asymptomatic carrier status (Plasmodium PCR-positive individuals) in the Principe population and cerebral malaria in the Angolan population was performed by logistic regression (adjusted for age and gender) using the SNPassoc v1.4-9 package (32) implemented in the R freeware (http://cran.r-project.org/). The results were considered suggestive below a conventional level of 0.05. Bonferroni corrections for multiple tests were carried out to exclude type I errors (the significance levels for 21 tests in the Principe population and 20 tests in the Angolan population are set at P < 2.38 × 10−3 and at P < 2.50 × 10−3, respectively). χ2 tests for HWE in the sample populations, allelic and haplotypic association, and linkage disequilibrium (LD) analysis were performed using Haploview 4.2 (33). Quantitative trait locus (QTL) analysis was performed to test the main effect of each tested NOS2 SNP on anti-CSP antibody titers (CSP QTL) and NO concentration (NO QTL) in the Principe population. At each SNP, anti-CSP antibody titers and the NO concentration were regressed onto genotype counts in a regression model that included gender and age as covariates. The SNPassoc v1.4-9 package (32) implemented in the R freeware was used for logistic regression analysis. Results with a P value of <0.05 were considered evidence for suggestive association.
RESULTS
Infection markers in apparently healthy individuals.
The sample collection of apparently healthy individuals was conducted in the Príncipe Island population in May 2005 when the disease transmission for malaria coursed at the mesoendemic level. Based on the criteria set in Materials and Methods, the final data set analyzed in the present study consisted of 1,168 subjects that had both genetic and phenotype data. Plasmodium carriage in apparently healthy individuals was evaluated by determining subpatent parasitemia scored by PCR, and susceptibility to infection was indirectly ascertained by quantifying antibodies against CSP that represent an immunological marker of repeated pre-erythrocytic infection (34). We found that 32.3% of the apparently healthy population carried Plasmodium DNA in peripheral blood (malaria asymptomatic carriers), while 79.8% of the population was positive for antibodies against CSP (Table 1 and Fig. 1). The asymptomatic carrier status was marginally dependent on age but, as expected, anti-CSP positivity was significantly influenced by age (odds ratio [OR] = 1.02, P = 8.00E–04), presumably reflecting the natural history to parasite exposure (Table 1). Nevertheless, parasite carriage was not dependent on declared ethnic ancestry or on demography, indicating that local epidemiological variables in Príncipe did not significantly influence the asymptomatic malaria status (see Table S1 in the supplemental material). Not surprisingly, CSP antibody titers were higher in parasite carriers, but the proportion of individuals negative for CSP antibodies among asymptomatic carriers (14%) suggested that asymptomatic infection did not prevent the detection of individuals lacking antibody markers of repeated pre-erythrocytic infection (see Fig. S1 in the supplemental material). These results suggested that these two infection markers represent different criteria in ascertaining malaria susceptibility.
TABLE 1.
Prevalence of infection markers in apparently healthy individuals
| Collection | Total no. of subjects | Age range in yrs (median) | No. male | No. female | OR (95% CI); Covariance P valuea |
|
|---|---|---|---|---|---|---|
| Age | Gender | |||||
| PCR+ | 378 | 0–75 (13.5) | 174 | 204 | 0.99 (0.98–0.99); 0.045 | 0.85 (0.67–1.09); NS |
| PCR− | 790 | 0–85 (16.0) | 332 | 457 | 0.99 (0.98–0.99); 0.045 | 0.85 (0.67–1.09); NS |
| CSP+ | 973 | 0–85 (17.0) | 419 | 553 | 1.02 (1.00–1.02); 8.0E–04 | 1.08 (0.79–1.49); NS |
| CSP− | 195 | 0–74 (8.0) | 87 | 108 | 1.02 (1.00–1.02); 8.0E–04 | 1.08 (0.79–1.49); NS |
Logistic regression odds ratios (OR) and 95% confidence intervals (CI) are indicated. NS, not significant.
FIG 1.
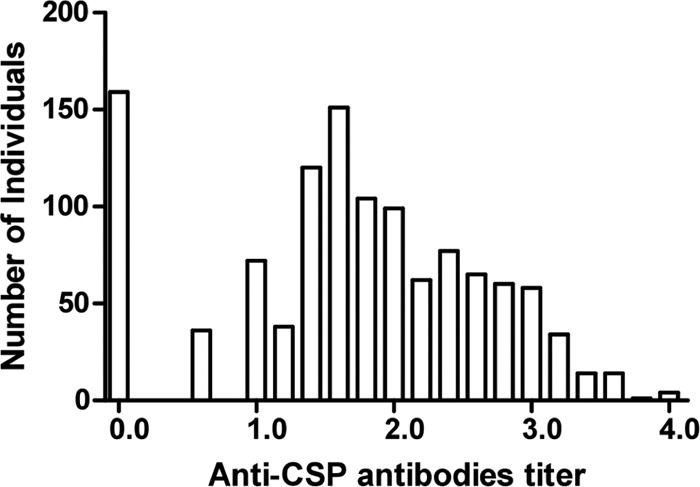
Distribution of plasma IgG antibody levels against Plasmodium circumsporozoite protein (CSP) in apparently healthy individuals. Antibody levels are presented in arbitrary units, and the positivity cutoff value is 0.90, as described in Materials and Methods.
In this mesoendemic setting, a significant proportion of the apparently healthy population carried parasitological markers of current infection. Nevertheless, a sizable fraction of the population with lifetime exposure does not show antibody markers of previous pre-erythrocytic infection. This suggested that host factors were influencing susceptibility to asymptomatic carrier status and persistent susceptibility to infection.
NOS2 variants are associated to the asymptomatic carrier status.
