Abstract
Previous studies revealed that the potential tumor suppressor EAF2 binds to and stabilizes pVHL, suggesting that EAF2 may function by disturbing the hypoxia signaling pathway. However, the extent to which EAF2 affects hypoxia and the mechanisms underlying this activity remain largely unknown. In this study, we found that EAF2 is a hypoxia response gene harboring the hypoxia response element (HRE) in its promoter. By taking advantage of the pVHL-null cell lines RCC4 and 786-O, we demonstrated that hypoxia-induced factor 1α (HIF-1α), but not HIF-2α, induced EAF2 under hypoxia. Subsequent experiments showed that EAF2 bound to and suppressed HIF-1α but not HIF-2α transactivity. In addition, we observed that EAF2 inhibition of HIF-1α activity resulted from the disruption of p300 recruitment and that this occurred independently of FIH-1 (factor inhibiting HIF-1) and Sirt1. Furthermore, we found that EAF2 protected cells against hypoxia-induced cell death and inhibited cellular uptake of glucose under hypoxic conditions, suggesting that EAF2 indeed may act by modulating the hypoxia-signaling pathway. Our findings not only uncover a unique feedback regulation loop between EAF2 and HIF-1α but also provide a novel insight into the mechanism of EAF2 tumor suppression.
INTRODUCTION
Aerobic organisms require oxygen for energy production, and thus, O2 homeostasis is critical for normal physiology and development (1). Hypoxia, a decreased O2 tension within living cells, can occur under several pathophysiological situations, including ischemia and solid tumor progression. Hypoxia produces significant stress that stimulates cellular adaptive responses such as glycolysis, erythropoiesis, angiogenesis, and vascular remodeling (2, 3). The hypoxia response is controlled by a family of hypoxia-induced factors (HIFs), composed of HIF-α and HIF-β (4). Whereas the expression of the HIF-β subunit is constant, the α subunit is rapidly degraded under normoxia but stabilized when the O2-dependent prolyl hydroxylases (PHDs) that target the O2-dependent degradation domain of HIF-α are inhibited (5, 6). Under hypoxia, HIF activation induces a vast array of genes whose products restore blood supply and nutrients for maintaining tissue integrity and homeostasis (7). During tumor initiation and progression, hypoxia appears in tumors as a consequence of the massive cellular expansion that distances cells from the oxygen-carrying vasculature, thereby causing HIF-α stabilization and activating genes involved in angiogenesis and energy production. As a result, the tumor cells re-establish cell survival and growth and even gain metastatic ability (8). In a wide range of cancers, expression of HIF is increased, linking to a poor clinical prognosis (2). Given the critical role of HIF in tumorigenesis, HIF is considered a key target for developing antitumor drugs (2), and understanding its regulation is a major topic of cancer research (3).
Posttranslational modification and protein-protein interactions regulate HIF-α transcriptional activity. For example, O2-dependent prolyl hydroxylation by PHDs marks HIF-α for ubiquitination by pVHL and subsequent proteolysis. In addition, asparaginyl hydroxylation by FIH (factor inhibiting HIF) impairs HIF transactivity by disrupting the interaction between HIF and the transcriptional coactivator CBP/p300. Moreover, some interaction proteins of HIF-α have been identified as being involved in regulating its transactivity via different mechanisms (5).
EAF2 (ELL-associated factor 2) was initially identified as a partner of ELL (eleven-nineteen lysine-rich leukemia), a fusion protein frequently associated with myeloid leukemia (9). Simultaneously, an independent study identified EAF2/U19 as a gene upregulated by androgen in rat and mouse prostate that exhibits tumor-suppressive function (10). While subsequent studies further supported its tumor suppressive activity in vivo (11, 12), the molecular mechanisms underlying this tumor suppression activity remain largely unclear. Recently, though, using a zebrafish model, we found that EAF2 and EAF1, a homolog of EAF2, can negatively regulate canonical Wnt/β-catenin signaling by binding to β-catenin, which may partially explain its tumor-suppressive function mechanistically (13).
Interestingly, we have demonstrated that EAF2 can bind to and stabilize pVHL, a well-defined tumor suppressor involved in HIF-degradation under normoxia. Given the importance of HIF-α in tumorigenesis, we sought to further evaluate the relationship between HIF-α and EAF2 as well as the behavior of EAF2 under hypoxic conditions. In this study, we found that HIF-1α, but not HIF-2α, transactivated the EAF2 gene. Moreover, the EAF2 protein bound to HIF-1α directly, but not to HIF-2α, and disrupted its interaction with p300 and subsequently its ability to induce transcription. In addition, EAF2 protected cells against hypoxia-induced cell death and affected cellular metabolism by inhibiting cellular glucose uptake in response to hypoxia stress. Our work may not only shed light on the molecular mechanisms of EAF2 in tumor suppression but also uncover a novel player in the hypoxia signaling pathway.
MATERIALS AND METHODS
Cell line and culture conditions.
HEK293T, HepG2, and 786-O cells were originally obtained from the ATCC. RCC4 and RCC4/VHL cells were kindly provided by Peter J. Ratcliffe. 786-O/WT8 and 786-O/PRC3 cells were kindly provided by William Kaelin. HEK293T, HepG2, and RCC4 cell lines were cultured in Dulbecco's modified Eagle medium (DMEM) (HyClone) with 10% fetal bovine serum (FBS). 786-O cells were cultured in RPMI 1640 (HyClone) with 10% FBS. EAF2 wild-type and EAF-null mouse embryo fibroblasts (MEFs) (12) were maintained in DMEM supplemented with sodium pyruvate, 10% FBS, 0.1 mmol/liter nonessential amino acids (Sigma) and 1% penicillin-streptomycin. All cells were grown at 37°C in a humidified incubator containing 5% CO2. Hypoxic conditions (1% O2) for cultured cells were achieved by using an incubator with O2 control filled with 5% CO2 and balanced with N2 (NBS Galaxy 48R).
Plasmid constructions.
The EAF2 promoter luciferase reporters were made by PCR amplification and subcloned into the pGL3-Basic vector (Promega). The p2.1 reporter was purchased from ATCC. The vascular endothelial growth factor gene (VEGF) promoter luciferase reporter (VEGF-Luc) was provided by Amato Giaccia. The Epo promoter luciferase reporter was provided by Eric Huang. The HRE reporter was provided by Navdeep Chandel. The PAI-1 promoter luciferase reporter was provided by Xin-Hua Feng. The SOD2 promoter luciferase reporter was PCR amplified from human genomic DNA and subcloned into pGL3-Basic vector. Human EAF2, HIF-1α, and FIH-1 genes were PCR amplified and subcloned into the pCMV-Myc, pCMV-HA, and pCGN-HAM vector. Human HIF-1α domains were subcloned into pCMV-Myc and pGEX-2B, and the human EAF2 gene was cloned into pET-32α. EAF2 was subcloned into the lentivirus vector pHAGE-CMV-MCS-IZsGreen. EAF2 short hairpin RNAs (shRNAs), HIF-1α shRNAs, HIF-2α shRNAs, FIH-1 shRNAs, and control shRNA (luciferase) were cloned into the lentivirus vector lentiLox3.7. HIF-1α and its domains were subcloned into PM vector (Clontech).
Antibodies and chemical reagents.
Anti-EAF2 antibody was raised by injecting glutathione S-transferase (GST)–EAF2 into rabbits or purchased from Epitomics (7118-1). Anti-Myc (9E10), anti-His (H-15), anti-p300 (N-15), anti-β-actin (sc-47778), anti-HIF-1α (H206), and anti-FIH-1 (sc-26219) antibodies were purchased from Santa Cruz. Antihemagglutinin (anti-HA) antibody was purchased from Covance. Anti-Flag (F1804) antibody was purchased from Sigma. Anti-α-tubulin (EPR1333) and anti-lactate dehydrogenase A (anti-LDHA) (2468-1) antibody was purchased from Epitomics. Anti-HIF-1α (NB100-105) and anti-HIF-2α (NB100-122) antibodies were purchased from Novus Biologicals. Anti-BNIP3L (ab8399) antibody was purchased from Abcam. Anti-SOD2 (EPR2560Y) antibody was purchased from GeneTex. Anti-PAI-1 (612025) antibody was purchased from BD Transduction Laboratories. Anti-pVHL polyclonal antibody was raised at Abmart (Shanghai, China). CoCl2 and DFX were purchased from Sigma. The glucose assay kit was purchased from BioVision. The glucose analog 6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) was purchased from Invitrogen. Anacardic acid was purchased from Sigma.
Virus production.
Lentiviruses for EAF2 overexpression or control were generated by transfecting HEK293T cells with a transducing vector and with the packaging vectors pMD.2 and pspAX. Lentiviruses for knockdown of EAF2, HIF-1α, HIF-2α, and FIH-1 and control (luciferase) were generated by transfecting HEK293T cells with transducing vector, packaging vectors VSVG, RSV-REV, and pMDL g/p RRE. After transfection for 48 h, virus particles in the medium were harvested, filtered, and transduced into target cells. The shRNA target sequences are as follows: control shRNA (luciferase shRNA), 5′-GTTGGCACCAGCAGCGCAC-3′; EAF2 shRNA-1, 5′-GAGTTGAAGGAAGCAGTAA-3′; EAF2 shRNA-2, 5′-GATTGCAAATCCTCTACTT-3′; HIF-1α shRNA-1, 5′-GAGCTTGCTCATCAGTTGC-3′; HIF-1α shRNA-2, 5′-GGGTTGAAACTCAAGCAAC-3′; HIF-2α shRNA-1, 5′-GCGCAAATGTACCCAATGA-3′; HIF-2α shRNA-2, 5′-GACAAGGTCTGCAAAGGGT-3′; FIH-1 shRNA-1, 5′-GACCTTGAATACCTGCAAG-3′; and FIH-1 shRNA-2, 5′-GCTTATTGAGAATGAGGAG-3′.
Luciferase reporter assays.
Cells were grown in 24-well plates and transfected with various amounts of plasmids by VigoFect (Vigorous Biotech, Beijing, People's Republic of China), as well as with pTK-Renilla used as an internal control. pFR-luciferase was purchased from Stratagene and used as a reporter for determining the transactivity of HIF-1α or HIF-1α domains fused with the Gal4-DNA binding domain. After the cells were transfected for 16 to 24 h, the luciferase activity was determined by the dual-luciferase reporter assay system (Promega).
