Abstract
Oxidative stress is a common hallmark of neuronal cell death associated with neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, as well as brain stroke/ischemia and traumatic brain injury. Increased accumulation of reactive species of both oxygen (ROS) and nitrogen (RNS) has been implicated in mitochondrial dysfunction, energy impairment, alterations in metal homeostasis and accumulation of aggregated proteins observed in neurodegenerative disorders, which lead to the activation/modulation of cell death mechanisms that include apoptotic, necrotic and autophagic pathways. Thus, the design of novel antioxidant strategies to selectively target oxidative stress and redox imbalance might represent important therapeutic approaches against neurological disorders. This work reviews the evidence demonstrating the ability of genetically encoded antioxidant systems to selectively counteract neuronal cell loss in neurodegenerative diseases and ischemic brain damage. Because gene therapy approaches to treat inherited and acquired disorders offer many unique advantages over conventional therapeutic approaches, we discussed basic research/clinical evidence and the potential of virus-mediated gene delivery techniques for antioxidant gene therapy.
Keywords: Antioxidant gene therapy, Brain ischemia, Neurodegenerative disorders, Oxidative stress, Reactive oxygen species, Reactive nitrogen species, Virus-mediated gene delivery
1. Introduction
Oxidative stress is a cellular condition induced by the de-regulated production of reactive species of oxygen (ROS) and nitrogen (RNS), which are highly reactive molecules generated by several biochemical and physiological processes of cellular metabolism under both normal and pathological conditions. The delicate balance between the production and elimination of ROS/RNS (redox homeostasis) determines the normal function of cells. However, when cells are unable to maintain redox homeostasis via the detoxification of these reactive species produced and/or repair the damage produced, oxidative stress prevails. During oxidative stress, many cellular functions are disturbed by the reaction of reactive species with cellular components such as amino acids, carbohydrates, DNA, RNA, lipids and proteins. ROS are produced upon incomplete reduction of oxygen (O2) by action of housekeeping enzymes and/or formed during the exposure to X-ray, γ or UV irradiation. RNS are generated under normal and pathological conditions by catalytic and non-catalytic reactions (Cooke et al., 2003; Olivares-Corichi et al., 2005; Stadtman et al., 2003; Tanaka et al., 2007; Yin et al., 2009).
Oxidative stress contributes to the etiology of metabolic disorders (Shibata et al., 2010) and neurodegenerative diseases (Patten et al., 2010), and it has also been established to have an important role in the acceleration of pre-existing conditions such as cell invasiveness in cancer (Shinohara et al., 2010). On the other hand, ROS/RNS are essential mediators of cellular processes such as redox signaling, immunological defense mechanism and protein folding. Over the years, the role of ROS and RNS as signaling molecules has been extensively documented. The key issue is the concentration at which these reactive species are present within the cell.
Considering the important role of oxidative stress in neuronal cell death (Franklin, 2011) and the growing knowledge about the protective role that antioxidant systems play, recent efforts have been directed to develop an efficient antioxidant approach to counteract the oxidative stress-induced neuronal cell death that is a hallmark in neurological diseases. Therefore, in this review we will discuss the advances in antioxidant gene therapy for neurodegenerative diseases as well as in brain ischemia and traumatic brain injury.
2. Oxidative stress and generation of ROS/RNS
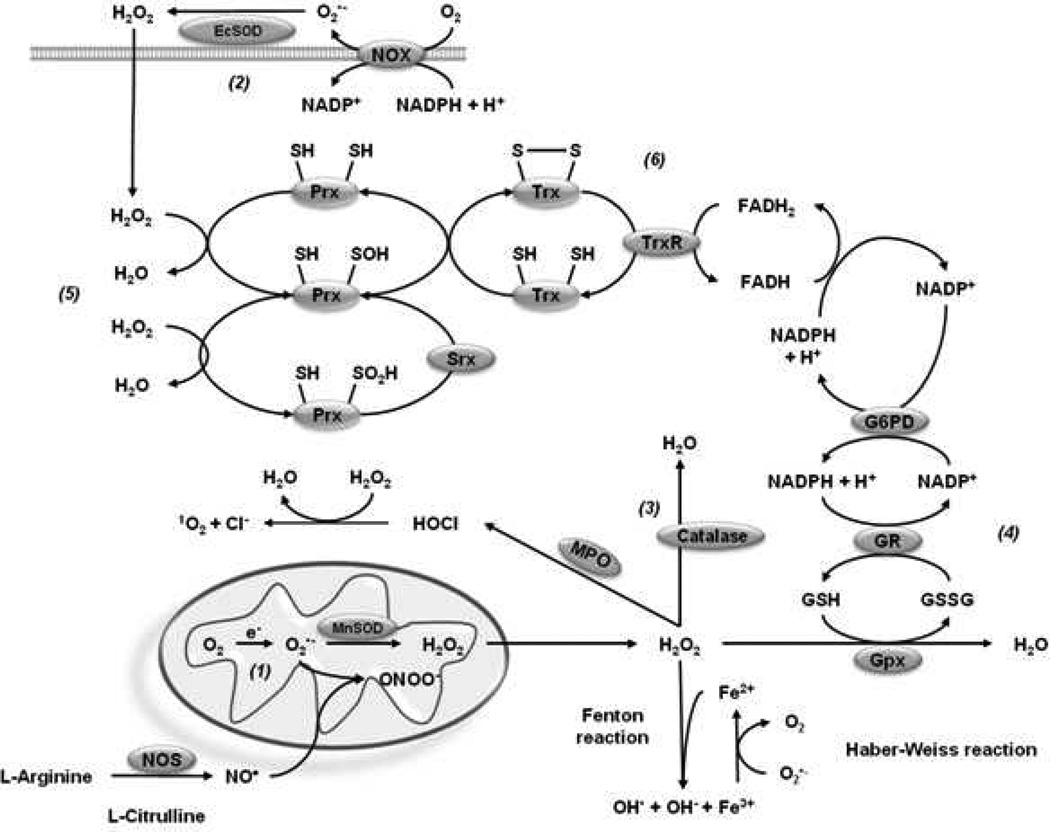
Within the cell, there are several organelles that have the ability to produce ROS such as peroxisomes (Schönfeld et al., 2009), the endoplasmic reticulum (Liu et al., 2004), autophagosomes/lysosomes (Kubota et al., 2010), endosomes (Li et al., 2011b) and the nucleus (Spencer et al., 2011). Notably, it has been amply demonstrated that one of the main sources of ROS is the mitochondria (Murphy, 2009). O2•− is produced by the one-electron reduction of O2 through the complex I (Grivennikova et al., 2006) and complex III (Chen et al., 2003) of the electron transport chain (ETC) and released to the mitochondrial matrix by complex I and to both the mitochondrial matrix and the inner membrane space (IMS) by complex III (Muller et al., 2004). A second important source of ROS production is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family (Nox enzymes). This family of enzymes catalyzes the production of O2•− from O2 and NADPH, and was originally described in polymorphonuclear neutrophils to provide host defense against bacteria via a rapid respiratory burst of O2•−. However, distinct Nox enzymes have also been reported in distinct brain regions (Infanger et al., 2006).
