Abstract
While the role of platelets in hemostasis is well characterized from a biological perspective, the biophysical interactions between platelets and their mechanical microenvironment are relatively unstudied. The field of cellular mechanics has developed a number of approaches to study the effects of extracellular matrix (ECM)-derived mechanical forces on various cells, and has elucidated that integrin-cytoskeleton-mediated force transduction governs many cellular processes. As platelets adhere and spread via similar molecular machinery that enables other cells to mechanosense and mechanotransduce forces from their biophysical microenvironment, platelets too are likely governed by the same overarching mechanisms. Indeed, recent platelet mechanobiology studies have revealed that key aspects of platelet physiology and activation are regulated by the mechanical and spatial properties of the ECM microenvironment. At the same time, there are also key differences that make platelets unique in the world of cells-- their size, origin as megakaryocyte fragments, unique αIIbβ3 integrin-- render their mechanosensing activities particularly interesting. The structurally “simple,” anucleate nature of platelets coupled with their high actin concentration (20% of total protein) and integrin density [1] seem to make them ideal for mechanical force generation and transmission. Further studies will enhance our understanding of the role of platelet mechanobiology in hemostasis and thrombosis, potentially leading to new categories of diagnostics that investigate the mechanical properties of clots to determine bleeding risk, as well as therapies that target the mechanotransduction signaling pathway to alter the stability of clots.
INTRODUCTION
Decades of research have elucidated the role of platelets in hemostasis and thrombus formation, and have identified key biological agonists that modulate these processes [2]. However, platelets also exist in and interact with a diverse and dynamic biophysical environment. Indeed, platelets are known to sense and mechanotransduce the hydrodynamic forces of the circulation into biological responses, but efforts have primarily focused on the effects of the shear environment on platelet aggregation and activation [3, 4]. In addition to a dynamic fluidic environment, platelets are also exposed to a myriad of extracellular matrices (ECM), including various collagen subtypes and fibrin/fibrinogen. While the platelet-ECM interface has been well characterized biologically, growing evidence suggests that these interactions may be biophysical in nature as well.
The nascent field of cellular mechanics has recently made remarkable strides in unraveling how cells biologically respond to physical forces. This interdisciplinary field seeks to quantitatively characterize the mechanobiology of how cells respond to dynamic forces, strains, and substrate geometries and rigidities in order to elucidate the underlying mechanisms by which cells sense and respond to the mechanical properties of their microenvironment. To those ends, researchers in this area have developed novel techniques and approaches to investigate mechanosensing and mechanotransduction for a wide range of cell types and cellular processes [5–7]. Now is an opportune time for experimental hematologists to leverage key cellular mechanical concepts and techniques to explore biophysical questions related to platelet physiology that were once technologically infeasible. In this review, we discuss how mechanobiology drives the underlying molecular machinery for mechanotransduction; several key cellular mechanics techniques and approaches, and their application to different cell types and processes; and how these concepts ultimately are relevant to platelet physiology and activation.
THE MOLECULAR MECHANISMS OF MATRIX MECHANOSENSING AND MECHANOTRANSDUCTION
Paramount to the communication between cells and the extracellular environment, both in regards to the biochemical and biophysical responses, is the cell cytoskeleton. The architecture of the cytoskeleton of a nucleated mammalian cell is comprised of three distinct polymers-- microtubules, actin filaments, and intermediary filaments—that enable cells to resist deformation. The dynamic assembly and disassembly of these polymers enable processes such as mitosis, motility, and shape change. While serving distinct functions independently, these polymers also work in concert to regulate and respond to mechanical forces. The ECM and its components are connected to and communicate with the cellular cytoskeleton via transmembrane heterodimers known as integrins. The integrin is a key player in mechanotransduction, as the beta-subunit binds actin filaments intracellularly via actin binding proteins such as talin, and their alpha-subunit binds extracellular ligands such as fibrinogen; integrins are involved with both outside-in and inside-out signaling. Ligand-bound integrins cluster into complexes known as focal adhesions (FAs) where additional structural and signaling proteins are concentrated [8]. The force transmission mechanism by integrins has been studied extensively by way of the traction force-- the tension that actin stress fibers bear at FAs. Importantly, it has been found that cell traction forces scale with FA size as well as the mechanical stiffness of the underlying ECM substrate [9, 10]. One downstream cell response to integrin-mediated ECM stiffness sensing is the restructuring of actin filaments within the cytoskeleton by a family of proteins known as GTPases [11]. RhoA GTP signaling controls the phosphorylation of the myosin light chain, thereby enhancing myosin contractility and controlling cytoskeletal tension [12]. This tension promotes the formation of stress fibers and focal adhesions by Rho associated kinase (ROCK) to maintain cell integrity. If myosin activity is inhibited, and force is applied externally, focal adhesions are nevertheless formed by way of a secondary Rho target, mDia1. This implies that external forces are sufficient to activate the Rho signaling pathway and cause cytoskeletal reorganization [13]. Another GTP binding protein, Rac, causes actin polymerization, which leads to the formation of lamellipodia, and allows for environmental sensing and motility [14].
