Abstract
Objective
High-density lipoprotein (HDL) promotes reverse cholesterol transport (RCT) from peripheral tissues to the liver for clearance. Reduced HDL-cholesterol (HDLc) is associated with atherosclerosis; however, as a predictor of cardiovascular disease, HDLc has limitations as it is not a direct marker of HDL functionality. Our objective was to develop a mass spectrometry based method for the simultaneous measurement of HDLc and ApoAI kinetics in mice using a single 2H2O tracer, and use it to examine genetic and drug perturbations on HDL turnover in vivo.
Approach and Results
Mice were given 2H2O in the drinking water and serial blood samples were collected at different time points. HDLc and ApoAI gradually incorporated 2H, allowing experimental measurement of fractional catabolic rates (FCR) and production rates (PR) for HDLc and ApoA1. ApoE−/− mice displayed increased FCR (p<0.01) and reduced PR of both HDLc and ApoAI (p<0.05) compared to controls. In human ApoAI transgenic mice, levels and PRs of HDLc and human ApoAI were strikingly higher than in wild type mice. Myriocin, an inhibitor of sphingolipid synthesis, significantly increased both HDL flux and macrophage-to-feces RCT, indicating compatibility of this HDL turnover method with the macrophage specific RCT assay.
Conclusions
2H2O-labeling can be used to measure HDLc and ApoAI flux in vivo, and to assess the role of genetic and pharmacological interventions on HDL turnover in mice. Safety, simplicity, and low cost of the 2H2O-based HDL turnover approach suggest that this assay can be scaled for human use to study effects of HDL targeted therapies on dynamic HDL function.
Keywords: HDL, ApoAI, cholesterol, heavy water, protein synthesis, isotopomer, mass spectrometry
Introduction
Plasma concentration of high density lipoprotein cholesterol (HDLc) and ApoAI, the principal protein of HDL, are inversely associated with the risk of atherosclerotic cardiovascular disease (CVD)1. Increasing plasma ApoAI by transgenic over expression or direct infusion of ApoAI decreases atherosclerotic lesions in several animal models of atherosclerosis2, 3. However, it has been challenging to treat atherosclerosis in humans using therapies aimed to increase HDLc and ApoAI4, 5. Anti-atherogenic properties of HDL are attributed to anti-oxidative, anti-inflammatory, anti-thrombotic, cytoprotective, and cholesterol efflux functions of this lipoprotein6. Therefore, simple measurements of circulating HDLc and ApoAI levels may not reflect HDL functionality, which also may be determined by many other factors, including oxidative stress and inflammation. In addition, static measurements of HDLc and ApoAI levels do not reveal the dynamic flux of cholesterol from peripheral tissues, including macrophage transfer to liver for clearance. This dynamic HDL function, called reverse cholesterol transport (RCT), consists of several steps involving efflux of free cholesterol from extrahepatic tissues to HDL, lecithin:cholesterol acyltransferase (LCAT) mediated cholesterol esterification, HDL remodeling, and scavenger receptor type B1 (SR-B1) assisted uptake of HDLc by the liver. In humans, but not mice, cholesterol ester transfer protein (CETP) can mediate the transfer of a portion of HDLc to LDL for uptake by hepatic LDL receptors7, 8.
HDL turnover has been extensively studied using dual radioactive and stable isotope methods9–13. The kinetics of ApoAI alone has been determined from the decay curve of injected 125I-labeled ApoAI in animals14. HDL double labeled with radioactive intracellular trapped tracers in both the ApoAI and cholesteryl ester moieties has been used to estimate both HDL uptake and turnover15, 16. This is a cumbersome method involving isolation of HDL from the donor and injection to the recipient animal after dual radioactive labeling17. Either bolus18 or primed/constant infusion of proteogenic (amino acids) and lipogenic (acetate) or cholesterol tracers were used to estimate the synthetic rate of ApoAI (and other apolipoproteins) and lipids in the lipoproteins of interests19. Combinations of stable isotopes, [13C]-acetate and [2H3]-leucine as labeled precursors of cholesterol/cholesterol ester and ApoAI, respectively, have been used to study HDL turnover in humans12. Aside from technical intricacies, these methods require large amounts of expensive tracers.
In this study we used an alternative heavy water (2H2O)-based metabolic labeling approach to measure HDL turnover. As a non-radioactive, safe and low cost tracer, 2H2O has been widely used to study lipid, protein and DNA turnover in free living organisms, including humans20–22. 2H2O rapidly equilibrates with total body water (including intracellular fluids) and it readily labels lipogenic precursors, e.g. acetyl-CoA/NADPH for lipid synthesis. Application of 2H2O as a tracer for cholesterol synthesis is well established23. Recently we demonstrated that the steady state 2H-labeling of most intracellular amino acids was achieved in rats within 60 min of an intraperitoneal bolus of 2H2O, suggesting that the transfer of amino acids to the polypeptide chain is the rate liming step in protein biosynthesis24. In addition, we have shown that amino acids labeling remained at steady-state throughout 60-day labeling experiment in rodents25. These studies validated the assumption that 2H2O is a true tracer precursor for protein synthesis. We have used this approach to quantify the rate of synthesis of plasma lipids, lipoproteins and acute response proteins under normal conditions and in response to dietary stress 24, 26–28. Recently we applied this technique to assess mitochondrial proteome dynamics in rat heart and liver and demonstrated that it can detect differences in the synthesis rates of spatially distinct cardiac mitochondrial subpopulations, i.e. subsarcolemmal and interfibrillar mitochondria29. Since 2H from 2H2O incorporates into both cholesterol23 and ApoAI24, here we utilized 2H2O as a single tracer to study HDL kinetics. In contrast to existing methods, 2H2O-based HDL turnover approach relies on proteomic analyses and allows discrimination and quantification of both human and mouse ApoAI kinetics individually. We applied this method to assess HDL dynamics in ApoE−/− and ApoAI transgenic mice. Further, we applied the HDL turnover method for the evaluation of HDL function simultaneously with an assay for macrophage-specific RCT method, allowing us to compare the results from these two assays that track different pools of cholesterol and different aspects of HDL metabolism. Finally, we assessed the effect of myriocin, an inhibitor of sphingolipid synthesis and modifier of HDL metabolism30, on HDL turnover and RCT. Myriocin treatment increased both HDL flux and macrophage-RCT, suggesting that 2H2O-based HDL turnover analysis may be used as a safe approach to measure HDL flux in humans and gain insight into factors relevant to RCT.
MATERIALS AND METHODS
Materials and methods are available in the online-only Supplement.