When O2•− suffers natural or enzymatic dismutation, hydrogen peroxide (H2O2) is arisen. The enzymatic generation of H2O2 is catalyzed by O2•− dismutases (SODs). H2O2 is thought to diffuse across membranes. In addition, it has been demonstrated that the diffusion of H2O2 is facilitated by members of the aquaporin family (Bienert et al., 2007; Bienert et al., 2006). H2O2 has a half-life of 1 millisecond, which allows it to react with several molecules or metals to produce the hydroxyl radical (OH•) by Fenton reaction (Christine C, 1995; Nappi et al., 1998; Reth, 2002).
Nitric oxide (NO•) is formed from L-arginine by the enzyme nitric oxide synthase (NOS) and is a small hydrophobic molecule that freely diffuses across membranes (Miersch et al., 2008). NO• has been recognized to act as a paracrine signaling molecule playing an important role as second messenger in processes as diverse as cell survival (Patel et al., 2010), proliferation (Magalhães et al., 2006), apoptosis (Wei et al., 2000) and neuronal differentiation (Ciani et al., 2004). O2•− reacts three times faster with NO• than with MnSOD leading to the production of the most oxidant specie peroxynitrite (ONOO−), which is able to cross membranes through the anion channel in the anionic form (ONOO−) and by passive diffusion in its protonated form, peroxynitrous acid (ONOOH) (Denicola et al., 1998). Three NOS genes have been described, all of which are found in distinct brain regions. Endothelial (eNOS) and neuronal NOS (nNOS) are classically calcium (Ca2+)/calmodulin-dependent and generate nanomolar concentrations of NO•, while inducible NOS (iNOS) can produce micromolar levels of NO• (Brown, 2010).
Myeloperoxidases (MPOs) produce hypochlorous acid (HOCl) from H2O2 and chloride anion (Cl−) using heme as a cofactor. MPOs also oxidize tyrosine to tyrosyl radical using H2O2 as an oxidizing agent. Until recently, phagocytic cells were thought to be the only cellular sources of MPOs. However, recent studies demonstrate that several cell types including neuronal cells, express MPOs under certain pathological conditions (Green et al., 2004; van der Veen et al., 2009). Cyclooxygenases (COXs) are also known to generate ROS as a byproduct of the metabolism of arachidonic acid. COXs metabolize arachidonic to prostaglandin G2 (PGG2) utilizing two O2 molecules and producing peroxyl radicals. COXs also possess a heme-containing active site that provides peroxidase activity, converting PGG2 to prostaglandin H2 (PGH2) by removing O2, which might be a source of oxygen radicals. In the presence of H2O2, the peroxide activity of COXs may oxidize various co-substrates such as NADH and glutathione (GSH), which could reduce O2 to •O2− (Im et al., 2006). Several studies have demonstrated COX-immunoreactivity in neuronal populations from different regions, where COX-2 is found in postsynaptic cell bodies and dendritic spines (Mancuso et al., 2006).
An increasing amount of evidence suggests that oxidative/nitrosative stress is linked to the pathophysiology of multiple human diseases. However, definitive evidence for this association has been controversial because of shortcomings found in methods available to assess oxidative stress in vivo. Measuring oxidative stress can be difficult because the biological half-life of free radicals and other reactive species is too short for direct detection. Therefore, evidence has to rely on indirect measurements. These indirect measurements are based on byproducts of oxidative damage to lipids, proteins and DNA, which provide an extensive array of potential biomarkers (Bast et al., 2013; Blumberg, 2004; Dalle-Donne et al., 2006; Halliwell, 2011). Lipid peroxidation generates mainly α,β-unsaturated reactive aldehydes, such as malondialdehyde (MDA), 4-hydroxy-2-nonenal (HNE), 2-propenal (acrolein) and isoprostanes. (Dalle-Donne et al., 2006; Devasagayam et al., 2003). It is important to mention that lipid hydroperoxides and aldehydes can also be absorbed from the diet, which can confound measurements of MDA and HNE in plasma or urinary samples (Dalle-Donne et al., 2006). The determination of isoprostanes is considered now the best available biomarker of lipid peroxidation, because of their stability (Dalle-Donne et al., 2006; Halliwell, 2011; Halliwell et al., 2004).
Protein damage by oxidative stress has been mostly determined by the formation of carbonyl compounds in vitro and in vivo. Protein carbonyls are chemically stable, which makes them suitable for postmortem samples analysis. However, carbonyls are not specific as markers of oxidative damage because bound aldehydes and glycated protein are also measured. (Dalle-Donne et al., 2006; Luo et al., 2009; Suzuki et al., 2010). Determination of 3-nitroyrosine (3-NT) is also widely used as an index of oxidative protein damage. However, its specificity is debated because nitration can occur independently of peroxynitrite exposure (Dalle-Donne et al., 2006; Duncan, 2003; Halliwell et al., 2004; Tsikas et al., 2005).
Oxidative DNA damage byproducts are also important markers assessed to evaluate oxidative stress in vivo. OH• generates a wide range of base and sugar modifications in DNA. However, the initial products of free radical attack undergo transformation into stable end products, whose abundance depends on reaction conditions and more importantly, none of these modifications can identify the location of the oxidative damage is located. 8-hydroxy-2’-deoxyguanosine (8OHdG) is commonly measured as an index of oxidative DNA damage. However, artifactual oxidative damage to DNA has been shown to occur during isolation, preparation and analysis of samples. To date, there is no agreement on basal levels of 8OHdG in cellular DNA, even when standard extraction procedures have been used. (Collins et al., 2004; Dalle-Donne et al., 2006; Gedik et al., 2005; Halliwell et al., 2004; Kryston et al., 2011).
