Abstract
M cells located in the follicle-associated epithelium of Peyer's patches (PP) are shown to be the principal sites for the sampling of gut luminal antigens. Thus, PP have long been considered the gatekeepers of the mucosal immune system. Here, we report a distinct gateway for the uptake of gut bacteria: clusters of non-follicle-associated epithelium-associated Ulex europaeus agglutinin (UEA)-1+ cells, which we have designated intestinal villous M cells. Interestingly, villous M cells are developed in various PP [or gut-associated lymphoid tissue (GALT)]-null mice, such as in utero lymphotoxin β receptor (LTβR)-Ig-treated, lymphotoxin α (LTα)-/-, tumor necrosis factor/LTα-/-, and inhibition of differentiation 2 (Id2)-/- mice. Intestinal villous M cells have been observed to take up GFP-expressing Salmonella, Yersinia, and Escherichia coli-expressing invasin, as well as gut bacterial antigen for subsequent induction of antigen-specific immune responses. Thus, the identified villous M cells could be an alternative and PP-independent gateway for the induction of antigen-specific immune responses by means of the mucosal compartment.
The huge intestinal surface area is physically protected by a layer of tightly joined epithelial cells, which prevent most enteric environmental antigens from penetrating the host (1). However, entry into the host is made possible by a special gateway, comprised of M cells, located over organized mucosal lymphoid follicles such as Peyer's patches (PP). The M cells, characterized by an irregular brush border and reduced glycocalyx, efficiently take up and transport a wide variety of macromolecules and microorganisms from the gut lumen to the inside of the PP (2–6), which contain all of the necessary lymphoid cells for the induction and regulation of antigen-specific IgA responses (7). However, the origin of M cells and the regulation of their development are not understood. A previous study (8) showed that i.v. injection of PP lymphocytes into severe combined immunodeficient mice resulted in formation of new lymphoid follicles and follicle-associated epithelium (FAE) with typical M cells. A similar phenomenon was seen by using in vitro studies in which coculture with B lymphocytes triggered the conversion of enterocyte cell lines into M cell-like cells (9). Further, B cells have recently been proposed to play a role in the organogenesis of the mucosal immune barrier (10). Two different B cell-null mice, lacking expression of either μ membrane exon or the JH segment of Ig genes, showed drastic reduction of FAE size and M cell numbers (10). In contrast, a recent study (11) demonstrated that the absence of mature T and B cells does not prevent the formation of FAE and M cells, and signaling of lymphotoxin (LT) α/β from non-B and non-T cells plays a critical role in formation of M cells in FAE of PP.
The common mucosal immune system (CMIS), which connects the inductive (e.g., PP) and effector (e.g., lamina propria; LP) sites, has been shown to be a central pathway for the induction of antigen-specific IgA immune responses in the gastrointestinal tract (7). For example, oral administration of Salmonella typhimurium leads to the transport of the bacterial antigen from the lumen of the intestinal tract into the PP by means of M cells for the initial priming of antigen-specific CD4+ T cells and IgA-committed B cells (12). These antigen-sensitized cells leave the PP and contribute to the subsequent induction of Salmonella-specific IgA response in the distant intestinal LP by means of CMIS. In addition to the well-characterized CMIS-dependent IgA induction pathway, recent evidence suggests the presence of an additional IgA induction pathway that is independently operated from the PP-originated CMIS (13–15). Interestingly, it also has been reported that induction of intestinal mucosal IgA against the commensal bacteria was independent from T cell help and organized lymphoid tissue (16). Further, our recent study (17) has demonstrated that antigen-specific IgA antibody responses can be induced in the absence of PP. These studies imply the existence of a PP-independent mucosal immune pathway for dietary antigen and bacteria uptake.
A recent study (18) has suggested that the invasion gene (SPI1)-deficient S. typhimurium can be disseminated from the intestinal epithelium to the systemic compartment in the absence of PP-associated M cells by means of the CD18-dependent pathway. Further, dendritic cells in the lamina propria of the small intestine expressing tight junction protein offer another possible antigen uptake site (19). Thus, intestinal DCs are capable of extending dendrites to the lumen side by opening the tight junction. However, the exact mechanism for inducing Ag-specific immune responses independently of PP requires further elucidation.
In this study, we have discovered intestinal villous M cells, which serve as an antigen gateway for the sampling of gut bacteria and subsequent induction of Ag-specific immune responses in a PP-independent manner. These lines of study are crucial for understanding the mechanisms of antigen uptake from the gut lumen, and for the rational design of effective mucosal vaccines and optimal drug delivery across the gut.
Experimental Procedures
Mice. BALB/c and C57BL/6 mice were purchased from CLEA Japan (Tokyo). LTβR-Ig fusion protein-treated and tumor necrosis factor (TNF) and LTα double knockout (TNF/LTα-/-; 129 × C57BL/6) mice were generated as described (20, 21). LTα-/- mice (C57BL/6) were obtained from The Jackson Laboratory. Inhibition of differentiation 2 (Id2)-/- mice (129/Sv) were generated as described (22).