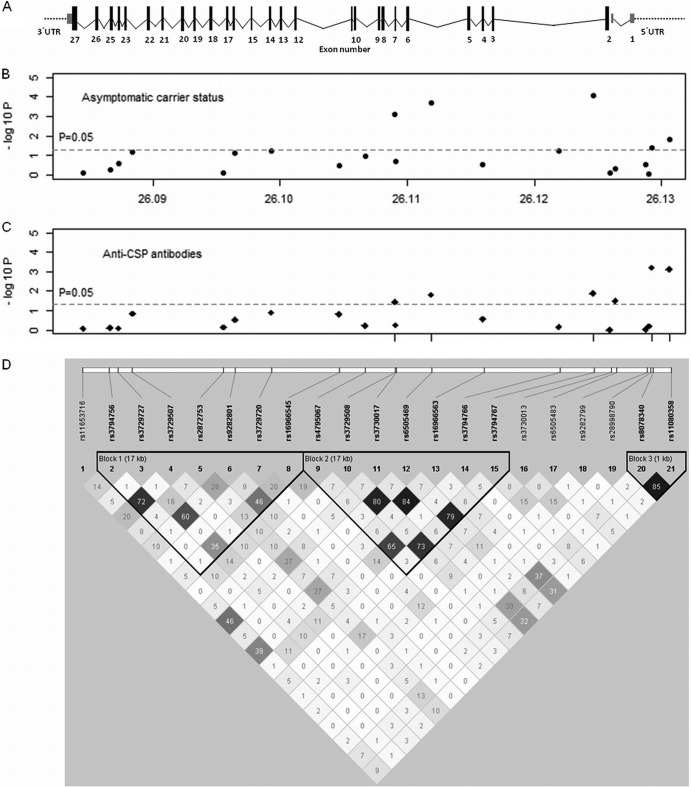
To test whether NOS2 gene variants were involved in malaria asymptomatic carrier status, we genotyped 1,168 individuals of the Príncipe collection for 21 NOS2 SNPs that spanned the promoter and the cistronic gene region (Fig. 2). We compared genotype frequencies in asymptomatic individuals against the general population. The age- and gender-corrected analysis under the genetic log-additive model indicated that three intronic SNP alleles (SNP 10/allele A, SNP 12/allele T, and SNP 15/allele T) were associated with susceptibility to malaria asymptomatic carrier status, with the highest association occurring at rs3794767 (P = 8.00E–05; OR = 1.53; 95% confidence interval [95% CI] = 1.24 to 1.88) (Fig. 2B and Table 2). In contrast, two other SNP alleles in the promoter region of NOS2 (SNP 20/allele A and SNP 21/allele A) were associated with decreased asymptomatic carrier susceptibility (P = 4.18E–2 and P = 1.51E–2, respectively), as denoted by OR < 1 (Fig. 2B and Table 2). Three of the five associated markers remained significantly associated after conservative Bonferroni correction for multiple testing (P ≤ 2.38E–3) (Table 2). The three intronic SNPs were in strong linkage disequilibrium (LD; r2 ≥ 0.73) and together with four other SNPs in this region compose a LD block (block 2 in Fig. 2D), which was separate from the LD block that included the two SNPs in the promoter region (block 3). Haplotypic association analysis comprising the SNPs in each of the three LD blocks (Fig. 2) did not identify haplotypes with a stronger association than the individual SNPs (data not shown). These results suggest that the major NOS2 effect implicated in increasing the risk of parasite carriage was mapping within the LD block that contains rs3794767.
FIG 2.
Genetic association of NOS2 SNPs with asymptomatic malaria and anti-CSP antibodies in the Príncipe population. (A) NOS2 gene structure scaled diagram. Exons are represented by boxes and numbered. (B and C) Plots of association tests for the healthy carrier status under the log-additive model (B) and QTL analysis of anti-CSP antibody levels (C) for the represented SNPs. The results are presented as the −log10 of the P value. (D) LD map for the tested SNPs obtained using Haploview 4.2 (values represent pairwise r2).
TABLE 2.