3. Oxidative stress and neuronal cell death
3.1. Neurodegenerative diseases
Neurodegenerative diseases are defined as hereditary and sporadic conditions that are characterized by progressive nervous system dysfunction. These disorders are often associated with atrophy of the affected central or peripheral structures of the nervous system and include: Alzheimer's Disease (AD) and other dementias, Parkinson's Disease (PD), Huntington's Disease (HD), Multiple Sclerosis, Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig's Disease), Prion diseases and others. Such cell death can be induced by exogenous factors such as neurotoxicants or mutations in key genes. During neuronal cell death, diverse mechanisms are involved. Solid evidence has demonstrated that oxidative stress plays a central role in the initiation of neuronal damage in neurodegeneration (Table 1).
Table 1.
Clinical and experimental (in vivo) findings linking oxidative stress to neurological diseases.
| Disease | Model | Findings | Reference |
|---|---|---|---|
| Alzheimer’s disease (AD) | Human postmortem brain tissues | Increase 3-NT in neurofibrillary tangles (NFTs) of postmortem brain tissues with respect to control cases lacking NFTs, where nitrotyrosine was not detected. | Good et al., 1996 |
| Increased 3-NT in neurons of brain tissues. 3-NT was undetectable in the cerebral cortex of age-matched control brains. The distribution is essentially identical to that of free carbonyls. | Smith et al., 1997 | ||
| Iron has been found accumulated in the hippocampus and cerebral cortex, colocalizing with AD lesions. RNA-bound iron plays a pivotal role in RNA oxidation. The cytoplasm of hippocampal neurons showed significantly higher redox activity and Fe2+ staining than age-matched controls and only AD rRNA contains 8-hydroxyguanosine in reverse transcriptase-PCR. | Su et al., 2008; Honda et al., 2005 | ||
| Elevated levels of labile Cu2+ that correlated with oxidative damage were found in AD brains in postmortem cortical tissue when compared with non-demented elderly controls. | James et al., 2012 | ||
| High Cu2+ concentrations are found within Aβ plaques; Aβ binds Cu2+ in AD tissue, and Aβ: Cu2+ complexes form a catalytic source of H2O2 that could enhance the production of •OH. | Su et al., 2008 | ||
| In vivo models | In vivo imaging using the fluorescent redox sensor roGFP in the APP/PS1 transgenic mice identifed susceptible neurons by their increased redox potential. The oxidative stress was most prevalent in neurites near plaques and propagated to cell bodies. | Xie et al., 2013 | |
| An oxidized redox state (NADPH/FAD), lower NADH regenerating capacity, lower GSH levels, and excessive ROS were found in 3xTg-AD compared with non-Tg neurons. | Ghosh et al., 2012 | ||
| Using triple transgenic AD mice a massive dysregulation of mitochondrial proteins mainly related to complexes I and IV of the oxidative phosphorylation system (OXPHOS) were found. These mitochondrial defects were associated with an increase of O2•−, as well as cytosolic ROS levels. | Rhein et al., 2009 | ||
| Alterations in the GSH/GSSG redox state and an increase of mixed-disulfide (Pr-SSG) were found in both brain tissues and blood samples of a double mutant AD transgenic mouse model. | Kahles et al., 2013 | ||
| Developmental exposure to Pb altered the levels, characteristics, and intracellular distribution of Aβ staining and plaques in the cortex of aged monkeys. These effects were accompanied by higher levels of oxidative damage to DNA. | Wu et al., 2008 | ||
| Parkinson’s Disease (PD) | Human postmortem brain tissues | A significant increase in protein carbonyl levels was found in brain areas associated with PD | Alam et al., 1997a; Floor and Wetzel 1998 |
| An increase in 8-hydroxyguanine (8-OHdG) was found in the SNpc of PD brain. |
Alam et al., 1997b Zhang et al., 1999 |
||
| Increased levels of malondialdehyde (MDA; an intermediate in the lipid peroxidation process) were found in PD SNpc compared with other brain regions and control tissue. | Dexter et al., 1989; Yoritaka et al., 1996 | ||
| Deficiency in the activity of the ETC in the substantia nigra of patients with PD. | Schapira et al., 1989, 2008 | ||
| Extensive and widespread accumulations of nitrated a-synuclein in the inclusions of PD, dementia with Lewy bodies and the Lewy body variant of AD. | Giasson et al., 2000; Hodara et al., 2004 | ||
| Mitochondria of SNpc from PD subjects showed a significant accumulation of α-synuclein, which was associated with impairment in complex I activity and increased oxidative stress. | Devi et al., 2008 | ||
| Postmortem analysis of PD brain tissues showed a considerable increase in total Fe, Zn, and Al content when compared with control tissues, with significantly lower GSH levels. | Uversky et al., 2001; Hirsch et al., 1991; Dexter et al., 1989, 1991; Riederer et al., 1989 | ||
| Parkin is S-nitrosylated in vivo in a mouse model of PD, and in brains of patients with PD and diffuse Lewy body disease. | Chung et al., 2004 | ||
| GSH levels were reduced in the PD SNpc (40% compared to control subjects). | Sian et al., 1994; Jenner et al., 1992; Dexter et al., 1994; Perry et al., 1982 | ||
| In vivo models | Mitochondrial oxidative stress was found increased in dopaminergic neurons within the SNpc compared to those of the ventral tegmental area (VTA), which are much less affected in PD. | Guzman et al., 2010 | |
| The loss of PINK1 impairs mitochondrial fission, which causes defective assembly of the ETC complexes, leading to oxidative stress and abnormal bioenergetics. | Liu et el., 2011; Morais et al., 2009 | ||
| A reduction in respiratory capacity of striatal mitochondria isolated from parkin −/− mice. Parkin −/− mice also exhibited decreased levels of proteins involved in protection from oxidative stress, serum antioxidant capacity and increased protein and lipid peroxidation. | Palacino et al., 2004 | ||
| Huntington’s Disease (HD) | Human postmortem brain tissues | HD patients had higher levels of lipid peroxidation in plasma levels and lower levels of GSH compared to age and sex-matched controls. | Klepac et al., 2007 |
| An Increase in protein-carbonyls was found in human brain postmortem samples obtained from striatum and cortex of patients with HD when compared to samples of age- and sex-matched controls. Carbonylated proteins found included enzymes involved in the glycolytic pathway and mitochondrial proteins related to ATP production. Oxidation resulted in decreased catalytic activity, in agreement with energy deficiency observed in HD. | Sorolla et al., 2008, 2010 | ||
| A signifcant increase in 8-OHdG in mtDNA was found in the parietal cortex of HD patients as compared to controls. | Polidori et al., 1999 | ||
| Serum 8-OHdG levels were markedly elevated in HD. | Hersch et al., 2006 | ||
| Leukocyte 8-OHdG and plasma MDA were elevated, and the activities of Cu/Zn-SOD and Gpx were reduced in 16 HD patients when compared to 36 age- and gender-matched controls. | Chen et al., 2007 | ||
| In vivo models | mtHtt is oxidized in cysteine residues proximal to the N-terminal domain promoting oligomerization and delayed clearance. | Fox et al., 2011 | |
| Increased concentrations of 8-OHdG were found in the urine, plasma and striatal microdialysates of HD mice. Increased concentrations of 8-OHdG were also observed in isolated brain DNA at 12 and 14 weeks of age. Immunocytochemistry showed increased 8-OHdG staining in late stages of the illness. | Bogdanov et al., 2001 | ||
