Imaging studies reveal activation of numerous brain regions in healthy individuals performing emotional empathy tasks. To identify regions critical for empathy, Hillis reviews studies of patients with impaired empathy after focal injury. Lesions to several areas disrupt empathy, but selective deficits in specific underlying cognitive processes are rarely reported.
Keywords: empathy, stroke, emotion, focal lesion studies
Abstract
Emotional empathy—the ability to recognize, share in, and make inferences about another person’s emotional state—is critical for all social interactions. The neural mechanisms underlying emotional empathy have been widely studied with functional imaging of healthy participants. However, functional imaging studies reveal correlations between areas of activation and performance of a task, so that they can only reveal areas engaged in a task, rather than areas of the brain that are critical for the task. Lesion studies complement functional imaging, to identify areas necessary for a task. Impairments in emotional empathy have been mostly studied in neurological diseases with fairly diffuse injury, such as traumatic brain injury, autism and dementia. The classic ‘focal lesion’ is stroke. There have been scattered studies of patients with impaired empathy after stroke and other focal injury, but these studies have included small numbers of patients. This review will bring together data from these studies, to complement evidence from functional imaging. Here I review how focal lesions affect emotional empathy. I will show how lesion studies contribute to the understanding of the cognitive and neural mechanisms underlying emotional empathy, and how they contribute to the management of patients with impaired emotional empathy.
Introduction
Most social interactions hinge on the degree to which the participants have or display empathy—the ability to share in, and make inferences about, another person’s cognitive and emotional state. From the cognitive neuroscience literature, two somewhat distinct models of empathy have emerged. One model specifies two dissociable systems: one developmentally and phylogenetically ‘early’ system for emotional contagion—the ability to recognize and share the feelings of another person—and one developmentally; and phylogenetically ‘later’ system for perspective-taking—the ability to make inferences about what another person is thinking or feeling (Shamay-Tsoory, 2011). These systems are seen to operate in parallel and depend on distinct neural structures. A competing model includes these same cognitive functions as stages or components of processing within a single system underlying empathy. That is, emotional empathy may require one to become aware of the emotional state of the other person and identify with it to some degree (emotional contagion), then ascribe the emotion to another agent and suppress one’s own perspective (perspective-taking) (Decety and Jackson, 2004).
I will not attempt to summarize the main points of this vast literature, but refer the reader to outstanding reviews from the cognitive neuroscience standpoint (Adolphs, 2003; Frith and Frith, 2003; Decety and Jackson, 2004; Decety, 2011) and from developmental psychology (Johnson, 2000; Bartsch 2002; Meltzoff and Decety, 2003). A third possibility that I will raise is a synthesis of these two models, in which both emotional contagion and perspective-taking are complex cognitive processes with a number of shared underlying cognitive mechanisms and neural substrates. For example, perspective-taking includes the ability to make both affective and non-affective (e.g. visuospatial) third-person judgements, which are dissociable (Schnell et al., 2011; Sebastian et al., 2012). I will refer to these processes as affective perspective-taking and cognitive perspective-taking. The difference between these functions is illustrated with the following example. You are told that your friend, David, had a dinner date with his fiancée, Catherine. Catherine called to say she could not make dinner, because she had to work late in surgery. David’s sister later informed David that she had seen Catherine that night having dinner at a restaurant with her ex-husband. You can (i) infer that David is likely to believe that Catherine lied to him (cognitive perspective-taking); (ii) infer that David is likely to feel angry and jealous (affective perspective-taking); and (iii) share David’s anger (emotional contagion). On the other hand, you might not only infer that David feels angry, you might feel sympathy (concern or pity, motivating kindness) towards David. Likewise, after brain damage, one might be able to share in the emotions of another without being able to make explicit inferences about the emotions of the other person, or vice versa. That is, these component cognitive processes are at least theoretically dissociable. However, ‘development’ of affective perspective-taking, and certainly ‘the development’ of emotional contagion, may depend on recognition of others’ emotions through facial expression and prosody. That is, it is difficult to imagine how one could learn that other people feel certain emotions in specific circumstances or learn to ascribe their own feelings to other people without recognizing those emotions in other people, at least on occasion.
Perhaps the most helpful definition of empathy has been provided by de Vignemont and Singer (2006), who propose that empathy exists if four criteria are met: (i) the individual is an affective state; (ii) the affective state is isomorphic to that of another person; (iii) the affective state is achieved by imagining or observing the affective state of the other person; and (iv) the individual attributes the source of their own affective state to the other person. Empathy is distinct from sympathy, because when one sympathizes, the affect (concern) the individual feels towards another person (or group) may not be the same as the affect of the other(s). Although de Vignemont and Singer (2006) also distinguish empathy from both emotional contagion and perspective-taking, my view is that emotional contagion and affective perspective-taking are components of emotional empathy. Emotional contagion (alone) meets the first three criteria, but the individual does not attribute the source of the affective state to the other person, so they cannot make inferences about the other person’s needs or likely actions [epistemological and prosocial roles of empathy described by de Vignemont and Singer (2006)]. Affective perspective-taking (alone) meets the last three criteria, but the person may not be in an affective state themselves. These criteria are not the same as distinct cognitive processes underlying empathy. Most investigators of neurologically impaired populations have studied only major components of empathy, such as emotional contagion and perspective-taking. However, each of these components likely requires a number of dissociable processes that might be individually affected by brain damage (Table 1). Emotional contagion involves suppression of one’s own affective state, (not necessarily conscious) awareness of the affective state of the other person, and adoption of the affective state of the other person. In our example, suppose you are pleased that Catherine may be getting back together with her ex-husband. To empathize with your friend, David, you suppress that pleasure, and share his anger at Catherine for lying to him. Affective perspective-taking requires suppressing your own perspective, recognizing the affective state of the other person through observation or imagination, and attributing the state to the other person. In our example, affective perspective-taking would entail recognizing that David’s anger (just by imagining the situation), and attributing anger to him. Integrating the two (the full system of emotional empathy) would entail all of the above; additionally, it would entail attributing the source of your feeling anger towards Catherine to your shared feelings with David. To feel angry at Catherine, you would also have to have some understanding of social concepts of honesty, faithfulness, and so on. You might even need to assign some value to these attributes. Some investigators have tried to dissect cognitive processes underlying one or the other of these components, either through functional imaging studies using paradigms that vary only by one process or by studying one or more patients with disruption in a single process. Some studies have even tried to identify particular areas of the brain responsible for a given process.
Table 1.
Cognitive processes underlying emotional empathy
| Emotional empathy | Emotional contagion | Affective perspective-taking |
|---|---|---|
| Contributing representations: meaning of social concepts (e.g. loyalty, honesty) | Suppression of one’s own (earlier) affective state | Suppression of one’s own perspective |
| Arousal and awareness (conscious or unconscious) of the affective state of the other person (through observation or imagination) | Recognition of the affective state of the other person (through observation or imagination) | |
| Adoption of a new affective state that is isomorphic to that of another person | Attribution of the affective state to the other person (requires cognitive flexibility, ‘mentalizing’) | |
| Integration of emotional contagion and affective cognitive perspective taking permits: | Attribution of the source of one’s newly adopted affective state to the other person | |
| Emotional regulation | ||
The purpose of this review is to examine how focal brain lesions affect emotional empathy—the ability to share and make inferences about the emotions of another person. I will show how the lesion literature contributes to the understanding of the cognitive and neural mechanisms underlying emotional empathy, as well as how these studies might contribute to the management of patients with impaired emotional empathy due to stroke and neurodegenerative disease. This review was motivated by a recent study from our Stroke Prevention and Recovery Centre (SPARC), in which stroke survivors and their caregivers were surveyed about the motor, sensory, cognitive and other sequelae that impacted quality of life (including problems with sleep, fatigue, pain, sex, swallowing, change in personality, walking and so on). Among caregivers of right hemisphere stroke survivors, the most frequent residual symptom identified as among the top five most important problems was ‘difficulty understanding the feelings of other people (loss of emotional empathy)’ reported by 50% of spouses or adult children (Urrutia et al., submitted for publication). This problem was rarely identified as an important problem by survivors themselves or by caregivers of left hemisphere stroke survivors. The clinical significance of loss of empathy has been more often recognized among clinicians and investigators of behavioural variant frontotemporal dementia (FTD) (Rankin et al., 2005; Eslinger et al., 2011; Rascovsky et al., 2011), and there have even been some preliminary studies of treatment to improve empathy in this population (Eslinger, 1998; Finger, 2011; Jesso et al., 2011). It has been proposed that neuromodulary interventions, such as transcranial magnetic stimulation or transcranial direct current stimulation might prove useful in treatment of empathy (Hétu et al., 2012), but these interventions would require a better understanding of the neural networks underlying the various cognitive functions that comprise empathy, to focus inhibitory or excitatory stimulation.
The neural mechanisms underlying empathy have been widely studied with functional imaging of healthy participants. These studies have converged in support of the proposal that medial prefrontal cortex, anterior insula, anterior cingulate, and amygdala, and temporoparietal junction are important for particular broad components of empathy, such as emotional contagion or cognitive perspective-taking. However, it is widely recognized that functional imaging studies reveal correlations between areas of activation and performance of a task, so that they can only reveal areas engaged in a task, rather than areas of the brain that are critical for the task (Robertson et al., 1993; Schoenfeld et al., 2002; Fellows et al., 2005; Squire et al., 2007). Lesion studies are useful as a complementary approach, to test whether regions activated during a task, such as a measure of cognitive or affective perspective-taking, are indeed necessary for that function. Impairments in empathy have been studied in a number of neurological disease states, but primarily in neurological diseases with fairly diffuse, bilateral (although often asymmetric) damage or dysfunction, such as autism (Dziobek et al., 2008), traumatic head injury (Eslinger, 1998; McDonald and Flanagan, 2004; Neumann et al., 2012), schizophrenia (Hooker et al., 2011; Lee et al., 2011) and FTD (Viskontas et al., 2007; Eslinger et al., 2011). The classic ‘focal lesion’ in the neurological literature is stroke. There have been scattered studies of impaired empathy after stroke and other focal injury, but these have had each had small numbers of patients. There have been no recent reviews of these studies, although together, they provide critical data for evaluating hypotheses from functional imaging. (Bird et al., 2004; Stuss and Anderson, 2004; Samson et al., 2007; Roldan Gerschcovich, et al., 2011; Couto et al., 2013), or have been studies with mixed lesion types (including only a few stroke patients).