Genotypic association of NOS2 SNPs to asymptomatic malariaa
| SNP no. | SNP reference | Position (Mb) | Gene regionb | Allelesc | Minor allele frequency | Genotypes | Asymptomatic carriers (n = 378) | Noncarriers (n = 790) | Padj | OR (95% CI) | Pcorr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs11653716 | 26084532 | Intron 26 | G/C | 0.274 | CC/GC/GG | 190/156/24 | 407/318/53 | 0.7856 | 0.97 (0.58–1.63) | NSd |
| 2 | rs3794756 | 26086629 | Intron 25 | T/C | 0.047 | CC/TC/TT | 304/34/0 | 653/57/4 | 0.5389 | 1.14 (0.75–1.74) | NS |
| 3 | rs3729727 | 26087316 | Intron 24 | A/G | 0.172 | GG/AG/AA | 272/90/15 | 531/233/24 | 0.2751 | 0.88 (0.70–1.11) | NS |
| 4 | rs3729507 | 26088432 | Intron 22 | C/G | 0.079 | GG/CG/CC | 308/66/2 | 681/96/9 | 0.0674 | 1.34 (0.98–1.81) | NS |
| 5 | rs2872753 | 26095562 | Intron 17 | G/A | 0.460 | AA/GA/GG | 102/201/75 | 219/418/53 | 0.7638 | 1.03 (0.86–1.23) | NS |
| 6 | rs9282801 | 26096473 | Intron 16* | T/G | 0.248 | GG/TG/TT | 235/116/27 | 430/309/50 | 0.0784 | 0.83 (0.68–1.02) | NS |
| 7 | rs3729720 | 26099324 | Intron 14* | T/C | 0.038 | CC/TC/TT | 357/19/1 | 723/66/1 | 0.0568 | 0.63 (0.38–1.03) | NS |
| 8 | rs16966545 | 26104622 | Intron 11 | A/T | 0.165 | TT/AT/AA | 273/92/12 | 535/233/17 | 0.3173 | 0.89 (0.70–1.13) | NS |
| 9 | rs4795067 | 26106675 | Intron 9 | G/A | 0.195 | AA/GA/GG | 261/102/15 | 505/245/39 | 0.1082 | 0.84 (0.67–1.04) | NS |
| 10 | rs3729508 | 26109030 | Intron 7* | A/G | 0.230 | GG/AG/AA | 191/152/25 | 489/253/36 | 7.90E–04 | 1.42 (1.16–1.75) | 1.66E–02 |
| 11 | rs3730017 | 26109102 | Exon 7 | T/C | 0.210 | CC/TC/TT | 242/123/12 | 481/274/35 | 0.2054 | 0.87 (0.70–1.08) | NS |
| 12 | rs6505469 | 26111886 | Intron 5 | T/A | 0.231 | AA/TA/TT | 189/152/27 | 490/259/33 | 1.90E-04 | 1.48 (1.21–1.82) | 3.99E–03 |
| 13 | rs16966563 | 26115949 | Exon 4 | C/T | 0.214 | TT/CT/CC | 240/124/14 | 483/263/42 | 0.2849 | 0.89 (0.72–1.10) | NS |
| 14 | rs3794766 | 26121921 | Intron 2 | T/C | 0.173 | CC/TC/TT | 275/81/15 | 524/228/30 | 0.0569 | 0.80 (0.63–1.01) | NS |
| 15 | rs3794767 | 26124605 | Intron 2 | T/C | 0.211 | CC/TC/TT | 198/146/23 | 515/231/30 | 8.00E–05 | 1.53 (1.24–1.88) | 1.68E–03 |
| 16 | rs3730013 | 26125918 | Intron 1* | T/C | 0.247 | CC/TC/TT | 211/151/16 | 434/311/40 | 0.7463 | 0.97 (0.78–1.19) | NS |
| 17 | rs6505483 | 26126345 | Intron 1 | A/G | 0.229 | GG/AG/AA | 217/139/20 | 473/273/40 | 0.4777 | 1.08 (0.88–1.33) | NS |
| 18 | rs9282799 | 26128728 | Promoter | T/C | 0.047 | CC/TC/TT | 333/38/1 | 712/68/0 | 0.2877 | 1.25 (0.83–1.88) | NS |
| 19 | rs28998790 | 26129014 | Promoter | A/G | 0.066 | GG/AG/AA | 327/46/2 | 684/98/3 | 0.9266 | 1.02 (0.72–1.44) | NS |
| 20 | rs8078340 | 26129212 | Promoter | A/G | 0.279 | GG/AG/AA | 213/130/28 | 390/317/68 | 4.18E–02 | 0.82 (0.67–0.99) | NS |
| 21 | rs11080358 | 26130596 | Promoter | A/G | 0.250 | GG/AG/AA | 226/128/17 | 421/309/53 | 1.51E–02 | 0.77 (0.63–0.95) | NS |
Association tests were performed using the log-additive model, and the results were adjusted for age and gender (Padj). Significant adjusted P values (Padj < 0.05) and significant Bonferroni-corrected P value (Pcorr < 0.05) are highlighted in boldface.
*, rs9282801 is located in intron 16 in a boundary region to exon 16 (at 88 bp), rs3729720 is located in intron 14 in a boundary region to exon 14 (at 10 bp), rs3729508 is located in intron 7 in a boundary region to exon 7 (at 11 bp), and rs3730013 is located in intron 1 in a boundary region to exon 2 (at 10 bp).
Minor/major frequency alleles are indicated in this column.
NS, not significant.
NOS2 variants are associated to susceptibility to pre-erythrocytic infection.
Next, we evaluated whether markers of pre-erythrocytic infection corroborated NOS2 association with asymptomatic malaria. QTL analysis of anti-CSP antibody plasma levels was performed in the Príncipe sample (1,168 individuals). Interestingly, we found that the three intronic SNP alleles (SNP 10/allele A, SNP 12/allele T, and SNP 15/allele T) that appeared to increase the risk of asymptomatic malaria were also significantly associated with elevated levels of anti-CSP antibodies (Fig. 2C and Table 3). Conversely, we found that the two SNP alleles in the promoter region (SNP 20/allele A and SNP 21/allele A) that were protecting subjects from parasite carrier status were strongly associated with low levels of anti-CSP antibodies (Table 3). Since anti-CSP levels were higher in asymptomatic carriers (see Fig. S1 in the supplemental material), we performed the same analysis, correcting for the malaria carrier status. Interestingly, only the association signals in promoter SNPs remained significant (Table 3), suggesting that the promoter region is a major factor in controlling susceptibility to pre-erythrocytic infection, as ascertained from the anti-CSP antibody levels. These results revealed that NOS2 variants exert a dual genetic control in susceptibility to malaria. (i) The promoter alleles are overrepresented in noncarriers and in individuals with low levels of anti-CSP antibodies, implying an association to reduced susceptibility to infection. (ii) On the other hand, the overrepresentation of intronic alleles in carriers and in individuals with higher levels of anti-CSP antibodies suggests their association with increased susceptibility to infection.
TABLE 3.