| Amyotrophic Lateral Sclerosis (ALS) | Human Patients samples | Derivatization analysis of oxidized carbonyl compounds performed on immunoprecipitated SOD1 identified a hyper-oxidized SOD1 that recapitulates mutant SOD1-like properties and damages mitochondria by forming a toxic complex with Bcl-2. | Guareschi et al., 2012 |
| The mean protein carbonyl level in the lumbar spinal cord from patients with sporadic motor neuron disease was increased by 119% (p < 0.02) when compared to normal control subjects and by 88% (p < 0.04) compared to the neurological disease control subjects. | Shaw et al., 1995 | ||
| Protein carbonyl and OH8dG levels were increased in sporadic ALS but not in autosomal dominant familial ALS patients. | Ferrante et al., 1997a | ||
| Immunoreactivity for 3-NT was detected in motor neurons of ALS but was not or was only minimally found in those of controls. The staining was also localized in the axons of motor neurons of ALS, but was not found in the corresponding controls. | Abe et al., 1995, 1997; Beal et al., 1997 | ||
| 4-HNE was elevated in the cerebrospinal fluid (CSF) of a patient with sporadic amyotrophic lateral sclerosis (sALS) compared with that of most patients with other neurological diseases | Smith et al., 1998; Simpson et al., 2004 | ||
| 8-OHdG levels were significantly elevated in plasma, urine, and cerebrospinal fluid (CSF) from subjects with ALS when compared to controls. Plasma and urine 8-OHdG levels increased significantly with time in the ALS group only. The rate of increase in urine 8-OHdG levels with time was significantly correlated with disease severity. | Bogdanov et al., 2000; Ihara et al., 2005 | ||
| In vivo models | The deletion of the Nox2, and to a lesser extent Nox1, can prolong survival in the SOD1 G93A transgenic mice. | Li et al., 2011b | |
| A significant increase in the concentrations of 3-NT in upper and the lower spinal cord and in the cerebral cortex of the SOD1 G93A transgenic mice was found. MDA was increased in cerebral cortex. 3-NT and MDA-modified protein immunoreactivities were increased throughout the transgenic mice spinal cord but particularly within motor neurons. | Ferrante et al., 1997b; Casoni et al., 2005 | ||
| Protein carbonyl content in 30-day-old SOD1-G93A mice was twice as high as the level found in age-matched nontransgenic mice. The levels in the SOD1-G93A mice increased dramatically (557%) between 100 and 120 days of age, compared with either the nontransgenic mice or transgenic animals that overexpress the wild-type human SOD1. | Andrus et al., 1998 | ||
| Transgenic mice expressing mutant SOD1-G93A show enhanced free radical content in spinal cord but not brain. This increase precedes motor neuron degeneration | Liu et al., 1998 | ||
| Brain Ischemia and Excitotoxicity | Human Patients | Multiple markers of oxidative damage are increased immediately after stroke and remain elevated for several days in 66 stroke subjects with respect to 132 control subjects. | Seet et al., 2011 |
| In vivo models | Nox inhibition or scavenging reduced brain injury H2O2 in a neonatal rat model of hypoxia/ischemia demonstrating that Nox contributes to oxidative stress mediating cerebral injury during ischemia/reperfusion. | Lu et al., 2012; Kahles et al., 2010; Kleinschnitz et al., 2010 | |
| Hypoxic-ischemic insult was produced in p10 mice. Administration of the complex I inhibitor pyridaben significantly decreased the extent of HI injury. Mitochondria isolated from the ischemic hemisphere in pyridaben-treated animals showed reduced H2O2 formation and decreased oxidative damage to the mitochondrial matrix. A protective effect of pyridaben administration was also observed when the reperfusion-driven oxidative stress was augmented by the exposure to 100% O2. | Niatsetskaya et al., 2012 | ||
| Traumatic Brain Injury (TBI) | In vivo models | Biochemical damage of the cerebral vasculature is initiated by the induction of the free radical-generating enzymes Nox1 and iNOS. Induction of these enzymes by shock-wave exposure increased oxidative and nitrosative damage. | Abdul-Muneer et al., 2013 |
| Levels of 4-HNE and 3-NT were significantly increased at 3 h post-exposure to blast and returned to control levels after 24 h. | Readnower et al., 2010 | ||
| Significant time-dependent changes in antioxidant levels were observed as early as 3 h post-trauma and paralleled increases in oxidants (4-HNEl, acrolein, and protein carbonyls), with peak values obtained at 24–48 h. | Ansari et al., 2008 | ||
| Lipid peroxidation in brain extracts were increased at 6 and 24 h after trauma compared with sham-operated controls. The total antioxidant reserves of brain homogenates and water-soluble antioxidant reserves as well as tissue concentrations of ascorbate, GSH, and protein sulfhydryls were reduced after TBI. | Tyurin et al., 2000 | ||
| Nox activity in the cerebral cortex and hippocampal CA1 region increases rapidly following controlled cortical impact in male mice, with an early peak at 1 h, followed by a secondary peak from 24–96 h after. In situ localization using oxidized hydroethidine and the neuronal marker, NeuN, revealed that the O2•− induction occurred in neurons at 1 h after TBI. | Zhang et al., 2012b | ||
| Selective inhibition of iNOS after trauma protected the injured brain by limiting ONOO− formation. | Gahm et al., 2006 |
3.1.1. Alzheimer’s Disease (AD)
An estimated of 35 million people worldwide have been diagnosed with AD, wich make it the most common neurodegenerative dementia. It is characterized by the impairment of behavioral and cognitive functions leading to death within 3 to 9 years after diagnosis. The principal risk factor for AD is age as its incidence doubles every 5 years after 65 years of age. Among the characteristics found in brains of AD patients are senile plaques, which contain amyloid-β peptide (Aβ) derived from the amyloid precursor protein (APP). Established genetic causes of AD include dominant mutations of genes encoding APP and presenilin 1 (PSEN1) and PSEN2. PSEN1 and PSEN2 mutations affect concentrations of Aβ1–42 because presenilin proteins form part of γ secretases, which cleave APP to produce Aβ. Potential risk genes for AD include apolipoprotein E (ApoE), which affects Aβ aggregation and clearance. Neurofibrillary tangles are also found in postmortem brains of AD patients containing the pathologically aggregated tau protein (Mena et al., 1995). Several post-translational modifications in tau have been reported such as ubiquitination, glycation and phosphorylation, among others. Tau mutations result in tauopathies, such as corticobasal degeneration and frontotemporal dementias, but not AD (Ballard et al., 2011; Holtzman et al., 2011; Querfurth et al., 2010; Selkoe, 2011). Post-mortem human studies demonstrate the accumulation of lipid peroxidation products in multiple brain regions and in cerebrospinal fluid (CSF) of AD subjects. Protein oxidation byproducts such as carbonyls and 3-nitrotyrosine are also found increased in the frontal and parietal lobes and in the hippocampus of AD and subjects with mild cognitive-impairment (MCI). Finally, oxidative modifications in nucleic acid bases are increased in AD and MCI brain samples (Good et al., 1996; Pratico, 2008). Several studies have indicated the presence of oxidative stress markers in the brain of most transgenic AD mouse models, suggesting that oxidative stress is an early event in the AD development that could promote the amyloid cascade by increasing Aβ synthesis and aggregation (reviewed in (Belkacemi et al., 2012).