For luciferase reporter assays in HEK293T cells with EAF2 knocked down, a shRNA targeting green fluorescent protein (GFP) (targeting sequence, 5′GCAAGCTGACCCTGAAGTTCAT3′) in pSUPER was used as a control, and transient transfection was employed for further assays.
Data were normalized to Renilla luciferase. Data are reported as means ± standard errors of the means (SEM) from three independent experiments performed in triplicate. The statistical analysis was performed using Graph Pad Prism 5 (unpaired t test) (GraphPad Software Inc., San Diego, CA).
Immunoprecipitation and Western blotting.
Coimmunoprecipitation and Western blot analysis were performed as described previously (14). Anti-Myc antibody-conjugated agarose beads were purchased from Sigma. Protein A/G-Sepharose beads were purchased from GE Company. The Fuji Film LAS4000 mini-luminescent image analyzer was used to photograph the blots. Multi Gauge V3.0 was used for quantifying the protein levels based on the band density obtained in Western blot analysis.
Chromatin immunoprecipitation.
The primers for amplifying the LDHA gene (LDHA) promoter region were: 5′-TTGGAGGGCAGCACCTTACTTAGA-3′ and 5′-GCCTTAAGTGGAACAGCTATGCTGAC-3′ (15). The primers for amplifying the PKM2 promoter region were 5′-TTCCTGCCTCTTGGTATGAC-3′ and 5′-CGGCTTGTTCCCTCCTAC-3′ (15). Chromatin immunoprecipitation (ChIP) assays were performed as described previously (16).
GST pulldown assays.
GST-tagged HIF-1α domains and His-tagged EAF2 were expressed in Escherichia coli BL21-Gold (DE3). GST resin (Novagen) and His resin (Novagen) were used for protein purification. The gels were stained by Coomassie blue or transferred to polyvinylidene difluoride (PVDF) membranes for Western blot assays.
Semiquantitative real-time PCR.
The total RNA was extracted by TRIzol reagent (Invitrogen). The cDNA synthesis was carried out using a first-strand cDNA synthesis kit (Fermentas). The primers for human EAF2 were 5′-GTGCGCTATGACTTCAAACCTGCT-3′ and 5′-ATGGTCACCTGTTCACCTTCACCA-3′. The primers for internal control 18S rRNA were 5′-TCAACTTCGATGGTAGTCGCCGT-3′ and 5′-TCCTTGGATGTGGTAGCCGTTCT-3′. Other primers were synthesized as described in previous reports (15, 17).
Detection of surviving cells and apoptotic cells.
EAF2+/+ and EAF2−/− MEFs were cultured in a hypoxia chamber (1% O2) for 16 to 24 h and then used for further assays. For detection of surviving cells, the cells were harvested, stained with trypan blue, and then counted using an automatic cell counter (TC10; Bio-Rad). For apoptotic-cell detection, the cells were stained by Hoechst 33342 (1 μg/ml) and counted under a fluorescence microscope (Nikon TE2000) (18).
Glucose uptake assays.
HepG2 cells were transduced with lentivirus encoding control shRNA or EAF2 shRNAs. Under hypoxic conditions (16 to 24 h), MEFs and transduced HepG2 cells were cultured in glucose-free DMEM for 24 h and then incubated with high-glucose DMEM for 1 h. The intracellular glucose level was measured by a fluorescence-based glucose assay kit (BioVision).
In addition, a 2-NBDG glucose uptake assay was also employed. Under hypoxic conditions, MEFs were cultured under normal conditions for 24 h, and 100 μM 2-NBDG (Invitrogen) was added to the medium for 1.5 h. For anacardic acid (AA) (p300 inhibitor) treatment, anacardic acid was added to culture medium (50 μM) for 18 h. Fluorescence was measured with a fluorescence-activated cell sorting (FACS) analyzer.
RESULTS
EAF2 is a hypoxia response gene.
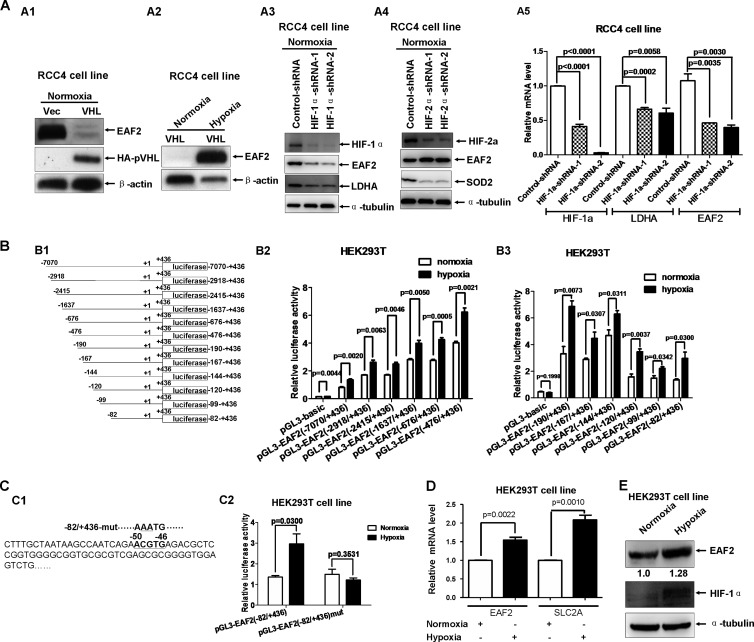
We showed previously that EAF2 binds to and stabilizes pVHL (12). To get a more complete picture of the relationship between EAF2 and pVHL, we took advantage of RCC4 cells, a pVHL-deficient clear-cell renal carcinoma cell line (19). When we compared the levels of EAF2 protein in mock-transfected RCC4 cells (RCC4/Vec) and cells stably expressing HA-tagged VHL (RCC4/HA-VHL), we found that EAF2 levels in RCC4/HA-VHL cells were much lower than levels in RCC4/Vec cells (Fig. 1A1). However, under hypoxic conditions (1% O2 for 16 to 24 h), EAF2 protein expression increased dramatically in RCC4/HA-VHL cells (Fig. 1A2), suggesting that EAF2 may play a role in the hypoxia signaling pathway. Because EAF2 can stabilize pVHL, we next evaluated whether pVHL could also affect EAF2 protein stability. We cotransfected HA-tagged EAF2 with increasing amounts of Myc-tagged VHL in HEK293T cells and found that EAF2 levels remained the same (see Fig. S1 in the supplemental material). Intriguingly, we had noted that the change in EAF2 protein levels in RCC4 cells was similar to that of HIF-α (HIF-1α or HIF-2α) reported previously (19). This similarity prompted us to hypothesize that EAF2 may be a hypoxia response gene transactivated by HIF-α. RCC4 cells contain both HIF-1α and HIF-2α (20). So to determine whether HIF-1α, HIF-2α, or both regulate EAF2 expression, we took advantage of the 786-O cell line, a pVHL-deficient clear cell renal cell carcinoma cell line expressing intact HIF-2α but not HIF-1α (20). Surprisingly, similar EAF2 protein levels were detected in mock-transfected 780-O cells (786-O/PRC3) and in HA-VHL stably transfected 786-O cells (786-O/WT8) (see Fig. S2A in the supplemental material) (21). In addition, hypoxic conditions did not change EAF2 protein levels in either 786-O/WT8 cells or 786-O/PRC3 cells (see Fig. S2B in the supplemental material). These results imply that EAF2 might be a target of HIF-1α but not of HIF-2α.
FIG 1.
EAF2 is a hypoxia-induced gene. (A1) Under normoxic conditions (21% O2), EAF2 was expressed in mock-transfected RCC4 cells (RCC4/Vec), but little was expressed in VHL stably transfected RCC4 cells (RCC4/VHL). (A2) Expression of EAF2 was detected in RCC4/VHL treated with hypoxia but was undetectable under normoxic conditions. The cells were exposed to normoxia (21% O2) or hypoxia (1% O2) for 16 to 20 h. (A3) Under normoxic conditions (21% O2), knockdown of HIF-1α by two different target site shRNAs of HIF-1α (HIF-1α shRNA-1 and HIF-1α shRNA-2) reduced EAF2 expression as well as LDHA expression. (A4) Under normoxic conditions (21% O2), knockdown of HIF-2α by two different target site shRNAs of HIF-2α (HIF-2α shRNA-1 and HIF-2α shRNA-2) reduced SOD2 expression but did not reduce EAF2 expression. An shRNA target luciferase was used as a control (Control-shRNA). (A5) The reduced expression of HIF-1α, LDHA, and EAF2 in RCC4 cells with HIF-1α knocked down was further confirmed by semiquantitative RT-PCR. (B1) Schematic of truncated EAF2 promoter constructs. (B2) The luciferase activities of different truncated EAF2 promoters induced by hypoxia (1% O2 for 16 to 20 h) were measured after being transiently transfected into HEK293T cells. pTK-Renilla served as an internal control. (B3) Fine mapping of the hypoxia response region in the EAF2 promoter. (C1) Schematic of one potential hypoxia responsive element (HRE) (ACGTG [in bold]) localized at the EAF2 promoter (−82 to +436). The mutated sites in the EAF2 promoter mutant (−82/+436-mut) are underlined. (C2) When the potential HRE site located in the EAF2 promoter (−82 to +436) was mutated, the induction of EAF2 promoter activity by hypoxia was abolished in HEK293T cells. (D) Semiquantitative real-time PCR analysis of mRNA levels of EAF2 and Glut-1 (SLC2A) under hypoxic conditions. HEK293T cells were treated with normoxia or hypoxia for 16 to 20 h. (E) The induction of EAF2 expression by hypoxia was confirmed by Western blotting in HEK293T cells. The cells were treated with hypoxia (1% O2) for 16 to 20 h. The relative intensity of EAF2 protein level was quantitated with Photoshop CS4 software and normalized to the α-tubulin protein level (internal control).