Here I systematically review all of the focal lesion studies of the neural correlates of emotional empathy that were reported in PubMed (using search terms ‘empathy’, ‘theory of mind’, or ‘perspective-taking’ in conjunction with ‘emotional’, ‘emotion’, or ‘affective’, and ‘lesion’, ‘tumor’, ‘CVA’, ‘resection’ or ‘stroke’), or cited in review papers found in that search. I will review only those that investigate specifically emotional empathy, and not all studies of ‘theory of mind’ or emotional recognition, and exclude studies that do not have imaging or autopsy evidence of lesion site and aetiology. I propose that, taken together, focal lesion studies confirm the results from functional imaging studies, indicating a critical role of medial prefrontal cortex, anterior insula, anterior cingulate, anterior temporal cortex, and amygdala, at least in the right hemisphere, in emotional empathy. Lesion studies also indicate that there are dissociable but overlapping networks underlying emotional contagion and affective perspective-taking aspects of emotional empathy (although there is likely no area specifically devoted to either of these constructs). These lesion studies show that some of the areas identified by functional imaging studies as engaged in emotional empathy are critical for one system or the other or both. More importantly, however, I show that lesion studies can highlight the importance of structures whose role may be underestimated by functional imaging studies, such as the right anterior temporal cortex. The data from various methodologies converge in support of a model of the neural mechanisms underlying empathy that partially arose from functional imaging, but is refined by the lesion studies. They indicate that the right anterior insula, anterior cingulate cortex, anterior temporal cortex, and amygdala may play a role not only in emotional contagion, but also in affective perspective-taking. The role of these structures may be to integrate information from somatosensory cortex and limbic structures with prefrontal cortex and inferior frontal cortex, that are important for assigning emotion to other versus self, assigning valence, suppressing one’s own emotion or perspective, and understanding social concepts. The results provide insights into assessment and management of individuals with damage to right hemisphere regions in frontal, temporal, parietal, cingulate and insular cortex and thalamus, including stroke and FTD.
Proposed model of the neural mechanisms underlying emotional empathy
One component of emotional empathy is the developmentally and phylogenetically ‘early’ system of emotional contagion system that may involve the right inferior frontal gyrus and orbitofrontal cortex. In a resting-state functional connectivity MRI study of healthy participants, Cox et al. (2012) identified greater connectivity between orbitofrontal cortex, amydala, ventral anterior insula, and anterior cingulate in individuals who scored higher (by self-rating) on Emotional Contagion compared with Perspective-Taking scales on the Inter-reactivity Index (IRI). This emotional contagion system may be closely linked or overlapping with areas of the brain necessary for recognizing emotions of others through prosody (tone of voice), facial expression, and gestures. For example, previous studies have indicated that right superior temporal sulcus, amygdala and fusiform cortex (Gorno-Tempini et al., 2001) are critical for recognizing facial expressions, whereas the right superior temporal cortex is critical for comprehension of affective prosody (Ross and Monnot, 2008).
A second major component of emotional empathy is a ‘later’ and higher level system for perspective-taking involving at least right prefrontal cortex. Emotional perspective-taking also likely involves a number of other cognitive functions such as cognitive flexibility (Decety and Jackson, 2004; Rankin et al., 2005), attention and working memory, abstract reasoning (Rankin et al., 2006), as well as belief attribution and suppression of one’s own perspective (Samson et al., 2004, 2005, 2007). Therefore, while this system depends on areas essential for affective perspective-taking in medial prefrontal cortex (Eslinger, 1998; Shamay-Tsoory et al., 2003), it also engages areas activated more generally in cognitive flexibility and belief attribution in temporoparietal junction and superior temporal sulcus (Frith and Frith, 2003; Saxe and Kanwisher, 2003; Samson et al., 2004; Schnell et al., 2011).
Integrating these two systems are structures engaged in both emotional contagion and affective perspective-taking that may involve von Economo neurons in the anterior insula and anterior cingulate cortex (Seeley, 2008) as well as closely related right anterior temporal cortex and amygdala.
Although it is likely that none of the brain regions mentioned above are specifically devoted to emotional empathy, I will focus on areas for which there is evidence from functional imaging and brain injured patients that they are particularly important for one or more aspects of empathy: anterior insula, anterior cingulate cortex, (ventromedial) prefrontal cortex, temporoparietal junction, orbitofrontal cortex, inferior frontal cortex, amygdala, anterior temporal cortex and right thalamus. As noted, functional imaging studies can only show areas of the brain where neural activation is correlated with performance on a task. Most of these studies compare two paradigms or one task to a control condition. It is impossible to be sure one is testing only what one wants to test; for example, if one condition is more challenging than the other, the activation may reveal areas of the brain associated with ‘challenging’ or stressful conditions more than the targeted process. Nearly all studies report only group data; rarely do all the individuals in the group show all of the peak activations shown by the group. In fact, it is possible that no individual in a study showed all of the peak activations; so it may be the case that some individuals show activation in one area and some in another area. Some areas are revealed only when the statistical threshold is lowered; these may be areas identified by chance alone, or areas associated with the process being investigated (but where there were too few participants to reveal the association). Given these caveats, here I report only results from the functional imaging literature that seem most robust—cases in which numerous functional imaging studies with different designs and populations of participants have converged in support of the hypothesis that one or more components of empathy engages the particular area of the brain. For brevity, I will not critique the individual functional imaging studies, but acknowledge that (like lesion studies), they each have weaknesses, and none has provided a comprehensive model of the neural networks underlying the distinct cognitive processes underlying empathy. I will also describe studies that have found lesions in other areas that have affected emotional empathy, including bilateral cerebellum, and other areas affected in frontotemporal dementia, because it is a relatively focal degenerative disease. I will not discuss studies of other neurodegenerative or psychiatric diseases that do not have focal lesions. I propose that the systems for emotional contagion and for affective cognitive perspective-taking are dissociable, because they have some distinct underlying cognitive processes that can be individually impaired by brain damage. However, they have partly overlapping neural substrates, both because they have some shared cognitive processes and require integration. Parts of the insula, anterior cingulate, anterior temporal cortex, and amygdala appear to be critical for both emotional contagion and affective perspective-taking, possibly because they are necessary for cognitive processes shared by both components (Table 1).
The lesion studies are summarized in Tables 2 and 3. Table 2 reports single case studies and small case series of patients who have been carefully studied (often with a variety of tests) to evaluate which cognitive processes or tasks are intact and which are spared. Some of these illustrate a double dissociation between impaired and spared processes. That is, one patient (or group) is impaired on one process and spared on the other; and the other patient (or group) shows the opposite pattern. Such double dissociations provide evidence that these are independent cognitive processes. However, these double dissociations do not permit one to assume that the lesions of the patient(s) with the impaired process are ‘the’ areas of the brain responsible for cognitive process impaired in each case. Structure-function associations are likely to be probabilistic (e.g. most but not all individuals are left hemisphere dominant for language); therefore statistical associations are required to identify lesion-deficit associations. Studies reporting statistically significant associations between lesion sites and some component of empathy are reported in Table 3.
Table 2.