RESULTS
Validation study
The key assumption in 2H2O-based metabolic experiment is that 2H2O rapid labels body water and transfers 2H from 2H2O to 2H-labeled amino acids, which incorporate into proteins, including ApoAI. This approach also excludes any possibility of ApoAI labeling through hydrogen/deuterium exchange in plasma with 2H2O after being secreted. In our previous studies we validated the assumption that tissue amino acids are rapidly labeled and attain a steady state24,25,27. The possibility of post-synthetic hydrogen/deuterium exchange was tested through the following experiment. Two ApoB depleted HDL samples were isolated from untreated wild type mice plasma. One of the samples (control) was saved until the analyses. The second sample was mixed with 10% 2H2O enriched water and incubated in a slow shaker for 7 days at room temperature. HDL proteins, including ApoAI were precipitated with 1 mL of cold acetone (−20 °C) from control and 2H2O-treated and proteins were analyzed as described below. The isotopic distribution of tryptic ApoAI peptide VAPLGAELQESAR (analyzed as M+2 ion with the m/z of 670.87) was analyzed to assess hydrogen/deuterium exchange with 2H2O. No measurable 2H-labeling was detected in tryptic ApoAI peptides, suggesting that the 2H-enrichment of HDL is exclusively related to metabolic labeling but not to hydrogen/deuterium exchange in plasma (Supplementary Figure 1).
HDL turnover studies in ApoE−/− and ApoAItg/tg mice
We previously demonstrated that bolus of 2H2O rapidly equilibrates with the total body water and intracellular amino acids within 30 min24. In this study we found that intraperitoneal bolus loading of 2H2O (22 μl/g body weight) followed with free access to drinking water enriched with 2H2O (6%) for up to 7 days maintains the body water at a steady state labeling of ~3.4%. No adverse effects on growth or food consumption were observed due to 2H2O administration.
We used 2H2O-metabolic labeling coupled with a mass spectrometry approach to assess HDLc and ApoAI turnover in mice. This approach is based on the rationale that after equilibration with total body water and all cell compartments, 2H2O readily labels acetyl-CoA and NADPH for cholesterol synthesis and amino acids for protein synthesis, respectively. After isolation of ApoB100 depleted plasma, lipids were extracted, and total HDLc and ApoAI were analyzed by GC-MS and high resolution LC-MS/MS, respectively. The integrated peaks ratios of HDLc/[2H6]-cholesterol internal standard, and an endogenous ApoAI peptide/heavy isotope labeled internal standard were used for quantification of the absolute amounts of HDLc and ApoAI. The changes in isotopomer distribution of HDLc and proteolytic ApoAI peptides at different time points were used to estimate HDLc and ApoAI FCR, PR and half life (t1/2) (Supplementary Figure 2). We performed a pilot experiment with one wild type mouse using minimal volume blood sampling (25 μl plasma) at selected time points and preparation of ApoB depleted plasma followed by mass spectrometry analyses to measure HDLc (Supplementary Figure 3A) and ApoAI turnover (Supplementary Figure 3B and 3C) as described above. ApoAI was analyzed using 3 different peptides. As expected each peptide reaches different asymptotic labeling since sequences are different (Supplementary Figure 3B). The maximal (plateau) labeling of a peptide depends on total body water labeling and the asymptotical number of exchangeable C-bound H atoms. Previously we have determined the asymptotical number of exchanged H atoms for each proteogenic amino acid and demonstrated that non-essential amino acids, alanine, glutamate, glutamine, glycine and serine are extensively labeled, meaning that they incorporate a higher number of 2H. Small, in some cases negligible, amounts of 2H label were found in most essential amino acids through transamination reaction24. Thus, peptides TQVQSVIDKASETLTAQ and VAPLGAELQESAR with several alanine (A), glutamine (Q) and glutamate (E) have higher labeling than that of peptide DFANVYVDAVK that has only two alanine and multiple essential amino acids (Supplementary Figure 3B). However, normalization of labeling at all time points to the maximum labeling for each peptide illustrates that they overlay and yield similar rate constants. (Supplementary Figure 3C). Thus, we determined that we could accurately measure the kinetics of ApoAI and HDLc in a single animal, and that the turnover kinetics of the three independent ApoAI peptides were similar (coefficient variation < 5%), with t1/2 values of ~ 19 hr (Supplemental Figure 2, similar to previous reports based on radioactive techniques14, 31. The t1/2 of HDLc was ~30 hr. Based on this pilot we chose to use the most abundant endogenous VAPLGAELQESAR peptide and its stable isotope labeled synthetic analog VAPL(13C6)GAEL(13C6)QESAR (as the internal standard) for our subsequent mouse ApoAI turnover studies.
Since ApoE-deficient mice are known to have lower HDLc levels compared to wild type mice 32, we applied this technique for a systematic comparison of HDL turnover in 12-week old female mice (n=6 of each strain). Consistent with previous studies, the total plasma cholesterol levels were significantly higher in ApoE−/− mice (p<0.05), however HDLc and ApoAI levels were significantly lower in ApoE−/− mice compared to wild type controls (p<0.05, Table 1). HDL turnover analysis revealed that this was accompanied with by an almost 2-fold increase in HDLc and ApoAI FCR (p<0.05, Table 1, Figure 1A, B), indicating shorter half lives. In addition, HDLc and ApoAI PR in ApoE-deficient mice were significantly lower than in wild type mice (2-fold and 1.4-fold, respectively, p<0.05, Table 1). RT-PCR analysis revealed that despite lower plasma ApoAI levels and ApoAI PR in ApoE−/− mice, mRNA of hepatic ApoAI in these animals were similar to wild type controls (Supplementary Figure 4A). Combined together, these findings suggest that ApoAI levels might be regulated by the post-transcriptional control of translation or post-translational modification, including by the degradation as it was demonstrated in this study. In contrast, hepatic SR-B1 mRNA and protein levels ascertained by RT-PCR and western blot revealed that higher turnover of HDL in ApoE−/− mice was associated with increased expression of SR-B1, the major receptor for hepatic uptake of HDL (Figure 2A,B). The increased HDL turnover and SR-B1 expression in ApoE−/− mice suggest that they may have higher hepatic HDLc uptake. Thus, our 2H2O-based turnover study demonstrated that ApoE deletion in mice significantly affected HDL metabolism.
Table 1.
Plasma levels and kinetics of HDL-cholesterol and ApoAI in mice HDL (n=6)
| Mice | Total Cholesterol | HDL Cholesterol | ApoAI | ||||
|---|---|---|---|---|---|---|---|
| Levels (mg/dL) | Levels (mg/dL) | FCR (%/hr) | PR (mg/kg/hr) | Levels (mg/dL) | FCR (%/hr) | PR (mg/kg/hr) | |
| Wild type | 111.6±22.7 | 52.8±17.2 | 2.7±0.7 | 0.6±0.2 | 95.9±12.3 | 3.3±0.4 | 1.3±0.2 |
| ApoE−/− | 493.0±97.5 | 17.5±4.6 | 4.1±0.8 | 0.3±0.1 | 32.7±8.5 | 6.4±1.1 | 0.9±0.1 |
| P value | <0.0001 | 0.001 | 0.01 | 0.01 | 0.0001 | 0.0004 | 0.01 |
Figure 1.