The amyloid cascade hypothesis suggests that the deposition of Aβ triggers neuronal dysfunction and death in the brain (Figure 1). Using in vivo imaging of a fluorescent indicator of oxidative stress in a transgenic mouse model, Xie et al., (2013) demonstrated that Aβ plaque formation precede and lead to oxidative stress in surrounding neurites, which propagates over time and leads to oxidation and degeneration of neuronal soma. These results implicate Aβ as the mediator of oxidative stress and subsequent neurodegeneration (Xie et al., 2013). It is important to state that amyloid-independent mechanisms have also been proposed to contribute to AD (Karran et al., 2011; Pimplikar et al., 2010). APP is an integral membrane protein expressed in many tissues and concentrated in the synapses of neurons. Its primary function is not known, though it has been implicated as a regulator of synapse formation, neuronal plasticity and iron export. Amyloidogenic processing is initiated by a β-secretase, the beta-site amyloid precursor protein–cleaving enzyme 1 (BACE-1), releasing a shortened sAPP. The C99 fragment is a γ-secretase substrate, generating Aβ and the amyloid precursor protein intracellular domain (AICD). Soluble Aβ is prone to aggregation. In a triple transgenic mouse model of AD (3xTg-AD) with human transgenes APP (SWE [swedish mutation]), PSEN1 (M146V) or PSEN2 (N141I), and Tau (P301L), an impaired oxidative phosphorylation, increased oxidative state and GSH depletion was reported to precede the onset of cognitive defects (Belkacemi et al., 2012; Ghosh et al., 2012; Rhein et al., 2009). Moreover, alterations in the glutathione redox state, an increase of GSSG together with a decrease of GSH/GSSG ratio, and an increase of mixed-disulfide (Pr-SSG) has been reported in brain tissues and blood samples at different disease stages in a double mutant AD transgenic mouse model (B6. Cg-Tg) carrying the APPswe and exon 9 deletion of the PSEN1 gene, suggesting that formation of Pr-SSG may be an early event, preceding amyloid plaque appearance (Zhang et al., 2012a).
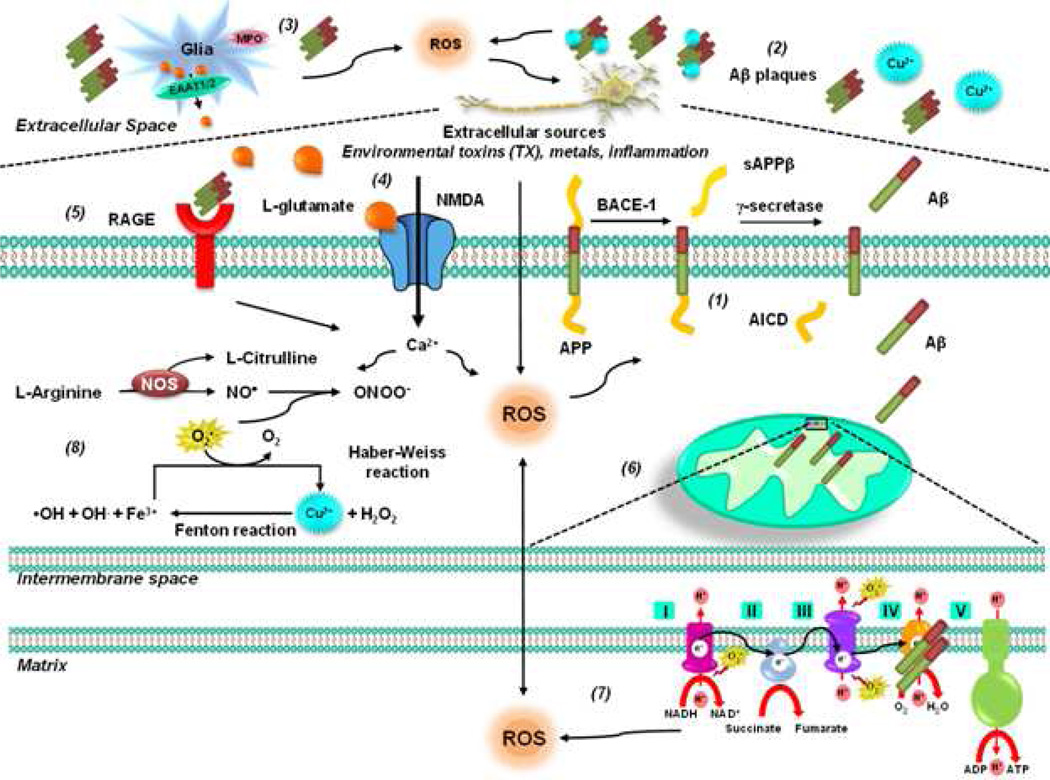
Figure 1. Oxidative stress in AD.