M Cell Staining. A standard lectin staining procedure was used for the detection of murine M cells (23). Mucus-free small intestine of naive BALB/c or C57BL/6 mice, with or without PP, was fixed in 4% paraformaldehyde for 1 h, washed, and then blocked with 10% FBS in PBS containing 0.1% glycine. A lectin-labeling experiment was performed with Ulex europaeus agglutinin (UEA) conjugated with tetramethylrhodamine B isothiocyanate (TRITC) (UEA-1-TRITC, Vector Laboratories) and wheat germ agglutinin (WGA) conjugated with FITC (WGA-FITC) at a concentration of 20 μg/ml for 2 hr. After being rinsed in PBS, samples were stored in a Tris-buffered solution containing 30% glycerol and 0.1% NaN3. The specimens were examined in a Bio-Rad MRC-600 confocal imaging system (Bio-Rad). Alkaline phosphatase activity and alcian blue staining were assessed on whole fixed small intestine as described (11). In addition, scanning and transmission electron microscopy analyses were performed for the characterization of M cells (see Supporting Experimental Procedures, which is published as supporting information on the PNAS web site).
Antigen Uptake in Situ. S. typhimurium PhoPc strain transformed with the pKKGFP plasmid was kindly provided by F. Niedergang (24, 25). Further, GFP-expressing Yersinia pseudotuberculosis, Escherichia coli-invasin, and E. coli were prepared by the method described (26, 27). Mice were anesthetized by i.p. injection of 2 mg of ketamine (Sigma) per mouse. Segments ≈10 cm long of the small intestine of TNF/LTα-/- mice and wild-type mice were ligated at both ends with surgical thread. GFP-expressing bacteria (5 × 108) were suspended in 1.0 ml and inoculated into the loop and incubated in situ. Ten minutes later, PP and the intestinal segments (without PP) were removed and extensively washed with cold PBS and RPMI medium 1640 including gentamycin (100 μg/ml). Intestinal epithelial cells (IECs) were isolated from PP and the intestinal segments as described (28), then fixed in 4% paraformaldehyde, washed with 10% FBS in PBS, and labeled with UEA-1-TRITC. The percentage of double-positive IECs was analyzed on a FACSCalibur flow cytometer (Becton Dickinson). In selected mice, whole-mounted small intestinal segments were processed for confocal microscopy as described above. To remove weakly adhered and/or extracellular bacteria, vigorous washing with cold PBS and RPMI medium 1640 containing gentamycin were adopted during the process of isolation of villous epithelium including M cells and epithelial cells after infection with bacteria. Gentamycin was selected as the antibiotic due to its lethal effects on Salmonella (29). Therefore, our present data include only Salmonella that had strongly adhered and was intracellular but not Salmonella that was weakly adhered and extracellular.
Immunization. The recombinant S. typhimurium BRD 847 strain used in the immunization study is a double aroA aroD mutant that expresses the nontoxic, immunogenic 50-kDa ToxC fragment of tetanus toxin from plasmid pTETnir15 under the control of the anaerobically inducible nirB promoter (rSalmonella-ToxC) (30). For the control, rSalmonella that are not expressing ToxC were adopted. Recombinant Salmonella organisms were resuspended in PBS to a concentration of 2.5 × 1010 bacteria per ml. Bacterial suspensions were orally administered by gavage (0.2 ml per mouse). Ab titers in serum were determined by ELISA, as described elsewhere (17).
Data Analysis. Data were expressed as mean ± SD and evaluated by the Mann–Whitney U test. P values of <0.05 were assumed to be statistically significant.
Results
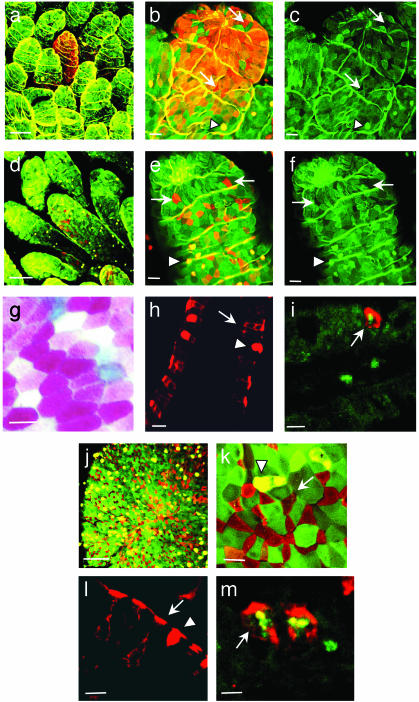
Identification of Clusters of UEA-1+ Cells in the Intestinal Villous Epithelium. M cells have been thought to be associated with, and to develop only in, the dome epithelium (or FAE) of mucosa-associated lymphoid tissues, e.g., PP. However, using confocal image analysis of whole-mount murine intestine stained with TRITC-conjugated UEA-1 and FITC-labeled WGA, we have found UEA-1+WGA- cells not only in the FAE region of PP (Fig. 1 j–l), but also in the villous epithelium (Fig. 1 a–i). UEA-1, which possesses specificity for carbohydrate structures containing α(1–2)-fucose, selectively binds to the entire plasma membrane of PP M cells but not to WGA+ columnar epithelial cells (23). To further confirm the specificity of UEA-1 staining, we have performed a blocking experiment using 50 mM soluble fucose. Preincubation of the UEA-1 with soluble fucose for 1 hr clearly blocked UEA-1 staining in fluorescence-activated cell sorter (FACS, Becton Dickinson) and immunohistochemistry analyses further indicating the specificity of the UEA-1 staining method (data not shown). Interestingly, two forms of villous UEA-1+WGA- cells, i.e., dense and diffuse, may be distinguished on the basis of the density of UEA-++WGA- cells (Fig. 1 a and b vs. d and e).
Fig. 1.