HDL turnover in ApoE-deficient (triangular symbols) and wild type (square symbols) mice assessed with 2H2O-metabolic labeling technique. Intraperitoneal bolus loading followed by free access to drinking water enriched with 2H2O (6%) led to a steady state body water labeling of ~3.4%. Time course enrichment of 2H incorporation into HDLc (A) and ApoA1 (B). Data show mean ± SD, n=6 per group.
Figure 2.
Effect of ApoE deletion on hepatic SR-B1 expression. A. RT-PCR of hepatic SR-B1 mRNA (means ± SD; *, p<0.05; N=6 per group). B. Western blot of hepatic SR-B1 from wild type and ApoE-deficient mice, with actin used as a loading control.
To assess the effect of human ApoAI over expression on HDL turnover we also performed a separate 2H2O-based HDL turnover study on six adult male ApoAI transgenic mice. Body water labeling was relatively stable during the 7 day of labeling experiment (Supplementary Figure 5). HDLc levels in these mice were 159±45 mg/dL, and as expected much higher than observed in wild type mice (53±17 mg/dL, wild type mouse data reported in Table 1 but no statistics are presented since these were separate experiments performed in different sexes). Proteomic analyses identified both murine and human ApoAI in the HDL of ApoAI transgenic mice. Consistent with previous reports31, the plasma level of murine ApoAI (0.44±0.06 mg/dL) was markedly decreased in ApoAI transgenics compared to wild type mice (95.9±12.3 mg/dL). As expected, because of the sequence differences of murine and human ApoAI peptides, these peptides reach different steady state 2H enrichment. The 2H2O-based kinetic analysis revealed that the FCR of HDLc in human ApoAI in transgenic mice (Figure 3A) vs. wild type mice (Table 1) was not altered (2.5+0.4 %/hr vs. 2.7+0.7 %/hr, respectively). Our method allowed the simultaneous measurement of the turnover of both human and murine ApoAI. The low levels of murine ApoAI in the transgenic mice were associated with both a 10-fold increase in FCR compared to wild type mice, and a 10-fold decrease in PR vs. wild type mice (compare Figure 3B with Table 1). These results are consistent with the results from previous HDL turnover studies using dual radioisotope approach suggesting that displacement of mouse ApoAI by human protein in ApoAI transgenic mice leads to increased renal clearance of mouse ApoAI33. Reduced PR of murine ApoAI was also paralleled with 4-fold reduction of hepatic ApoAI mRNA determined by RT-PCR in these animals (Supplementary Figure 4B) suggesting feedback inhibition of mouse ApoAI production due to increased expression of human protein in these animals. This finding contradicts to previous report, which based on Northern blot analyses concluded that mouse ApoAI expression is identical in transgenic and wild type animals34. The FCR of human ApoAI in the transgenic mice was 11.1±0.6 %/hr (Figure 3B). Normalization of the data at all-time points for the maximum plateau labeling of mouse and human ApoAI peptides illustrates clear differences in the turnover rates of mouse and human protein (Supplementary Figure 6). The PR of human ApoAI in the transgenic mice was very high (46.9.0±5.8 mg/kg/hr, Figure 3B) indicating that the elevated plasma HDLc in these mice is a direct consequence of the production of more HDL particles.
Figure 3.
HDL turnover in ApoAI transgenic mice liver. A. Time course of 2H incorporation into HDLc. B. Time course of 2H incorporation into human (triangular symbols) and mouse (square symbols) ApoAI. To account for any variations in the total body water labeling, the net labeling of ApoAI at each time point was normalized to water labeling. Insets give the corresponding levels, FCR, and PR for HDL-c and human and mouse ApoAI (mean ± SD, N=6 per group).
HDL turnover and macrophage-specific RCT studies in wild type mice: Effect of myriocin
To investigate the relationship between HDL turnover and RCT we used myriocin as a modifier of sphingolipid and HDL metabolism. We used the 2H2O-metabolic labeling approach and the macrophage-specific RCT method in the same animals during a 3-day time course. This study, in contrast to the prior studies had two minor differences: 1) a lower level of 2H2O enrichment of body water was used; and 2) a shorter 3-day time course was used to be compatible with the RCT study. Myriocin consumption in diet (~0.3 mg/kg body weight/day) for 2 weeks did not affect food intake or body weight. Body water enrichment was stable and equivalent in the control and myriocin treated groups at ~ 1.6% (Supplemental Figure 7). Although myriocin led to ~15% increases in total plasma cholesterol and HDLc, these changes were not statistically significant (Table 2). However, the HDLc FCR (3.2±0.2 %/pool vs. 2.5±0.3 %/pool, p=0.01) and PR (0.8±0.1 mg/kg/h vs. 0.6±0.1 mg/kg/h, p=0.01) were significantly increased in response to myriocin treatment (Figure 4A and Table 2). Myriocin significantly increased both plasma levels (160.8±9.2 mg/dL vs 198.9±15.1 mg/dL, p=0.01) and PR (3.7±0.3 vs. 4.4±0.2, p=0. 01) of ApoAI without any effect on FCR (Figure 4B and Table 2).
Table 2.
Effect of myriocin on HDL turnover (n=5)
| Group | Total Cholesterol | HDL Cholesterol | ApoAI | ||||
|---|---|---|---|---|---|---|---|
| Levels (mg/dL) | Levels (mg/dL) | FCR (%/hr) | PR (mg/kg/hr) | Levels (mg/dL) | FCR (%/hr) | PR (mg/kg/hr | |
| Control | 84.6±7.3 | 48.2±9.1 | 2.5±0.3 | 0.6±0.1 | 160.8±9.2 | 5.0±0.6 | 3.7±0.3 |
| Myriocin | 98.3±17.5 | 55.4±2.2 | 3.2±0.2 | 0.8±0.1 | 198.9±15.1 | 4.9±0.2 | 4.4±0.2 |
| P value | NS | NS | 0.01 | 0.01 | 0.01 | NS | 0.01 |
Figure 4.
Effect of myriocin on HDL turnover in wild type mice. Time course of 2H incorporation into HDLc (A) and ApoA1 (B) for control (square symbols) and myriocin treated (triangular symbols) mice (mean ± SD, N=5 control group and N=4 myriocin group).