QTL analysis of NOS2 SNPs that control anti-CSP antibody levels in the Principe populationa
| SNP no. | SNP reference | Gene region | Genotypes | Genotype nb (n = 1168) | Mean antibody level | Mean difference (95% CI) | Padj | Mean difference (95% CI) | Padj |
|---|---|---|---|---|---|---|---|---|---|
| 10 | rs3729508 | Intron 7* | GG/AG/AA | 680/405/61 | 1.645/1.764/1.780 | 0.098 (0.007–0.189) | 3.51E–02 | 0.033 (−0.050–0.116) | 0.4412 |
| 12 | rs6505469 | Intron 5 | AA/TA/TT | 679/411/60 | 1.635/1.768/1.817 | 0.112 (0.022–0.203) | 1.55E–02 | 0.040 (−0.043–0.124) | 0.3445 |
| 15 | rs3794767 | Intron 2 | CC/TC/TT | 713/377/53 | 1.634/1.791/1.755 | 0.118 (0.025–0.212) | 1.33E–02 | 0.040 (−0.046–0.126) | 0.3622 |
| 20 | rs8078340 | Promoter | GG/AG/AA | 603/447/96 | 1.760/1.661/1.433 | −0.146 (−0.23–0.063) | 6.25E–04 | −0.111 (−0.187–0.034) | 4.56E–03 |
| 21 | rs11080358 | Promoter | GG/AG/AA | 647/437/70 | 1.757/1.656/1.378 | −0.151 (−0.238–0.063) | 7.57E–04 | −0.106 (−0.186–0.026) | 9.34E–03 |
Analysis was performed using the log-additive model, and the results were adjusted for age and gender (first Padj column) and for age, gender, and asymptomatic status (second Padj column). Significant P values (Padj < 0.05) are highlighted in boldface. Mean difference refers to the mean antibody level difference. Introns denoted by asterisks are discussed in Table 2, footnote b.
NOS2 variants associated to Plasmodium infection susceptibility control NO plasma levels.
We sought to test whether the five NOS2 SNPs associated with malaria susceptibility were also controlling NOS2 activity. NO plasma levels were measured in a random subset of participants, 132 of which were noncarriers (mean ± the standard deviation [SD] = 51.9 ± 28.4 μM) and 84 of which were asymptomatic carriers (NO mean ± SD = 63.3 ± 40.5 μM). This difference in NO plasma levels (P = 2.30E–02) raised the possibility that NOS2 regulation was conditioned by malaria infection and justified a separate analysis of asymptomatic and noncarrier individuals. QTL analysis revealed that the two SNP alleles in the promoter region (SNP 20/allele A and SNP 21/allele A) conferring a reduced risk of parasite carriage and pre-erythrocytic infection were also associated with lower levels of plasma NO in noncarriers (Table 4). In contrast, the three intronic SNP alleles (SNP 10/allele A, SNP 12/allele T, and SNP 15/allele C) associated with an increased risk of Plasmodium carriage and pre-erythrocytic infection also conferred higher plasma NO levels among asymptomatic carriers (Table 5).
TABLE 4.
QTL analysis of NO plasma levels control by NOS2 SNPs in noncarriersa
| SNP no. | SNP reference | Gene region | Allelesb | Genotypes | Genotype nb (n = 132) | Mean NO level | Mean difference (95% CI) | Padj |
|---|---|---|---|---|---|---|---|---|
| 10 | rs3729508 | Intron 7* | A/G | GG/AG/AA | 76/47/5 | 48.55/59.41/41.79 | 5.22 (–3.62–14.05) | 0.2498 |
| 12 | rs6505469 | Intron 5 | T/A | AA/TA/TT | 71/52/7 | 47.92/59.36/36.98 | 3.49 (–4.90–11.87) | 0.4163 |
| 15 | rs3794767 | Intron 2 | T/C | CC/TC/TT | 76/46/6 | 49.32/59.25/36.12 | 2.39 (–6.25–11.02) | 0.5890 |
| 20 | rs8078340 | Promoter | A/G | GG/AG/AA | 71/48/9 | 55.65/48.97/34.01 | –8.61 (–16.55–0.66) | 3.57E–02 |
| 21 | rs11080358 | Promoter | A/G | GG/AG/AA | 74/48/7 | 56.16/47.19/38.64 | –8.67 (–16.93–0.41) | 4.17E–02 |
Analysis was performed using the log-additive model, and the results were adjusted for age and gender (Padj). Significant P values are highlighted in boldface. Introns denoted by asterisks are discussed in Table 2, footnote b.
Indicated as minor/major frequency alleles.
TABLE 5.
QTL analysis of NO plasma levels control by NOS2 SNPs in asymptomatic carriersa
| SNP no. | SNP reference | Gene region | Allelesb | Genotypes | Genotype nb (n = 84) | Mean NO level | Mean difference (95% CI) | Padj |
|---|---|---|---|---|---|---|---|---|
| 10 | rs3729508 | Intron 7* | A/G | GG/AG/AA | 42/31/9 | 57.06/60.27/101.09 | 16.30 (4.28–28.31) | 9.49E–03 |
| 12 | rs6505469 | Intron 5 | T/A | AA/TA/TT | 41/32/9 | 55.39/62.32/101.09 | 17.04 (5.03–29.06) | 6.80E–03 |
| 15 | rs3794767 | Intron 2 | T/C | CC/TC/TT | 45/30/6 | 57.56/65.43/99.23 | 14.70 (1.50–27.9) | 3.21E–02 |
| 20 | rs8078340 | Promoter | A/G | GG/AG/AA | 41/30/12 | 65.82/63.27/55.81 | –5.43 (–17.01–6.14) | 0.3602 |
| 21 | rs11080358 | Promoter | A/G | GG/AG/AA | 41/36/6 | 66.22/61.68/55.17 | –5.99 (–19.34–7.35) | 0.3813 |
Analysis was performed using the log-additive model, and the results were adjusted for age and gender (Padj). Significant P values are highlighted in boldface. Introns denoted by asterisks are discussed in Table 2, footnote b.