Summary of case studies and small series of emotional empathy
| Brain region | Reference | Cases with lesion site n | Time post-injury | Type of injury | Conclusions |
|---|---|---|---|---|---|
| Prefrontal and orbitofrontal hypoperfusion | Kemp et al., 2013 | 1 | 3–4 years | Caudate haemorrhage | Prefrontal and orbitofrontal hypoperfusion → impaired affective perspective-taking and impaired recognition of negative emotions |
| Right medial prefronal and orbitofrontal | Fisher et al., 2011 | 1 | 1 and 8 years | Trauma | Right medial prefrontal lesion → acute deficits in a variety of social and executive functions; but full recovery and normal affective perspective taking by 8 years post-injury |
| Left medial frontal and orbitofrontal with bilateral frontal hypoperfusion by SPECT | Grattan and Eslinger, 1992 | 1 | 26 years | Intracerebral haemorrhage | Left medial and orbitofrontal subarachnoid haemorrhage at age 7 with bilateral hypoperfusion → impaired empathy and cognitive flexibility at age 33 |
| Medial prefrontal | Bird et al., 2004 | 1 | 87 and 101 days | Anterior cerebral artery stroke | Subacute bilateral medial prefrontal stroke → no clear deficits in empathy, but relative weakness in affective perspective-taking |
| Right dorsomedial prefrontal | Herbet et al., 2013 | 5 | Immediately post-op, 3 months | Slow-growing tumour, surgical | Lesions caused empathy deficits immediately postoperatively, but patients recovered 3 months later |
| Right prefrontal | Leigh et al., 2013 | 3 | Within 48 h | Stroke | Acute right prefrontal lesions → impaired affective perspective-taking |
| Right dorsolateral prefrontal | Eslinger et al., 1997 | 1 | 5–15 years | Trauma | Right prefrontal lesion → impaired emotional empathy but intact cognitive flexibility 5–15 years after lesion age 3 |
| Right inferior frontal gyrus and STG | Samson et al., 2005 | 1 | 8 months | Stroke | Right inferior frontal gyrus (and superior tempoiral gyrus lesion) → impaired inhibition of self-perspective |
| Right inferior frontal gyrus, temporal pole, and insula | Herbet et al., 2013 | 5 | Immediately post-op, 3 months | Slow-growing tumour, surgical | Lesions caused perspective-taking deficits immediately post-op, but recovered 3 months later |
| Right temporal pole, medial orbitofrontal | Narvid et al., 2009 | 1 | 5+ years | FTD | Right and medial orbitofrontal, temporal atrophy → impaired emotional recognition and emotional empathy |
| Right temporal pole | Perry et al., 2001 | 2 | FTD | Both with right temporal pole atrophy → impaired empathy (IRI) and impaired recognition of emotion through prosody and facial expression | |
| Amygdala | Stone et al., 2003 | 2 | 12 years (1) | 1 surgical | Bilateral amygdala → impaired theory of mind, affective perspective-taking |
| 1 herpes | |||||
| Right amygdala | Leigh et al., 2013 | 27 total, 4 amygdala lesions | Within 48 h | Stroke | All with right amygdala lesions → impaired affective perspective-taking |
| Bilateral amygdala | Hurlemann et al., 2010 | 2 | Urbach-Wiethe disease | Bilateral amygdala lesions → deficits in emotional but not cognitive empathy | |
| Anterior insula, anterior cingulate | Gu et al., 2012 | 3 anterior insula, 3 anterior cingulate cortex, 6 other | 3–32 months | Glioma | Anterior insula lesion → impaired implicit and explicit perception of another person’s pain (emotional contagion) |
| Anterior cingulate cortex | Baird et al., 2006 | 3 | Not stated | Glioma, surgery, haematoma | Right anterior cingulate cortex → impaired emotional recognitionBilateral anterior cingulate cortex → impaired theory of mind |
| Right anterior insula, or its connections | Couto et al., 2013 | 2 | 18 months | Stroke | Right insular damage → no impairment; Sub-insular damage → empathy impairment |
| Cerebellum | Roldan Gersch-covich et al., 2011 | 1 | 3 months | Stroke | Bilateral cerebellar lesions → impaired empathy on a variety of tests |
STG = superior temporal gyrus.
Table 3.
Summary of group lesion studies of emotional empathy
| Brain region | Reference | Number of patients | Time post-injury | Type of injury | Conclusions |
|---|---|---|---|---|---|
| Ventrolmedial prefrontal | Shamay-Tsoory & Aharon-Peretz, 2007 | 49 with prefrontal lesions (10 ventro-medial) | Mixed | Heterogeneous | Ventromedial prefrontal lesions were associated with impaired affected perspective taking (but not cognitive perspective taking) relative to controls or dorslateral prefrontal lesions |
| 44 controls | |||||
| Ventro-medial prefrontal | Leopold et al., 2012 | 8 Left, 7 right | Many years | Penetrating traumatic brain injury; combat | Patients with bilateral lesions performed worse than those with left lesions; those with bilateral or left lesions performed worse than right lesions or controls, on empathy tests |
| 15 bilateral | |||||
| Frontal and non-frontal lesions | Grattan et al., 1994 | 20 with frontal lesions; 20 with non-frontal lesions | Within 1 year of lesion | 36 stroke; 4 tumour | Right or left dorsolateral prefrontal lesions → impaired empathy and cognitive flexibility. Mesial frontal lesion→ impaired cognitive flexibility only |
| Inferior frontal gyrus | Shamay-Tsoory et al., 2009 | 8 inferior frontal gyrus, 11 pre frontal, 11 posterior | Heterogeneous | Heterogeneous | Inferior frontal gyrus lesion → impaired emotional contagion |
| Inferior frontal gyrus/orbitofrontal cortex | Spikman et al., 2012 | 28 | 6–26 years | Traumatic brain injury | Inferior frontal gyrus/orbitofrontal lesion → impaired empathy, theory of mind, recognition of emotions; only recognition of emotions associated with orbitofrontal cortex damage |
| Frontal and non-frontal lesions | Grattan et al., 1994 | 20 with frontal lesions; 20 with non-frontal lesions | Within 1 year of lesion | 36 stroke; 4 tumour | Orbitofrontal lesions → impaired empathy (self-report scale) compared to non-frontal and dorsolateral prefrontal lesions but spared cognitive flexibility |
| Right temporal pole | Leigh et al., 2013 | 27 total, 7 with right temporal pole lesions | Within 48 h | Stroke | All with right temporal pole lesions→ impaired affective perspective-taking. Severity of affective perspective-taking deficit correlated with volume of lesion in right temporal pole |
| Right temporal pole | Rankin et al., 2005 | 123 | Chronic progressive | FTD, PSP, CBD, AD | Right temporal pole atrophy correlated with severity of impaired emotional contagion and affective perspective-taking |
| Anterior insula | Leigh et al., 2013 | 27 total; 8 with anterior insula lesions; 3 with anterior cingulate cortex lesions | Within 48 h | Stroke | Right anterior insula or anterior cingulate cortex lesion → impaired affective perspective-taking. Volume of anterior insula lesion correlated with severity of deficit |
| Frontal, temporal, parietal, amygdala, caudate | Eslinger et al., 2011 | 36 | Chronic progressive | FTD | IRI empathetic concern scores correlated right medial prefrontal, left supplementary motor area; Perspective-taking scores correlated volume in right dorsolateral prefrontal, frontal pole, parietal, amygdala, and caudate, left supplementary motor area and superior temporal gyrus |
AD = Alzheimer’s disease; CBD = corticobasal degeneration; PSP = progressive supranuclear palsy.
Prefrontal cortex
Numerous functional imaging studies have implicated the prefrontal cortex in affective perspective-taking (Amodio and Frith, 2006; Hooker et al., 2008). For example, prefrontal cortex is activated in concert with other regions when participants are asked to make inferences about how another person would feel if they were to have full understanding of the situation, as in a false belief task (Hooker et al., 2008). Similarly, activation of ventromedial prefrontal cortex was revealed when affective perspective-taking was contrasted and cognitive perspective-taking tasks (Sebastian et al., 2012). A voxel-based morphometry study of individuals with schizophrenia demonstrated an association between three measures of ‘theory of mind’, including affective perspective-taking, and grey matter volume in ventromedial prefrontal cortex (Hooker et al., 2011). Worse perspective-taking skills were related to grey matter loss in prefrontal cortex, even after controlling for global cognitive function.
Focal lesions of prefrontal cortex and affective empathy
Single cases and case series
Kemp et al. (2013) studied a man years after a right caudate haemorrhage, when he still showed right orbitofrontal and prefrontal hypoperfusion by single photon emission computed tomography (SPECT). He was significantly impaired on a variety of tasks that required affective perspective-taking and recognition of sadness or fear. A woman studied 1 year after extensive right prefrontal and orbitofrontal damage as a result of trauma had marked deficits across social and executive functions. However, when studied 7 years later, her performance had recovered on tests of executive functions and affective perspective-taking, including the faux pas recognition test (Fisher et al., 2011). The faux pas test (Baron-Cohen et al., 1997) was designed to evaluate the appreciation of the difference between the knowledge of the speaker and knowledge of the listener (cognitive theory of mind or perspective-taking) and to recognize the emotional impact on the listener (affective theory of mind or perspective-taking). Another female studied 87 and 101 days after a bilateral anterior cerebral artery stroke, affecting bilateral medial prefrontal cortex, showed no perspective-taking deficits, despite persistent marked deficit in planning and memory. Her performance on the faux pas test was at the lower end of the normal range, indicating that affective perspective-taking may have been a relative weakness (Bird et al., 2004). Another individual, tested 26 years after an extensive left medial prefrontal and orbitofrontal lesion and bilateral frontal hypoperfusion on SPECT had persistent deficits on tasks of empathy and executive functions (Grattan and Eslinger, 1992). In a series of acute stroke patients studied within 48 h of onset of acute right hemisphere ischaemic stroke on a task of affective perspective-taking that required making inferences about the emotions of another person from a story or a video, all three patients with acute infarcts in right prefrontal cortex had impaired performance on affective perspective-taking (Leigh et al., 2013). In a longitudinal study of 10 patients with right frontal slow growing tumours, two were impaired on empathy tasks before surgery; five with surgical lesions centred on the right dorsomedial prefrontal cortex and five with lesions involving the right inferior frontal gyrus, insula and temporal pole were impaired in affective perspective-taking tasks immediately after surgery, but mostly recovered 3 months later (Herbet et al., 2013).
In summary, in all of these cases, all patients with prefrontal lesions (usually right, sometimes left or bilateral) tested immediately after the lesion had deficits in emotional empathy. Affective perspective-taking was always impaired, if this component was differentially tested. The patients showed variable recovery, tested 87 days to 33 years later. Right or bilateral frontal hypoperfusion was associated with poor recovery, in the few cases it was reported.
Group studies
An early study by Eslinger et al. (1996) of 37 adults with diverse brain injuries (including trauma, encephalitis, ruptured aneurysm, and multiple sclerosis) evaluated with an empathy scale that evaluates role-taking and perspective-taking ability and a questionnaire of emotional empathy found no significant association between cognitive perspective-taking and emotional empathy whether family members or patients’ ratings were used. Although they provided no direct evidence in the form of statistical associations, they speculated that dorsolateral prefrontal lesions were responsible for deficits in cognitive empathy (cognitive perspective-taking), whereas orbitofrontal lesions might be responsible for deficits in emotional empathy, on the basis of the cognitive tasks with which the separate scores were correlated.