While the overall mechanobiological cell response to integrin binding has been characterized, the immediate force sensing mechanism at the integrin level is less clear. Several general mechanisms for integrin mechanosensing have been proposed [15]: catch bonds, whereby the ECM-integrin bond strengthens in the presence of force [16]; channel opening, in which membrane stretching leads to an increase in the intracellular concentration of an ion, such as Ca2+, and results in increased traction force [10]; enzyme regulation, where the increase in the activity of an enzyme such as focal adhesion kinase mechanically regulates the cytoskeletal response [17]; exposure of phosphorylation sites, in which membrane stretching causes a conformational change in proteins that allows for their phosphorylation [18]; and exposure of binding sites, whereby membrane stretching enhances the binding of proteins, such as vinculin, to cytoskeletal proteins [19]. Depending on the cell type and ECM composition, it is plausible that a number of these mechanisms act in concert or in succession in response to ECM rigidity. For instance, Moore et al proposed that force induced cytoskeletal organization could begin with integrin binding to the ECM causing phosphorylation sites on Src kinases to be exposed [15]. The subsequent activation of Src kinases would lead to integrin-cytoskeletal binding via talin [20], at which point the ECM-integrin catch bond would stretch the cell cytoskeleton, change the conformation of talin, and increase vinculin binding [19]. Finally, by way of cytoskeleton bound vinculin there would be a localized increase in structurally reinforced actin filaments [21]. The integrin connections between the cellular microenvironment and the cell are thus force mechanosensors: the rigidity of the ECM directly translates into a cascade of molecular responses that are known to regulate diverse cellular functions such as metastasis, differentiation, proliferation, and apoptosis. Importantly, as several of these signaling pathways are already known to be involved in platelet activation, such as Rho A being instrumental in platelet shape and Src kinase activation for platelet spreading [22, 23], it is plausible that the same integrin-ECM mechanotransduction mechanisms exist in platelets as well.
TOOLS OF THE TRADE
A number of tools and techniques have been developed to study cellular mechanics and mechanotransduction in a variety of biological systems. While some are conceptually simple, others are technologically complex. These techniques all aim to determine the relationship between internally and externally derived forces and their corresponding cellular biological responses.
Matrix Manipulation
The simplest means to alter a cell’s biophysical environment is by controlling the substrate with which it interacts. For example, simply changing the geometry of the substrate in shape and/or dimension can direct cell differentiation, proliferation, and apoptosis [6, 24]. The microenvironmental geometry of the cellular environment can be manipulated by microcontact printing of ECM proteins, which conceptually is similar to using stamps and inkpads, but at the micro/nanoscale. These techniques typically leverage micropatterned templates that are fabricated via soft lithography, in which microscopic patterns with desired shapes and sizes are transferred from a silicon wafer to a silicone polymer, namely polydimethylsiloxane (PDMS), and the resulting PDMS pattern is used to stamp ECM proteins onto a flat surface, such as a glass coverslip or gel [9, 25] (Figure 1A). Numerous papers have described how simple geometric constraint of the ECM patterns mediates key cellular processes. For example, when mesenchymal stem cells (MSCs) that are driven to differentiate into osteoblasts in the control condition are geometrically confined, they differentiate instead into adipocytes. This process is dependent on mechanical signaling through the RhoA pathway, as RhoA is more highly expressed in the spread osteoblasts, and the exogenous addition of active RhoA causes differentiation into osteoblasts regardless of the presence of adipocyte specific soluble differentiation factors [26].