Indicated as minor/major frequency alleles.
These findings strongly suggest that the major genetic determinants of NO production controlled by NOS2 are distinct in parasite carriers and noncarriers. These results also show that the effects of promoter and intronic NOS2 SNP alleles on the susceptibility to Plasmodium infection paralleled their effects on NO plasma levels. Together, the data indicate that increased NO bioavailability correlates with higher susceptibility to parasite carriage and pre-erythrocytic infection, supporting the notion that NO plays an anti-inflammatory role in the context of malaria infection and decreases the efficacy of antiparasite responses.
NOS2 variants governing NO production are associated with clinical malaria progression.
We used hospital-based samples to investigate whether NOS2 SNP variants control progression of clinical malaria manifestations, namely, from uncomplicated malaria to cerebral malaria. We compared the genotype frequencies of 20 NOS2 SNPs in a sample collection of 269 Angolan children (see Table S2 in the supplemental material) that presented with uncomplicated malaria (n = 142) or developed cerebral malaria (n = 127).
We found that three SNP alleles (SNP 11/allele T, SNP 12/allele T, and SNP 13/allele C) conferred protection against cerebral malaria, as denoted by an OR of <1 (Table 6). This association signal overlapped the gene region that confers susceptibility to infection (Tables 2 and 3) and increased NO levels in asymptomatic carriers (Table 5). Specifically, SNP 12 (rs6505469) allele T was associated with increased NO levels and susceptibility to infection in Príncipe but protected against cerebral malaria in Angolan children. On the other hand, the SNP alleles in the promoter region did not show an association with clinical malaria.
TABLE 6.
Association of NOS2 SNPs with cerebral malariaa
| SNP no. | SNP reference | Gene region | Allelesb | Minor allele frequency | Genotypes | Genotype no. |
CM vs UM |
||
|---|---|---|---|---|---|---|---|---|---|
| CM (n = 127) | UM (n = 142) | Padj | OR (95% CI) | ||||||
| 1 | rs11653716 | Intron 26 | G/C | 0.328 | CC/GC-GG | 66/60 | 59/82 | 0.07533 | 0.64 (0.39–1.05) |
| 2 | rs3794756 | Intron 25 | T/C | 0.086 | CC/TC-TT | 106/20 | 121/21 | 0.92323 | 1.03 (0.53–2.03) |
| 3 | rs3729727 | Intron 24 | A/G | 0.214 | GG/AG-AA | 77/50 | 87/55 | 0.86357 | 1.04 (0.63–1.72) |
| 4 | rs3729507 | Intron 22 | C/G | 0.089 | GG/CG-CC | 105/22 | 121/21 | 0.70311 | 1.14 (0.59–2.2) |
| 5 | rs2872753 | Intron 17 | G/A | 0.44 | AA/GA-GG | 45/81 | 38/104 | 0.12158 | 0.66 (0.39–1.12) |
| 6 | rs9282801 | Intron 16* | T/G | 0.271 | GG/TG-TT | 60/66 | 79/63 | 0.15144 | 1.43 (0.88–2.34) |
| 7 | rs3729720 | Intron 14* | T/C | 0.056 | CC/TC-TT | 109/18 | 131/11 | 0.06581 | 2.09 (0.94–4.66) |
| 8 | rs16966545 | Intron 11 | A/T | 0.174 | TT/AT-AA | 81/45 | 99/42 | 0.25747 | 1.35 (0.8–2.28) |
| 9 | rs4795067 | Intron 9 | G/A | 0.155 | AA/GA-GG | 87/39 | 107/34 | 0.26219 | 1.36 (0.79–2.35) |
| 10 | rs3729508 | Intron 7* | A/G | 0.188 | GG/AG-AA | 91/36 | 85/56 | 0.05503 | 0.61 (0.36–1.01) |
| 11 | rs3730017 | Exon 7 | T/C | 0.24 | CC/TC-TT | 83/44 | 72/70 | 1.89E–02 | 0.56 (0.34–0.91) |
| 12 | rs6505469 | Intron 5 | T/A | 0.167 | AA/TA-TT | 96/31 | 91/51 | 4.33E–02 | 0.58 (0.34–0.99) |
| 13 | rs16966563 | Exon 4 | C/T | 0.236 | TT/CT-CC | 88/39 | 69/73 | 7.90E–04 | 0.43 (0.26–0.71) |
| 14 | rs3794766 | Intron 2 | T/C | 0.153 | CC/TC-TT | 86/38 | 109/32 | 0.18941 | 1.45 (0.83–2.52) |
| 15 | rs3794767 | Intron 2 | T/C | 0.167 | CC/TC-TT | 92/32 | 90/49 | 0.11435 | 0.65 (0.38–1.11) |
| 16 | rs3730013 | Intron 1* | T/C | 0.262 | CC/TC-TT | 66/61 | 79/63 | 0.65862 | 1.12 (0.69–1.81) |
| 17 | rs6505483 | Intron 1 | A/G | 0.199 | GG/AG-AA | 79/48 | 90/50 | 0.55685 | 1.17 (0.7–1.95) |
| 18 | rs9282799 | Promoter | T/C | 0.034 | CC/TC-TT | NA/NA | NA/NA | NA | NA |
| 20 | rs8078340 | Promoter | A/G | 0.269 | GG/AG-AA | 70/55 | 70/69 | 0.44726 | 0.83 (0.51–1.35) |
| 21 | rs11080358 | Promoter | A/G | 0.225 | GG/AG-AA | 79/47 | 82/59 | 0.52927 | 0.85 (0.52–1.4) |
Association tests were performed using the dominant model, and the results were adjusted for age and gender (Padj). Significant adjusted P values (Padj < 0.05) are highlighted in boldface. rs16966563 (SNP 13) is still significant after Bonferroni correction (P = 1.58E–02). Introns denoted by asterisks are discussed in Table 2, footnote b. NA, not available.