Confocal view of UEA-1+ cells in villous epithelium (a–i) and FAE of PP (j–m) isolated from naive BALB/c mice. M cell- and columnar epithelial cell-specific UEA-1-TRITC and WGA-FITC, respectively, were applied to the whole-mount preparation of the small intestine (a–f, j, and k). M cells were stained by UEA-1 (red, arrow), enterocytes by WGA (green), and goblet cells by UEA-1 and WGA (yellow, arrowhead). Villous M cells were found as two different distribution forms, dense (a and b) and diffuse (d and e) types. In contrast to the epithelial and goblet cells, M cells in the villous epithelium were completely negative to the WGA staining (c and f). Frozen sections were prepared and stained with UEA-1-TRITC alone (h and l) or with UEA-1-TRITC and B220 mAb-FITC (i and m) and the M cells were shown to have a pocket membrane and pocket lymphocytes (arrow) whereas the goblet cells do not (arrowhead). M cells were doubly negative cells for alkaline phosphatase activity demonstrated by red/pink color substrate, and alcian blue staining (white; g). The scale bar for a, d, and j is 50 μm; for b, c, e, f, h, and l is 20 μm; and for g, i, k, and m is 10 μm.
Our study revealed that these newly identified villous UEA-++WGA- cells share features with PP M cells but differ from goblet and columnar epithelial cells. Analysis of frozen sections of intestinal villi stained with TRITC-UEA-1 reveals that the villous UEA-1+ cells possess the characteristic feature of M cells in PP FAE, i.e., a unique subdomain of the basolateral membrane, also known as the pocket membrane (Fig. 1h). The pocket lymphocytes were further confirmed by the staining with TRITC-UEA-1 and FITC-B220 mAb in villous UEA-1+ cells (Fig. 1i) as well as in PP M cells (Fig. 1m).
Although they possess some affinity for UEA-1, goblet cells, unlike M cells, are capable as well of binding to WGA, making them doubly positive cells (UEA+ WGA+; Fig.1 b, e, and k). In contrast to the epithelial and goblet cells, UEA-1+ cells in the villous epithelium were completely negative for WGA staining (Fig. 1 c and f). Further, goblet cells are morphologically distinguished from M cells in that they do not possess the characteristic pocket membrane (Fig. 1 h and i). Intestinal columnar epithelial cells have high alkaline phosphatase (ALP) activity demonstrating with red or pink color, as do goblet cells stained with alcian blue, but M cells have neither of these features (11). Like PP M cells, villous UEA-1+ cells in whole-mount intestinal samples were found to be negative for ALP activity and alcian blue staining (Fig. 1g). Thus, the UEA-1+ cells, shown by our study to be analogous to PP M cells, have been designated villous M cells.
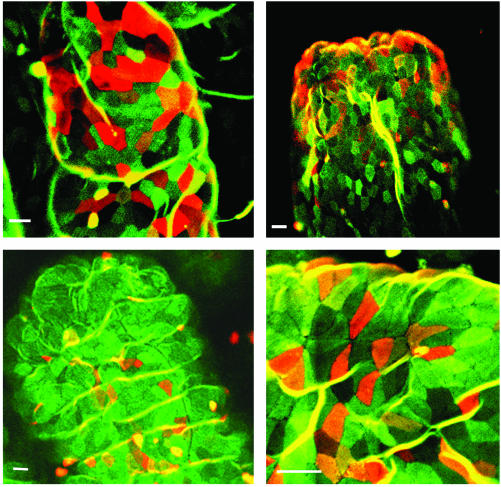
Development of Clusters of Villous UEA-1+ M Cells in the Various PP-Null Mice. To further support this view, we examined whether villous M cells can develop in PP [or gut-associated lymphoid tissue (GALT)]-deficient mice, such as in utero LTβR-Ig-treated (21), LTα-/- (31), TNF/LTα-/- (20), and Id2-/- mice (22). We found M cells with the characteristic UEA-1+ WGA- staining in the tip regions of intestinal villi of all PP-deficient mice (Fig. 2), thus documenting the presence of an FAE-independent M cell developmental pathway. This view was further supported by the presence of M cells in TNF/LTα-/- mice lacking newly described isolated lymphoid follicle (ILF) in addition to PP (data not shown). To define the distribution and number of villous M cell population in wild-type mice and GALT-null mice, we determined the frequency of the dense type of villous M cells in whole small intestine using the confocal imaging system. Approximately 40–50 dense-type villous M cell clusters were found per whole small intestine of wild-type mice. Similarly, ≈50–60 villous M cell clusters were found in the whole small intestine of TNF/LTα-/- mice, one of the representative GALT-null mice. The finding of similar numbers of villous M cells in the GALT-null and wild-type mice could suggest that the development of villous M cells is completely independent of GALT and FAE.
Fig. 2.
The presence of villous M cells in PP-null mice, such as in utero LTβR-Ig-treated C57BL/6 mice (Upper Left), LTα-/- mice of C57BL/6 background (Upper Right), TNF/LTα-/- mice of 129 × C57BL/6 background (Lower Left), and Id2-/- mice of 129×Sv background (Lower Right). The scale bar for all pictures is 10 μm. The whole-mount preparations of small intestine were stained with FITC-WGA and TRITC-UEA-1.