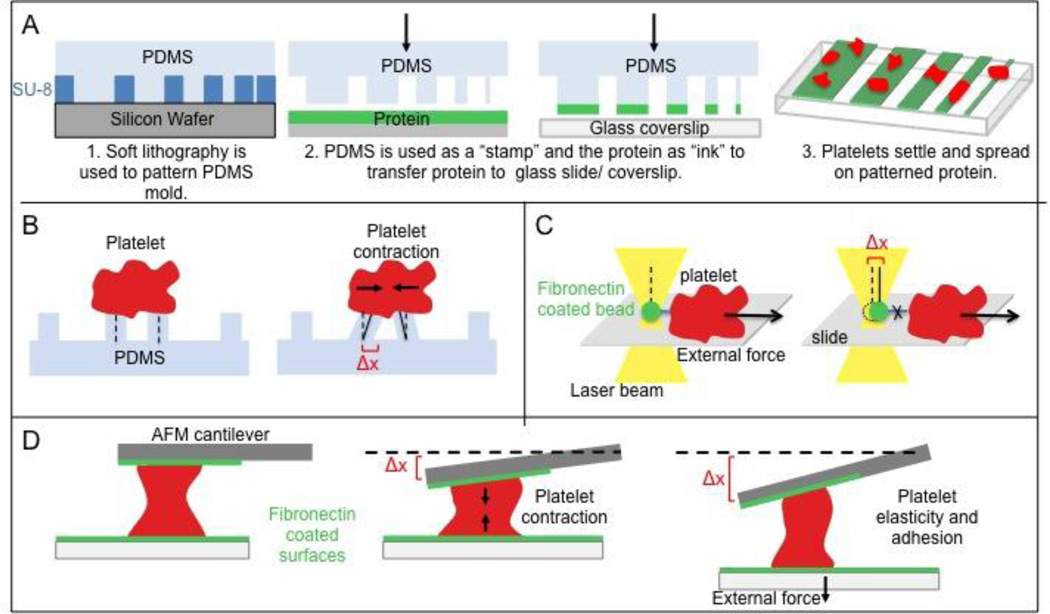
Figure 1. Tools of cell mechanics.
(A) Microstamping. Proteins are micropatterned to determine the dependency of a platelet on microenvironment geometry. (B) Platelet micropillar deflection. Platelets settle on PDMS pillars and pillar deflection (Δx) is used to compute platelet contractile force via Hooke’s Law, which states that force is directly proportional to Δx. (C) Optical tweezers or optical traps. A fibronectin-coated microbead held in a laser beam gradient is brought into contact with a platelet to allow for integrin binding. As the platelet is moved away from the bead, the bead deflection (Δx) at the point of bond rupture gives the strength of the αIIbβ3 – fibronectin bond. (D) Atomic force microscopy. Deflection of the AFM cantilever (Δx) gives platelet contractile force, or alternatively, platelet elasticity and adhesion if the bottom boundary is moved.
Likewise, by mechanically straining a cell substrate uniaxially, biaxially, or equiaxially the phenotype and biochemical response of cells is affected [27]. For example, when exposed to cyclical strain, smooth muscle cells display an upregulation in contractile markers [28]. Kurpinski et al further showed that MSCs express smooth muscle markers when exposed to stretch parallel, but not perpendicular, to the direction of cell growth [27]. This implies that force sensing for differentiation is directionally dependent.
Finally, as mentioned above, the mechanical properties of the ECM itself can mediate cell adhesion, differentiation, spreading, and motility [5]. This phenomenon can be replicated experimentally by conjugating controlled amounts of ECM proteins atop gels comprised of polyacrylamide or alginate. The stiffness can be modified by varying the gel crosslinking, while the surface ECM chemistry remains unchanged. Thus this technique enables the uncoupling of the mechanical and biochemical properties of the ECM [29]. Alternately, the stiffness can be tuned in a concentration independent manner by exposing the matrix to external stress [30]. Further, Kotlarchyk et al devised a system by which a stiffness gradient can be created within a single fibrin gel [31].
Researchers have utilized this technique to study how different cell types respond to different ECM rigidities. For example, Engler et al reported that MSC differentiation is controlled by the elasticity of the matrix they are grown on. In identical serum, the stiffest collagen coated polyacrylamide gels gave rise to an osteogenic phenotype, while the most elastic resulted in neurogenic cells. The ability of non-muscle myosin II inhibitors to block differentiation suggests that focal adhesions and the cytoskeleton are responsible for this stiffness sensing [32]. Recently, it was also found that as the cellular matrix becomes more stiff, lamin-A is more highly expressed in the nucleus. Additionally, the presence of lamin-A seems to direct differentiation by enabling myosin and actin activity [33].
AFM – atomic force microscopy
Conventionally, atomic force microscopy (AFM) allows for precise high-resolution imaging of substrates down to the nanometer scale by probing the surface with a mechanical cantilever. The applications for AFM have been expanded to mechanical measurements whereby a cantilever of known spring constant is brought into contact with a substrate, and its subsequent deflection is related to force. AFM has thus been used to measure elasticity and stiffness of various cell components under varied environmental conditions [34, 35]. Using AFM, Rotsch et al found the stiffness of cell protrusions to be similar to that of the edge of the cell, thereby showing that filopodia are active sites of actin polymerization [36]. Chaudhuri et al further found that branched actin networks initially stiffen with applied force, but progressively soften as the load increases. A reduction in force returns the network to its original stiffness, suggesting that actin polymers buckle under stress but can “unbuckle” because the overall branching actin network is still present. This action enables dynamic remodeling of the actin cytoskeleton in response to stress [37].