The amyloid cascade hypothesis suggests that deposition of Aβ triggers neuronal dysfunction and death in the brain. APP is an integral membrane protein concentrated in the synapses of neurons. (1) Amyloidogenic processing is initiated by β-secretase beta-site amyloid precursor protein–cleaving enzyme 1 (BACE-1), releasing a shortened sAPP. The C99 fragment is a γ-secretase substrate, generating Aβ and AICD. PSEN1 and PSEN2 mutations affect concentrations of Aβ1–42 because presenilin proteins form part of γ secretase, which cleaves APP to produce Aβ. (2) Soluble Aβ is prone to aggregation. In addition, Aβ interacts with several metal ions affecting its solubility and leading to fibrillization and cellular toxicity. The Aβ-metal complex triggers ROS production. (3) Activation of glial cells has also been proposed to contribute to ROS formation via MPO. (4) In addition, accumulation of extracellular levels of the exicitatory amino acid glutamate leads to perturbations in neuronal Ca2+ homeostasis that have the potential to trigger mitochondrial dysfunction and ROS formation. (5) The RAGE has also been proposed to mediate Aβ's pro-oxidant effects. (6) Aβ has been shown to be transported into mitochondria via the outer membrane (TOM) complex and localized at the mitochondrial cristae. (7) In the mitochondria, Aβ inhibits mitochondrial cytochrome oxidase (complex IV) and the key Krebs-cycle enzymes (α-ketoglutarate and pyruvate dehydrogenase) impairing ETC, ATP production, oxygen consumption, and mitochondrial membrane potential. Dysfunctional mitochondria produce ROS and mtDNA oxidative damage. Conversely, mitochondrial ROS also trigger increased Aβ production. (8) Aβ induces an increase in iNOS expression and NO• ONOO− generation in glial cells, whereas it inhibits the activity of nNOS and eNOS in neuronal-like cells.
Aβ interacts with several metal ions affecting its solubility and leading to fibrillization and cellular toxicity (Benilova et al., 2012; O'Brien et al., 2011). Elevated levels of labile copper (Cu+2) that correlated with oxidative stress were found in AD brains (James et al., 2012). The morphology of Aβ aggregates is modified in a concentration-dependent manner by metal ions as iron (Fe+2), Cu+2 and zinc (Zn+2). Once Aβ is bound to metal ions such as Fe+2 and Cu+2, the Aβ-metal complexes can mediate the production of H2O2 and OH• (Su et al., 2008). It has been suggested that AD pathogenesis may be influenced by early life exposures to toxic metals such as lead, which are related to oxidative damage as well (Wu et al., 2008a; Wu et al., 2008b).
Mitochondrial dysfunction is a hallmark observed in AD and has been related to the accumulation of Aβ within mitochondria. The Aβ is transported into mitochondria via the translocase of the outer membrane (TOM) complex and localized at the mitochondrial cristae (Hansson Petersen CA, 2008). Aβ inhibits mitochondrial enzymes in the brain and in isolated mitochondria such as cytochrome oxidase (complex IV) and the key Krebs-cycle enzymes (α-ketoglutarate and pyruvate dehydrogenase) impairing ETC, ATP production, oxygen consumption, and mitochondrial membrane potential. Dysfunctional mitochondria produce ROS and as a consequence, mtDNA oxidative damage. Conversely, current evidence shows that mitochondria-derived ROS are sufficient to trigger increased Aβ production both in vitro and in vivo, and thereby initiate a vicious cycle further impairing mitochondrial function (Leuner et al., 2012). In the mitochondria, Aβ also interacts with the 17-β-hydroxysteroid dehydrogenase X (HSD17B10) also known as Aβ peptide-binding alcohol dehydrogenase (ABAD), an enzyme that catalyzes the oxidation of a wide variety of fatty acids, alcohols, and steroids, exacerbating neuronal cell death by mitochondrial dysfunction and oxidative stress (Yao et al., 2011).
The receptor for advanced glycation end products (RAGE) has also been proposed to mediate Aβ's pro-oxidant effects (Yan et al., 1996). In addition, perturbations in neuronal Ca2+ homeostasis induced by Aβ have the potential to trigger mitochondrial dysfunction and ROS formation (Bezprozvanny et al., 2008). Aβ has a critical methionine residue at position 35, which is thought to be associated with Aβ peptide toxicity. Oxidation of Met35 produces methionine sulfoxide or methionine sulfone via irreversible oxidation. However, contradictory reports exist regarding the role of Met35 residue in Aβ toxicity and oxidative stress (Butterfield et al., 2010; Butterfield et al., 2007; Maiti et al., 2010). MPOs have been found increased in AD brains (Green et al., 2004), and the overexpression of the MPO-463G allele in astrocytes increases lipid peroxidation in a transgenic mouse model of AD (Maki et al., 2009). COX-2 inhibition is reported to improve the memory and synaptic plasticity in AD models (Jang et al., 2005; Kotilinek et al., 2008).
Increased levels of nitrated proteins are found in AD brains (Smith et al., 1997). Aβ induces an increase in iNOS expression and NO•/ONOO− generation in glial cells (Akama et al., 1998; Akama et al., 2000; Combs et al., 2001; Xie et al., 2002). However, contradictory results exist regarding the effect of iNOS knockout in AD transgenic mouse models (Colton et al., 2006; Wilcock et al., 2008). Similarly, microglial cells from ApoE epsilon 4 allele (ApoE4), a risk factor in AD, produce higher levels of NO• (Brown et al., 2002; Colton et al., 2002; Ramassamy et al., 1999). In contrast, Aβ inhibits the activity of nNOS and eNOS in neuronal-like cells (Venturini et al., 2002).
3.1.2. Parkinson’s Disease (PD)
PD is a neurodegenerative disorder characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta (SNpc). The accumulation of α-synuclein is a crucial step in the pathogenesis of PD and a key constituent of intraneuronal proteinaceous inclusions known as Lewy bodies. There is no current treatment to stop neuronal cell death and/or to cure PD. Current evidence supports a role for mitochondrial dysfunction, oxidative stress, and abnormal protein accumulation as early triggers of neuronal death in PD pathogenesis. Existing therapies available only delay the onset of PD and/or ameliorate motor symptoms by addressing dopamine deficit (Levy et al., 2009; Yao et al., 2009). A fraction of PD occurrence is related to mutations in genes such as those of α-synuclein (SNCA), DJ-1 (PARK7), PTEN-induced putative kinase 1 (PINK1), leucine rich repeat kinase 2 (LRRK2) and parkin (PARK2). However, 90% of PD cases occur in a sporadic (idiopathic) form without a defined genetic basis. The major risk factor identified for PD is aging as its occurrence increases exponentially from ages 65 to 90. Epidemiological evidence shows an increase in the risk of developing PD upon the exposure to environmental toxicants such as pesticides, metals, polychlorinated biphenyls, as well as early life inflammatory processes (Gao et al., 2011a). Thus, it is now being considered that PD arises from the convergence of genetic susceptibility, environmental exposures, and aging.