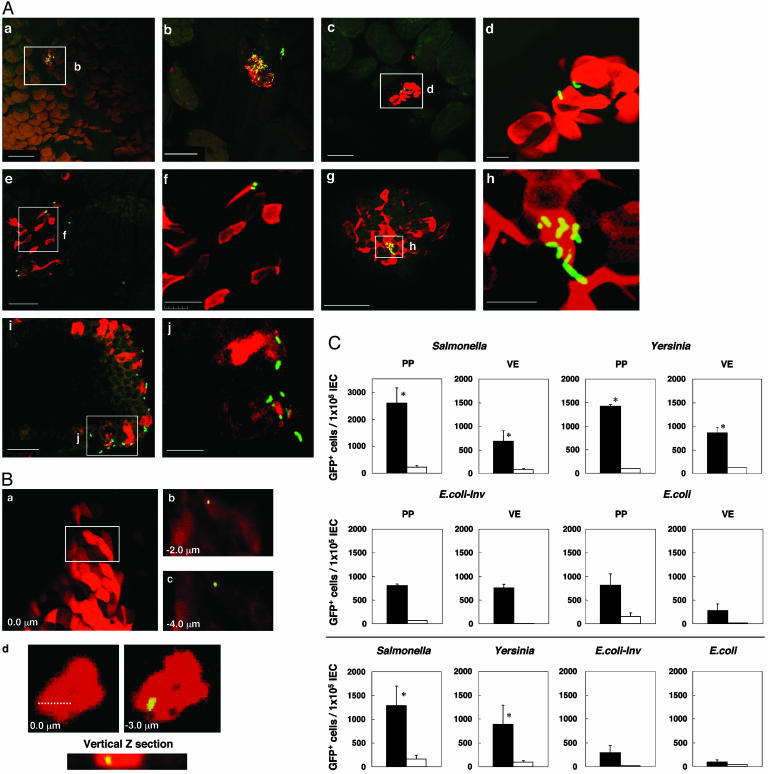
Attachment and Internalization of Bacteria by Villous M Cells. A further experiment was performed to gauge the ability of villous M cells to take up pathogenic microorganisms. Ligated small intestinal loops from wild-type mice were inoculated with rSalmonella typhimurium expressing green fluorescence (rSalmonella-GFP), Yersinia pseudotuberculosis (Yersinia-GFP), E. coli-expressing Yersinia invasin (E. coli-invasin-GFP), and wild-type E. coli-GFP. After a 10-min incubation with each bacteria in situ, sequential immunohistologic analyses of ligated small intestinal loops directly demonstrated the presence of rSalmonella-GFP in UEA-1+ cells in the villous epithelium of wild-type mice (Fig. 3 Aa and Ab) and TNF/LTα-/- mice (Fig. 3 Ae and Af). In addition, Yersinia-GFP was also specifically adhered to villous UEA-1+ cells of wild-type mice (Fig. 3 Ac and Ad) and TNF/LTα-/- mice (Fig. 3 Ag and Ah). Immunohistologic analyses using frozen sections showed that rSalmonella-GFP was located in the apical membrane regions of villous UEA-1+ cells (Fig. 3 Ai and Aj). To show the ability of villous UEA-1+ cells to take up bacteria, we performed an ileal loop infection experiment using rSalmonella-GFP and analyzed the localization of bacteria with sequential confocal microscopy (Fig. 3B). Sequential Z plans of whole mount staining revealed the localization of rSalmonella-GFP in the intracellular region (Fig. 3 B a–c). In addition, rSalmonella-GFP was found in the intracellular region of villous UEA-1+ cells prepared by cytospin (Fig. 3Bd).
Fig. 3.
(A) Immunohistochemistry for antigen uptake by UEA-1+ villous M cells. Each panel shows histological features for sampling of GFP-expressing Salmonella (a, b and e, f) and Yersinia (c, d and g, h)byUEA+ cells in the small intestine of wild-type (a–d) and PP-null TNF/LTα-/- mice (e–j). Whole mount (a–d and e–h) and frozen sections of small intestine after exposure of GFP-expressing Salmonella were prepared and stained with UEA-1-TRITC (i and j). The scale bars are as follows: for a, c, and g,50 μm; for b, e, and i,20 μm; and for d, f, h, and j,10 μm. (B) Localization of GFP-expressing Salmonella in the intracellular region of UEA-1+ villous M cells. An ileal loop infection experiment using rSalmonella-GFP was performed for 30 min, and whole-mount tissues and UEA-1+ IEC cells were analyzed by sequential confocal planar microscopy. Sequential Z plans of whole-mount staining revealed the localization of rSalmonella-GFP in the intracellular region of villous UEA-1+ cells (a, b, and c). Further, the cytospin analysis revealed that rSalmonella-GFP also existed in the intracellular region of villous UEA-1+ cells (d). (C) Antigen uptake by UEA-1+ villous M cells. Cells (5 × 108) of GFP-expressing S. typhimurium PhoPc (Salmonella), Y. pseudotuberculosis (Yersinia), E. coli-invasin (E. coli-Inv), and E.-coli were administered into a 10-cm loop of the small intestine of naive wild-type mice (Top and Middle) or TNF/LTα-/- mice (Bottom). After 10 min of incubation in situ, IECs were isolated from PP and villous epithelium. After being fixed with 4% paraformaldehyde, IECs were stained by UEA-1-TRITC, and uptake efficiency was analyzed by fluorescence-activated cell sorter (FACS). Data demonstrate the frequency of GFP+ cells in the UEA+ (filled bar) and UEA- (open bar) cells isolated from PP and villous epithelium (VE). The results represent the mean values ± SD from three separate experiments (three mice per group). *, P < 0.05 vs. the UEA- IEC group.