To confirm that EAF2 is a specific target of HIF-1α but not of HIF-2α, we knocked down HIF-1α by using two HIF-1α shRNAs (HIF-1α shRNA-1 and HIF-1α shRNA-2, which target different sites in HIF-1α) in RCC4 cells and examined EAF2 protein levels as well as levels of LDHA (a well-defined HIF-1α target [17], designed as a positive control). Similar to that of LDHA, the EAF2 protein level was diminished when HIF-1α was knocked down (Fig. 1A3). However, when HIF-2α was knocked down in RCC4 cells, the EAF2 protein level was not reduced, but the level of SOD2 protein, a well-defined target of HIF-2α (22), was reduced (Fig. 1A4). Moreover, semiquantitative RT-PCR analysis indicated that mRNA levels of EAF2 and LDHA were also decreased when HIF-1α was knocked down (Fig. 1A5). Additionally, in 786-O cells, when HIF-2α was knocked down, the protein level of PAI-1, a target of HIF-2α (17), was diminished, but the EAF2 protein level remained the same (see Fig. S2C in the supplemental material). Semiquantitative RT-PCR analysis further confirmed that the mRNA level of POU5F1, a target of HIF-2α, was indeed reduced when HIF-2α was knocked down, but the EAF2 mRNA level was not affected (see Fig. S2D in the supplemental material). Collectively, these data suggest that EAF2 is a specific target of HIF-1α but not of HIF-2α.
To determine whether EAF2 is indeed a hypoxia response gene, we subsequently carried out a series of promoter assays. A 7.5-kb promoter of EAF2 was PCR amplified (−7070 to +436; the transcription initial site is designated +1) and subcloned into the pGL3-Bacsic vector (Promega) (Fig. 1B1). To map hypoxia response elements (HRE) in the EAF2 promoter, we made other 11 deletion mutants (Fig. 1B1). All EAF2 promoter luciferase reporters were effectively induced by hypoxia in HEK293T cells (Fig. 1B2 and B3). In a search of the sequence of the shortest EAF2 promoter construct (−82 to + 436), one potential HRE site was identified between nucleotides −50 and −46 (ACGTG) (Fig. 1C1). Mutating CG to AA (AAATG) in this reporter completely abolished the hypoxia-induced activity (Fig. 1C2). Using HEK293T cells, we confirmed that hypoxia induced an increase in EAF2 mRNA similar to that of SLC2A (Glut-1) (Fig. 1D), a well-defined hypoxia-induced gene (15). In addition, hypoxia also led to increased levels of endogenous EAF2 protein (1.28-fold) (Fig. 1E). Notably, the EAF2 protein level induced by hypoxia in HEK293T cells (Fig. 1E) was much lower than that in RCC4/HA-VHL cells induced by hypoxia (Fig. 1A2). This inconsistency might be due to the difference in pVHL protein levels between these two cell lines. The stably transfected RCC4/HA-VHL cell line expressed high levels of exogenous pVHL, but HEK293T cells expressed lower levels of endogenous pVHL, which might not fully block HIF-1α transactivity, resulting in higher basal EAF2 protein levels in HEH293T cells under normoxic conditions. However, the tendency for EAF2 to be induced by hypoxia in HEK293T cells was still obvious (Fig. 1E). Taken together, these results indicate that EAF2 is a hypoxia response gene specifically induced by HIF-1α in response to hypoxia.
FIG 2.
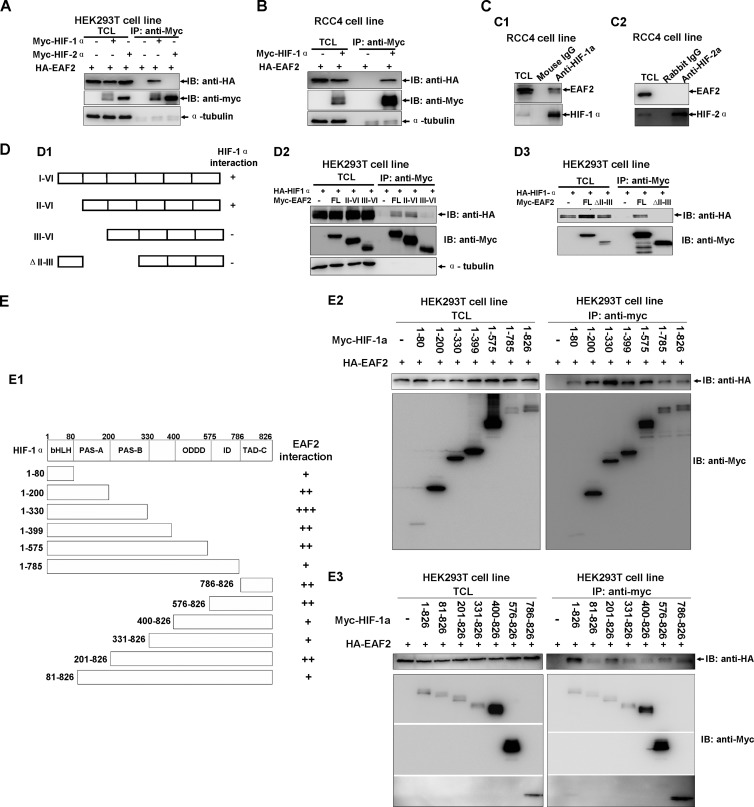
EAF2 interacts with HIF-1α in vitro and in vivo. (A) EAF2 interacts with HIF-1α but not HIF-2α, as revealed by coimmunoprecipitation assays. Myc-HIF-1α, Myc-HIF-2α, and HA-EAF2 were transiently transfected into HEK293T cells, and anti-Myc antibody-conjugated agarose beads were used for immunoprecipitation. (B) Coimmunoprecipitation between transfected Myc-HIF-1α and HA-EAF2 in RCC4 cells. (C) Endogenous EAF2 interacted with endogenous HIF-1α in RCC4 cells, but EAF2 did not interact with HIF-2α in RCC4 cells. (D1) Schematic of EAF2 domains. (D2 and D3) Coimmunoprecipitation between transfected Myc-EAF2 domains and HA-HIF-1α in HEK293T cells. (E1) Schematic of HIF-1α domains. The extent of the interaction between EAF2 and HIF-1α domain is indicated by the number of plus signs. (E2 and E3) Coimmunoprecipitation between transfected Myc-HIF-1α domains and HA-EAF2 in 293T cells. TCL, total cell lysate; IP, immunoprecipitation; IB, immunoblot.
EAF2 interacts with HIF-1α in vivo and in vitro.
To determine the potential role of EAF2 in the hypoxia signaling pathway, we evaluated whether EAF2 could interact with HIF-1α or HIF-2α. We cotransfected HA-tagged EAF2 (HA-EAF2) together with Myc-tagged HIF-1α (Myc-HIF-1α) or Myc-HIF-2α into HEK293T cells and performed coimmunoprecipitation assays. Myc-HIF-1α could pull down HA-EAF2 effectively, but Myc-HIF-2α did not (Fig. 2A), indicating that HIF-1α interacts with EAF2. Because both HIF-1α and EAF2 bind to pVHL (12), pVHL might serve as a bridge to mediate the interaction between HIF-1α and EAF2. To rule out this possibility, we performed coimmunoprecipitation assays in RCC4 cells (VHL null). As shown in Fig. 2B, HIF-1α also bound to EAF2 in these cells. To test whether these proteins interact endogenously, we performed coimmunoprecipitation assays in RCC4 cells using a monoclonal anti-HIF-1α antibody and found that HIF-1α could indeed interact with endogenous EAF2 (Fig. 2C1). In contrast, endogenous HIF-2α could not interact with endogenous EAF2 as revealed by coimmunoprecipitation assays using anti-HIF-2α antibody (Fig. 2C2).
Subsequently, we performed domain mapping to determine which region of EAF2 could bind to HIF-1α. The results showed that the exon 2 (for domain II; amino acids [aa] 36 to 49) of EAF2 might be crucial for HIF-1α interaction, because an EAF2 III-VI mutant (domains III to VI; aa 50 to 260) and an EAF2 II-III deletion mutant (aa 36 to 113 deleted) were undetectable for binding to HIF-1α in this assay (Fig. 2D1 to D3). Furthermore, we mapped the domain of HIF-1α for EAF2 interaction by coimmunoprecipitation assays. All domains of HIF-1α could bind to EAF2 with varied binding affinity (Fig. 2E1 to E3). To determine whether EAF2 could bind to HIF-1α directly, we performed GST pulldown assays. His-tagged EAF2 and GST-tagged HIF-1α domains were bacterially expressed and purified. As shown in Fig. S3 in the supplemental material, EAF2 could almost bind to all domains of HIF-1α with varied binding affinity except for the region of aa 786 to 826, which could not be detected for binding to HIF-1α, different from that revealed by above in vivo coimmunoprecipitation assays. This inconsistence might be resulted from the variation of binding affinity between in vivo coimmunoprecipitation assays and in vitro coimmunoprecipitation assays. It appeared that all domains of HIF-1α could bind to EAF2. Together, these results suggest that EAF2 binds to HIF-1α directly but not to HIF-2α.
Given the fact that EAF2 protein level is increased under hypoxic conditions and EAF2 binds to HIF-1α, this raised that possibility that EAF2 not only serves as a downstream target of HIF-1α but also is stabilized by HIF-1α. To test this possibility, we cotransfected Myc-tagged EAF2 with increasing amounts of Myc-tagged HIF-1α in HEK293T cells and found that the protein level of Myc-EAF2 remained the same (see Fig. S4A in the supplemental material). In addition, the protein stability of EAF2 was not affected by hypoxia treatment (see Fig. S4B in the supplemental material). To further determine the effect of HIF-1α on EAF2 protein stability, we cotransfected Myc-tagged EAF2 together with Myc empty vector or Myc-tagged HIF-1α. After transfection for 18 to 20 h, the cells were treated with cycloheximide (CHX; 10 μg/ml) for different times to block new protein synthesis. As shown in Fig. S4C in the supplemental material, the protein stability of EAF2 was not affected by HIF-1α cotransfection. Thus, HIF-1α binding cannot alter the protein stability of EAF2.
EAF2 inhibits HIF-1α transcriptional activity.