Oxidative damage to lipids, proteins and DNA is found in post-mortem PD brains (Alam et al., 1997a; Alam et al., 1997b; Dexter et al., 1991; Dexter et al., 1989; Dexter et al., 1994; Floor et al., 1998; Hirsch et al., 1991; Jenner et al., 1992; Perry et al., 1982; Riederer et al., 1989; Sian et al., 1994; Yoritaka et al., 1996; Zhang et al., 1999). SNpc dopaminergic cells have high levels of basal oxidative stress compared to other dopaminergic neuronal populations (Guzman et al., 2010). A decrease in the activity of the mitochondrial ETC is found in the SNpc of patients with PD (Henchcliffe et al., 2008; Schapira, 2008; Schapira et al., 1989) suggesting that oxidative stress is linked to mitochondrial dysfunction (Figure 2). Environmental pesticides such as paraquat and rotenone have been proposed to mediate mitochondrial ROS generation via complex I and III (Castello et al., 2007; Drechsel et al., 2009; Sherer et al., 2003; Sherer et al., 2007).
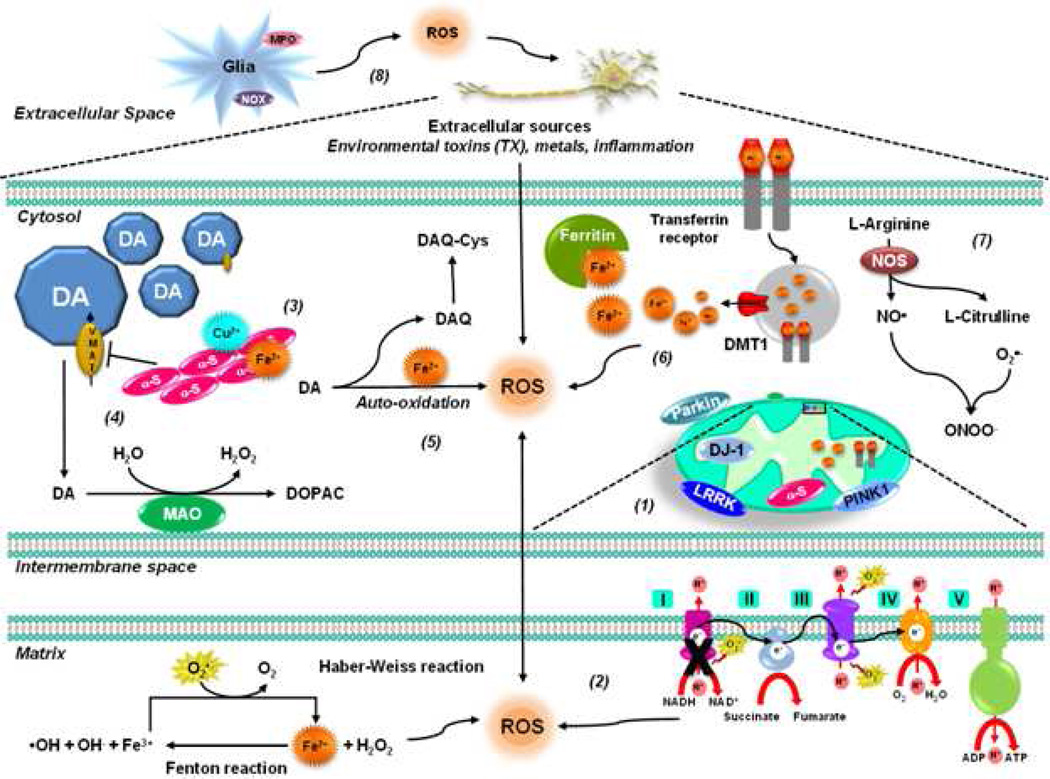
Figure 2. Oxidative stress in PD.
(1) A fraction of PD cases are related to mutations in genes such as those encoding α-synuclein, DJ-1, PINK1, LRRK2 and parkin. Most of these gene products have been found to interact with mitochondrial components to different extent and under pathological conditions, impair its function leading to ROS formation (see text for further details). (2) Oxidative stress in PD is linked primarily to mitochondrial dysfunction. Decreased activity of mitochondrial complex I is found in the SNpc of PD patients. (3) Metal ions accelerate α-synuclein oligomerization / aggregation, Cu2+ being the one that induces it faster. (4) Mutant α-synuclein and environmental toxins impair VMAT2 augmenting cytosolic dopamine, which dopamine is metabolized by MAO or auto-oxidized generating ROS and DAQ. (5) DAQs are highly reactive products which can inactivate proteins by reacting with protein thiols. (6) Increased iron deposition and free iron concentration have been found in the SNpc of PD brains, which leads to increased generation of •OH via Fenton and Haber-Weiss reactions. Low levels of ferritin in PD patients have been reported. Alterations in DMT1 function and transferrin receptors have also been proposed to contribute to PD. (7) RNS also play an important role in PD. Parkinsonian mimetics have been shown to mediate NO• and ONOO− generation as well as nitration of α-synuclein that promotes its oligomerization and aggregation. (8) Environmental toxins have also been shown to induce oxidative damage through Nox and MPO activation in glial cells.