Intestinal epithelial cells were further isolated from villous epithelium and PP after ileal loop injection of the microorganism expressing GFP, and then counterstained with TRITC-UEA-1 for flow cytometry analysis. We found a higher frequency of rSalmonella-GFP-, Yersinia-GFP-, or E. coli-invasin-GFP-containing cells in the fraction of UEA-1+ cells than in the UEA-1- cells isolated from villous epithelium (Fig. 3C), and similar patterns were noted for the UEA-1+ and UEA-1- cells isolated from the dome region of PP. In addition, high numbers of rSalmonella-GFP-, Yersinia-GFP-, or E. coli-invasin-GFP-containing cells were also recovered from UEA-1+ but not UEA-1- cells isolated from the villous epithelium of TNF/LTα-/- mice lacking GALT (Fig. 3C). Taken together, these results indicate that villous M cells have the ability to take up several different bacteria from the lumen known to be taken up by FAE-M cells.
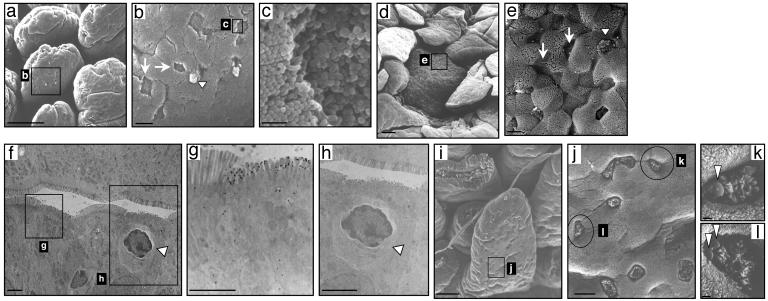
Scanning and Transmission Electron Microscope Analysis of Villous M Cells. Scanning electron microscopy (SEM) of the villous M cells revealed a hallmark feature of M cells, i.e., a depressed surface with short and irregular microvilli (Fig. 4 a, b, and c), seen also in the M cells of PP (Fig. 4 d and e). Transmission electron microscopy analysis also showed the binding of gold particle-conjugated UEA-1+ cells in the villous M cells (Fig. 4 f and g). Further, the presence of infiltrating mononuclear cells was also seen in the pocket of villous M cells (Fig. 4h). The SEM also demonstrated the binding of bacteria to the membrane of villous M cells in the small intestine of FAE-null TNF/LTα-/- mice after intestinal exposure of Salmonella (Fig. 4 i–l). These findings provide supportive evidence that the newly identified villous M cell formed an alternative gateway for antigen sampling and/or entry from the lumen of intestinal villous epithelium. These findings provide evidence that M cells are developed and localized in the villous epithelium as well as in the FAE of PP.
Fig. 4.
Scanning and transmission electron microscopy of M cells in villous epithelium and FAE. Scanning electronic microscopy demonstrates that the M cells (arrow) in villous epithelium (a–c) and PP (d and e) are distinguished from enterocytes and goblet cells (arrowhead) by their relatively depressed and dark brush border. A transmission electron microscopy view of villous M cells shows short stub-like microvilli (f, g, and h) and the presence of infiltrating mononuclear cells in the pocket of villous M-cells (h; arrowhead). (i–l) The presence of villous M cells and the uptake of bacteria in the villous epithelium (arrowhead) after intestinal exposure of Salmonella (see Fig. 3 legend) in PP-deficient TNF/LTα-/- mice. The scale bars are as follows: for a, d, and i,50 μm; for b, e, f, and j,5 μm; for g and h, 1.0 μm; and for c, k, and l, 0.5 μm.
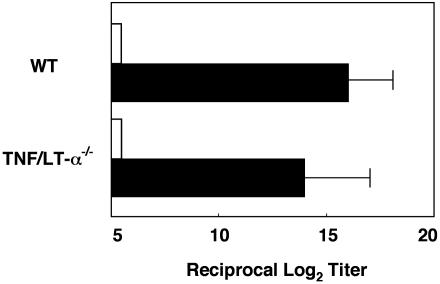
Induction of Ag-Specific Immune Responses in PP-Deficient Mice. Our next experiments sought to examine whether antigen-specific antibody responses could be induced in GALT-deficient mice by means of the villous M cells. When GALT-null mice with TNF/LTα gene deficiency and wild-type mice were immunized orally with rS. typhimurium BRD 847 expressing a 50-kDa ToxC fragment of tetanus toxin (rSalmonella-ToxC), titers of tetanus toxoid (TT)-specific serum IgG antibodies were as high in the serum of the TNF/LTα-/- mice as in orally immunized wild-type mice (Fig. 5). Expectedly, levels of TT-specific serum IgG antibody titers were not detectable when wild-type and TNF/LTα-/- mice were orally immunized with rSalmonella not expressing ToxC (under the 5 of reciprocal log2 titer in Fig. 5). These findings suggest that the villous M cells are an important antigen-sampling site for the induction of antigen-specific immune responses to gastrointestinal environmental antigens.
Fig. 5.
Induction of Ag-specific immune responses in PP-deficient mice. Shown are PP-deficient (TNF/LTα-/-) and wild-type mice, which were orally immunized with rSalmonella-ToxC (filled bar) or rSalmonella alone (open bar). Serum samples were obtained 21 days after oral immunization for the assessment of tetanus toxoid (TT)-specific antibody responses by ELISA. The results represent the mean values ± SD from three separate experiments (three mice per group). There is no statistically significant difference between TNF/LTα-/- and wild-type mice analyzed by unpaired Mann–Whitney U test.