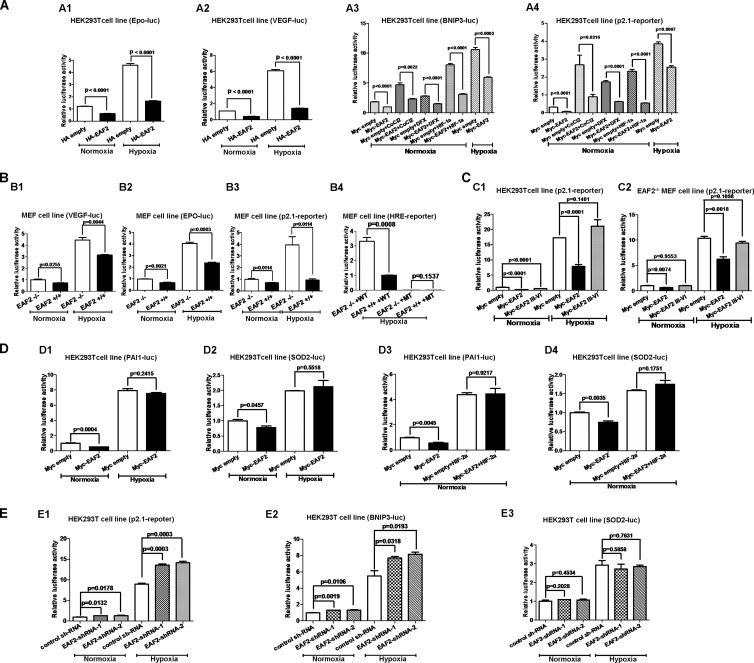
To determine the functional importance of EAF2 binding to HIF-1α, we tested the effect of EAF2 on the promoter activities of four well-known hypoxia-induced genes, including Epo, VEGF, BNIP3, and p2.1 (23–26). Overexpression of EAF2 in HEK293T cells inhibited the promoter activities of these genes under both normoxic and hypoxic conditions (Fig. 3A). Under hypoxia-mimetic conditions as achieved by treatment with cobalt chloride (CoCl2) (100 μM), the hydroxylase inhibitor desferrioxamine (DFX) (100 μM), or exogenous HIF-1α expression, the induction of promoter activity of BNIP3 and p2.1 was also suppressed by EAF2 overexpression (Fig. 3A3 and A4). Furthermore, the promoter activity of VEGF (VEGF-luciferase [VEGF-luc]), Epo (Epo-luc), p2.1 (p2.1-luc), and multiple HRE (hypoxia response element) repeat reporter (HRE reporter) was much lower in EAF2 wild-type MEFs (EAF2+/+) than in EAF2-null MEFs (EAF2−/−) under normoxic and hypoxic conditions (Fig. 3B). As expected, overexpression of the truncated EAF2 mutant (domains III to VI) without HIF-1α binding ability could not suppress p2.1 promoter luciferase reporter activity in HEK293T cells and EAF2−/− MEFs (Fig. 3C1 and C2). In contrast, overexpression of EAF2 in HEK293T cells had no effect on the promoter activity of two well-defined HIF-2α downstream targets, PAI-1 and SOD2 (Fig. 3D) (17, 22), even when HIF-2α was overexpressed (Fig. 3D3 and D4). Consistently, knockdown of EAF2 by two shRNAs (EAF2 shRNA-1 and EAF2 shRNA-2, which target two different sites on EAF2) in HEK293T cells enhanced promoter activity of p2.1 and BNIP3 under normoxic and hypoxic conditions (Fig. 3E1 and E2). However, knockdown of EAF2 in HEK293T cells had no effect on the promoter activity of SOD2 under normoxic and hypoxic conditions (Fig. 3E3). The efficiency of EAF2 shRNA-mediated EAF2 knockdown in HepG2 cells and HEK293T cells was confirmed by semiquantitative real-time RT-PCR or Western blot assays (see Fig. S5 in the supplemental material).
FIG 3.
EAF2 inhibits HIF-1α transactivity. (A) The promoter activities of four well-defined hypoxia-induced genes were suppressed by overexpression of EAF2 in HEK293T cells. (A1) Epo promoter activity was suppressed by overexpression of EAF2. (A2) VEGF promoter activity was suppressed by overexpression of EAF2. (A3) Under normoxic conditions (21% O2), the induction of the BNIP3 promoter reporter by cobalt chloride (100 μM) and DFX (100 μM) and HIF-1α overexpression in HEK293T cells were suppressed by overexpression of EAF2; the induction of BNIP3 promoter reporter by hypoxia (1% O2) was also suppressed by overexpression of EAF2. (A4) Under normoxic conditions (21% O2), the induction of the p2.1 reporter by cobalt chloride (100 μM) and DFX (100 μM) and HIF-1α overexpression in HEK293T cells were suppressed by overexpression of EAF2; the induction of the p2.1 reporter by hypoxia (1% O2) was also suppressed by overexpression of EAF2. (B) The HIF reporter activity was increased in EAF2 knockout (EAF2−/−) mouse embryonic fibroblasts (MEFs) compared with wild-type MEFs (EAF2+/+) under normoxic and hypoxic conditions. (B1) VEGF promoter reporter activity under normoxic and hypoxic conditions. (B2) Epo promoter reporter activity under normoxic and hypoxic conditions. (B3) p2.1 reporter activity under normoxic and hypoxic conditions. (B4) HIF reporter (HRE) activity under hypoxic conditions. WT, wild-type HRE reporter; MT, mutated HRE reporter. (C) The truncated mutant of EAF2 (EAF2 III-VI) without the ability to bind to HIF-1α did not suppress HIF-1α transactivity. (C1) Compared to overexpression of full-length EAF2, overexpression of the truncated EAF2 III-VI mutant in HEK293T cells did not suppress p2.1 promoter luciferase reporter activity under hypoxic conditions. (C2) Compared to overexpression of full-length EAF2, overexpression of EAF2 III-VI in EAF2-null MEFs (EAF2−/−) did not suppress p2.1 promoter luciferase reporter activity. (D) EAF2 has no effect on HIF-2α transactivity. (D1) Overexpression of EAF2 in HEK293T cells did not affect PAI-1 promoter luciferase reporter activity under hypoxic conditions. (D2) Overexpression of EAF2 in HEK293T cells did not affect SOD2 promoter luciferase reporter activity under hypoxic conditions. (D3) Overexpression of EAF2 in HEK293T cells did not affect HIF-2α induced PAI-1 promoter luciferase reporter activity under normoxic conditions. (D4) Overexpression of EAF2 in HEK293T cells did not affect HIF-2α-induced SOD2 promoter luciferase reporter activity under normoxic conditions. (E) The promoter activities of two HIF-1α downstream genes (p2.1 and BNIP3) were enhanced by knockdown of EAF2 in HEK293T cells, but the promoter activity of one HIF-2α downstream gene (SOD2) was not affected by knockdown of EAF2 in HEK293T cells under hypoxic conditions. (E1) p2.1 promoter activity was enhanced by EAF2 knockdown in HEK293T cells under normoxic and hypoxic conditions. (E2) BNIP3 promoter activity was enhanced by EAF2 knockdown in HEK293T cells under normoxic and hypoxic conditions. (E3) SOD2 promoter activity was not affected by EAF2 knockdown in HEK293T cells under normoxic and hypoxic conditions. pTK-Renilla was used as an internal control, and the luciferase activity was determined 18 to 24 h after transfection.
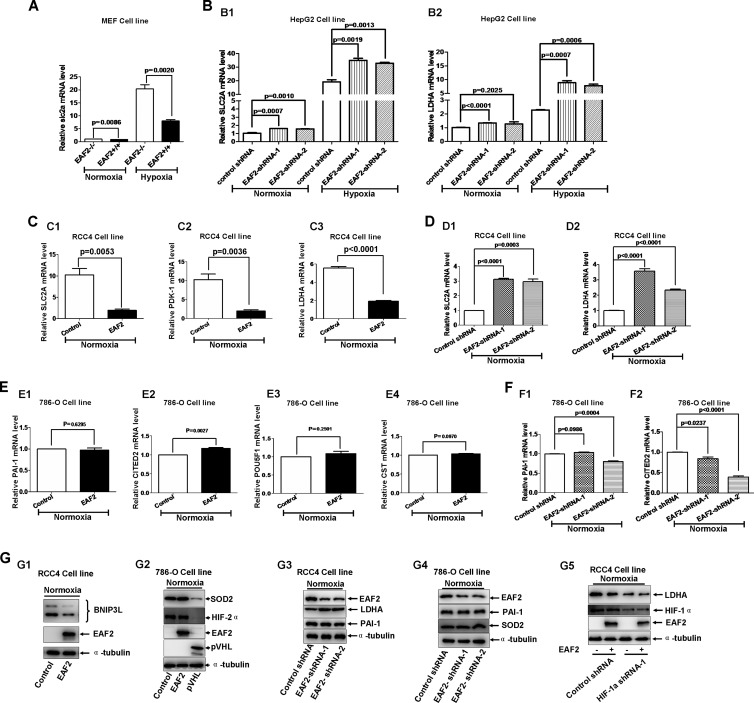
We next examined hypoxia-induced gene expression in different cell lines under different treatments by semiquantitative real-time RT-PCR. As shown in Fig. 4A, expression of mouse slc2a was much lower in EAF2 wild-type MEFs than in EAF2-null MEFs under normoxic and hypoxic conditions. Knockdown of EAF2 by EAF2 shRNAs caused increases in mRNA levels of SLC2A and LDHA in HepG2 cells under normoxic and hypoxic conditions (Fig. 4B). In RCC4 cells, overexpression of EAF2 suppressed expression of SLC2A, PDK-1, and LDHA under normoxic conditions (Fig. 4C) (15). As expected, knockdown of EAF2 caused increased levels of SLC2A and LDHA mRNAs in RCC4 cells under normoxic conditions (Fig. 4D). In contrast, overexpression of EAF2 in 786-O cells did not alter expressions of PAI-1, CITED2, POU5F1, and CST, four well-defined HIF-2α target genes (Fig. 4E) (17). Additionally, knockdown of EAF2 in 786-O cells did not enhance expressions of PAI-1 and CITED2 (Fig. 4F). These data indicate that EAF2 suppresses transcriptional activity of HIF-1α but not that of HIF-2α, consistent with the above observations that EAF2 binds to HIF-1α but not to HIF-2α.
FIG 4.