Shamay-Tsoory et al. (2003) have carried out a series of detailed studies of affective and cognitive empathy in patients with focal lesions. All have included patients with traumatic brain injury (with and without haematoma), meningioma, glioma, and a few strokes. An early study showed that 25 patients with left or right prefrontal lesions were impaired on empathy tasks, whereas 17 patients with right posterior lesions were impaired on empathy and emotional recognition (Shamay-Tsoory et al., 2003). A later study demonstrated that 36 patients with prefrontal lesions were impaired relative to controls and relative to 15 patients with parietal lesions, on empathy (Shamay-Tsoory et al., 2004). Furthermore, patients with right, but not left, parietal lesions, were also impaired on empathy. Another study revealed that right ventromedial, but not dorsolateral, prefrontal lesions, were significantly impaired in affective perspective-taking (Shamay-Tsoory et al., 2007).
Another study (Shamay-Tsoory et al., 2009) of 30 patients included 11 with (mostly right) ventromedial prefrontal lesions, compared with eight patients with (mostly left) inferior frontal lesions, and 11 patients with right posterior lesions involving the superior temporal gyrus and temporoparietal junction, and healthy controls, using the IRI self-ratings (Davis, 1983). This test is generally self-administered, and includes four question types, designed to evaluate affective empathy (empathetic concern and personal distress) and cognitive empathy (perspective-taking and fantasy scales). Patients with (right) ventromedial prefrontal cortex lesions were significantly more impaired than other groups on the perspective-taking only, whereas patients with inferior frontal cortex lesions were more impaired on emotional contagion; and the posterior lesion group showed no difference from normal control subjects. Thus, right ventromedial prefrontal lesions can cause impairments in perspective-taking, whereas inferior frontal lesions can cause impairments in emotional contagion.
In a study of 34 patients with right, left or bilateral prefrontal surgical lesions, using self- and informant-report measures to evaluate emotion recognition, empathy, antisocial behaviour, social conformity, and sociability, Bramham et al. (2009) found that right unilateral prefrontal lesions were associated with impaired recognition of emotion in others. The right prefrontal lesion group had significantly lower insight regarding this emotional recognition difficulty in comparison with the left unilateral lesion group, indicated by comparing self to informant measures.
In contrast with the previous studies, Leopold et al. (2012) found that left, rather than right, ventromedial prefrontal cortex lesions were more associated with affective perspective-taking impairments. They compared performance of eight patients with left lesions, seven with right lesions and 15 with bilateral lesions, all involving ventromedial prefrontal cortex caused by penetrating traumatic brain injuries, to two comparison groups (one without brain injuries, one normal control) on the faux pas recognition task. Those with bilateral lesions were the most impaired, but performance was not significantly different from those with left lesions. Those with left lesions had significantly more impaired performance than those with right lesions. Another study, of patients studied within a year post-lesion, found that left or right dorsolateral prefrontal lesions affected empathy more than mesial prefrontal lesions, although orbitofrontal lesions had the greatest effect on empathy (Grattan et al., 1994).
Summary
Most of these studies report that right (and often left) prefrontal lesions are associated with impairments in affective perspective-taking. The apparently conflicting evidence reported by Bird et al. (2004) (a single case of bilateral prefrontal lesion with minimal impairment in affective perspective taking) and Leopold et al. (2012) (no significant difference between patients with right traumatic lesions and controls) illustrates a caveat in lesion studies. Lesion studies generally assume that if an area is necessary for a function, then damage to that area should cause impairment of that function. However, this reasoning is based on the (false) assumption that the structure-function relationships in the brain are static. We know that, to varying degrees, reorganization occurs, so that other areas of the brain assume that function of the damaged area (Jenkins and Merzenich, 1987; Xerri et al., 1998). Therefore, if a patient is studied long after onset of the lesion, they may have at least partially recovered from the initial impairment caused by the lesion. Therefore, the lack of a deficit cannot be interpreted as evidence against the hypothesis that that the area is normally critical for the function being studied. In cases of slow growing tumours (menigioma, glioma), the reorganization may occur slowly over time, so that if the tumour is resected, reorganization may have already occurred, leaving the patient with no deficit, even if the tumour was in an area that would have normally been critical to a particular function. Therefore, the lack of a deficit after tumour resection (or in the presence of a slow growing tumour) or a long time after stroke cannot be taken as evidence that the area of lesion is not normally critical for a specific function. Note that only two of the patients with right frontal slow growing tumours in the Herbet et al. (2013) study had empathy deficits before surgery. However, when some of the surrounding tissue was resected, they had deficits immediately postoperatively. Many were able to recover within 3 months, indicating right prefrontal cortex is not necessary for recovery of empathy. Likewise in the Leopold et al. (2012) study, all patients were tested many years after penetrating traumatic brain injury, so some (with right lesions) may have recovered from previous deficits. The longitudinal studies demonstrate recovery from initial deficits in affective perspective-taking deficits caused by right prefrontal lesions. Studying patients at the onset of an acute lesion, such as stroke (or immediately postoperatively), allows one to study the effects of lesions before substantial reorganization or recovery. Acute lesions consistently resulted in affective perspective-taking deficits in the available studies.
These studies also reveal some challenges in studying empathy with neurologically impaired individuals. Lesions that affect empathy may also impair insight or recognition of one’s deficits, or cause other deficits that complicate assessment. Lesions that affect recognition of one’s own deficits, for example, raise questions about testing of empathy using standardized tools that often rely on self-assessment, such as the IRI. Individuals with impaired insight or awareness of their deficits may respond as they would have responded before the lesion. Patients with frontal lesions tend to show an increase in self-report of empathy impairment 6 months after injury, whereas their caregivers report a decrease in impairment (Grattan and Eslinger, 1990); perhaps because the patients themselves improve in insight over that time). Likewise, large right hemisphere lesions are likely to include areas that affect the ability to recognize emotion from facial cues or prosody (stress or tone of voice used to convey emotion) (Pell, 2006; Ross and Monnot, 2008; Dara et al., 2013a). In the Leigh et al. (2013) study, we were unable to identify double dissociation between impaired prosody and impaired affective empathy; all patients with impaired empathy had impaired affective prosody (but the reverse was not the case). Recognition of facial expressions, gestures and affective prosody (changes in loudness, pitch and rhythm of voice to convey emotion) might be necessary for developing emotional contagion, but intact recognition of affective prosody was not necessary for making inferences about others’ emotions through videos or stories. Nevertheless, deficits in recognition and/or expression of affective prosody caused by right hemisphere lesions might be misinterpreted as reduced emotional concern or impaired affective perspective-taking. Impaired affective prosody and impaired recognition of facial cues are common deficits after right hemisphere stroke (Dara et al., 2013a), and if mistaken for impaired emotional empathy might have substantial (and potentially preventable) negative influence on social and personal relationships.
Studying patients with progressive impairments, such as FTD, allows one to study the effect of relatively focal atrophy in the absence of recovery or (presumably) reorganization. However, there is often widespread cerebral dysfunction beyond the focal atrophy. Patients with FTD also have impaired insight and recognition of their deficits, making it problematic to assess empathy using standardized self-assessment tools. Therefore, caregivers’ assessments of empathy have been used instead in this population (Rankin et al., 2005; Eslinger et al., 2011). However, it is difficult to be certain that caregivers’ assessments are accurate, in part because they also may misinterpret impairments in recognition or production of prosody or facial expression as a lack of emotional empathy.
In both patients with stroke and FTD, it is important to control for mood disorders as well as impairments in prosody when evaluating empathy. Depression and other mood disorders are common in both stroke and dementia, and may complicate assessment of empathy (Lee et al., 2001). Some studies have found a negative correlation between depression and empathy scores (O’Keeffe et al., 2007), whereas others have found no correlation (Eslinger et al., 2011). It may be harder for a depressed person to suppress their own affect to adopt that of the other person.
In summary, functional imaging studies and most focal lesion studies converge in support of the hypothesis that (ventromedial) prefrontal cortex seems to be necessary for affective perspective-taking before reorganization of structure-function relationships. There is somewhat more evidence that right than left prefrontal lesions cause affective perspective-taking deficits, but more comparison studies are needed to resolve this issue.
Orbitofrontal and inferior frontal gyrus
Several functional imaging studies have shown activation in inferior frontal gyrus in association with specific components of emotional empathy or emotional recognition (Chakrabarti et al., 2006; Dapretto et al., 2006; Gazzola et al., 2006). Most of these studies point to a role for the inferior frontal gyrus and orbitofrontal gyrus in emotional contagion (Jabbi et al., 2007). These regions, especially on the right, are also important for recognition and expression of emotion through prosody (Ross and Monnot, 2008) and facial expression. It has been proposed that the mirror neuron system in this area is important for recognizing facial expressions and mimicking them, and that this mimicry forms an important part of developing emotional contagion (Bodini et al., 2004; Keysers and Gazzola 2006; Shamay-Tsoory, 2011). However, others have challenged the role of mirror neurons in any component of empathy (Decety, 2011). The orbitofrontal cortex may have a more general role in determining the valence or importance; medial orbitofrontal cortex activation seems to be correlated with reward value, whereas activation in lateral orbitofrontal cortex activity is correlated with value of punishment (Kringelbach and Rolls, 2004). This area may be important for modulating the level of empathy, depending on factors such as familiarity between the empathizer and the target of the empathy, common traits or experiences, gender, and potential consequences.