Discussion
Because there is currently no reliable identified gene and corresponding antigen marker that positively identified M cells, the phenotype of M cells is defined by a combination of criteria including (3, 4) (i) the presence of the fucose epitope defined by the lectin UEA on M cell membrane, (ii) short and irregular microvilli, (iii) endocytic activity and ability to take up bacteria as well as macromolecules, and (iv) an intraepithelial pocket that allows a cluster of lymphocytes to be located in the epithelium. Based on our present results, villous M cells share all of the identifying features necessary to identify M cells found in the FAE of PP. Although M cell development has been thought to depend on FAE in organized mucosal lymphoid tissues, our results provide evidence that it can occur in the villous epithelium even in the absence of FAE. Further, these villous M cells are a gateway for entry or sampling of bacteria (e.g., Salmonella-, Yersinia-, and E.-coli-expressing invasin) for the subsequent induction of antigen-specific immune responses.
M cells have been identified and documented only in the FAE-associated epithelium and occasionally on villi immediately adjacent to the lymphoid follicle (4, 32). A previous study (32) indicated that isolated M cells were found in the villous epithelium near the PP of the rabbit small intestine. In addition, clusters of UEA-1+ cells in the small intestinal villi of conventional mice have been reported (33). These two studies suggested the existence of UEA-1+ cells in the small intestinal villi of rabbit and mouse but did not address their identity or biological function. In this regard, our present study provides evidence of the existence of M cells in the villous epithelium away from PP of not only wild-type mice but also GALT-null mice. Further, our present results directly demonstrate the functional aspect of the villous M cells as a gateway for bacteria. Thus, we have substantially advanced the case that villous M cells are distinct from FAE-associated M cells in PP and have further shown that these villous M cells are a biologically important component of the mucosal immune system.
As discussed above, M cells reportedly are occasionally found in the villous epithelium adjacent to rabbit PP (32); however, we stress that villous M cells are located quite a distance from PP. Although the exact source of M cells has yet to be pinpointed, it is widely held that their development and localization are always associated with the organized lymphoid tissue of mucosal surfaces (e.g., PP). Our findings presented here, however, challenge this common assumption by providing evidence that M cells can be developed in villous epithelium in the absence of the FAE thought to be necessary to their development in the organized mucosal lymphoid tissue. The diverse cellular phenotypes in the intestinal epithelium arise from crypt stem cells whose differentiation pathways can be modified by endogenous and exogenous influences (33–37). A previous study (34) demonstrated that Streptococcus pneumoniae-treated FAE tissues showed a marked increase in both IEL and epithelial cells with morphological and functional features of M cells. Further, enterocytes located in the peripheral of the FAE were converted into operational M cells as early as one hour after in vivo exposure to S. pneumoniae (32). Interestingly, expression of α1,2-linked fucosylated glyconjugates in the ileal epithelium was induced by the flora (33). Further, our unpublished data indicate that significantly increased numbers of UEA-1+ cells in the villous epithelium of both wild-type and PP-null mice were detected after in vivo exposure to S. typhimurium. Therefore, it is possible that newly identified villous M cells can be developed from epithelial cells in response to foreign antigens and/or pathogens in the gut lumen. An interesting possibility would be that these UEA-1+ crypt cells could be programmed to develop into the villous UEA-1+ M cells after exposure to the exogenous microorganisms.
M cells in the FAE provide an entry site for pathogens, such as S. typhimurium, Mycobacterium bovis, Shigella flexneri, Y. enterocolitica and retroviruses (4, 38–40). It is well known that the invasion genes of the Salmonella pathogenicity island (SPI1) are necessary for the entry of S. typhimurium into FAE-M cells and epithelial cells (38, 41). However, SPI1-deficient Salmonella is transported from the gastrointestinal tract to the blood stream by CD18-expressing phagocytes, and CD18-deficient mice were shown to be resistant to orally administered Salmonella (18). Overall, it seems likely that several cell types, including M cells, epithelial cells, and CD18-expressing macrophages, are involved in permitting the penetration of Salmonella. On the other hand, previous studies have showed that Y. enterocolitis selectively and specifically invades the FAE of PP by means of M cells but not by means of other cells (42, 43). Interestingly, it has been suggested α4β1 integrin is expressed on the apical membranes of M cells but not on villous or dome epithelial enterocytes, implying that this integrin may be exploited by Yersinia to attach to and invade the M cells (44). Further, invasin mediates uptake of Y. pseudotuberculosis into mammalian cells through binding with β1-chain integrins with high affinity (45, 46). In light of these complexities, the fact that villous M cells and FAE-associated M cells in PP sampled GFP-expressing Salmonella, Yersinia, and E. coli-invasin suggests that villous M cells likely possess a capacity of playing as professional bacteria translocating cells.
A recent study (13) has provided new evidence that IgA-specific B cell responses including isotype-switching can be induced in intestinal lamina propria without the influence of PP. In addition, our recent study showed that ILF in the small intestine are structurally and functionally similar to the PP and contain M cells on their FAE region (47). To eliminate the possible role of M cells associated with the ILF for antigen sampling, we used TNF/LTα-/- mice, which lack both PP (17) and ILF (unpublished data). Interestingly, high numbers of GFP-expressing Salmonella, Yersinia, and E. coli-invasin were recovered from UEA-1+ but not UEA-1- cells isolated from the villous epithelium of TNF/LTα-/- mice although the total uptake of GFP+ eukaryotic cells was less pronounced than in wild-type mice (Fig. 3C). Together with the data for the induction of antigen-specific antibody responses after oral immunization in these PP- and ILF-deficient mice (Fig. 5), our results provide a strong case that villous M cells are an alternative gateway of antigen entry for the mucosal immune system. On the other hand, a recent study (19) showed that antigen sampling occurs across the non-FAE by mucosal intraepithelial dendritic cells. Thus, it is still possible that antigen-specific immune responses seen in TNF/LTα-/- mice could be initiated by means of these intraepithelial dendritic cells.