EAF2 inhibits HIF-1α target gene expression. (A) Semiquantitative real-time PCR analysis showed that the expression of SLC2A (Glut-1) was reduced in EAF2 wild-type MEFs (EAF2+/+) compared to that in EAF2-null MEFs (EAF2−/−) under normoxic and hypoxic conditions. (B) Knockdown of EAF2 in HepG2 cells enhanced HIF-1α target gene expression. (B1) Semiquantitative real-time PCR analysis showed that the expression of SLC2A was enhanced in EAF2 knockdown HepG2 cells under normoxic and hypoxic conditions. (B2) Semiquantitative real-time PCR analysis showed that the expression of LDHA was enhanced in EAF2 knockdown HepG2 cells under hypoxic conditions. (C) Overexpression of EAF2 in RCC4 cells reduced expression of the HIF-1α target genes SLC2A (Glut-1) (C1), PDK-1 (C2), and LDHA (C3) under normoxic conditions, as determined by semiquantitative real-time PCR analysis. (D) Knockdown of EAF2 in RCC4 cells enhanced expression of The HIF-1α target genes SLC2A (Glut-1) (D1) and LDHA (D2) under normoxic conditions, as determined by semiquantitative real-time PCR analysis. (E) Overexpression of EAF2 in 786-O cells did not alter levels of the HIF-2α target genes PAI-1 (E1), CITED2 (E2), POU5F1 (C3), and CST (E4) under normoxic conditions, as determined by semiquantitative real-time PCR analysis. (F) Knockdown of EAF2 in 786-O cells mediated by EAF2 shRNA did not enhance expressions of the HIF-2α target genes PAI-1 (F1) and CITED2 (F2) under normoxic conditions, as determined by semiquantitative real-time PCR analysis. (G1) The BNIP3L protein level was decreased when EAF2 was overexpressed in RCC4 cells. (G2) Overexpression of EAF2 in 780-O cells did not affect HIF-2α downstream target SOD2 protein level; overexpression of pVHL was used as a positive control. (G3) Knockdown of EAF2 in RCC4 cells enhanced HIF-1α target LDHA protein level but did not affect HIF-2α target PAI-1 protein level. (G4) Knockdown of EAF2 in 786-O cells did not affect HIF-2α target PAI-1 and SOD2 protein levels. (G5) When HIF-1α was kept intact in RCC4 cells, overexpression of EAF2 reduced expression of HIF-1α target LDHA (left two lanes), but when HIF-1α was knocked down by HIF-1α shRNA in RCC4 cells, overexpression of EAF2 did not affect HIF-1α target LDHA protein level (right two lanes).
To further determine the suppressive effect of EAF2 on HIF-1α transcriptional activity, we first examined the protein level of BNIP3L, encoded by a hypoxia-induced gene (27), in RCC4 cells with EAF2 overexpression. As shown in Fig. 4G1, BNIP3L was suppressed when EAF2 was overexpressed. However, overexpression of EAF2 in 786-O cells did not affect SOD2 protein levels (Fig. 4G2). As a positive control, overexpression of VHL in 786-O cells resulted in a decrease in SOD protein level (Fig. 4G2). Subsequently, we knocked down EAF2 in RCC4 cells and checked expression of the HIF-1α target (LDHA) and HIF-2α target (PAI-1) (Fig. 4G3). As expected, the expression of LDHA was enhanced, but the expression of PAI-1 was not altered (Fig. 4G3). In 786-O cells, knockdown of EAF2 did not alter the expression of PAI-1 and SOD2 (Fig. 4G4). Moreover, in RCC4 cells, when HIF-1α was kept intact, overexpression of EAF2 suppressed LDHA expression (Fig. 4G5, left two lanes). However, when HIF-1α was knocked down, overexpression of EAF2 had no effect on LDHA expression (Fig. 4G5, right two lanes). Together, these data further implicate a specific suppressive role of EAF2 on HIF-1α.
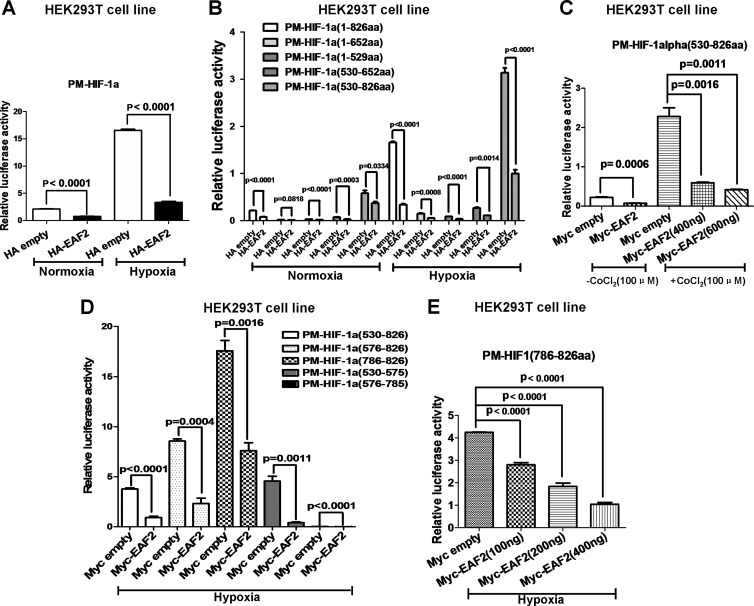
To determine which domain of HIF-1α was responsible for EAF2 inhibition, we fused the Gal4 DNA-binding domain (PM vector; Promega) to different HIF-1α domains to make fusion proteins and then tested the effect of EAF2 on the transactivity of these fusion proteins (14). pFR-luciferase (Stratagene) was used as a reporter (14). Consistent with the above observations, overexpression of EAF2 inhibited the transactivity of the full-length HIF-1α fusion protein (Fig. 5A). As previously reported, HIF-1α contains a DNA-binding domain, an oxygen-dependent domain, and a transactivation domain (TAD) (2). Overexpression of EAF2 reduced the transactivity of all HIF-1α domains (Fig. 5B). The TAD domain (aa 530 to 826), which exhibited the highest transactivity, was suppressed by EAF2 in a dose-dependent manner in HEK293T cells treated with cobalt chloride (CoCl2, 100 μM) (Fig. 5C). More precise mapping showed that the C terminus of TAD (aa 786 to 826) (C-TAD) displayed the highest activity, which again was suppressed by EAF2 in a dose-dependent manner (Fig. 5D and E).
FIG 5.
EAF2 inhibits transactivity of HIF-1α. (A) The transactivity of HIF-1α was inhibited by overexpression of EAF2 in HEK293T cells. (B) The transactivity exhibited in HIF-1α transactivation domain (aa 530 to 826) was inhibited by overexpression of EAF2 in HEK293T cells. (C) The transactivity of HIF-1α transactivation domain induced by CoCl2 (100 μM) treatment was inhibited by overexpression of EAF2 in a dose-dependent manner. (D) Fine mapping of the HIF-1α transactivation domain (aa 530 to 826) inhibited by overexpression of EAF2. (E) The transcriptional activity of HIF-1α C-TAD (aa 786 to 826) was suppressed by EAF2 in a dose-dependent manner under hypoxic conditions.
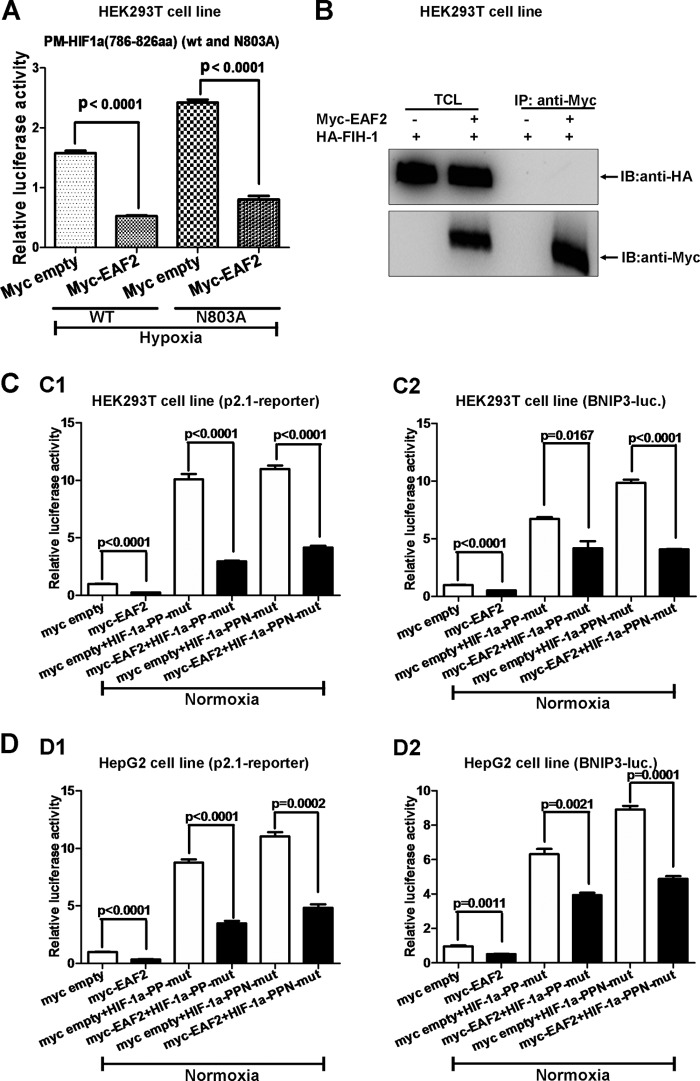
Among the inhibitory factors of HIF-1α reported, FIH-1 binds to the 575 to 826 aa region and inhibits HIF-1α transactivity by hydroxylating residue N803 (26). In this study, we observed that EAF2 exhibited behavior similar to that of FIH. This similarity led us to speculate that FIH-1 might mediate EAF2's inhibitory function on HIF-1α. Therefore, we made a mutant C-TAD fusion in which the Asn in site 803 is mutated to Ala (PM-HIF-1α aa 786 to 826; N803A) and tested the effect of EAF2 on it. Overexpression of EAF2 suppressed the transactivity of the mutant as well as that of the wild-type C-TAD (Fig. 6A). In addition, EAF2 could not interact with FIH-1 when the two proteins were co-overexpressed (Fig. 6B). Thus, the inhibition by EAF2 of HIF-1α appears to be independent of FIH-1. Moreover, to determine whether the hydroxylation sites targeted by PHDs in HIF-1α were affected by EAF2, we examined the effect of EAF2 on the transcriptional activity of the PPN mutant (HIF-1α containing P402A, P564A, and N803A) versus the PP mutant (HIF-1α containing P402A and P564A) by promoter assays in HEK293T cells. As shown in Fig. 5C, overexpression of EAF2 suppressed the promoter activity of p2.1 and BNIP3 induced by both the PP and PPN mutants. Similar results were obtained with HepG2 cells (Fig. 5D). These observations imply that hydroxylation residues targeted by both PHDs and FIH-1 were not responsible for the suppressive effect of EAF2 on HIF-1α.