Focal lesions of inferior frontal gyrus and orbitofrontal gyrus and affective empathy
Case study
A patient studied 8 months after right inferior frontal cortex (and superior temporal gyrus) stroke was reported to be impaired specifically in suppressing his own perspective. He was unimpaired in recognizing the beliefs, emotions and perspectives of another, as long as he did not hold a strong belief of his own. This case provides evidence that suppressing one’s own belief dissociates from perspective-taking, although the association with inferior frontal cortex is less clear (Samson et al., 2005).
Group studies
In a study of 30 patients, eight with inferior frontal lesions had impaired emotional contagion (measured with the IRI), compared with patients with lesions involving the prefrontal cortex (who had impaired perspective-taking) and patients who had posterior lesions (who were unimpaired) (Shamay-Tsoory et al., 2009). A group of 28 patients 6–26 years after moderate to severe traumatic brain injury, some of whom had focal lesions involving orbitofrontal or dorsolateral frontal cortex damage, were significantly impaired on tests of empathy (faux pas test), theory of mind, and recognition of emotions. However, only impaired recognition of emotions correlated with damage to orbitofrontal cortex (Spikman et al., 2012).
Summary
As in many of the previous studies, limitations of these studies include the fact patients were studied at quite heterogeneous time periods after injury, so that some may have undergone extensive reorganization or recovery. Also traumatic brain injury [included in both the Shamay-Tsoory et al. (2009) study in a few cases and all of the cases in the Spikman et al. (2012) study] often has diffuse effects on the brain, beyond the focal damage seen on CT or MRI. Nevertheless, the significant correlation between severity of deficit in recognizing emotions and degree of damage to orbitofrontal cortex indicates that this area may be important in recognizing emotions of others.
In sum, the functional imaging literature provides evidence for a role of inferior frontal gyrus and orbitofrontal gyrus in emotional contagion. Lesion studies provide evidence that these areas are critical for emotional contagion, or at least one aspect, emotional recognition. The right inferior frontal gyrus may also be critical for suppressing one’s own perspective.
Amygdala
Two quantitative, coordinate-based Activation Likelihood Estimation meta-analysis (Turkeltaub et al., 2002), including one analysis of 112 functional MRI studies of emotional empathy (Bzdok et al., 2012) and one analysis of 32 functional MRI studies of empathy for pain (Gu et al., 2012) identified activation in amygdala in association with empathy. In the analysis by Bzdok et al. (2012), the common area of activation associated with emotional empathy was confined to the right amygdala. One recent functional imaging study specifically focused on the role of the amygdala in empathy by evaluating the effects of intranasal vasopressin on activation in amygdala and other brain regions during empathy in a functional MRI study of emotional empathy (Brunnlieb et al., 2013). Results indicated that vasopressin modulated right amygdala activation associated with emotional empathy and increased connectivity between right amygdala and medial prefrontal cortex as well as inferior parietal cortex during the emotional empathy task.
Lesions of the amygdala and emotional empathy
Single cases/case series
Two females with selective damage to bilateral amygdala caused by Urbach-Wiethe disease were found to have deficits in emotional but not cognitive empathy (Hurlemann et al., 2010). These patients showed normal learning of an association task when non-social re-inforcers, but not when social re-inforcers were used for training. Furthermore, intranasal oxytocin, a neuropeptide that affects amygdala function, increased emotional but not cognitive empathy, in healthy males, and improved their learning performance on the association task when social, but not non-social, re-inforcers were used (the opposite pattern to the females with bilateral amygdala lesions).
In the Leigh et al. (2013) study of acute stroke lesions that affect emotional empathy, right amygdala was one of the areas where acute infarct was consistently associated with impaired affective perspective-taking. Stone et al. (2003) also reported that two patients with acquired bilateral amygdala lesions were impaired on two affective perspective-taking tasks. Errors were unrelated to level of difficulty of the items, indicating it was not a general impairment, but specific to empathy.
Summary
The amygdalae are involved in a variety of emotional processes; lesions in right or bilateral amydala consistently interfere with performance on emotional empathy tasks, whether they tap primarily emotional contagion or affective perspective-taking. A proposed role of the amydala is in its contribution to the assessment of emotional salience (Critchley, 2009).
Right temporal pole
Functional imaging studies have typically revealed temporal pole activation in association with ‘mentalizing’ or cognitive perspective taking (Meyer et al., 2013). However, voxel-based morphometry studies of behavioural variant FTD, a neurodegenerative disease that is manifest primarily by changes in comportment and social behaviour (Rascovsky et al., 2011) reveals a specific role of right temporal pole in emotional empathy—both in emotional contagion and affective perspective-taking. Emotional empathy has been reported to be more impaired in patients with behavioural variant FTD than in other dementias (Rankin et al., 2005). Voxel-based morphometry allows identification of structure–function relationships by determining the correlation between volumes of grey matter (loss) in particular areas and performance on behaviours that depend on those areas. Rankin et al. (2006) carried out a voxel-based morphometry study of 123 patients with Alzheimer’s disease, progressive supranuclear palsy, corticobasal degeneration, and FTD using caregivers’ ratings on the IRI (Davis, 1983) to evaluate empathy. They found that impairments in empathy measured by the sum of empathetic concern (reflecting emotional contagion) and perspective-taking significantly correlated with the volume of grey matter right temporal pole, fusiform gyrus and medial inferior frontal region. The empathetic concern subscale alone correlated with volume of grey matter in the right temporal pole, caudate/subcallosal gyrus and inferior frontal gyrus; whereas perspective-taking correlated with atrophy in the right temporal pole, right and posterior fusiform, and right caudate/ subcallosal gyrus. Therefore, the right temporal pole might be critical for both emotional contagion and affective perspective-taking, or integrating the two, or it might have a more general role in representing social concepts, which enables one to understand the emotions of others in a social interaction. For, in the example of David hearing about Catherine at the restaurant, to recognize David’s anger or jealousy, one would have to have access to social representations of tact, honour, and so on. A specific role of the superior right temporal pole in representing social concepts has been demonstrated both with functional imaging study of healthy control subjects (Zahn et al., 2007) and in a PET study of individuals with frontotemporal lobar degeneration (Zahn et al., 2009). In the latter study, hypometabolism in right superior segment of anterior temporal cortex (Brodmann areas 38 and 22) was associated with impairment in understanding social concepts or traits (e.g. loyal, tactless, and honourable) relative to animal concepts or traits (e.g. trainable, useful, and nutritious).
Focal lesions of right temporal pole
Case studies
Two patients with FTD had impaired empathy measured by caregiver ratings on the IRI and impaired recognition of emotions through prosody and facial expression, associated with right temporal pole atrophy (Perry et al., 2001). A detailed report (Narvid et al., 2009) revealed a significant impairment in emotional comprehension and contagion, perspective-taking, and attribution of intentions despite generally preserved cognitive abilities associated with right and medial orbital frontal and anterior temporal regions, but sparing of dorsolateral frontal cortex.
Group study
The area most strongly associated with acute stroke among 27 patients in the Leigh et al. (2013) study described earlier was the right temporal pole. All six patients with right temporal pole lesions had impaired empathy. There was also a significant correlation between the volume of lesion in the right temporal pole and the percentage of errors on the affective perspective-taking task.
Summary
Evidence from voxel-based morphometry in focal neurodegenerative disease and one recent focal lesion study together indicate that right anterior temporal cortex likely plays a critical role in emotional empathy. It either has a role in both emotional contagion and affective perspective taking or a more general process, such as representing social concepts (Zahn et al., 2007, 2009).
Anterior insula and anterior cingulate cortex
There is ample evidence from functional imaging studies that perception of another person’s feelings, at least negative emotions and pain, engage anterior insula and anterior cingulate cortex (Singer et al., 2004; Chakrabarti et al., 2006; Jabbi et al., 2007; Lamm et al., 2011; Bernhardt and Singer, 2012; Gu et al., 2012). Other functional imaging studies have emphasized a role of right anterior insula in integrating and coordinating awareness of feelings or disgust (Brown et al., 2011). A plausible role of the insula in integrating the two systems underlying emotional empathy is supported by its widespread connections between the orbitofrontal, prefrontal, anterior cingulate, and temporal pole, and amygdala (Mesulam and Mufson, 1982; Viskontas et al., 2007; Bernhardt and Singer, 2012). Likewise, the anterior cingulate has dense connections not only to the insula, but also to orbitofrontal cortex and amygdala. Von Economo neurons, found in anterior cingulate and anterior insula, may be selectively targeted in behavioural variant FTD, a neurodegenerative disease in which impaired empathy is a major characteristic (Seeley et al., 2006). A loss of Von Economo neurons and fork cells in the right anterior anterior insular cortex correlated with severity of clinical disease in behavioural variant FTD (Kim et al., 2012).
Focal lesions of insula and anterior cingulate and affective empathy
Single cases/case series
Gu et al. (2012) tested whether three patients with anterior insula lesion or three with anterior cingulate lesions had deficits in perception of empathetic pain. All six patients had resections of gliomas. Patients with anterior insular lesions, but not patients with anterior cingulate cortex lesions, showed impairments in both implicit and explicit empathy for pain. A detailed study of one patient with a large insular lesion (tested 18 months after onset) and another with a lesion involving the putamen, claustrum and external capsule, reported that only the patient with the subcortical lesion was impaired on empathy (Couto et al., 2013). The authors argued that the subcortical lesion interrupted the frontotemporal connections from the insula, which are more critical for emotional recognition and empathy than the insula itself. Likewise, of three patients with chronic medial frontal lobe lesions primarily involving the anterior cingulate, compared with control subjects, only the one with bilateral cingulate lesion was impaired in perspective-taking (Baird et al., 2006). However, normal performance of patients with chronic insular or unilateral cingulate lesions cannot be taken as evidence that these areas are not normally critical for empathy, as those with unilateral lesions may have recovered.