Indicated as minor/major frequency alleles.
The Angolan population showed a LD structure similar to the Principe population (see Fig. S2 in the supplementary material and Fig. 2, respectively). Specifically, the three intronic SNPs (SNPs 10, 12, and 15) in strong LD (r2 ≥ 0.67), together with SNPs (SNPs 9, 11, 13, and 14) composed comparable LD blocks. Likewise, the two SNPs in the promoter region showed strong LD (r2 = 0.79). Haplotypic analysis of association with progression to cerebral malaria, comprising the SNPs in each of the three LD blocks, did not identify haplotypes with a higher significant association than the individual SNPs. We thus identified here two distinct regions in the NOS2 gene that control susceptibility to malaria in different stages of infection and demonstrated that the control of NO plasma levels by these two gene regions is dependent on the infection status.
DISCUSSION
We analyzed the role of NOS2 polymorphisms in governing NO plasma levels and the risk of Plasmodium carriage, susceptibility to pre-erythrocytic infection, and cerebral malaria. We found that two NOS2 SNP alleles in the promoter region that were associated with decreased NO plasma levels in noncarriers also conferred decrease susceptibility to pre-erythrocytic infection, as ascertained from the anti-CSP antibody levels. This suggests that decreased NO bioavailability controlled by the NOS2 promoter acts to decrease susceptibility to Plasmodium productive infection. Conversely, we identified a gene region associated with high NO plasma levels in asymptomatic carriers that conferred susceptibility to Plasmodium carriage and pre-erythrocytic infection but afforded protection against cerebral malaria. This implies that a distinct NOS2 gene region contributing to the control of NO bioavailability acts in infected individuals and impacts infection progression and disease severity.
Several genetic studies on the association of NOS2 polymorphisms with malaria in different populations have focused on genetic variation in the promoter region and demonstrated association signals influencing the outcome of infection (6, 8–12, 35). Furthermore, NOS2 promoter variants have been shown to drive NO production in malaria patients (11, 12, 36). In the present study, we identified two distinct regions in the NOS2 gene that govern NO plasma levels. The data indicate that the NOS2 promoter region significantly contributed to NO plasma levels in the absence of Plasmodium infection but did not significantly influence the clinical outcome of infection.
Remarkably, we found that in infected individuals the main region controlling NO plasma levels mapped within the cistronic region encompassing introns 2 to 7. These results introduce the notion that cis-acting elements in this region that control NOS2 gene expression/activity are sensitized by Plasmodium infection through mechanisms yet to be elucidated. Our genetic study raises the possibility of a dual regimen of NOS2 gene expression control, entailing a promoter region that provides “basal” NO levels in the absence of infection and an intronic region that induces higher levels of NOS2 expression upon parasite infection.
The role of NO in malaria pathogenesis is controversial, and both detrimental and beneficial effects have been considered (37). It has been proposed that NO is produced at high levels to kill Plasmodium parasites and that elevated NO levels may impair neuronal signaling and generate oxidant damage and red blood cell damage that leads to anemia, contributing to the clinical features of malaria (38–40). Nevertheless, NO anti-inflammatory properties have been claimed to prevent cerebral malaria (3, 41), and epidemiological evidence is accumulating that NO plasma levels are decreased in cerebral malaria (6, 11, 12, 38).
Our genetic analysis in two different populations from the West coast of Africa entailed different designs to collect samples from healthy exposed individuals or clinical malaria cases but revealed conserved LD structure and comparable allelic frequencies in the analyzed NOS2 region. The data cohesively suggest that genetic factors decreasing the NO level production provide decreased susceptibility to productive infection while genetic factors increasing NO plasma levels (namely, the T allele at SNP12, rs6505469) are associated with increased susceptibility to infection and protect against cerebral malaria. Since ascertainment of asymptomatic malaria cases may depend on the time point of collection, we also used pre-erythrocytic antibodies as an infection susceptibility marker. Finding that the presence of parasite DNA or antiparasite antibodies was associated with the same NOS2 SNP alleles reinforced the notion that susceptibility to infection is partially controlled by NOS2 variants.
Although we do not have access to measurements of local NO bioavailability, our data are consistent with the hypothesis that NO operates through anti-inflammatory properties to impair antiparasite responses, increasing susceptibility to infection, but also protecting against stronger inflammatory responses involved in progression to cerebral malaria. This complex pattern of NOS2 genetic control was revealed through combining results of the genetic analysis across the NOS2 gene in apparently healthy individuals and in clinical malaria cases. Although we analyzed individuals from two distinct African populations, further studies are needed to confirm these observations in other populations. Nevertheless, this work suggests that NO bioavailability attributable to NOS2 is subjected to a dual regimen of genetic control conditioned by infection. This may in part explain the apparently controversial results on the role of NOS2 polymorphisms and NO bioavailability in malaria.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from the Fundação Gulbenkian de Ciência and the Fundação para a Ciência e Tecnologia (grant HMSP-CT/SAU-ICT/0068/2009). M.D.J.T. and M.M. were funded by FCT fellowships SFRH/BPD/35062/2007 and SFRH/BPD/29354/2006, respectively). This research was also supported in part by the Intramural Research Program of the National Institutes of Health.
Footnotes
Published ahead of print 30 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01070-13.