Optical traps/tweezers
Optical traps employ a highly focused laser beam to hold and manipulate dielectric particles; and the intensity gradient of the laser physically holds the particle at its center [38]. Force application causes displacement of the particle from the beam center, and at small displacements, the restoring force of the beam can be modeled as a Hookean spring. By attaching a dielectric bead to the object of interest (DNA, cell, etc), trapping the bead in the beam, and manipulating the bead position, mechanical quantities such as elasticity and bond force can be measured from subnanometer deflections yielding precise force measurements down to the piconewton level [39]. For example, using fibronectin-coated beads in contact with fibroblasts, the integrin-fibronectin binding force at different stiffnesses was measured, and the researchers found that fibroblasts sense matrix stiffness and respond with proportional force at the integrins [16]. In a later study, preferential and increased binding of integrins to fibronectin beads at the leading edge of fibroblast lamellipodia was observed, indicating that saturating ligand-cytoskeleton-matrix binding at the leading edge allows cells to pull maximally on the matrix for motility [40].
Microdevices and Microfluidics
While AFM and optical tweezers provide precise measures of nano-scale forces, the measurement of individual events using these tools are time intensive and suffer from low throughput. Researchers have thus turned to microfabrication to increase measurement throughput. Using techniques from the microelectronics field led to a process known as soft lithography, where micron sized features can be patterned from silicon wafers onto PDMS microdevices that can be coated with adhesive proteins and biomolecules [41]. To measure the traction forces of individual cells, cells are allowed to settle on PDMS micropillars, of which the mechanical properties are known a priori. When the cells adhere and contract, the deflections of the pillars, which now act as springs, are measured and the resultant forces can be calculated [42] (Figure 1B). Further, by placing magnetic wires in the pillars, one can apply an external force using a magnetic field and measure the cell’s response. Using this device, Sniadecki et al found that there is a size increase in focal adhesions at the location of the applied force. Cell contractility, on the other hand, was affected more globally, as decreased forces were measured along the periphery of the cell [43].
For measuring hydrodynamic forces as well as adhesion to a substrate under flow, channels can be patterned into the PDMS, bonded to glass, and the resulting “microfluidic channels” can be used to interrogate biological properties. Microfluidics allow for the use of small sample volumes and provide maximal control of environmental factors when studying in vivo processes [44]. Yap and Kamm used microfluidic channels to show that neutrophils become mechanically distorted in narrow, high shear channels. The time to pseudopod projection (activation of neutrophils) was inversely correlated with increasing shear stress, implying that the response is modulated to the magnitude of mechanical force. Additionally, it was found that post-deformation, the cells nearly regained their resting cytoskeleton mechanical properties but in a deformed geometry, suggesting a role for actin depolymerization during shear activation [45]. Microfluidic channels can also be combined with micropatterning to simultaneously investigate multiple means of mechanical force sensing [46]. Indeed, microfluidics have recently entered the experimental hematology sphere, and have enabled novel studies of platelet aggregation and cellular interactions in sickle cell disease under physiological hemodynamic conditions, but these studies are beyond the scope of this review [47–50]. This technique has recently been further modified to enable endothelial cells to be cultured within the microchannels, improving physiologic relevance of these systems. Several groups have reported “endothelialization” of microfluidics to recapitulate the microvasculature for system level studies of thrombosis, sickle cell disease, angiogenesis, and other disease processes [50–52].
PLATELET MECHANICS AND THE ECM MICROENVIRONMENT
As described above, recent work in the field of cellular mechanics have begun to unravel the molecular machinery for mechanotransduction in a variety of mammalian nucleated cells. The sensing mechanisms across cell types are generally integrin mediated and involve restructuring of the cytoskeleton by actin reorganization. As platelets adhere and spread via similar mechanobiological machinery, it is not unreasonable to posit that platelets too physiologically respond to their external environment. To that end, several groups have applied techniques described above to quantitatively investigate the cellular mechanics of how platelets respond to their matrix microenvironment. These and future studies using similar approaches will yield new biophysical insight in hemostasis and thrombosis.