FIG 6.
The repression of HIF-1α by EAF2 is independent of FIH-1. (A) The transcriptional activities of HIF-1α C-TAD (aa 786 to 826) and the N803A mutant were suppressed by EAF2 overexpression. (B) Coimmunoprecipitation assays showed that EAF2 could not interact with FIH-1 when the two proteins were overexpressed in HEK293T cells. (C) EAF2 suppressed the transcriptional activity of the p2.1 reporter (C1) and the BNIP3 promoter reporter (C2) induced by the HIF-1α PP mutant (P402A P564A) and PPN mutant (P402A P564A N803A) in HEK293T cells under normoxic conditions. (D) EAF2 suppressed the transcriptional activity of the p2.1 reporter (D1) and the BNIP3 promoter reporter (D2) induced by HIF-1α PP and PPN mutants in HepG2 cells under normoxic conditions.
Taken together, these results suggest that EAF2 suppresses HIF-1α transcriptional activity independently of FIH-1 and PHDs.
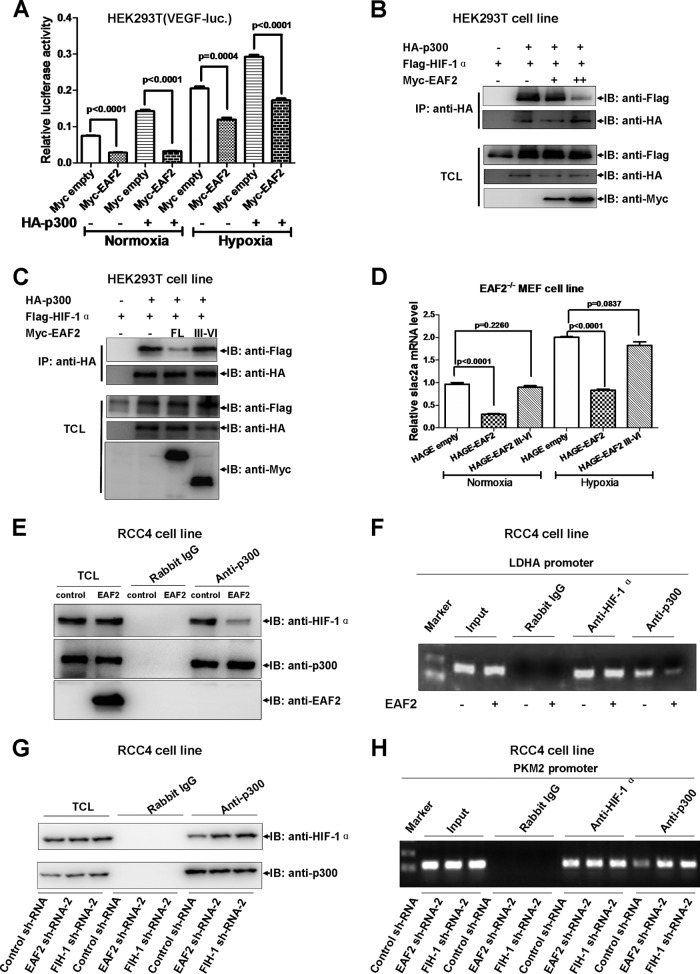
EAF2 represses HIF-1α transcriptional activity by blocking CBP/p300 recruitment.
As reported previously, CBP/p300 activates HIF-1α target gene expression by enhancing HIF-1α transactivity through binding to the C-TAD domain (28). This led us to determine whether EAF2 inhibited HIF-1α transactivity by disrupting the CBP/p300 interaction. To test this possibility, we first measured the effect of EAF2 on the ability of p300 to stimulate VEGF luciferase reporter activity. As shown in Fig. 7A, cotransfection of p300 expression vector could indeed enhance VEGF promoter activity under both normoxic and hypoxic conditions, but this was suppressed by EAF2 overexpression. To further confirm whether EAF2's effect is due to the disruption of HIF-1α and p300, we performed binding assays. As expected, increased EAF2 levels weakened the binding between HIF-1α and p300 (Fig. 7B). Moreover, the EAF2 III-VI mutant, which could not bind to HIF-1α, lost its ability to disrupt the binding between HIF-1α and p300 (Fig. 7C; compare columns 3 and 4, top row). Subsequently, we examined the suppressive role of the EAF2 III-VI mutant in EAF2−/− MEFs. As shown in Fig. 7D, overexpression of the EAF2 III-VI mutant did not suppress slc2a expression.
FIG 7.
EAF2 binding disturbs p300 interaction with HIF-1α. (A) Under normoxic and hypoxic conditions, the enhancement of VEGF promoter activity by cotransfection of HA-p300 was suppressed by overexpression of EAF2 in HEK293T cells (P < 0.0001). (B) Increased levels of EAF2 reduced p300 binding to HIF-1α when the two proteins were overexpressed in HEK293T cells. (C) Overexpression of full-length EAF2 reduced p300 binding to HIF-1α, but overexpression of EAF2 III-VI (which cannot bind to HIF-1α) did not do so. (D) Overexpression of EAF2 in EAF2-null MEFs reduced slc2a expression, but overexpression of EAF2 III-VI did not reduce slc2a expression under normoxia (P = 0.226) and hypoxia (P = 0.0837). (E) Overexpression of EAF2 reduced endogenous p300 binding to endogenous HIF-1α in RCC4 cells. (F) Overexpression of EAF2 reduced HIF-1α-mediated recruitment of p300 to the LDHA promoter, a target of HIF-1α, as revealed by chromatin immunoprecipitation assays (ChIP). Anti-HIF-1α and anti-p300 were used for ChIP assays, and rabbit IgG was used as control. (G) Knockdown of EAF2 enhanced endogenous p300 binding to endogenous HIF-1α in RCC4 cells. (H) Knockdown of EAF2 and FIH-1 enhanced HIF-1α-mediated recruitment of p300 to the PKM2 promoter, a target of HIF-1α, as revealed by chromatin immunoprecipitation assays (ChIP). Anti-HIF-1α and anti-p300 were used for ChIP assays, and rabbit IgG was used as the control.
To further determine the effect of EAF2 on the interaction between HIF-1α and p300, we performed endogenous coimmunoprecipitation assays using anti-p300 antibody in RCC4 cells and confirmed that EAF2 could indeed disrupt interaction between HIF-1α and p300 (Fig. 7E). In addition, we noticed that overexpression of EAF2 did not change the protein level of either HIF-1α or p300 (see Fig. S6 in the supplemental material). To determine whether the effect of EAF2 on the interaction between HIF-1α and p300 can affect HIF-1α's function in vivo, we checked the binding ability of p300 on the LDHA promoter in the presence of EAF2 by chromatin immunoprecipitation assays. As shown in Fig. 7F, overexpression of EAF2 in RCC4 cells diminished p300 binding to LDHA promoter. In contrast, knockdown of EAF2 in RCC4 cells enhanced interaction between HIF-1α and p300, similar to FIH-1 knockdown (designed as a positive control) (26) (Fig. 7G). The efficiency of FIH-1 shRNA-mediated FIH-1 knockdown was confirmed by Western blotting assays (see Fig. S7 in the supplemental material). As expected, knockdown of EAF2 enhanced p300 binding to the PKM2 (a target of HIF-1α [15]) promoter region, similar to FIH-1 knockdown (Fig. 7H).
As reported previously, Sirt1 inactivates HIF-1α by blocking p300 recruitment, consequently repressing HIF-1 target genes (29). To determine whether EAF2 inhibited HIF-1α activity via Sirt1, we made two constructs, a GAL4-DNA binding domain fused with wild-type HIF-1α (aa 576 to 785) and a mutated C-TAD in which the Sirt1 deacetylating site, lysine 674, was mutated to arginine (K674R). We observed that overexpression of EAF2 had similar suppressive effects on these two constructs (see Fig. S8 in the supplemental material), implying that Sirt1 might not mediate EAF2's suppressive effect on HIF-1α.
Taken together, these results suggest that EAF2 might disturb the recruitment of p300 by HIF-1α, resulting in a reduction of HIF-1α transcriptional activity.
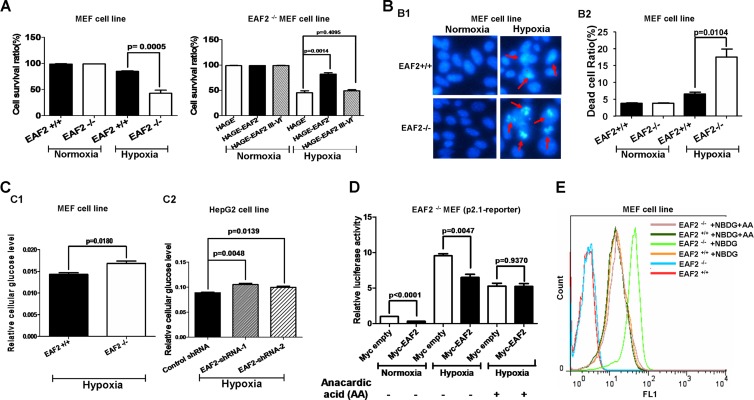
EAF2 protects against hypoxia-induced cell death and inhibits cellular uptake of glucose under hypoxic conditions.
To determine the biological consequence of HIF-1α suppression by EAF2, we evaluated hypoxia-induced cell death in EAF2-null MEFs. Based on an automatic cell counter, we saw no difference in the cell survival rate between wild-type MEFs (EAF2+/+) and EAF2-null MEFs (EAF2−/−) under normoxic conditions. However, under hypoxic conditions, the cell survival rate of EAF2+/+ cells was higher than that of EAF2−/− cells (Fig. 8A). Additionally, under normoxic conditions, overexpression of full-length EAF2 as well as that of exons 3 to 6 (domains III to VI) of EAF2 in EAF2-null MEFs (EAF2−/−) did not exhibit any effect on cell survival rate compared that of control. Nevertheless, under hypoxic conditions, overexpression of full-length EAF2 enhanced cell survival rate significantly. In contrast, overexpression of exon 3 to 6 (domains III to VI) of EAF2 had no effect on the cell survival rate. Furthermore, we used Hoechst staining to detect dead cells. As shown in Fig. 8B, the dead cell ratio of EAF2−/− cells was higher than that of EAF2+/+ cells under hypoxic conditions. Even through the numbers obtained in the cell counter assay and the Hoechst staining assay were not closely correlated, probably due to differences in sensitivity between these two assays; the tendency was quite similar.