REFERENCES
- 1.Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. 2011. Genetic polymorphisms linked to susceptibility to malaria. Malar. J. 10:271. 10.1186/1475-2875-10-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA. 2010. Mechanisms of genetically based resistance to malaria. Gene 467:1–12. 10.1016/j.gene.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 3.Miller LH, Ackerman HC, Su XZ, Wellems TE. 2013. Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 19:156–167. 10.1038/nm.3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet LR. 2001. Nitric oxide in parasitic infections. Int. Immunopharmacol. 1:1457–1467. 10.1016/S1567-5769(01)00090-X [DOI] [PubMed] [Google Scholar]
- 5.Gudo ES, Prista A, Jani IV. 2013. Impact of asymptomatic Plasmodium falciparum parasitemia on the immunohematological indices among school children and adolescents in a rural area highly endemic for Malaria in southern Mozambique. BMC Infect. Dis. 13:244. 10.1186/1471-2334-13-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit MR. 2009. The CCTTT pentanucleotide microsatellite in iNOS promoter influences the clinical outcome in Plasmodium falciparum infection. Parasitol. Res. 104:1315–1320. 10.1007/s00436-009-1329-9 [DOI] [PubMed] [Google Scholar]
- 7.Parikh S, Dorsey G, Rosenthal PJ. 2004. Host polymorphisms and the incidence of malaria in Ugandan children. Am. J. Trop. Med. Hyg. 71:750–753 [PubMed] [Google Scholar]
- 8.Cramer JP, Mockenhaupt FP, Ehrhardt S, Burkhardt J, Otchwemah RN, Dietz E, Gellert S, Bienzle U. 2004. iNOS promoter variants and severe malaria in Ghanaian children. Trop. Med. Int. Health 9:1074–1080. 10.1111/j.1365-3156.2004.01312.x [DOI] [PubMed] [Google Scholar]
- 9.Ohashi J, Naka I, Patarapotikul J, Hananantachai H, Looareesuwan S, Tokunaga K. 2002. Significant association of longer forms of CCTTT Microsatellite repeat in the inducible nitric oxide synthase promoter with severe malaria in Thailand. J. Infect. Dis. 186:578–581. 10.1086/341779 [DOI] [PubMed] [Google Scholar]
- 10.Burgner D, Usen S, Rockett K, Jallow M, Ackerman H, Cervino A, Pinder M, Kwiatkowski DP. 2003. Nucleotide and haplotypic diversity of the NOS2A promoter region and its relationship to cerebral malaria. Hum. Genet. 112:379–386. 10.1007/s00439-002-0882-4 [DOI] [PubMed] [Google Scholar]
- 11.Hobbs MR, Udhayakumar V, Levesque MC, Booth J, Roberts JM, Tkachuk AN, Pole A, Coon H, Kariuki S, Nahlen BL, Mwaikambo ED, Lal AL, Granger DL, Anstey NM, Weinberg JB. 2002. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360:1468–1475. 10.1016/S0140-6736(02)11474-7 [DOI] [PubMed] [Google Scholar]
- 12.Planche T, Macallan DC, Sobande T, Borrmann S, Kun JF, Krishna S, Kremsner PG. 2010. Nitric oxide generation in children with malaria and the NOS2G-954C promoter polymorphism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299:R1248–R1253. 10.1152/ajpregu.00390.2010 [DOI] [PubMed] [Google Scholar]
- 13.Levesque MC, Hobbs MR, O'Loughlin CW, Chancellor JA, Chen Y, Tkachuk AN, Booth J, Patch KB, Allgood S, Pole AR, Fernandez CA, Mwaikambo ED, Mutabingwa TK, Fried M, Sorensen B, Duffy PE, Granger DL, Anstey NM, Weinberg JB. 2010. Malaria severity and human nitric oxide synthase type 2 (NOS2) promoter haplotypes. Hum. Genet. 127:163–182. 10.1007/s00439-009-0753-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutlis CS, Hobbs MR, Marsh RL, Misukonis MA, Tkachuk AN, Lagog M, Booth J, Granger DL, Bockarie MJ, Mgone CS, Levesque MC, Weinberg JB, Anstey NM. 2003. Inducible nitric oxide synthase (NOS2) promoter CCTTT repeat polymorphism: relationship to in vivo nitric oxide production/NOS activity in an asymptomatic malaria-endemic population. Am. J. Trop. Med. Hyg. 69:569–573 [PubMed] [Google Scholar]
- 15.Mombo LE, Ntoumi F, Bisseye C, Ossari S, Lu CY, Nagel RL, Krishnamoorthy R. 2003. Human genetic polymorphisms and asymptomatic Plasmodium falciparum malaria in Gabonese schoolchildren. Am. J. Trop. Med. Hyg. 68:186–190 [PubMed] [Google Scholar]
- 16.Le Port A, Cot M, Etard JF, Gaye O, Migot-Nabias F, Garcia A. 2008. Relation between Plasmodium falciparum asymptomatic infection and malaria attacks in a cohort of Senegalese children. Malar. J. 7:193. 10.1186/1475-2875-7-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Males S, Gaye O, Garcia A. 2008. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin. Infect. Dis. 46:516–522. 10.1086/526529 [DOI] [PubMed] [Google Scholar]
- 18.Hoyer S, Nguon S, Kim S, Habib N, Khim N, Sum S, Christophel EM, Bjorge S, Thomson A, Kheng S, Chea N, Yok S, Top S, Ros S, Sophal U, Thompson MM, Mellor S, Ariey F, Witkowski B, Yeang C, Yeung S, Duong S, Newman RD, Menard D. 2012. Focused screening and treatment (FSAT): a PCR-based strategy to detect malaria parasite carriers and contain drug resistant P. falciparum, Pailin, Cambodia. PLoS One 7:e45797. 10.1371/journal.pone.0045797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD. 2013. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin. Infect. Dis. 57:40–47. 10.1093/cid/cit174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babiker HA, Gadalla AA, Ranford-Cartwright LC. 2013. The role of asymptomatic Plasmodium falciparum parasitaemia in the evolution of antimalarial drug resistance in areas of seasonal transmission. Drug Resist. Update 16:1–9. 10.1016/j.drup.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Bottius E, Guanzirolli A, Trape JF, Rogier C, Konate L, Druilhe P. 1996. Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 90:15–19. 10.1016/S0035-9203(96)90463-0 [DOI] [PubMed] [Google Scholar]