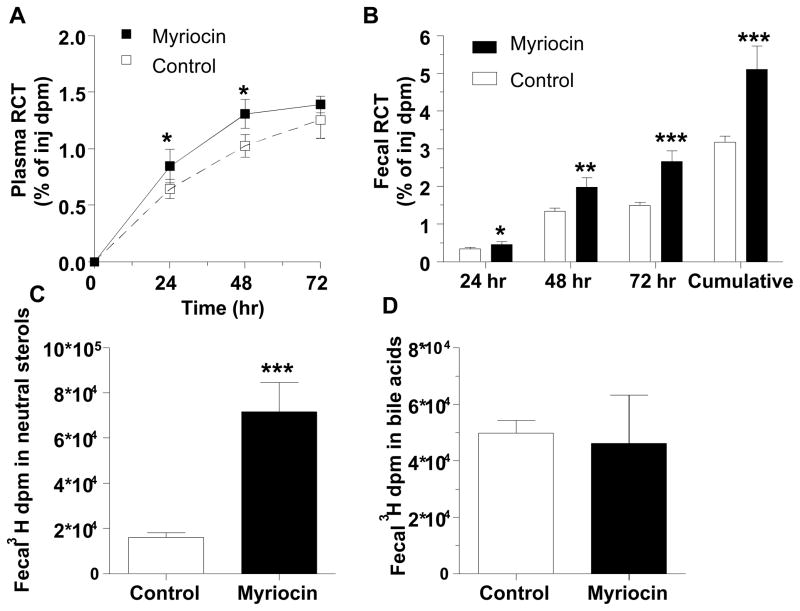
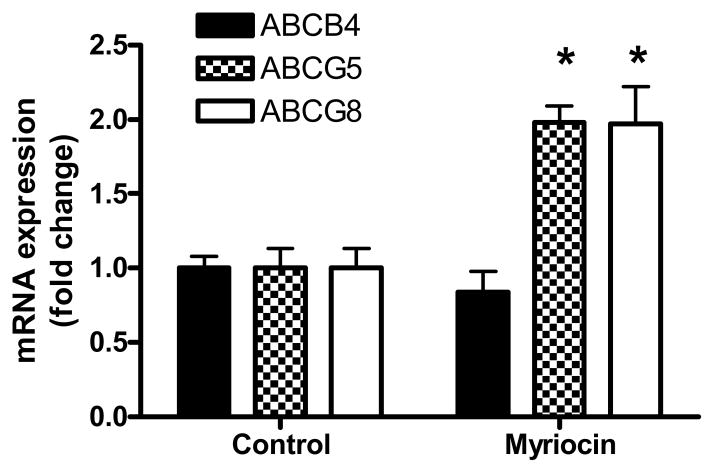
Myriocin also significantly increased macrophage-specific RCT to plasma at 24 and 48 hrs (p<0.05) (Figure 5A), however this treatment did not affect hepatic uptake of cholesterol (Supplementary Figure 8). The most pronounced effect of myriocin was on RCT to the fecal compartment, which was significantly increased in the myriocin treated group at each daily time point, leading to a 1.61-fold increase in fecal RCT cumulatively over the 3-day time course (p<0.001, Figure 5B). Thus, myriocin caused higher mobilization of macrophage cholesterol into plasma coupled with increased excretion of sterols to feces. Fecal sterol composition analysis revealed that myriocin led to an ~3 fold increase in excreted [3H]neutral sterols (Figure 5C) without altering [3H]bile acid excretion (Figure 5D). Increased fecal excretion of sterols also was associated with 2-fold induction of hepatic mRNA levels of ATP-binding cassette transporters (Abc) Abcg5 and Abcg8, the half-transporters involved in hepatobillary elimination of cholesterol (Figure 6). In contrast, myriocin did not have any significant effect on hepatic gene expression of bilary phospholipid transporter Abcb4 (same as Mdr2).
Figure 5.
Effect of myriocin on macrophage-specific RCT. A. Time course of RCT to the plasma (% of injected [3H]cholesterol) after subcutaneous injection of cholesterol-labeled macrophages. B. Daily and cumulative fecal RCT. C. Fecal [3H] dpm in neutral sterols. D. Fecal [3H] dpm in bile acids. For all panels, open symbols and bars represent control group and closed symbols and bars for myriocin group, mean ± SD, N=5 control group and N=3 myriocin group (one mouse eliminated from myriocin group due to loss of labeled donor foam cells); *. p<0.05; **, p<0.01; ***, p<0.001.
Figure 6.
Effect of myriocin on hepatic RNA expression of ATP-binding cassette transporters involved in bilary cholesterol excretion (mean ± SD, N=5 control group and N=4 myriocin group); *p<0.001.
We also assessed the effect of myriocin on hepatic cholesterol metabolism. Hepatic total cholesterol pool did not change due to myriocin treatment (Figure 7A). However, myriocin significantly increased FCR of hepatic cholesterol (1.2% vs. 0.7%/h, p<0.001, Figure 7B) and cholesterol PR almost 2 fold (Figure 7C). FCR of hepatic cholesterol was estimated using the precursor/product relationship based on 2H-enrichments of total cholesterol and body water. However, it is important to note that calculation of hepatic FCR would be more accurate if tissue samples were collected at the early hours, during the semi-linear rise of cholesterol labeling. This was not possible in this study due to terminal sacrifice of all animals after 3 days of 2H2O-metabolic labeling. Therefore, most likely that hepatic FCR and PR values reported in this study are underestimated, although this will not alter our conclusions on effect of myriocin on hepatic cholesterol metabolism. The myriocin effect on increasing hepatic cholesterol FCR is consistent with increased cholesterol excretion into feces that we observed in the RCT study (Figure 5). The myriocin mediated increase in cholesterol excretion suggests that the observed increase in hepatic cholesterol PR may be due in part to a compensatory up regulation of cholesterol biosynthesis. Thus, combined together these results demonstrate that myriocin increased RCT from macrophage to feces and this was associated with increased HDLc turnover.
Figure 7.
Effect of myriocin on hepatic cholesterol metabolism. A. Hepatic cholesterol content. B. Hepatic cholesterol FCR. C. Hepatic cholesterol PR. For all panels, open bars represent control group and closed bars for myriocin group, mean ± SD, N=5 control group and N=4 myriocin group; *. p<0.01.
DISCUSSION
We present a novel simple method to measure HDL turnover in vivo. This method is based on a 2H-metabolic labeling approach for simultaneous measurements of HDLc and ApoAI kinetics using a single heavy water tracer. As a proof of principle we applied this technique to estimate HDL turnover in genetically modified mice with a large range of HDLc levels. Measured ApoAI turnover rates were within the range of the values reported in the literature using different methods and consistent with the expectations based on genetic modifications in these animals14, 35, 36. For example, the human ApoAI FCR we measured in ApoAI transgenic mice of 11.1 ± 0.6 %/hr agrees well with the human ApoAI FCR values of 11 ± 1 %/hr obtained in similar mice by radioiodination and following turnover after i.v. injection 35. In addition, the mouse ApoAI FCR of 3.3 ± 0.4 %/hr we determined is similar to the previously measured mouse ApoAI FCR of 4.3 %/hr that was assessed after injection of radioiodinated mouse ApoAI 14. We also demonstrated that myriocin, a drug known to diminish atherosclerosis in mice, stimulated HDL turnover which was associated with increased macrophage-to-feces RCT. Thus, as a simple and safe approach, the 2H2O-based HDL turnover method could be widely applied to study HDL turnover and give insights into HDL functionality in RCT.