Fibrinogen-Integrin Bond Strength
As mentioned above, the ECM-integrin interaction is key in cellular mechanics and recent work has focused on the mechanics of the binding between the platelet αIIbβ3 integrin and fibrinogen. For instance, Litvinov et al used optical tweezers to measure the binding strength and activation state of individual integrin-fibrinogen pairs for purified proteins as well as for activated platelets (Figure 1C). Importantly, αIIbβ3 integrin was found to be present in either an on or an off state, where the probability of binding was increased with an increase in “on” integrins, but the adhesion strength of 80–90 pN of the pair was always conserved for molecule and cell surface experiments. Likewise, both a weak agonist (ADP) and a strong agonist (TRAP) produced the same adhesion strength, implying that different agonists result in the same affinity for αIIbβ3 binding to fibrinogen [53].
Platelet Contractile Force
Platelets contract after initial aggregation leading to clot retraction and stabilization. To measure the overall force of platelet aggregates, Liang et al. allowed platelets to bind to microfabricated pillars, stimulated them with thrombin, and visualized pillar deflection by the formed microclots. It was found that the contractile force was similar on fibrinogen and fibronectin coated pillars, implicating the role of the αIIbβ3 ligand. Interestingly, without the presence of a fibrin network, individual platelet force was estimated as 2.1 nN, ~1000-fold higher than previous measurements, yet still ~100-fold lower than adherent cells [54]. However, when force per cell volume is considered, platelet force is found to be an order of magnitude higher. Single platelet contractile force and elasticity measurements have also been reported using a modified side view AFM technique, which also enables kinetic measurements of platelet contraction at then single cell level. In this technique, the platelet is positioned between the fibrinogen-coated AFM tip and a fibrinogen-coated substrate, modeling the interaction between two platelets or a single platelet intermeshed within a fibrin network. Upon adherence and spreading onto both surfaces, actin fibers spanned between them and single platelet contraction occurred nearly instantaneously reaching its average maximum force of ~29 nN after ~10– 15 minutes. Furthermore, as the stiffness of the cantilever was increased, mirroring an increase in stiffness of the clot microenvironment, the contraction force increased as well. Additionally, platelet stiffness and adhesion increased as the platelet contractile force increased. These results suggest that platelets mechanosense the microenvironment and act to ensure uniform clot contraction, as well as provide additional structural support to clots, corroborating previous reports that platelet-rich clots are stiffer than those that are platelet-free [55]. Overall, these studies collectively reveal that platelet contraction is mediated by mechanotransduction of the mechanical microenvironment.
Platelet Adhesion and Aggregation
Mechanotransduction of forces in platelets has previously been limited to thrombus formation in response to fluid shear. Standard flow chamber assays for platelet adhesion under flow have been translated to microfluidics, where total required blood volume is reduced [56], and seminal studies using these novel platforms have recently been published. For example, using microfluidics with geometric characteristics that model a stenosis, the shear gradient (the change from high shear rate at the stenosis to low shear rate directly thereafter) has been observed to be the main effector of platelet aggregation, independent of platelet agonists. At the point of high shear rate, membrane tethers are formed which allow for transient binding of platelets, while the low shear region directly following causes restructuring of tethers that allows for more stable thrombi formation. Soluble agonists were then found to stabilize the already formed clots. This supports clinical data showing that although aspirin and other agonist inhibitors greatly reduce thrombus formation, they are not able to prevent thrombosis altogether [57].
To investigate the biological response of platelets to physical and spatial cues, collagen and fibrinogen microstamps can be micropatterned to yield different geometries, and platelet adhesion and spreading on those geometrically constrained protein patterns can be monitored (Figure 1A and Figure 2). For example, platelet spreading is confined to patterned islands of fibrinogen “microdots” [58]. Further, using precisely dimensioned micropatterned protein stripes separated by blocked glass, it was found that, similar to other cell types, platelets extend filopodia to sense their environment, migrate to sites of detected protein, and spread on protein surfaces (both collagen and fibrinogen) with remarkable fidelity to pattern geometry. Stripe separations of < 5 µm (filopodia length of ~3 µm) caused platelet spreading to span the stripes. Additionally, platelets were able to adhere to protein patterns >0.6 µm, and at protein stripes of >5 µm, platelet surface area was confined to the patterned stamp. Biologically, actin was localized at the edge of the protein stripes, whereas the distribution of tubulin was unaffected, thereby indicating that spatial cues regulate specific biologic responses (Figure 2) [59]. Van de Walle et al monitored the aggregation of platelets on similarly patterned surfaces under hemodynamic flow conditions in a microfluidic device. They found that on stripes separated by ~5 µm, platelet aggregates were initially limited to the stripe geometry, but then readily bridged the gap; whereas at ~30 µm separation, bridging of aggregates was rare; in both cases, platelet filopodia were often seen to extend perpendicular to the patterned substrates. When length of patterns was decreased in the downstream direction to < 6µm, platelet adhesion was significantly decreased. αIIbβ3 integrin binding to fibrinogen was confirmed to be the cause of platelet aggregation in this system, as adhesion did not occur in the presence of a blocking antibody [60].