- 22.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. 2002. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am. J. Trop. Med. Hyg. 66:641–648 [DOI] [PubMed] [Google Scholar]
- 23.Nkoghe D, Akue JP, Gonzalez JP, Leroy EM. 2011. Prevalence of Plasmodium falciparum infection in asymptomatic rural Gabonese populations. Malar. J. 10:33. 10.1186/1475-2875-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinetz JM, Gilman RH. 2002. Asymptomatic Plasmodium parasitemia and the ecology of malaria transmission. Am. J. Trop. Med. Hyg. 66:639–640 [DOI] [PubMed] [Google Scholar]
- 25.Billo MA, Johnson ES, Doumbia SO, Poudiougou B, Sagara I, Diawara SI, Diakite M, Diallo M, Doumbo OK, Tounkara A, Rice J, James MA, Krogstad DJ. 2012. Sickle cell trait protects against Plasmodium falciparum infection. Am. J. Epidemiol. 176(Suppl 7):S175–S185. 10.1093/aje/kws323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawaly YR, Sakuntabhai A, Marrama L, Konate L, Phimpraphi W, Sokhna C, Tall A, Sarr FD, Peerapittayamongkol C, Louicharoen C, Schneider BS, Levescot A, Talman A, Casademont I, Menard D, Trape JF, Rogier C, Kaewkunwal J, Sura T, Nuchprayoon I, Ariey F, Baril L, Singhasivanon P, Mercereau-Puijalon O, Paul R. 2010. Heritability of the human infectious reservoir of malaria parasites. PLoS One 5:e11358. 10.1371/journal.pone.0011358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambo MR, Trovoada MJ, Benchimol C, Quinhentos V, Goncalves L, Velosa R, Marques MI, Sepulveda N, Clark TG, Mustafa S, Wagner O, Coutinho A, Penha-Goncalves C. 2010. Transforming growth factor beta 2 and heme oxygenase 1 genes are risk factors for the cerebral malaria syndrome in Angolan children. PLoS One 5:e11141. 10.1371/journal.pone.0011141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood BM, Armstrong JR. 1991. Comparison of two simple methods for determining malaria parasite density. Trans. R. Soc. Trop. Med. Hyg. 85:186–188. 10.1016/0035-9203(91)90015-Q [DOI] [PubMed] [Google Scholar]
- 29.Snounou G. 1996. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol. Biol. 50:263–291 [DOI] [PubMed] [Google Scholar]
- 30.Plassmeyer ML, Reiter K, Shimp RL, Jr, Kotova S, Smith PD, Hurt DE, House B, Zou X, Zhang Y, Hickman M, Uchime O, Herrera R, Nguyen V, Glen J, Lebowitz J, Jin AJ, Miller LH, MacDonald NJ, Wu Y, Narum DL. 2009. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J. Biol. Chem. 284:26951–26963. 10.1074/jbc.M109.013706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afonso RA, Fernandes AB, Santos C, Ligeiro D, Ribeiro RT, Lima IS, Patarrao RS, Videira PA, Caldeira J, Macedo MP. 2012. Postprandial insulin resistance in Zucker diabetic fatty rats is associated with parasympathetic-nitric oxide axis deficiencies. J. Neuroendocrinol. 24:1346–1355. 10.1111/j.1365-2826.2012.02341.x [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez JR, Armengol L, Sole X, Guino E, Mercader JM, Estivill X, Moreno V. 2007. SNPassoc: an R package to perform whole genome association studies. Bioinformatics 23:644–645. 10.1093/bioinformatics/btm025 [DOI] [PubMed] [Google Scholar]
- 33.Barrett JC, Fry B, Maller J, Daly MJ. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- 34.Druilhe P, Pradier O, Marc JP, Miltgen F, Mazier D, Parent G. 1986. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect. Immun. 53:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kun JF, Mordmuller B, Lell B, Lehman LG, Luckner D, Kremsner PG. 1998. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet 351:265–266. 10.1016/S0140-6736(05)78273-8 [DOI] [PubMed] [Google Scholar]
- 36.Kun JF, Mordmuller B, Perkins DJ, May J, Mercereau-Puijalon O, Alpers M, Weinberg JB, Kremsner PG. 2001. Nitric oxide synthase 2(Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 184:330–336. 10.1086/322037 [DOI] [PubMed] [Google Scholar]
- 37.Sobolewski P, Gramaglia I, Frangos J, Intaglietta M, van der Heyde HC. 2005. Nitric oxide bioavailability in malaria. Trends Parasitol. 21:415–422. 10.1016/j.pt.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 38.Anstey NM, Granger DL, Hassanali MY, Mwaikambo ED, Duffy PE, Weinberg JB. 1999. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am. J. Trop. Med. Hyg. 61:249–252 [DOI] [PubMed] [Google Scholar]
- 39.Stevenson MM, Riley EM. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4:169–180. 10.1038/nri1311 [DOI] [PubMed] [Google Scholar]
- 40.Clark IA, Cowden WB. 2003. The pathophysiology of falciparum malaria. Pharmacol. Ther. 99:221–260. 10.1016/S0163-7258(03)00060-3 [DOI] [PubMed] [Google Scholar]
- 41.Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. 2006. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 12:1417–1422. 10.1038/nm1499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.