Several in vivo and in vitro labeling approaches using radioactive and stable isotopes have been developed to assess HDL turnover and RCT12, 37. Because of safety concerns, radioactive methods are now largely limited to animal studies. Previous human studies of HDL turnover with stable isotopes relied on precursor-product relationships and involve administration of two different tracers, i.e. a labeled amino acid and acetate or cholesterol. In addition to the inconvenience related to long-term oral consumption or intravenous infusion of tracers and the difficulties in determining the intracellular precursor enrichment, these methods require specialized gas-chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) for the measurement of the low isotopic enrichment of the product 12, 38. In this study we used a single 2H2O tracer and a simple protocol to measure both HDLc and ApoAI turnover in ApoB100-depleted plasma. In contrast to other tracers, 2H2O rapidly equilibrates with total body water, intracellular amino acids and lipogenic substrates (acetyl-CoA and NADPH), thus eliminating the need for assessment of the true intracellular precursor enrichment, a critical step in a tracer study.
In addition, incorporation of multiple copies of 2H results in amplification of isotopic enrichment in ApoAI and HDLc, enhancing measurement sensitivity during the 3 to 7 day labeling experiments we performed in mice. Serial blood sampling allowed us to measure HDL kinetics within each mouse. This protocol also allowed us to combine the measurement of HDL turnover with the macrophage-specific RCT in the same experiment. Furthermore, as a safe, non-radioactive tracer, 2H2O can be administered in drinking water to free living organisms making this method easily adaptable for use in human studies. Based on previous studies with 2H2O 21, we envision that 0.5% 2H2O enrichment of total body water for ~15 days in humans would allow accurate quantification of both HDLc and ApoAI turnover. It is relatively inexpensive (~$500–$1000/person) compared to traditional cholesterol, acetate and amino acid tracers ($3000–$5,000/person). The price of the 2H2O-metabolic labeling experiment in mice is mainly associated with the analyses, as the cost of the 2H2O tracer per mouse is less than $10.
It is important to note that the exponential rise modeling utilized in this 2H2O-metabolic labeling study requires several days for accurate projection of a plateau enrichment of a product. However, it will not be practical to apply this approach to other proteins and lipids with slower turnover rates or when accurate projection to plateau is impossible. In addition, the exponential rise modeling requires serial sampling for accurate fitting. An alternative approach would be a short-term experiment that would require analyses only two samples, i.e. the baseline sample before 2H2O administration and another sample collected during the semi-linear rise of the product enrichment. In this case the FCT can be calculated using precursor/product relationship. This short-term experiments would necessitate the knowledge of the product’s theoretical plateau labeling based on body water enrichment and the number of labeled C-H sites in the product26.
We applied the 2H2O-metabolic labeling approach for the assessment of HDL turnover in wild type and ApoE−/− mice. Mice lacking ApoE are characterized with reduced plasma HDLc and ApoAI levels, which spontaneously develop atherosclerosis in response to their high levels of non-HDLc32. The kinetic analysis revealed that HDLc and ApoAI FCR was higher, and their PR lower in ApoE−/− mice compared to wild type controls, which accounted for their reduced HDLc and ApoAI levels. Despite lower ApoAI PR, hepatic mRNA of ApoAI in ApoE−/− mice was similar to wild type controls. The discrepancy between mRNA and ApoAI levels demonstrated in this study highlights the importance of protein kinetics measurements. The increased HDLc FCR in ApoE−/− mice was accompanied by increased hepatic expression of SR-B1, the receptor for HDLc uptake by the liver. The increased SR-BI expression in ApoE−/− mice could be one of the potential mechanisms of accelerated HDL turnover. Although the increased HDL turnover and SR-B1 expression in ApoE−/− mice suggest higher hepatic HDLc uptake, further studies are warranted to determine whether HDL turnover method could be used to measure selective uptake of HDL cholesteryl ester.
Previously, Tall and colleagues measured cholesteryl-ether and radioionated HDL clearance in control and ApoE-deficient mice39. They also observed increased hepatic SR-B1 expression in ApoE-deficient mice; however, the clearance of [3H]cholesteryl-ether HDL from the plasma was slower in these ApoE-deficient mice39. The 2H2O labeling method we used labels all pools of HDLc, and does so endogenously without disturbing HDL structure or function. However, the radioactive tracer method used ex vivo labeling of HDL can have profound effects on HDL structure and function. Thus, the current method of 2H-metabolic labeling to measure HDL kinetics may be more accurate since it may not disturb endogenous HDL function.
In contrast to ApoE-deficient mice, ApoAI transgenic mice with over expression of human ApoAI have high plasma HDLc and ApoAI, and are protected against atherosclerosis40. We demonstrated that HDLc PR and ApoAI FCR and PR were increased in ApoAI transgenic mice2. Presumably the larger pool of HDLc and ApoAI observed in these animals drives HDL-mediated net cholesterol removal, which is consistent with previous macrophage-specific RCT studies41. In addition, our results demonstrate clear relationship between HDL levels and turnover. Combining the data in Tables 1 and 2, we observed that ApoAI levels were directly related to it’s PR (r2= 0.9, p=0.03, Figure 8 D). Similar, but not significant association was observed between HDLc levels and PR. (r2= 0.9, p=0.06, Figure 8 B). However, there were no consistent inverse correlations between both HDLc and ApoAI levels, and their FCRs (Figure 8 A,C). Yet, these findings are specific to the models under study, and it is possible that other genetic and/or environmental factors modulating ApoAI or HCLc levels could act primarily through FCR. For example, in apoAI turnover studies in normolipidemic humans, ApoAI and HDLc levels were associated with ApoAI PR, and not FCR42. However, in studies combining normolipidemic and hyperlipidemic humans, HDLc was associated with HDL size and ApoAI FCR43.
Figure 8.
The relationships between concentration and turnover of HDLc (A and B) and ApoAI (C and D). The data are derived from Tables 1 and 2 (means ± SD). The levels of ApoAI were directly related to PR (D). Similar, but not significant association was observed between HDLc levels and its PR (B). There were not significant inverse relationships between HDLc and ApoAI levels and their FCR (A and C).