FIG 8.
EAF2 protects against hypoxia-induced cell death and inhibits cellular uptake of glucose under hypoxic conditions. (A, left) Under hypoxic conditions (1% O2 for 24 h), EAF2+/+ MEFs had a significantly higher cell survival rate than EAF2−/− MEFs (P = 0.0005). (Right) Under hypoxic conditions (1% O2 for 24 h), overexpression of full-length EAF2 in EAF2−/− MEFs enhanced the cell survival rate (P = 0.0014), but overexpression of domains III to VI of EAF2 did not do so (P = 0.4095). Cells were counted using a Bio-Rad cell counter after trypan blue staining. (B1) The representative pictures of EAF2+/+ and EAF2−/− MEFs stained with Hoechst 33342 after treatment by normoxia (21% O2) or hypoxia (1% O2) for 24 h. The dying cells are marked by arrows. (B2) Quantitative analysis for Hoechst staining. EAF2−/− MEFs had a significantly higher dead cell ratio than EAF2+/+ MEFs (P = 0.0104). Data are means and SEM representing nine randomly selected fields of three wells in a six-well plate. (C1) EAF2−/− MEFs had significantly higher intracellular glucose levels than EAF2+/+ MEFs. The glucose level was measured by a glucose assay kit (BioVision). (C2) Knockdown of EAF2 in HepG2 cells caused an increase in cellular uptake of glucose under hypoxia (P = 0.0048 and P = 0.0139). (D) Inhibition of p300 activity abolished the suppressive role of EAF2 on p2.1 promoter luciferase activity in EAF2−/− MEFs (P = 0.9370 versus P = 0.0047). Anacardic acid (AA) was added to the culture medium (50 μM) for 18-h treatment. Data are means and SEM of three independent experiments performed in triplicate. (E) Anacardic acid treatment (50 μM) abrogated the effect of EAF2 on cellular glucose uptake. Without anacardic acid treatment, EAF2−/− MEFs had higher intracellular glucose levels than EAF2+/+ MEFs (green curve versus orange curve). With anacardic acid treatment, the intracellular glucose levels in both EAF2−/− MEFs and EAF2+/+ MEFs dropped to the same level as that of EAF2+/+ MEFs without anacardic acid (AA) treatment (purple, dark green, and orange curves versus green curve). Glucose uptake was measured by FACS, following 1.5 h exposure to 2-NBDG (100 μM).
The role of HIF-1α in cell metabolism has been widely investigated (15, 30–35). For example, studies have shown that HIF-1α promotes glucose uptake (35). In this study, we showed that EAF2 modulated the induction of several cellular glucose metabolism-related genes downstream of HIF-1α, such as SLC2A (Glut-1), PDK-1, and LDHA. Thus, we investigated whether EAF2 can also affect cellular glucose metabolism by measuring cellular glucose uptake in the presence or absence of EAF2 with a Biovision glucose uptake assay kit, as employed by previous investigators, following the protocol provided by the manufacturer (15, 35, 36). Under hypoxic conditions, EAF2−/− cells has a higher cellular glucose level than EAF2+/+ cells (Fig. 8C1), suggesting that EAF2 suppressed cellular glucose uptake. To further explore this possibility, we knocked down EAF2 in HepG2 cells and measured cellular glucose uptake. As indicated in Fig. 8C2, EAF2 knockdown resulted in a significant increase in cellular glucose uptake under hypoxia (P = 0.0048 and P = 0.0139).
To further determine whether the effect of EAF2 on cellular glucose metabolism consisted of disrupting p300 recruitment of HIF-1α, we took advantage of the p300 inhibitor anacardic acid to block p300 function in cells (37–40) and then checked intracellular glucose levels by 2-NBDG glucose uptake assays (35) (41, 42). Initially, we examined the effect of anacardic acid on EAF2's suppressive role. As shown in Fig. 8D, anacardic acid treatment (50 μM, 18 h) abolished the suppressive role of EAF2 on p2.1 promoter luciferase reporter activity in EAF2−/− MEFs, implying that anacardic acid could indeed inhibit p300 activity and that the suppressive effect of EAF2 on HIF-1α transactivity might occur by disrupting p300 recruitment. Subsequently, we carried out 2-NBDG glucose uptake assays. As shown in Fig. 8E, without anacardic acid (AA) treatment, EAF2−/− MEFs had higher intracellular glucose levels than EAF2+/+ MEFs (compare the green curve and the orange curve); however, with anacardic acid (AA) treatment, the intracellular glucose levels in both EAF2−/− MEFs and EAF2+/+ MEFs dropped to the same level as that of EAF2+/+ MEFs without anacardic acid (AA) treatment (Fig. 8E; compare the purple, dark green, and orange curves with the green curve). These results not only reinforce the EAF2's function on cellular glucose metabolism but also reinforce the idea that the disruption of p300 recruitment by EAF2 constitutes a major mechanism of EAF2's suppression of the hypoxia signaling pathway. Taken together, these results indicate that EAF2 can affect HIF-1α's biological function as well.
Based on the above observations, we propose a model for EAF2's action in hypoxia signaling pathway (see Fig. S9 in the supplemental material).
DISCUSSION
In this study, we identified EAF2 as a hypoxia response gene which is specifically stimulated by HIF-1α but not by HIF-2α. As key regulators of low-oxygen stress response, HIF-1α and HIF-2α activate the expression of overlapping as well as unique transcriptional targets, and their induction can have distinct biological effects (43–46). Even though the N-terminal transactivation domain has been suggested to confer target gene specificity of HIF-1α and HIF-2α (46), so far, no difference has been identified for the hypoxia response element (HRE) localized in the promoters of hypoxia response genes in response to the induction by HIF-1α and HIF-2α. Thus, coactivators might take in charge for this distinct regulation, which needs to be further investigated.
Moreover, we also found that EAF2 inhibits the transactivity of HIF-1α but not that of HIF-2α via protein-protein interaction under hypoxia. The distinct functions of HIF-1α and HIF-2α, particularly in tumorigenesis, are widely recognized (47–55). In addition, several proteins have been identified as binding to and regulating either HIF-1α or HIF-2α differentially. For example, HAF, an E3 ubiquitin ligase, binds and ubiquitinates HIF-1α but not HIF-2α (56). Here, we find that EAF2 not only is upregulated by HIF-1α under hypoxia but also specifically binds to and inhibits HIF-1α transactivity, uncovering a unique feedback regulation loop between EAF2 and HIF-1α. Given that EAF2 binds to and stabilizes pVHL, as revealed previously (12), EAF2 might suppress HIF-1α activity under both hypoxia and normoxia via different pathways. Under hypoxia, EAF2 could directly binds to and inactivate HIF-1α by disrupting p300 recruitment; under normoxia, EAF2 may bind to and stabilize pVHL to enhance HIF-1α proteasomal degradation or directly inhibit HIF-1α activity, as was seen under hypoxic conditions. In this study, we find that EAF2 can suppress the expressions of HIF-1α target genes under both normoxic and hypoxic conditions, which may provide evidence to support this notion. Of note, the suppressive effect of EAF2 on HIF-1α transactivity under normoxic conditions was sometimes not significant compared to that under hypoxic conditions, but the tendency was reproducible. This might indicate that EAF2 can affect HIF-1α activity in normoxic cells as well as that in hypoxic cells. Probably, due to the lower protein level of HIF-1α in normoxic cells, the effect of EAF2 on suppression of HIF-1α activity in these normoxic cells is not always significant, resulting in less important biological consequences.
The disruption of HIF-1α activity under normoxia has been well characterized. In the presence of oxygen, prolyl hydroxylases (PHDs) modify HIF-α at two conserved prolines, resulting in an interaction between HIF and pVHL and the subsequent degradation of HIF by the proteasome (6). However, the negative regulation of HIFs under hypoxia is still unclear. To date, some of negative regulators of HIF-1α under hypoxia have been identified, including IPAS, Sirt-1, Sirt-6, MCM, ARD1, and FHL1-3 (29, 35, 57–60). Among these, the protein levels of Sirt-1, MCM, and ARD1 are downregulated by hypoxia, while FHL is upregulated, similar to EAF2 (29, 35, 57–59). Thus, the identification of EAF2 as a hypoxia-induced negative regulator of HIF-1 may provide a revealing insight into the mechanisms of HIF-1 inhibition under hypoxia.
The role of HIF-1α in tumorigenesis has been widely investigated (2, 61–63). HIF-1 upregulates the expression of genes that are involved in crucial aspects of tumor initiation and progression, including angiogenesis, cell survival, glucose metabolism, invasion, and metastasis (8). However, the mechanisms of EAF2 as a potential tumor suppressor remain largely unknown. Here, we report that EAF2 can bind to and suppress HIF-1, which may explain its function in tumor suppression. Actually, as a classic tumor suppressor, p53 has also been demonstrated to bind to and inhibit HIF-1α transactivity (64).
Even though we have provided evidence to support the idea that EAF2 can disrupt p300 recruitment of HIF-1α for diminishing HIF-1α activity, EAF2 may act through other mechanisms to affect the hypoxia signaling pathway. Further investigations of the molecular mechanisms of EAF2 in the hypoxia signaling pathway may shed new light on developing drugs for cancer treatment.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Peter J. Ratcliffe, William Kaelin, Amato Giaccia, Eric Huang, Xin-Hua Feng, and Navdeep Chandel for the generous gifts of reagents. We also thank Laura E. Pascal for critical reading.
W.X. is supported by 973 grant 2010CB126306, CAS Major Scientific and Technological Project XDA08010208, and NSFC grant 31071212, 91019008. This work was also supported in part by NIH R37 DK51193 (Z.W.).
Footnotes
Published ahead of print 13 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00718-13.