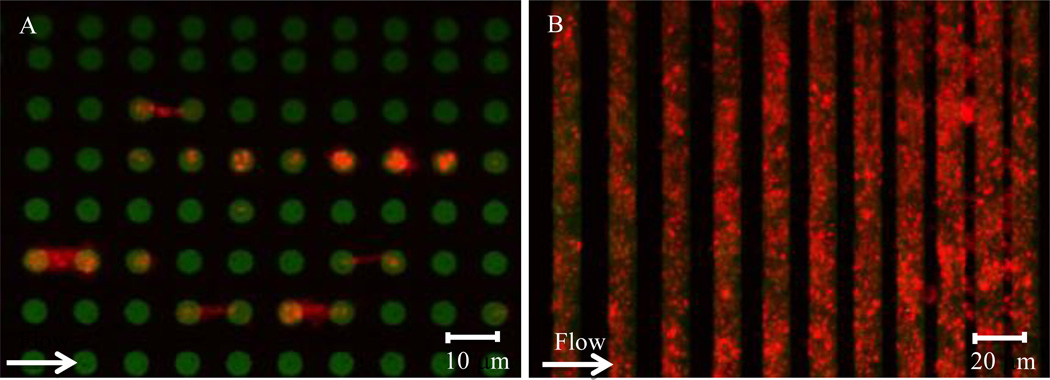
Figure 2. Platelet adhesion to collagen under flow.
(A) Platelets (red) adhere to and spread between 6 µm collagen micropatterned dots (green). (B) Platelets (red) adhere to collagen stripes (green) under flow with high geometric fidelity. As the gaps between the collagen stripes decrease in size, flowing platelets “bridge” over those stripes.
DISCUSSION
Akin to other cells and cellular cytoskeletal processes, platelet adhesion and spreading is regulated by integrins and the actin cytoskeleton, and is mediated by similar signaling pathways. Platelets, however, are especially interesting due to their small, anucleate nature, the shortened time scale on which they produce forces, the high proportion of actin in their cytoskeleton, as well as their unique integrin, αIIbβ3. The structural simplicity of platelets likely allows for their highly efficient production and transduction of mechanical force and stimulus. Therefore, the techniques and concepts that were initially developed to study general cell mechanics should be applied to platelets to further our insight into how platelets interact with their mechanical and spatial microenvironment. As platelet mechanosensing occurs in the absence of gene expression, the platelet also decouples cellular mechanical processes that require genetic expression from those that only require existing cytoskeletal components and proteins. Importantly, a better understanding of platelet mechanotransduction will lead to a mechanical picture of the role of platelets in hemostasis and thrombosis, and may lead to new therapeutic targets. For instance, drugs that are found to reduce platelet contractility without totally impairing platelet function may help to render clots more susceptible to natural hemolysis. Further, knowledge of platelet response to mechanical cues will inform the design of surgical implants that reduce platelet deposition and clot formation, and thereby improve the lifetime and efficacy of the implant. Additionally, assays for the study of platelet mechanics could readily form the basis of future diagnostic and drug delivery platforms. Indeed, a recently introduced novel nanoparticle drug delivery technique draws inspiration from platelet shear activation. In this platform, microparticles break into nanoparticles when exposed to high shear stress, delivering therapeutics specifically to sites of vessel occlusion and minimizing overall drug dosages [61].
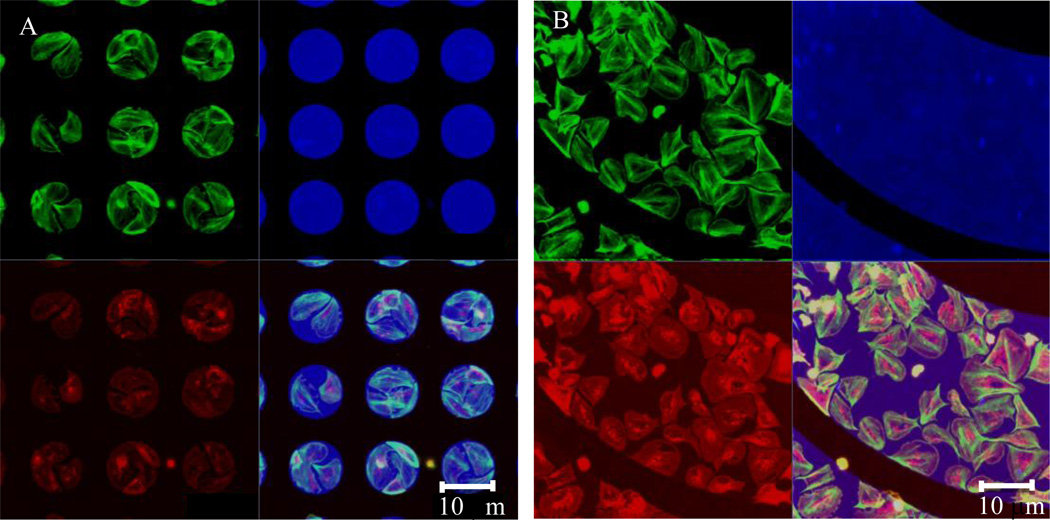
Figure 3. Actin localization to geometric boundaries.