Group studies
A voxel-based lesion-symptom mapping study of 192 Vietnam combat veterans who had all had focal penetrating traumatic brain injuries many years previously, tested on a self-reported emotional empathy scale found self-reported reductions on emotional empathy correlated with lesion volumes in ventrolateral prefrontal cortex, left and right posterior temporal lobes, and insula. (Driscoll et al., 2012). Leigh et al. (2013) found that acute infarction of the anterior insula was significantly associated with impaired affective perspective-taking among 27 patients studied within 48 h of stroke onset. Volume of right insular lesion was also significantly correlated with errors on the emotional empathy task. All patients with right anterior cingulate lesions had also impaired affective perspective-taking.
Summary
Functional imaging studies indicate that anterior insula and anterior cingulate cortex are activated in both tasks of emotional contagion and affective perspective-taking (at least in taking the perspective of a loved one; Cheng et al., 2010). Lesion studies are mixed, but most studies indicate lesions involving right anterior insula and right anterior cingulate are associated with deficits in emotional contagion (Gu et al., 2012) and/or affective perspective-taking (Leigh et al., 2013) or emotional empathy in general (Driscoll et al., 2012). Results are consistent with the proposal that these regions are involved in both of these components (or a cognitive process shared by the two components) and serve to integrate them.
Temporoparietal junction
Results from many functional imaging studies converge in support of the hypothesis that an area of the temporoparietal junction, near the superior temporal sulcus, is consistently activated when making inferences about what another person believes, or when attributing a belief to another person (Saxe and Kanwisher, 2003; Sebastian et al., 2012). Most of these studies indicate that this area has an important role in mentalizing and third-person perspective-taking, but not specifically for affective perspective-taking (Schnell et al., 2011; Sebastian et al., 2012).
Focal lesions of temporoparietal junction and deficits in emotional empathy
In a case series of three patients with damage to left temporoparietal junction, tested on the false-belief test described above, Samson et al. (2004) reported that all three patients were impaired in perspective-taking abilities, or making inferences about someone else’s belief. This deficit was not specific to making inferences about another person’s emotions or feelings, however. This deficit was contrasted to a deficit in suppressing one’s own belief, which was impaired after damage to inferior frontal gyrus (Samson et al., 2005). Similar deficits in cognitive perspective-taking associated with lesions in or around temporoparietal junction have been reported by Shamay-Tsoory et al. (2004, 2009).
Summary
Functional imaging and lesion studies provide evidence that temporoparietal junction bilaterally is engaged in cognitive perspective-taking, but not specifically emotional empathy.
Right thalamus
Although a role of the thalamus has not been emphasized in the functional imaging literature or previous reviews of the neural basis of empathy, meta-analyses of functional imaging studies of affective empathy have revealed that right thalamus is one of the areas that most consistently shows activation in association with perception of another person’s pain or negative emotions (Lamm et al., 2011; Gu et al., 2012).
Focal lesions of the right thalamus and deficits in emotional empathy
In the study of 27 acute stroke patients by Leigh et al. (2013), 4 of 14 patients with acutely impaired empathy had lesions of the right thalamus. The right thalamic lesion was the only lesion in two of these patients, indicating that it was likely responsible for the deficit.
Summary
Right thalamus may play a role in the network of brain regions underlying emotional empathy (emotional contagion or affective perspective-taking).
Cerebellum
Some of the meta-analyses of functional MRI studies of emotional empathy, particularly for other people’s feelings of pain have shown activation of cerebellum in association with the emotional empathy task (Singer et al., 2004; Gu et al., 2012).
Focal lesions of the cerebellum and emotional empathy
Case report/case series
A detailed case report describes a deficit in emotional empathy after an extensive, bilateral cerebellar stroke (Roldan Gerschcovich et al., 2011). The authors argue that his empathy impairment was because of impaired connections between medial and lateral posterior cerebellum and prefrontal, parietal and temporal association cortex as well as anterior cingulate and insula, amygdala and other limbic and autonomical structures. Deficits in empathy are also listed among impairments that result from lesions of the ‘limbic cerebellum’ (vermis and fastigial nucleus), but without performance on specific assessments of empathy (Schmahmann et al., 2007).
Summary
Limbic cerebellum may play a role in the network of brain regions underlying emotional empathy (emotional contagion or affective perspective-taking), but more detailed studies and groups studies are needed to define its role.
Frontotemporal dementia
One voxel-based morphometry study of FTD and other focal neurodegenerative disease revealed that both emotional contagion and affective perspective-taking scores correlated with grey matter loss in right temporal pole (Rascovsky et al., 2011). Although this result is consistent with other focal lesion studies and some functional imaging studies, not all voxel-based morphometry studies of FTD confirm the functional MRI results. Eslinger et al. (2011) also completed a voxel-based morphometry study of 26 patients with FTD, and obtained both patients’ and caregivers’ ratings of the patients on the IRI. They found no difference between patients and healthy control subjects on the IRI when using the patients’ scores, but found that patients were more impaired than controls on the empathetic concern and perspective-taking subscales, when caregivers’ rating were used. These scores correlated with Theory of Mind test and executive function tests. Empathetic concern scores correlated with grey matter volume in right medial prefrontal and left supplementary motor area, whereas perspective-taking scores correlated with volume in right dorsolateral prefrontal, frontal pole, parietal, amygdala, and caudate, as well as the left supplementary motor area and superior temporal gyrus. Despite the fact that patients with FTD have insular, anterior cingulate, and orbitofrontal atrophy, volume of these areas was not correlated with impairments in either component of empathy. This result is surprising, in view of the prominent role of these areas indicated by functional imaging studies (Carr et al., 2003; Gu et al., 2012) and their prominent atrophy in FTD (Rosen et al., 2005; Viskontas et al., 2007).
One possible account for the discrepancy between the voxel-based morphometry studies and the functional imaging studies is that the two voxel-based morphometry studies of FTD relied on caregivers’ ratings of empathy; the patients’ self-ratings of empathy on the IRI were normal. It is possible that caregivers overestimate impairments of empathy, especially in patients with prefrontal and superior temporal cortex atrophy, as these are areas that can cause impaired production and recognition of prosody (changes in stress and intonation of voice) and facial expression to convey emotion. Consistent with this hypothesis, Perry et al. (2001) described patients with FTD who both had impaired empathy as indicated by caregiver ratings on the IRI and impaired recognition of emotions through prosody and facial expression, associated with right temporal pole atrophy. That is not to say that caregivers always over-estimate impairments in empathy, as there were no differences in ratings by caregivers and 50 patients with a variety of cerebral lesions on a scale of emotional empathy (Grattan and Eslinger, 1989). Rather, caregivers or others might mistake impaired prosody for impaired emotional empathy. As mentioned above, impaired prosody can be mistaken for impaired empathy; and patients with FTD are known to be impaired in recognition of prosody and facial expression of emotion (Viskontas et al., 2007; Dara et al., 2013b). Leigh et al. (2013) reported that impairments of prosody were more common than impairments of empathy after right hemisphere lesions. Trinkler et al. (2013) have also claimed that patients with Huntington’s disease are impaired in recognition and expression of emotion through prosody and facial expression, but spared in empathy, or making inferences about the emotions of others. Snowden et al. (2003) concluded that both patients with Huntington’s disease and those with FTD are impaired in empathy, but loss of empathy in Huntington’s disease is because of impaired emotional processing (with relatively spared cognitive perspective-taking), whereas impaired empathy in FTD is a result of a failure in attributing mental state to others, as part of a more general executive dysfunction. Given that atrophy in FTD can be relatively localized to left or right frontal or temporal regions, individual patients may have distinct causes of their empathy impairments. Some of the areas of atrophy that correlated with scores on the IRI by caregivers of FTD correspond to areas important for the comprehension and production of affective prosody (Ross and Monnot, 2008). Of course, these may also overlap with the areas important for emotional empathy. It is likely that some patients with FTD have impaired empathy and prosody (as well as impaired insight about their deficits), whereas others have primarily impaired recognition and production of prosody and facial expression of emotion (mistaken for impaired empathy by caregivers). Consistent with some variability in empathy in FTD, Gregory et al. (1997) reported that only 74% of patients with FTD were impaired on the faux pas test (which evaluates affective perspective-taking). Patients with medial prefrontal cortex atrophy were especially impaired on this test.
Conclusions
The studies reviewed above converge in support of a few general conclusions. First, several studies have shown a double dissociation between measures of emotional contagion and measures affective perspective-taking. However, the studies have fallen short of providing evidence that all of the proposed cognitive processes underlying emotional contagion and affective perspective-taking listed in Table 1 can be individually impaired by brain damage. They provided less strong evidence for specific roles of particular areas of the brain in each of these processes. However, taken together, they provide enough data to propose the following hypotheses. These data indicate that right inferior frontal cortex and orbitofrontal cortex are critical for some component of emotional contagion, whereas (at least right) prefrontal cortex is critical for some component of affective perspective-taking. At least right anterior insula, anterior cingulate, amygdala, and temporal pole are critical for both systems, and may integrate the two systems. One or more (perhaps all) of these areas could also have a more general role in social concepts or emotions, rather than being specific to emotional empathy. The bilateral temporoparietal junction does seem to be engaged non-specifically in perspective-taking and other ‘mentalizing’. On these hypotheses, lesions in right anterior insula, anterior cingulate, amygdala or temporal pole would cause deficits in both emotional contagion and affective perspective-taking. On the other hand, damage to right prefrontal cortex would cause impaired affective perspective-taking only, whereas damage to inferior frontal gyrus or orbitofrontal gyrus would affect emotional contagion. The evidence for these hypotheses comes from single cases and group studies, but the evidence is not as strong as one would like. Strong evidence would come from double dissociations between impairments in emotional contagion (as a result of lesions in right inferior frontal gyrus or orbitofrontal gyrus) and affective perspective-taking (because of lesions in right prefrontal cortex) or their underlying cognitive processes, tested with the same measures across patients/studies, with adequate numbers to demonstrate a statistically significant association between the lesion site and the impaired process. Few studies have carefully dissected the various components of emotional empathy (but see Samson et al., 2004, 2005, 2007). Group studies have rarely reported double dissociations, and when they have done so, they have not reported statistically significant associations between each impairment and a specific lesion site.