In summary, our observations indicate that typical M cells can develop without the influence of the FAE associated with mucosal lymphoid tissue such as PP and in fact are present in villous epithelium. Moreover, villous M cells may be an alternative gateway for the penetration of pathogenic microorganisms as well as an additional antigen-sampling site for the induction of antigen-specific immune responses by means of the mucosal tissues.
Supplementary Material
Acknowledgments
We thank Mr. Takashi Wada for helpful technical assistance with scanning electron microscopy. We thank Drs. William R. Brown and Kimberly McGhee for editing the manuscript and Drs. Ichiro Takahashi, Jean-Pierre Kraehenbuhl, and Satoshi Fukuyama for helpful discussions and suggestions. This work was supported by grants from Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation (JST), the Ministry of Education, Science, Sports, and Culture, and the Ministry of Health and Welfare in Japan, as well as by SRC fund to IRC at the University of Ulsan from Korea Science and Engineering Foundation (KOSEF) and the Korean Ministry of Science and Technology.
Abbreviations: PP, Peyer's patches; FAE, follicle-associated epithelium; LT, lymphotoxin; TNF, tumor necrosis factor; WGA, wheat germ agglutinin; UEA, Ulex europaeus agglutinin; TRITC, tetramethylrhodamine B isothiocyanate; IEC, intestinal epithelial cell; ILF, isolated lymphoid follicle; GALT, gut-associated lymphoid tissue; Id2, inhibition of differentiation 2.
References
- 1.Madara, J. L. (1998) Annu. Rev. Physiol. 60, 143-159. [DOI] [PubMed] [Google Scholar]
- 2.Frey, A., Giannasca, K. T., Weltzin, R., Giannasca, P. J., Reggio, H., Lencer, W. I. & Neutra, M. R. (1996) J. Exp. Med. 184, 1045-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraehenbuhl, J. P. & Neutra, M. R. (2000) Annu. Rev. Cell Dev. Biol. 16, 301-332. [DOI] [PubMed] [Google Scholar]
- 4.Neutra, M. R., Frey, A. & Kraehenbuhl, J. P. (1996) Cell 86, 345-348. [DOI] [PubMed] [Google Scholar]
- 5.Neutra, M. R., Mantis, N. J., Frey, A. & Giannasca, P. J. (1999) Semin. Immunol. 11, 171-181. [DOI] [PubMed] [Google Scholar]
- 6.Owen, R. L. (1977) Gastroenterology 72, 440-451. [PubMed] [Google Scholar]
- 7.Mestecky, J., Blumberg, R. S., Kiyono, H. & McGhee, J. R. (2003) in Fundamental Immunology, ed. Paul, W. E. (Lippincott Williams & Wilkins), 5th Ed., p. 965.
- 8.Savidge, T. C. & Smith, M. W. (1995) Adv. Exp. Med. Biol., 371, 239-241. [DOI] [PubMed] [Google Scholar]
- 9.Kerneis, S., Bogdanova, A., Kraehenbuhl, J. P. & Pringault, E. (1997) Science 277, 949-952. [DOI] [PubMed] [Google Scholar]
- 10.Golovkina, T. V., Shlomchik, M., Hannum, L. & Chervonsky, A. (1999) Science 286, 1965-1968. [DOI] [PubMed] [Google Scholar]
- 11.Debard, N., Sierro, F., Browning, J. & Kraehenbuhl, J. P. (2001) Gastroenterology 120, 1173-1182. [DOI] [PubMed] [Google Scholar]
- 12.VanCott, J. L., Kobayashi, T., Yamamoto, M., Pillai, S., McGhee, J. R. & Kiyono, H. (1996) Vaccine 14, 392-398. [DOI] [PubMed] [Google Scholar]
- 13.Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. (2001) Nature 413, 639-643. [DOI] [PubMed] [Google Scholar]
- 14.Hiroi, T., Yanagita, M., Iijima, H., Iwatani, K., Yoshida, T., Takatsu, K. & Kiyono, H. (1999) J. Immunol. 162, 821-828. [PubMed] [Google Scholar]
- 15.Hiroi, T., Yanagita, M., Ohta, N., Sakaue, G. & Kiyono, H. (2000) J. Immunol. 165, 4329-4337. [DOI] [PubMed] [Google Scholar]
- 16.Macpherson, A. J., Gatto, D., Sainsbury, E., Harriman, G. R., Hengartner, H. & Zinkernagel, R. M. (2000) Science 288, 2222-2226. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto, M., Rennert, P., McGhee, J. R., Kweon, M. N., Yamamoto, S., Dohi, T., Otake, S., Bluethmann, H., Fujihashi, K. & Kiyono, H. (2000) J. Immunol. 164, 5184-5191. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez-Torres, A., Jones-Carson, J., Baumler, A. J., Falkow, S., Valdivia, R., Brown, W., Le, M., Berggren, R., Parks, W. T. & Fang, F. C. (1999) Nature 401, 804-808. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno, M., Urbano, M., Valzasina, B., Francolini, M., Rotta, G., Bonasio, R., Granucci, F., Kraehenbuhl, J. P. & Ricciardi-Castagnoli, P. (2001) Nat. Immunol. 2, 361-367. [DOI] [PubMed] [Google Scholar]