REFERENCES
- 1.Semenza GL. 2012. Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408. 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3:721–732. 10.1038/nrc1187 [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. 2012. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol. Med. 18:534–543. 10.1016/j.molmed.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greer SN, Metcalf JL, Wang Y, Ohh M. 2012. The updated biology of hypoxia-inducible factor. EMBO J. 31:2448–2460. 10.1038/emboj.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majmundar AJ, Wong WJ, Simon MC. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40:294–309. 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. 2001. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3. 10.1016/S0092-8674(01)00518-9 [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. 2009. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol. Cell 35:856–867. 10.1016/j.molcel.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai YP, Wu KJ. 2012. Hypoxia-regulated target genes implicated in tumor metastasis. J. Biomed. Sci. 19:102. 10.1186/1423-0127-19-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simone F, Luo RT, Polak PE, Kaberlein JJ, Thirman MJ. 2003. ELL-associated factor 2 (EAF2), a functional homolog of EAF1 with alternative ELL binding properties. Blood 101:2355–2362. 10.1182/blood-2002-06-1664 [DOI] [PubMed] [Google Scholar]
- 10.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z. 2003. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 63:4698–4704 http://cancerres.aacrjournals.org/content/63/15/4698.long [PubMed] [Google Scholar]
- 11.Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, Cai X, Hahn J, Liu J, Pins M, Doglio L, Dhir R, Gingrich J, Wang Z. 2008. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene 27:1536–1544. 10.1038/sj.onc.1210786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao W, Ai J, Habermacher G, Volpert O, Yang X, Zhang AY, Hahn J, Cai X, Wang Z. 2009. U19/Eaf2 binds to and stabilizes von Hippel-Lindau protein. Cancer Res. 69:2599–2606. 10.1158/0008-5472.CAN-08-2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu JX, Zhang D, Xie X, Ouyang G, Liu X, Sun Y, Xiao W. 2013. Eaf1 and Eaf2 negatively regulate canonical Wnt/beta-catenin signaling. Development 140:1067–1078. 10.1242/dev.086157 [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Feng X, Ban B, Liu J, Wang Z, Xiao W. 2009. Elongation factor ELL (eleven-nineteen lysine-rich leukemia) acts as a transcription factor for direct thrombospondin-1 regulation. J. Biol. Chem. 284:19142–19152. 10.1074/jbc.M109.010439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. 2011. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145:732–744. 10.1016/j.cell.2011.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie XW, Liu JX, Hu B, Xiao W. 2011. Zebrafish foxo3b negatively regulates canonical Wnt signaling to affect early embryogenesis. PLoS One 6:e24469. 10.1371/journal.pone.0024469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlus MR, Wang L, Ware K, Hu CJ. 2012. Upstream stimulatory factor 2 and hypoxia-inducible factor 2alpha (HIF2alpha) cooperatively activate HIF2 target genes during hypoxia. Mol. Cell. Biol. 32:4595–4610. 10.1128/MCB.00724-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng X, Liu X, Zhang W, Xiao W. 2011. p53 directly suppresses BNIP3 expression to protect against hypoxia-induced cell death. EMBO J. 30:3397–3415. 10.1038/emboj.2011.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275. 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 20.Khan MN, Bhattacharyya T, Andrikopoulos P, Esteban MA, Barod R, Connor T, Ashcroft M, Maxwell PH, Kiriakidis S. 2011. Factor inhibiting HIF (FIH-1) promotes renal cancer cell survival by protecting cells from HIF-1alpha-mediated apoptosis. Br. J. Cancer 104:1151–1159. 10.1038/bjc.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr 2001. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468. 10.1126/science.1059817 [DOI] [PubMed] [Google Scholar]
- 22.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. 2009. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324:1289–1293. 10.1126/science.1169956 [DOI] [PubMed] [Google Scholar]
- 23.Wang GL, Semenza GL. 1993. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. U. S. A. 90:4304–4308. 10.1073/pnas.90.9.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruick RK. 2000. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc. Natl. Acad. Sci. U. S. A. 97:9082–9087. 10.1073/pnas.97.16.9082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahon PC, Hirota K, Semenza GL. 2001. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15:2675–2686. 10.1101/gad.924501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. 2001. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 61:6669–6673 http://cancerres.aacrjournals.org/content/61/18/6669.full [PubMed] [Google Scholar]
- 28.Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. 1998. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 17:6573–6586. 10.1093/emboj/17.22.6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. 2010. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 38:864–878. 10.1016/j.molcel.2010.05.023 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Lei L, Liu D, Jovin I, Russell R, Johnson RS, Di Lorenzo A, Giordano FJ. 2012. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1alpha-dependent function. Proc. Natl. Acad. Sci. U. S. A. 109:17478–17483. 10.1073/pnas.1209281109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB, Caffrey T, Yu F, Johnson KR, Powers R, Hollingsworth MA, Singh PK. 2012. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A. 109:13787–13792. 10.1073/pnas.1203339109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. 2011. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harbor Symp. Quant. Biol. 76:347–353. 10.1101/sqb.2011.76.010678 [DOI] [PubMed] [Google Scholar]
- 33.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. 2011. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell 19:416–428. 10.1016/j.ccr.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rey S, Luo W, Shimoda LA, Semenza GL. 2011. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood 117:4988–4998. 10.1182/blood-2010-11-321190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. 2010. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140:280–293. 10.1016/j.cell.2009.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agbor TA, Cheong A, Comerford KM, Scholz CC, Bruning U, Clarke A, Cummins EP, Cagney G, Taylor CT. 2011. Small ubiquitin-related modifier (SUMO)-1 promotes glycolysis in hypoxia. J. Biol. Chem. 286:4718–4726. 10.1074/jbc.M110.115931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogiwara H, Ui A, Otsuka A, Satoh H, Yokomi I, Nakajima S, Yasui A, Yokota J, Kohno T. 2011. Histone acetylation by CBP and p300 at double-strand break sites facilitates SWI/SNF chromatin remodeling and the recruitment of non-homologous end joining factors. Oncogene 30:2135–2146. 10.1038/onc.2010.592 [DOI] [PubMed] [Google Scholar]
- 38.Cui L, Miao J, Furuya T, Fan Q, Li X, Rathod PK, Su XZ. 2008. Histone acetyltransferase inhibitor anacardic acid causes changes in global gene expression during in vitro Plasmodium falciparum development. Eukaryot. Cell 7:1200–1210. 10.1128/EC.00063-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. 2003. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 278:19134–19140. 10.1074/jbc.M301580200 [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Jiang X, Chen S, Price BD. 2006. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 580:4353–4356. 10.1016/j.febslet.2006.06.092 [DOI] [PubMed] [Google Scholar]
- 41.Nitin N, Carlson AL, Muldoon T, El-Naggar AK, Gillenwater A, Richards-Kortum R. 2009. Molecular imaging of glucose uptake in oral neoplasia following topical application of fluorescently labeled deoxy-glucose. Int. J. Cancer 124:2634–2642. 10.1002/ijc.24222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, Reue K, Christofk H, Mischel PS, Pajonk F. 2011. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl. Acad. Sci. U. S. A. 108:16062–16067. 10.1073/pnas.1106704108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordan JD, Simon MC. 2007. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr. Opin. Genet. Dev. 17:71–77. 10.1016/j.gde.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. 2003. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 23:9361–9374. 10.1128/MCB.23.24.9361-9374.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. 2006. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol. Cell. Biol. 26:3514–3526. 10.1128/MCB.26.9.3514-3526.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. 2007. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol. Biol. Cell 18:4528–4542. 10.1091/mbc.E06-05-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. 2002. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell 1:247–255. 10.1016/S1535-6108(02)00044-2 [DOI] [PubMed] [Google Scholar]
- 48.Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, Fukumura D, Moreno-Murciano MP, Herbert JM, Burger A, Riedel J, Elvert G, Flamme I, Maxwell PH, Collen D, Dewerchin M, Jain RK, Plate KH, Carmeliet P. 2005. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell 8:131–141. 10.1016/j.ccr.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 49.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. 2005. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 25:5675–5686. 10.1128/MCB.25.13.5675-5686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Pahlman S. 2006. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10:413–423. 10.1016/j.ccr.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 51.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH. 2008. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene 27:5354–5358. 10.1038/onc.2008.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qing G, Simon MC. 2009. Hypoxia inducible factor-2alpha: a critical mediator of aggressive tumor phenotypes. Curr. Opin. Genet. Dev. 19:60–66. 10.1016/j.gde.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. 2009. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Invest. 119:1159–1166. 10.1172/JCI38499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. 2010. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 120:2699–2714. 10.1172/JCI39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, Jacks T, Chodosh LA, Kissil JL, Simon MC, Keith B. 2010. HIF-2alpha deletion promotes Kras-driven lung tumor development. Proc. Natl. Acad. Sci. U. S. A. 107:14182–14187. 10.1073/pnas.1001296107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koh MY, Darnay BG, Powis G. 2008. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol. Cell. Biol. 28:7081–7095. 10.1128/MCB.00773-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hubbi ME, Luo W, Baek JH, Semenza GL. 2011. MCM proteins are negative regulators of hypoxia-inducible factor 1. Mol. Cell 42:700–712. 10.1016/j.molcel.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. 2002. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 111:709–720. 10.1016/S0092-8674(02)01085-1 [DOI] [PubMed] [Google Scholar]
- 59.Hubbi ME, Gilkes DM, Baek JH, Semenza GL. 2012. Four-and-a-half LIM domain proteins inhibit transactivation by hypoxia-inducible factor 1. J. Biol. Chem. 287:6139–6149. 10.1074/jbc.M111.278630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. 2001. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414:550–554. 10.1038/35107085 [DOI] [PubMed] [Google Scholar]
- 61.Rohwer N, Zasada C, Kempa S, Cramer T. 2013. The growing complexity of HIF-1alpha's role in tumorigenesis: DNA repair and beyond. Oncogene 32:3569–3576. 10.1038/onc.2012.510 [DOI] [PubMed] [Google Scholar]
- 62.Lu X, Kang Y. 2010. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin. Cancer Res. 16:5928–5935. 10.1158/1078-0432.CCR-10-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo YG, Hayashi M, Christensen J, Huang LE. 2009. An essential role of the HIF-1alpha-c-Myc axis in malignant progression. Ann. N. Y. Acad. Sci. 1177:198–204. 10.1111/j.1749-6632.2009.05043.x [DOI] [PubMed] [Google Scholar]
- 64.Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L. 1998. p53 inhibits hypoxia-inducible factor-stimulated transcription. J. Biol. Chem. 273:11995–11998. 10.1074/jbc.273.20.11995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.