A somewhat similar HDLc turnover study was performed in mice by Dietschy and colleagues44; although, this study did not detect a relationship between HDLc and centripetal cholesterol flux. However, there are some key design differences between their study and ours. For example, they typically administered 3H-water and determined cholesterol turnover during ~ 1 hour, limiting their findings to the early rapid label incorporation phase, while in our studies the measured changes in cholesterol turnover reflect the integration of events over several days. In addition, their study used a CETP transgene to modulate mouse HDL while we used an ApoAI transgene. We conclude that the 2H2O-based turnover method described here extends the classical studies of Dietschy and others45, 46 thereby allowing one to simultaneously examine lipid and protein kinetics which could presumably yield novel insight regarding lipid trafficking in the context of modulating atherosclerosis.
Traditionally plasma level of HDLc has been considered as a marker of the RCT. As it has been shown by Goodman and colleagues HDLc (and ApoAI) levels are not quantitatively important determinants of the mass of slowly exchanging body cholesterol pool47. This finding disproves the hypothesis that high levels of plasma HDLc are associated with reduced cholesterol in peripheral organs. RCT is a complex process involving ABCA1 and ABCG1 mediated efflux of free cholesterol from extrahepatic tissues to HDL, LCAT and CETP catalyzed HDL remodeling, SR-B1 dependent selective uptake of HDLc by the liver and ABCG5/ABCG8 facilitated excretion into feces8. Activation or suppression of each these steps may have concordant or discordant effects on HDLc levels and RCT. For example, hepatic over expression of SR-B1 in mice reduces atherosclerosis which is also associated with increased RCT, despite the fact that HDLc levels were reduced41. Thus, HDLc levels are not always predictive of HDL functionality, as the static measure of the HDL pool does not reflect the flow of cholesterol through RCT. In this study we tested the relationship between global flux of HDL and RCT through direct comparison of these two methods in same animals. In these experiments we used myriocin as a pharmacological tool to modulate sphingolipid and HDL metabolism. It is known that oral administration of myriocin protects against atherosclerosis in ApoE−/− mice, and several activities of myriocin such as impaired cholesterol absorption, induced hepatic ApoAI mRNA, and reduced hepatic mRNAs for cholesterol biosynthetic enzymes, may play a role in myriocin’s protective effect against atherosclerosis30, 48, 49. In our study, performed in wild type mice, myriocin treatment improved macrophage-specific RCT to plasma and feces, however it did not affect hepatic uptake of cholesterol. It is known that cholesterol excretion is closely associated with phospholipid secretion into the bile and the deficiency of ATP-dependent translocation of phosphopilids in Mdr2−/− mice results in impaired cholesterol secretion50. Interestingly increased cholesterol excretion due to myriocin treatment in this study was associated with increased hepatic gene expression of the-half-transporters Abcg5/Abcg8, but not Mdr2 (Abcb4). Abcb4 is involved in phospholipid secretion and it is coupled to cholesterol excretion. These results suggest dissociation of cholesterol and phospholipid secretion due to myriocin treatment. Although the mechanisms of this uncoupling is not clear, similar Mdr2-independent cholesterol secretion previously has been reported51.
Higher mobilization of macrophage cholesterol into plasma coupled with increased hepatic excretion of neutral sterols into feces and reduced cholesterol reabsorption could explain our observed increased in fecal RCT with no changes in hepatic cholesterol uptake and levels. Our study expands the information about myriocin’s beneficial activities to include increased HDL turnover and macrophage-specific RCT, and demonstrates that increased ApoAI levels were associated with increased ApoAI production. Overall these results demonstrate that HDL turnover method could be used to assess the flow of HDLc, the dynamic HDL function. In addition the HDL turnover method is compatible with the measures of macrophage-specific RCT. However, in contrast to RCT assay that measures macrophage-specific HDL flux, the HDL turnover method presented in this study estimates the turnover rates of total HDLc and cannot discriminate specific fluxes from different organs, including macrophages. In our study the labeling of cholesterol in key peripheral organs has not been measured, therefore we could not evaluate the role of HDL in removal of the specific pool of peripheral cholesterol. As it has been shown by Schwartz et al., ~80% of esterified cholesterol in HDL originates from LDL, suggesting their hepatic origin52. This also is in agreement with our findings on hepatic cholesterol metabolism in myriocin study which demonstrates that the terminal labeling of hepatic cholesterol in this study is very similar to the labeling of HDLc (data are not presented), suggesting that HDL flux closely associated with the hepatic cholesterol metabolism. However, the future studies are warranted to determine the contribution of different organs to HDLc flux. Since the influx of cholesterol from macrophages into the plasma is very small compared to the flux from the liver, it is less likely that the HDL turnover method could detect the changes in HDLc turnover due to macrophage-specific RCT. However, it is noteworthy that the HDL turnover and RCT assays can be done simultaneously, as it was demonstrated in this study. In some circumstances the data will be aligned in these two assays and compliment each other.
Another limitation of this study is that HDLc turnover analysis is based on total cholesterol, i.e. free cholesterol plus esterified cholesterol. This was due to restricted plasma (25 μl) samples availability from a mouse at each time point. A separate analysis of free and cholesteryl esters is feasible in larger animals and humans with multiple ~50–100 μl plasma collection. Distinction of free and esterified cholesterol in HDL in combination of cholesterol turnover analyses in LDL and VLDL particles and multicompartmental data analysis would yield useful information on the activities of CETP, HDL remodeling and cholesterol/cholesterol ester exchange between different particles.
Recently, a newly developed assay was described that uses a cholesterol stable isotope dilution method to assess “total RCT” in humans in vivo38. This method requires extensive (~24–32 h) and expensive [2,3-13C2]-cholesterol infusion, followed by multicompartmental modeling to measure tissue cholesterol efflux and plasma cholesterol esterification rates. The non-absorbable tracer, 2H4-sitastonol, is also required for assessment of stool recovery for measuring total fecal sterol excretion. This approach assumes the complete equilibrium of the infused tracer with hepatic cholesterol. As discussed previously, it is difficult to prove this assumption in humans53. In addition, this method estimates peripheral cholesterol efflux based on the rate of label appearance in total plasma cholesterol and cholesterol esters without distinguishing among the various lipoprotein classes. In contrast, the HDL turnover method presented in this study makes no assumptions about tracer equilibration, since 2H2O, an inexpensive tracer, equilibrates rapidly with total body water and the precursors of lipid and protein biosynthesis 24, 54. In addition, our method specifically measures the flux of cholesterol in the HDL compartment, which may be a useful measure of dynamic HDL function relevant to its role in RCT.
In conclusion, we have developed a safe and simple stable isotope based method for estimating HDL turnover that is compatible with the radioactive macrophage-specific RCT assay. Oral 2H2O administration has been previously used in human studies to measure gluconeogeneis, protein synthesis, and lipogenesis 21, 23, 55. Thus, we propose that our method can be easily adapted to measure HDL turnover in humans, and be used to study genetic, environmental, and pharmacological effects on HDL turnover and its implications for HDL function, RCT and atherosclerosis.