- 20.Eugster, H. P., Muller, M., Karrer, U., Car, B. D., Schnyder, B., Eng, V. M., Woerly, G., Le Hir, M., di Padova, F., Aguet, M., Zinkernagel, R., Bluethmann, H. & Ryffel, B. (1996) Int. Immunol. 8, 23-36. [DOI] [PubMed] [Google Scholar]
- 21.Rennert, P. D., Browning, J. L., Mebius, R., Mackay, F. & Hochman, P. S. (1996) J. Exp. Med. 184, 1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokota, Y., Mansouri, A., Mori, S., Sugawara, S., Adachi, S., Nishikawa, S. & Gruss, P. (1999) Nature 397, 702-706. [DOI] [PubMed] [Google Scholar]
- 23.Gebert, A., Fassbender, S., Werner, K. & Weissferdt, A. (1999) Am. J. Pathol. 154, 1573-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins, S. A., Niedergang, F., Corthesy-Theulaz, I. E. & Kraehenbuhl, J. P. (2000) Cell. Microbiol. 2, 59-68. [DOI] [PubMed] [Google Scholar]
- 25.Niedergang, F., Sirard, J. C., Blanc, C. T. & Kraehenbuhl, J. P. (2000) Proc. Natl. Acad. Sci. USA 97, 14650-14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolin, I. & Wolf-Watz, H. (1984) Infect. Immun. 43, 72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosqvist, R., Skurnik, M. & Wolf-Watz, H. (1988) Nature 334, 522-524. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto, M., Fujihashi, K., Kawabata, K., McGhee, J. R. & Kiyono, H. (1998) J. Immunol. 160, 2188-2196. [PubMed] [Google Scholar]
- 29.Elsinghorst, E. A. (1994) Methods Enzymol. 236, 405-420. [DOI] [PubMed] [Google Scholar]
- 30.Chatfield, S. N., Charles, I. G., Makoff, A. J., Oxer, M. D., Dougan, G., Pickard, D., Slater, D. & Fairweather, N. F. (1992) Biotechnology (N.Y.) 10, 888-892. [DOI] [PubMed] [Google Scholar]
- 31.De Togni, P., Goellner, J., Ruddle, N. H., Streeter, P. R., Fick, A., Mariathasan, S., Smith, S. C., Carlson, R., Shornick, L. P., Strauss-Schoenberger, J., et al. (1994) Science 264, 703-707. [DOI] [PubMed] [Google Scholar]
- 32.Borghesi, C., Taussig, M. J. & Nicoletti, C. (1999) Lab. Invest. 79, 1393-1401. [PubMed] [Google Scholar]
- 33.Bry, L., Falk, P. G., Midtvedt, T. & Gordon, J. I. (1996) Science 273, 1380-1383. [DOI] [PubMed] [Google Scholar]
- 34.Borghesi, C., Regoli, M., Bertelli, E. & Nicoletti, C. (1996) J. Pathol. 180, 326-332. [DOI] [PubMed] [Google Scholar]
- 35.Meynell, H. M., Thomas, N. W., James, P. S., Holland, J., Taussig, M. J. & Nicoletti, C. (1999) FASEB J. 13, 611-619. [DOI] [PubMed] [Google Scholar]
- 36.Sierro, F., Pringault, E., Assman, P. S., Kraehenbuhl, J. P. & Debard, N. (2000) Gastroenterology 119, 734-743. [DOI] [PubMed] [Google Scholar]
- 37.Slack, J. M. (2000) Science 287, 1431-1433. [DOI] [PubMed] [Google Scholar]
- 38.Jones, B. D., Ghori, N. & Falkow, S. (1994) J. Exp. Med. 180, 15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassef, J. S., Keren, D. F. & Mailloux, J. L. (1989) Infect. Immun. 57, 858-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf, J. L., Rubin, D. H., Finberg, R., Kauffman, R. S., Sharpe, A. H., Trier, J. S. & Fields, B. N. (1981) Science 212, 471-472. [DOI] [PubMed] [Google Scholar]
- 41.Galan, J. E. & Curtiss, R., 3rd (1989) Proc. Natl. Acad. Sci. USA 86, 6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Autenrieth, I. B. & Firsching, R. (1996) J. Med. Microbiol. 44, 285-294. [DOI] [PubMed] [Google Scholar]
- 43.Autenrieth, I. B., Vogel, U., Preger, S., Heymer, B. & Heesemann, J. (1993) Infect. Immun. 61, 2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark, M. A., Hirst, B. H. & Jepson, M. A. (1998) Infect. Immun. 66, 1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isberg, R. R. & Leong, J. M. (1990) Cell 60, 861-871. [DOI] [PubMed] [Google Scholar]
- 46.Isberg, R. R., Voorhis, D. L. & Falkow, S. (1987) Cell 50, 769-778. [DOI] [PubMed] [Google Scholar]
- 47.Hamada, H., Hiroi, T., Nishiyama, Y., Takahashi, H., Masunaga, Y., Hachimura, S., Kaminogawa, S., Takahashi-Iwanaga, H., Iwanaga, T., Kiyono, H., et al. (2002) J. Immunol. 168, 57-64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.