This review also indicates that specific nuclei of the right thalamus also likely play an important role in relaying processed sensory information about affective prosody and emotional facial expression to cortical regions to shape inferences about others’ emotions. However, although meta-analyses of functional imaging studies show activation in bilateral or right thalamus in association with emotional empathy (Lamm et al., 2011; Bzdok et al., 2012; Gu et al., 2012), acute lesions of right thalamus inconsistently result in impaired affective perspective-taking (Leigh et al., 2013), indicating that particular nuclei of the thalamus may serve a critical role in the neural network(s) supporting emotional empathy.
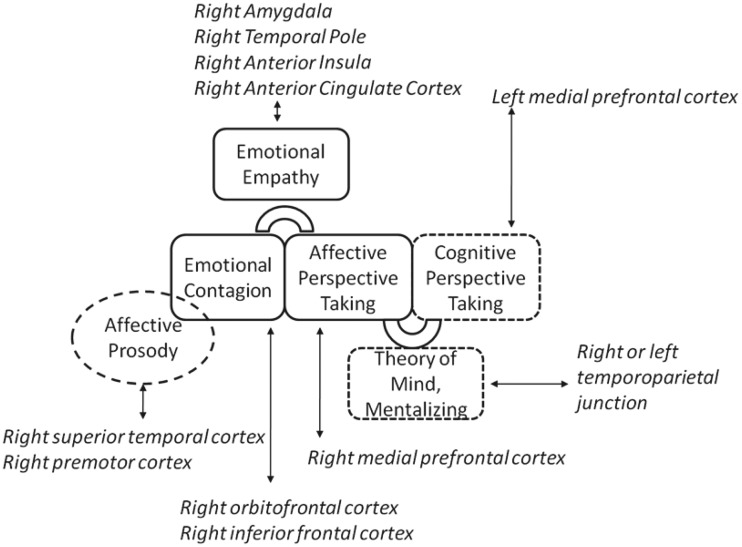
In summary, there are a number of cognitive processes underlying the ability to share in and make inferences about another person’s emotions that may depend on overlapping networks of brain regions. The right anterior insula, anterior cingulate, temporal pole and amygdala may have a critical role in integrating various components of these networks, within and across systems supporting emotional contagion and affective perspective-taking. Patients with focal lesions help refine our understanding of distinct cognitive processes that comprise empathy. The results I have reviewed provide evidence for a functional-anatomical model that is a synthesis of the ‘parallel’ and ‘two-stage’ models of empathy, shown in Fig. 1. This model does not distinguish all of the postulated cognitive processes involved in empathy, delineated in Table 1, because the available studies have not provided sufficient data to show that these can all be independently disrupted by brain damage, much less the precise neural regions responsible for each of these processes. Rather, the lesion studies have shown more gross double dissociations between performance on tasks or questionnaires that assess emotional contagion versus affective perspective-taking.
Figure 1.
A schematic of some of the proposed cognitive and neural mechanisms underlying emotional empathy (in solid borders) and associated cognitive processes (in dashed borders). Right amygdala, temporal pole, anterior insula, and anterior cingulate cortex are hypothesized to be critical for both emotional contagion and affective perspective-taking. In contrast, right orbitofrontal cortex and inferior frontal cortex are hypothesized to be selectively important for emotional contagion, and right medial prefrontal cortex is hypothesized to be selectively critical for affective perspective-taking.
One of the clinical implications that emerged from this review is that an apparent mismatch in the areas of atrophy associated with impaired emotional empathy in FTD versus lesions associated with impaired emotional empathy in other diseases. This mismatch may be explained by the interpretation of impaired prosody and comprehension of prosody and facial expressions as impaired emotional empathy by spouses of patients with FTD. That is, areas of atrophy associated with ‘impaired empathy’ in FTD (as measured by spouses’ ratings on the IRI) correspond to areas associated with impaired comprehension of facial expressions and prosody (emotional tone of voice and stress) that are known to be impaired in FTD. This possible account of the discrepancy needs further investigation, but it is critical to distinguish between patients’ inability to recognize emotions through facial expression and prosody, versus inability to share emotions of another or make inferences about emotions of another, given information about their situation. All of these are critical to human interaction, and may share some neural substrates, but can be selectively impaired by brain damage, and would require different management strategies. As illustrated previously, although recognition of emotions is likely a prerequisite for developing emotional contagion and affective perspective-taking (both components of emotional empathy), it is not necessary later for affective perspective-taking. Neither is affective perspective-taking necessary for emotional recognition. A few studies have reported dissociations: impaired recognition of emotion from prosody and intact affective perspective-taking (Dara et al., 2013b; Leigh et al., 2013), or impaired affective perspective-taking with intact prosody together yielding a double dissociation using the same tasks. If either of these functions is impaired, it is important to counsel the family or caregiver that the deficit is caused by the neurological disease, and to provide education regarding how to compensate for or treat the problem. For example, if an individual is impaired in recognizing emotions from faces or prosody, it is essential for others to state their emotions explicitly to the person. Both social support therapy and music therapy have been used to improve emotional empathy with some success reported in a small randomized trial (Eslinger, 1998), as has a cognitive-oriented approach reported in a case study (Grattan and Eslinger, 1991). Medication may also improve emotional and social function. In a double-blind, placebo-controlled trial in 20 individuals with FTD, Jesso et al. (2011) showed that a single dose of intranasal oxytocin was associated with improvement in some aspects of social function. Oxytocin has also been associated with improved social perception in schizophrenia (Fischer-Shofty et al., 2013) and emotional empathy more generally in healthy males or females with amygdala lesions (Hurlemann et al., 2010). Future research is required to identify effective ways to improve affective perspective-taking. Focused neuromodulary treatments, such as transcranial magnetic stimulation or transcranial direct current stimulation, which have been useful in rehabilitation of motor and cognitive deficits as a result of stroke and focal dementias (Hétu et al., 2012) may be useful, with the knowledge of the neural networks that underlie affective perspective-taking. Simply understanding the precise nature of the individual’s deficits, and the relationship to the brain lesion, provides a first step in educating the patient and caregiver, as well as in developing new interventions. Future investigations should also design experiments to assess the status of each of the cognitive processes underlying emotional contagion and affective perspective-taking, to determine if each of the proposed processes can be independently impaired by focal lesions. Finally, standard assessments of each cognitive process will yield adequate numbers of patients with focal lesions and deficits to allow us to test more specific hypotheses about the areas of the brain critical for each of the cognitive processes underlying emotional empathy.
Funding
This work was supported by: National Institute of Neurological Disorders and Stroke, grant # [RO1NS47691].