Supplementary Material
Significance.
Although epidemiological studies show that HDLc is associated with decreased risk of coronary artery disease, recent drug and genetic studies have shed doubt on whether HDLc is causal in protecting against atherosclerosis or if it is just an associated biomarker. It is now well established that not all HDL is equally functional and that oxidation and glycation can create dysfunctional HDL with impaired reverse cholesterol transport and anti-inflammatory activities. Thus, it may be more important to measure HDL function and flux than static levels of HDLc. Here we describe an elegant heavy water single stable isotope based method to simultaneously measure the turnover of HDLc and ApoAI in vivo. We show that this method can be used in mice to assess turnover changes due to genetic and pharmacological perturbations. In addition, we show that this method is compatible with the widely used method to assess macrophage to feces reverse cholesterol transport. Finally, we suggest that this method may be adaptable for use in human studies to measure the flux of HDLc and ApoAI, which may be useful as surrogates of HDL function.
Acknowledgments
We thank Dr. Chandramouli at Case Western Reserve University School of Medicine for access to the GC-MS instrument in his lab.
Sources of Funding:
This work was supported by The National Institutes of Health grants PO1 HL098055 (SLH and JDS), 5R21RR025346-03 (TK) and The American Heart Association grant 13IRG14700011 (TK).
Abbreviations
- 2H2O
heavy water
- RCT
reverse cholesterol transport
- HDLc
HDL cholesterol
- FCR
fractional catabolic rate
- PR
production rate
- Fmoc
9-fluorenylmethoxycarbonyl derivative
- LTQ-Orbitrap-MS
linear trap Orbitrap mass spectrometry
- GC-MS
gas chromatography mass spectrometry
- SIM
selected ion monitoring
- MPE
molar percent enrichment
Footnotes
Disclosures:
Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens. Dr. Smith reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Smith reports having been paid as a consultant for Esperion, and receiving research funds from Esperion. Dr. Smith reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab. and Esperion.
References
- 1.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 3.Hoang A, Drew BG, Low H, Remaley AT, Nestel P, Kingwell BA, Sviridov D. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. Eur Heart J. 2012;33:657–665. doi: 10.1093/eurheartj/ehr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: A systematic review. JAMA. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glomset JA. The plasma lecithins:Cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 8.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the hdl receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 10.Jolley CD, Woollett LA, Turley SD, Dietschy JM. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J Lipid Res. 1998;39:2143–2149. [PubMed] [Google Scholar]
- 11.Spady DK, Woollett LA, Meidell RS, Hobbs HH. Kinetic characteristics and regulation of hdl cholesteryl ester and apolipoprotein transport in the apoA-I−/− mouse. J Lipid Res. 1998;39:1483–1492. [PubMed] [Google Scholar]
- 12.Ouguerram K, Krempf M, Maugeais C, Maugere P, Darmaun D, Magot T. A new labeling approach using stable isotopes to study in vivo plasma cholesterol metabolism in humans. Metabolism: Clin Expl. 2002;51:5–11. doi: 10.1053/meta.2002.29006. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Melchior GW, Castle CK, Murray RW, Blake WL, Dinh DM, Marotti KR. Apolipoprotein A-I metabolism in cholesteryl ester transfer protein transgenic mice. Insights into the mechanisms responsible for low plasma high density lipoprotein levels. J Biol Chem. 1994;269:8044–8051. [PubMed] [Google Scholar]
- 15.Glass C, Pittman RC, Civen M, Steinberg D. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro. J Biol Chem. 1985;260:744–750. [PubMed] [Google Scholar]
- 16.Rinninger F, Pittman RC. Regulation of the selective uptake of high density lipoprotein-associated cholesteryl esters. J Lipid Res. 1987;28:1313–1325. [PubMed] [Google Scholar]
- 17.de Beer FC, Connell PM, Yu J, de Beer MC, Webb NR, van der Westhuyzen DR. HDL modification by secretory phospholipase a(2) promotes scavenger receptor class B type I interaction and accelerates HDL catabolism. J Lipid Res. 2000;41:1849–1857. [PubMed] [Google Scholar]
- 18.Patterson BW, Hachey DL, Cook GL, Amann JM, Klein PD. Incorporation of a stable isotopically labeled amino acid into multiple human apolipoproteins. J Lipid Res. 1991;32:1063–1072. [PubMed] [Google Scholar]
- 19.Foster DM, Barrett PH, Toffolo G, Beltz WF, Cobelli C. Estimating the fractional synthetic rate of plasma apolipoproteins and lipids from stable isotope data. J Lipid Res. 1993;34:2193–2205. [PubMed] [Google Scholar]
- 20.Schoenheimer R, Rittenberg D. Deuterium as an indicator in the study of intermediary metabolism. Science. 1935;82:156–157. doi: 10.1126/science.82.2120.156. [DOI] [PubMed] [Google Scholar]
- 21.Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR. Quantifying rates of protein synthesis in humans by use of 2H2O: Application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab. 2004;286:E665–672. doi: 10.1152/ajpendo.00271.2003. [DOI] [PubMed] [Google Scholar]
- 22.Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Prot. 2007;2:3045–3057. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- 23.Jones PJ, Scanu AM, Schoeller DA. Plasma cholesterol synthesis using deuterated water in humans: Effect of short-term food restriction. J Lab Clin Med. 1988;111:627–633. [PubMed] [Google Scholar]
- 24.Li L, Willard B, Rachdaoui N, Kirwan JP, Sadygov RG, Stanley WC, Previs S, McCullough AJ, Kasumov T. Plasma proteome dynamics: Analysis of lipoproteins and acute phase response proteins with 2H2O metabolic labeling. Mol Cell Proteomics. 2012;11:1–16. doi: 10.1074/mcp.M111.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasumov T, Dabkowski ER, Shekar KC, Li L, Ribeiro RF, Jr, Walsh K, Previs SF, Sadygov RG, Willard B, Stanley WC. Assessment of cardiac proteome dynamics with heavy water: Slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2013;304:H1201–14. doi: 10.1152/ajpheart.00933.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasumov T, Ilchenko S, Li L, Rachdaoui N, Sadygov RG, Willard B, McCullough AJ, Previs S. Measuring protein synthesis using metabolic 2H labeling, high-resolution mass spectrometry, and an algorithm. Analytical biochemistry. 2011;412:47–55. doi: 10.1016/j.ab.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rachdaoui N, Austin L, Kramer E, Previs MJ, Anderson VE, Kasumov T, Previs SF. Measuring proteome dynamics in vivo: As easy as adding water? Mol Cell Proteomics. 