(A) Platelets (red) adhere to and spread on 10 µm fibrinogen micropatterned dots (blue). Actin (green) is concentrated at the dot boundaries. (B) Similar platelet activity occurs on curved geometries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris HE, Weeds AG. Platelet actin: sub-cellular distribution and association with profilin. FEBS letters. 1978;90:84–88. doi: 10.1016/0014-5793(78)80303-2. [DOI] [PubMed] [Google Scholar]
- 2.Rivera J, Lozano ML, Navarro-Nunez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doggett TA, Girdhar G, Lawshe A, Schmidtke DW, Laurenzi IJ, Diamond SL, et al. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIb(alpha)-vWF tether bond. Biophysical Journal. 2002;83:194–205. doi: 10.1016/S0006-3495(02)75161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody NA, King MR. Platelet adhesive dynamics. Part II: high shear-induced transient aggregation via GPIbalpha-vWF-GPIbalpha bridging. Biophysical Journal. 2008;95:2556–2574. doi: 10.1529/biophysj.107.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 6.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 7.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. Journal of cell science. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 10.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 12.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. The Journal of Cell Biology. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. The Journal of Cell Biology. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 15.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Developmental Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Okura M, Imamoto A. Focal adhesions require catalytic activity of Src family kinases to mediate integrin-matrix adhesion. Molecular and Cellular Biology. 2002;22:1203–1217. doi: 10.1128/MCB.22.4.1203-1217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Science. Vol. 323. New York, NY: 2009. Stretching single talin rod molecules activates vinculin binding; pp. 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP. Selective regulation of integrin--cytoskeleton interactions by the tyrosine kinase Src. Nature Cell Biology. 1999;1:200–206. doi: 10.1038/12021. [DOI] [PubMed] [Google Scholar]
- 21.Felsenfeld DP, Choquet D, Sheetz MP. Ligand binding regulates the directed movement of beta1 integrins on fibroblasts. Nature. 1996;383:438–440. doi: 10.1038/383438a0. [DOI] [PubMed] [Google Scholar]
- 22.Aslan JE, McCarty OJ. Rho GTPases in platelet function. Journal of thrombosis and haemostasis: JTH. 2013;11:35–46. doi: 10.1111/jth.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, et al. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. The Journal of Cell Biology. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cukierman E, Pankov R, Stevens DR, Yamada KM. Science. Vol. 294. New York, NY: 2001. Taking cell-matrix adhesions to the third dimension; pp. 1708–1712. [DOI] [PubMed] [Google Scholar]
- 25.von Philipsborn AC, Lang S, Bernard A, Loeschinger J, David C, Lehnert D, et al. Microcontact printing of axon guidance molecules for generation of graded patterns. Nature Protocols. 2006;1:1322–1328. doi: 10.1038/nprot.2006.251. [DOI] [PubMed] [Google Scholar]
- 26.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 27.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birukov KG, Shirinsky VP, Stepanova OV, Tkachuk VA, Hahn AW, Resink TJ, et al. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Molecular and Cellular Biochemistry. 1995;144:131–139. doi: 10.1007/BF00944392. [DOI] [PubMed] [Google Scholar]
- 29.Pathak A, Kumar S. Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integrative biology: quantitative biosciences from nano to macro. Integr Biol. 2011;3:267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials. 2010;31:1875–1884. doi: 10.1016/j.biomaterials.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Kotlarchyk MA, Shreim SG, Alvarez-Elizondo MB, Estrada LC, Singh R, Valdevit L, et al. Concentration independent modulation of local micromechanics in a fibrin gel. PloS One. 2011;6:e20201. doi: 10.1371/journal.pone.0020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radmacher M, Fritz M, Kacher CM, Cleveland JP, Hansma PK. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophysical Journal. 1996;70:556–567. doi: 10.1016/S0006-3495(96)79602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophysical Journal. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotsch C, Jacobson K, Radmacher M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:921–926. doi: 10.1073/pnas.96.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashkin A, Dziedzic JM. Science. Vol. 235. New York, NY: 1987. Optical trapping and manipulation of viruses and bacteria; pp. 1517–1520. [DOI] [PubMed] [Google Scholar]