Glossary
Abbreviations
- FTD
frontotemporal dementia
- IRI
inter-reactivity index
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontalcortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baird A, Dewar BK, Critchley H, Dolan R, Shallice T, Cipolotti L. Social and emotional functions in three patients with medial frontal lobe damage including the anterior cingulate cortex. Cogn Neuropsychiatry. 2006;11:369–88. doi: 10.1080/13546800444000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: Evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry. 1997;38:813–22. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Bartsch K. The role of experience in children’s developing folk epistemology: review and analysis from the theory-theory perspective. New Ideas Psychol. 2002;20:145–61. [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Ann Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘theory of mind’ and cognition. Brain. 2004;127:914–28. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Bodini B, Iacoboni M, Lenzi GL. Acute stroke effects on emotions: an interpretation through the mirror system. Curr Opin Neurol. 2004;17:55–60. doi: 10.1097/00019052-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Bramham J, Morris RG, Hornak J, Bullock P, Polkey CE. Social and emotional functioning following bilateral and unilateral neurosurgical prefrontal cortex lesions. J Neuropsychol. 2009;3:125–43. doi: 10.1348/174866408X293994. [DOI] [PubMed] [Google Scholar]
- Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M. Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. Neuroimage. 2011;58:250–8. doi: 10.1016/j.neuroimage.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnlieb C, Münte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013;1499:29–42. doi: 10.1016/j.brainres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–96. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc Neurosci. 2006;1:364–84. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, Decety J. Love hurts: an fMRI study. Neuroimage. 2010;51:923–9. doi: 10.1016/j.neuroimage.2010.02.047. [DOI] [PubMed] [Google Scholar]
- Couto B, Sedeño L, Sposato LA, Sigman M, Riccio PM, Salles A, et al. Insular networks for emotional processing and social cognition: comparison of two case reports with either cortical or subcortical involvement. Cortex. 2013;49:1420–34. doi: 10.1016/j.cortex.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc Cogn Affect Neurosci. 2012;7:727–37. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dara C, Gottesman RF, Hillis AE. International Stroke Conference. 2013a. Right hemisphere dysfunction is more accurately detected by prosody impairment than by hemispatial neglect. Abstract. [Google Scholar]
- Dara C, Kirsch-Darrow L, Ochfeld E, Slenz J, Agranovich A, Vasconcellos-Faria A, et al. Impaired emotion processing from vocal and facial cues in frontotemporal dementia compared to right hemisphere stroke. Neurocase. 2013b;19:521–9. doi: 10.1080/13554794.2012.701641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–26. [Google Scholar]
- Decety J. Dissecting the neural mechanisms mediating empathy. Emotion Rev. 2011;3:92–108. [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Driscoll DM, Dal Monte O, Solomon J, Krueger F. Empathic deficits in combat veterans with traumatic brain injury: a voxel-based lesion-symptom mapping study. Cogn Behav Neurol. 2012;25:160–6. doi: 10.1097/WNN.0b013e318280cf4e. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) J Autism Dev Discord. 2008;38:464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Neurological and neuropsychological bases of empathy. Eur Neurol. 1998;39:193–9. doi: 10.1159/000007933. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Biddle KR, Grattan LM. Cognitive and social development in children with prefrontal cortex lesions. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of prefrontal cortex: evolution, neurobiology, and behavior. Baltimore: Brookes; 1997. pp. 295–335. [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2011;23:74–82. doi: 10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Satish U, Grattan LM. Alterations in cognitively- and affectively-based empathy after cerebral damage. J Int Neuropsychol Soc. 1996;2:15. [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: an empirical study of impact in cognitive neuroscience. J Cogn Neurosci. 2005;17:850–8. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Finger EC. New potential therapeutic approaches in frontotemporal dementia: oxytocin, vasopressin, and social cognition. J Mol Neurosci. 2011;45:696–701. doi: 10.1007/s12031-011-9550-2. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Brüne M, Ebert A, Shefet D, Levkovitz Y, Shamay-Tsoory SG. Improving social perception in schizophrenia: the role of oxytocin. Schizophr Res. 2013;146:357–62. doi: 10.1016/j.schres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Fisher T, Shamay-Tsoory S, Eran A, Aharon-Peretz J. Characterization of recovery and neuropsychological consequences of orbitofrontal lesion: a case study. Neurocase. 2011;17:285–93. doi: 10.1080/13554794.2010.536955. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond Ser B. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–9. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Grattan LM, Bloomer RH, Archambault FX, Eslinger PJ. Cognitive flexibility and empathy after frontal lobe lesion. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:251–9. [Google Scholar]
- Grattan LM, Eslinger PJ. Higher cognition and social behavior: changes in cognitive flexibility and empathy after cerebral lesions. Neuropsychology. 1989;3:175–85. [Google Scholar]
- Grattan LM, Eslinger PJ. Influence of cerebral lesion site upon the onset and progression of interpersonal deficits following brain injury. J Clin Exp Neuropsychol. 1990;12:33. [Google Scholar]
- Grattan LM, Eslinger PJ. Characteristics of favorable recovery from frontal lobe damage. Neurology. 1991;41(suppl 1):266. [Google Scholar]
- Grattan LM, Eslinger PJ. Long-term psychological consequences of childhood frontal lobe lesion in patient DT. Brain Cogn. 1992;20:185–95. doi: 10.1016/0278-2626(92)90068-w. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Orrell M, Sahakian B, Hodges JR. Can frontotemporal dementia and Alzheimer’s disease be differentiated using a brief battery of tests? Int J Geriatr Psychiatry. 1997;12:375–83. doi: 10.1002/(sici)1099-1166(199703)12:3<375::aid-gps518>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–35. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Bonnetblanc F, Moritz-Gasser S, Duffau H. Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow-growing lesion. Cortex. 2013 doi: 10.1016/j.cortex.2013.08.003. in press. [DOI] [PubMed] [Google Scholar]
- Hétu S, Taschereau-Dumouchel V, Jackson PL. Stimulating the brain to study social interactions and empathy. Brain Stimulation: Basic, translational, and clinical research in neuromodulation. 2012;5:95–102. doi: 10.1016/j.brs.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry. 2011;70:1169–78. doi: 10.1016/j.biopsych.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. Soc Cogn Affect Neurosci. 2008;3:204–17. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–66. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- Johnson SC. The recognition of mentalistic agents in infancy. Trends Cogn Sci. 2000;4:22–8. doi: 10.1016/s1364-6613(99)01414-x. [DOI] [PubMed] [Google Scholar]
- Jesso S, Morlog D, Ross S, Pell MD, Pasternak SH, Mitchell DG, et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain. 2011;134(Pt 9):2493–501. doi: 10.1093/brain/awr171. [DOI] [PubMed] [Google Scholar]
- Kemp J, Berthel MC, Dufour A, Després O, Henry A, Namer IJ, et al. Caudate nucleus and social cognition: Neuropsychological and SPECT evidence from a patient with focal caudate lesion. Cortex. 2013;49:559–71. doi: 10.1016/j.cortex.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Towards a unifying neural theory of social cognition. Prog Brain Res. 2006;156:379–401. doi: 10.1016/S0079-6123(06)56021-2. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Sidhu M, Gaus SE, Huang EJ, Hof PR, Miller BL, et al. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex. 2012;22:251–9. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lee HS, Brennan PF, Daly BJ. Relationship of empathy to appraisal, depression, life satisfaction, and physical health in informal caregivers of older adults. Res Nurs Health. 2001;24:44–56. doi: 10.1002/1098-240x(200102)24:1<44::aid-nur1006>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011;41:2297–304. doi: 10.1017/S0033291711000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R, Oishi K, Hsu J, Lindquist M, Gottesman RF, Jarso S, et al. Acute lesions that impair affective empathy. Brain. 2013;136:2539–49. doi: 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A, Krueger F, Dal Monte O, Pardini M, Pulaski SJ, et al. Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Soc Cogn Affect Neurosci. 2012;7:871–80. doi: 10.1093/scan/nsr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Flanagan S. Social perception deficits after traumatic brain injury: interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. 2004;18:572–9. doi: 10.1037/0894-4105.18.3.572. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Decety J. What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Philos Trans R Soc Lond Ser B. 2003;358:491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, Wang C, Shi Z, Eisenberger NI, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc Cogn Affect Neurosci. 2013;8:446–54. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvid J, Gorno-Tempini ML, Slavotinek A, Dearmond SJ, Cha YH, Miller BL, et al. Of brain and bone: the unusual case of Dr. A. Neurocase. 2009;15:190–205. doi: 10.1080/13554790802632967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Zupan B, Babbage DR, Radnovich AJ, Tomita M, Hammond F, et al. Affect recognition, empathy, and dysosmia after traumatic brain injury. Arch Phys Med Rehabil. 2012;93:1414–20. doi: 10.1016/j.apmr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- O’Keeffe FM, Murray B, Coen RF, Dockree PM, Bellgrove MA, Garavan H, et al. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain. 2007;130(Pt 3):753–64. doi: 10.1093/brain/awl367. [DOI] [PubMed] [Google Scholar]
- Pell MD. Judging emotion and attitudes from prosody following brain damage. Prog Brain Res. 2006;156:303–17. doi: 10.1016/S0079-6123(06)56017-0. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, Miller BL. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–60. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer J, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cog Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Xie S, et al. Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Knight RT, Rafal R, Shimamura AP. Cognitive neuropsychology is more than single-case studies. J Exp Psychol Learn Mem Cogn. 1993;19:710–17. doi: 10.1037/0278-7393.19.3.710. [DOI] [PubMed] [Google Scholar]
- Roldan Gerschcovich E, Cerquetti D, Tenca E, Leiguarda R. The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase. 2011;17:270–5. doi: 10.1080/13554791003730618. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED, Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008;104:51–74. doi: 10.1016/j.bandl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Humphreys GW. Error analyses reveal contrasting deficits in ‘theory of mind’: neuropsychological evidence from a 3-option false belief task. Neuropsychologia. 2007;45:2561–9. doi: 10.1016/j.neuropsychologia.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: a case of a selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–11. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in ‘Theory of Mind’. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg J, Sherman C. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage. 2011;54:1743–54. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Noesselt T, Poggel D, Tempelmann C, Hopf JM, Woldorff MG, et al. Analysis of pathways mediating preserved vision after striate cortex lesions. Ann Neurol. 2002;52:814–24. doi: 10.1002/ana.10394. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Fontaine NM, Bird G, Blakemore SJ, Brito SA, McCrory EJ, et al. Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Soc Cogn Affect Neurosci. 2012;7:53–63. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol. 2008;21:701–7. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–7. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45:3054–67. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007;90:274–83. doi: 10.1016/j.schres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy:a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Lester H, Chisin R, Israel O, Bar-Shalom R, Peretz A, et al. The neural correlates of understanding the other’s distress: a positron emission tomography investigation of accurate empathy. Neuroimage. 2005a;27:468–72. doi: 10.1016/j.neuroimage.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired ‘affective theory of mind’ is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005b;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Goldsher D, Berger BD, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. J Clin Exp Neuropsychol. 2004;26:1113–27. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Firth CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Gibbons ZC, Blackshaw A, Doubleday E, Thompson J, Craufurd D, et al. Social cognition in frontotemporal dementia and Huntington’s disease. Neuropsychologia. 2003;41:688–701. doi: 10.1016/s0028-3932(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Spikman JM, Timmerman ME, Milders MV, Veenstra WS, van der Naalt J. Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J Neurotrauma. 2012;29:101–11. doi: 10.1089/neu.2011.2084. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder A, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia. 2003;41:209–20. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Anderson V. The frontal lobes and theory of mind: developmental concepts from adult focal lesion research. Brain Cogn. 2004;55:69–83. doi: 10.1016/S0278-2626(03)00271-9. [DOI] [PubMed] [Google Scholar]
- Trinkler I, Cleret de Langavant L, Bachoud-Lévi AC. Joint recognition-expression impairment of facial emotions in Huntington’s disease despite intact understanding of feelings. Cortex. 2013;49:549–58. doi: 10.1016/j.cortex.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–45. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79:2119–48. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, et al. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132:604–16. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–5. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]