2009;8:2653–2663. doi: 10.1074/mcp.M900026-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunengraber DZ, McCabe BJ, Kasumov T, Alexander JC, Chandramouli V, Previs SF. Influence of diet on the modeling of adipose tissue triglycerides during growth. Am J Physiol Endocrinol Metab. 2003;285:E917–925. doi: 10.1152/ajpendo.00128.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kasumov T, Dabkowski ER, Shekar KC, Li L, Ribeiro RF, Jr, Walsh K, Previs SF, Sadygov RG, Willard B, Stanley WC. Assessment of cardiac proteome dynamics with heavy water: Slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria. American Am J Physiol Heart Circ Physiol. 2013;304:H1201–14. doi: 10.1152/ajpheart.00933.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park TS, Panek RL, Rekhter MD, Mueller SB, Rosebury WS, Robertson A, Hanselman JC, Kindt E, Homan R, Karathanasis SK. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in apoe knockout mice. Atherosclerosis. 2006;189:264–272. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 31.Chajek-Shaul T, Hayek T, Walsh A, Breslow JL. Expression of the human apolipoprotein a-i gene in transgenic mice alters high density lipoprotein (HDL) particle size distribution and diminishes selective uptake of HDL cholesteryl esters. Proc Natl Acad Sci U S A. 1991;88:6731–6735. doi: 10.1073/pnas.88.15.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dansky HM, Shu P, Donavan M, Montagno J, Nagle DL, Smutko JS, Roy N, Whiteing S, Barrios J, McBride TJ, Smith JD, Duyk G, Breslow JL, Moore KJ. A phenotype-sensitizing apoe-deficient genetic background reveals novel atherosclerosis predisposition loci in the mouse. Genetics. 2002;160:1599–1608. doi: 10.1093/genetics/160.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh A, Ito Y, Breslow JL. Apolipoprotein A-I gene expression in transgenic mice. Biotechnology. 1991;16:227–235. [PubMed] [Google Scholar]
- 34.Rubin EM, Ishida BY, Clift SM, Krauss RM. Expression of human apolipoprotein a-i in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991;88:434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayek T, Chajek-Shaul T, Walsh A, Azrolan N, Breslow JL. Probucol decreases apolipoprotein A-I transport rate and increases high density lipoprotein cholesteryl ester fractional catabolic rate in control and human apolipoprotein A-I transgenic mice. Arterioscler Thromb Vasc Biol. 1991;11:1295–1302. doi: 10.1161/01.atv.11.5.1295. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Li W, Wang SP, Mendoza V, Rosa R, Hubert J, Herath K, McLaughlin T, Rohm RJ, Lassman ME, Wong KK, Johns DG, Previs SF, Hubbard BK, Roddy TP. Quantifying apoprotein synthesis in rodents: Coupling LC-MS/MS analyses with the administration of labeled water. J Lipid Res. 2012;53:1223–1231. doi: 10.1194/jlr.D021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner S, Voogt J, Davidson M, Glass A, Killion S, Decaris J, Mohammed H, Minehira K, Boban D, Murphy E, Luchoomun J, Awada M, Neese R, Hellerstein M. Measurement of reverse cholesterol transport pathways in humans: In vivo rates of free cholesterol efflux, esterification, and excretion. JAMA. 2012;1:e001826. doi: 10.1161/JAHA.112.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arai T, Rinninger F, Varban L, Fairchild-Huntress V, Liang CP, Chen W, Seo T, Deckelbaum R, Huszar D, Tall AR. Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc Natl Acad Sci U S A. 1999;96:12050–12055. doi: 10.1073/pnas.96.21.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein A-I. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class b type i (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Oliveira e Silva ER, Kong M, Han Z, Starr C, Kass EM, Juo SH, Foster D, Dansky HM, Merkel M, Cundey K, Brinton EA, Breslow JL, Smith JD. Metabolic and genetic determinants of HDL metabolism and hepatic lipase activity in normolipidemic females. J Lipid Res. 1999;40:1211–1221. [PubMed] [Google Scholar]
- 43.Brinton EA, Eisenberg S, Breslow JL. Human HDL cholesterol levels are determined by apoA-I fractional catabolic rate, which correlates inversely with estimates of HDL particle size. Effects of gender, hepatic and lipoprotein lipases, triglyceride and insulin levels, and body fat distribution. Arterioscler Thromb Vasc Biol. 1994;14:707–720. doi: 10.1161/01.atv.14.5.707. [DOI] [PubMed] [Google Scholar]
- 44.Osono Y, Woollett LA, Marotti KR, Melchior GW, Dietschy JM. Centripetal cholesterol flux from extrahepatic organs to the liver is independent of the concentration of high density lipoprotein-cholesterol in plasma. Proc Natl Acad Sci U S A. 1996;93:4114–4119. doi: 10.1073/pnas.93.9.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turley SD, Andersen JM, Dietschy JM. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981;22:551–569. [PubMed] [Google Scholar]
- 46.Dietschy JM, Spady DK. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984;25:1469–1476. [PubMed] [Google Scholar]
- 47.Blum CB, Dell RB, Palmer RH, Ramakrishnan R, Seplowitz AH, Goodman DS. Relationship of the parameters of body cholesterol metabolism with plasma levels of HDL cholesterol and the major HDL apoproteins. J Lipid Res. 1985;26:1079–1088. [PubMed] [Google Scholar]
- 48.Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, Garner B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J Lipid Res. 2008;49:324–331. doi: 10.1194/jlr.M700261-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Park TS, Li Y, Pan X, Iqbal J, Lu D, Tang W, Yu L, Goldberg IJ, Hussain MM, Jiang XC. Serine palmitoyltransferase (SPT) deficient mice absorb less cholesterol. Biochim Biophys Acta. 2009;1791:297–306. doi: 10.1016/j.bbalip.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smit JJ, Schinkel AH, Oude Elferink RP, et al. Homozygous disruption of the murine MDR2 p-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 51.Oude Elferink RP, Ottenhoff R, van Wijland M, Frijters CM, van Nieuwkerk C, Groen AK. Uncoupling of biliary phospholipid and cholesterol secretion in mice with reduced expression of MDR2 p-glycoprotein. J Lipid Res. 1996;37:1065–1075. [PubMed] [Google Scholar]
- 52.Schwartz CC, Berman M, Vlahcevic ZR, Swell L. Multicompartmental analysis of cholesterol metabolism in man. Quantitative kinetic evaluation of precursor sources and turnover of high density lipoprotein cholesterol esters. J Clin Invest. 1982;70:863–876. doi: 10.1172/JCI110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popjak G, Beeckmans ML. Synthesis of cholesterol and fatty acids in foetuses and in mammary glands of pregnant rabbits. Biochemical J. 1950;46:547–561. doi: 10.1042/bj0460547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol. 1997;273:E1209–1215. doi: 10.1152/ajpendo.1997.273.6.E1209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.