- 39.Moffitt JR, Chemla YR, Smith SB, Bustamante C. Recent advances in optical tweezers. Annual Review of Biochemistry. 2008;77:205–228. doi: 10.1146/annurev.biochem.77.043007.090225. [DOI] [PubMed] [Google Scholar]
- 40.Nishizaka T, Shi Q, Sheetz MP. Position-dependent linkages of fibronectin-integrin-cytoskeleton. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:692–697. doi: 10.1073/pnas.97.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annual review of biomedical engineering. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 42.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sniadecki NJ, Anguelouch A, Yang MT, Lamb CM, Liu Z, Kirschner SB, et al. Magnetic microposts as an approach to apply forces to living cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14553–14558. doi: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Koo LY, Wang WM, Lauffenburger DA, Griffith LG, Jensen KF. Microfluidic shear devices for quantitative analysis of cell adhesion. Analytical Chemistry. 2004;76:5257–5264. doi: 10.1021/ac049837t. [DOI] [PubMed] [Google Scholar]
- 45.Yap B, Kamm RD. Journal of Applied Physiology. Vol. 98. Bethesda, Md: 2005. Mechanical deformation of neutrophils into narrow channels induces pseudopod projection and changes in biomechanical properties; pp. 1930–1939. (1985). [DOI] [PubMed] [Google Scholar]
- 46.Nalayanda DD, Kalukanimuttam M, Schmidtke DW. Micropatterned surfaces for controlling cell adhesion and rolling under flow. Biomedical Microdevices. 2007;9:207–214. doi: 10.1007/s10544-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 47.Neeves KB, Maloney SF, Fong KP, Schmaier AA, Kahn ML, Brass LF, et al. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. Journal of thrombosis and haemostasis: JTH. 2008;6:2193–2201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 48.Welsh JD, Colace TV, Muthard RW, Stalker TJ, Brass LF, Diamond SL. Platelet-targeting sensor reveals thrombin gradients within blood clots forming in microfluidic assays and in mouse. Journal of thrombosis and haemostasis: JTH. 2012 doi: 10.1111/j.1538-7836.2012.04928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood DK, Soriano A, Mahadevan L, Higgins JM, Bhatia SN. A biophysical indicator of vaso-occlusive risk in sickle cell disease. Science Translational Medicine. 2012;4:123ra26. doi: 10.1126/scitranslmed.3002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. The Journal of Clinical Investigation. 2012;122:408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers DR, Sakurai Y, Tran R, Ahn B, Hardy ET, Mannino R, et al. Endothelialized microfluidics for studying microvascular interactions in hematologic diseases. Journal of visualized experiments: JoVE. 2012 doi: 10.3791/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischel LL, Young EW, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Litvinov RI, Shuman H, Bennett JS, Weisel JW. Binding strength and activation state of single fibrinogen-integrin pairs on living cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7426–7431. doi: 10.1073/pnas.112194999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang XM, Han SJ, Reems JA, Gao D, Sniadecki NJ. Platelet retraction force measurements using flexible post force sensors. Lab on a Chip. 2010;10:991–998. doi: 10.1039/b918719g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nature materials. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarvepalli DP, Schmidtke DW, Nollert MU. Design considerations for a microfluidic device to quantify the platelet adhesion to collagen at physiological shear rates. Annals of Biomedical Engineering. 2009;37:1331–1341. doi: 10.1007/s10439-009-9708-z. [DOI] [PubMed] [Google Scholar]
- 57.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nature Medicine. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 58.Corum LE, Eichinger CD, Hsiao TW, Hlady V. Using microcontact printing of fibrinogen to control surface-induced platelet adhesion and activation. Langmuir: The ACS Journal of Surfaces and Colloids. 2011;27:8316–8322. doi: 10.1021/la201064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kita A, Sakurai Y, Myers DR, Rounsevell R, Huang JN, Seok TJ, et al. Microenvironmental geometry guides platelet adhesion and spreading: a quantitative analysis at the single cell level. PloS One. 2011;6:e26437. doi: 10.1371/journal.pone.0026437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van de Walle AB, Fontenot J, Spain TG, Brunski DB, Sanchez ES, Keay JC, et al. The role of fibrinogen spacing and patch size on platelet adhesion under flow. Acta Biomaterialia. 2012;8:4080–4091. doi: 10.1016/j.actbio.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, et al. Science. Vol. 337. New York, NY: 2012. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels; pp. 738–742. [DOI] [PubMed] [Google Scholar]