PD-associated genes have also been shown to modulate or induce oxidative stress in dopaminergic cells. α-Synuclein is a 140 amino acid brain protein mainly localized in pre-synaptic terminals, and gene multiplications or point mutations are associated with familial PD. Oxidative stress promotes α-synuclein oligomerization and aggregation (Giasson et al., 2000; Meloni et al., 2011). Accumulation of α-synuclein has also been suggested to trigger mitochondrial oxidative stress (Devi et al., 2008) and alterations in Ca2+ permeability (Furukawa et al., 2006; Goldberg et al., 2012). Extracellular α-synuclein also triggers microglia activation and Nox-mediated ROS formation (Zhang et al., 2005), which in turn increase α-synuclein aggregation (Cristovao et al., 2012). It has been proposed that α-synuclein exerts protective effects (Hashimoto et al., 2002), while the A53P mutant α-synuclein sensitizes cells upon oxidative damage (Ko et al., 2000). DJ-1 oxidation induces its mitochondrial translocation (Canet-Aviles et al., 2004), and DJ-1 knockdown or mutants render cells more susceptible to parkinsonian toxins and oxidative stress (Kim et al., 2005; Taira et al., 2004). In addition, DJ-1 has been reported to regulate GSH levels (Liu et al., 2008). Interestingly, DJ-1 seems to be primarily expressed in astrocytes (Bandopadhyay et al., 2004). PINK1 protein is a serine/threonine kinase localized in the mitochondria and the cytosol. Loss of PINK1 has been shown to induce mitochondrial dysfunction, oxidative stress and mitochondrial turnover via mitophagy (Dagda et al., 2009; Gandhi et al., 2009; Gegg et al., 2009; Heeman et al., 2011; Liu et al., 2011; Morais et al., 2009). Parkin deficiency induces mitochondrial dysfunction and oxidative stress (Palacino et al., 2004), as well as an increase in monoamine oxidase (MAO) expression (Jiang et al., 2012). On the other hand, Parkin protects cells against dopamine toxicity (Jiang et al., 2004). LRKK2 also protects against oxidative stress (Liou et al., 2008). However, gain-of-function of LRRK2 mutations disturb mitochondrial dynamics leading to increased ROS formation (Niu et al., 2012).
Metal-induced ROS generation has been postulated to contribute to oxidative damage in PD. However, controversy still exists regarding the occurrence of alterations in the levels of Fe+2 or Cu+2 in PD brains (Mariani et al., 2012). Metal ions accelerate the oligomerization/aggregation of α-synuclein, being Cu+2 the one that induces it faster (Paik et al., 1999; Rasia et al., 2005; Rose et al., 2011; Uversky et al., 2001; Wang et al., 2010b; Wright et al., 2009). α-synuclein-Cu2+ complexes have been shown to induce ROS accumulation and dopamine oxidation (Meloni et al., 2011; Wang et al., 2010a). The accumulation of Mn+2 in basal ganglia structures is associated with the neurodegenerative disorder commonly referred to as manganism, a condition that shares many similarities with PD, which is also linked to increased oxidative damage (Milatovic et al., 2011). However, Mn+2-induced parkinsonism does not seem to involve degeneration of the nigrostriatal dopaminergic system (Guilarte, 2010).
In the brain, Fe+2 is most abundant in areas rich in dopaminergic neurons. Increased Fe+2 deposition and increased free Fe+2 concentrations have been found in the SNpc of PD brains, which may lead to increased generation of •OH via Fenton and Haber-Weiss reactions (Sian-Hulsmann et al., 2011). Autopsy studies found an increased total Fe+2 content in the SNpc of PD patients compared to age-matched controls but other studies did not confirm these findings. Fe+2 content in the SNpc seems to be primarily affected in advanced PD patients suggesting that it is not involved in early disease development. Alterations in Fe+2 distribution are also found in PD. Contrary to normal subjects, where more Fe+2 is deposited in the SN pars reticulata, in PD Fe+2 is deposited abundantly in the SNpc containing pigmented neurons (Sian-Hulsmann et al., 2011). Together with paraquat, Fe+2 enhances dopaminergic neurodegeneration (Peng et al., 2007). Low levels of ferritin in the SNpc of PD patients and incidental Lewy body disease cases were reported. Overexpression of ferritin decreases oxidative stress and neuronal cell death induced by parkinsonian mimetics (Kaur et al., 2010; Lee et al., 2010; Shi et al., 2010). Neuromelanin pigmented neurons in SNpc are preferentially lost in PD, and Fe+2 accumulation in the SNpc is deposited within neuromelanin granules. A specific haplotype of the divalent metal transporter 1 (DMT1/SLC11A2) gene was found to occur at greater frequencies in PD patients suggesting that alterations in DMT1 function may contribute to PD (Dusek et al., 2012), whereas 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic cell death was associated with an increase in DMT1 and transferrin receptor levels (Kalivendi et al., 2003; Salazar et al., 2008). The levels of Fe+2 “handling” proteins such as ceruloplasmin have also been reported altered in PD (Hochstrasser et al., 2005; Jin et al., 2011). In the presence of Fe+2, α-synuclein induces OH• formation (Turnbull et al., 2001), whereas in other studies, oxidative damage induced by Fe+2 was exacerbated primarily by mutant, but not wild type (WT) α-synuclein (Chew et al., 2011; Martin et al., 2003; Ostrerova-Golts et al., 2000).
Oxidative stress in PD is also associated with the pro-oxidant properties of dopamine. Mutant α-synuclein down-regulates the vesicular monoamine transporter (VMAT2) augmenting the cytosolic dopamine levels (Lotharius et al., 2002a; Lotharius et al., 2002b), which is either metabolized by monoamineoxidase to generate H2O2, or auto-oxidized in the presence of Fe+2 generating O2•−, H2O2 and dopamine-quinone species (DAQ) (Abou-Sleiman et al., 2006). α-synuclein also interacts with the dopamine transporter (DAT), although its effects in dopamine uptake and toxicity are unclear (Lee et al., 2001; Wersinger et al., 2003). Furthermore, dopaminergic cell death induced by rotenone and paraquat has been proposed to depend on dopamine oxidation (Kang et al., 2009; Liu et al., 2005). Plasma membrane NADPH- oxidases, mainly Nox1, also mediate ROS generation in dopaminergic and microglial cells upon MPP+, rotenone or paraquat treatment (Cristovao et al., 2009; Gao et al., 2003; Gao et al., 2011b; Goldberg et al., 2012; Wu et al., 2003; Wu et al., 2005). MPOs and COX-2 have also been shown to participate in MPTP-induced oxidative stress and neurotoxicity (Choi et al., 2005; Teismann et al., 2003; Wang et al., 2005).
RNS also play an important role in oxidative stress. Both NO• and ONOO− have been shown to participate in 6-OHDA, MPP+/MPTP and rotenone toxicity, which are prevented by inhibition/knockout of NO• synthases (Ara et al., 1998; Broom et al., 2011; Dehmer et al., 2000; Hantraye et al., 1996; He et al., 2003; LaVoie et al., 1999; Liberatore et al., 1999; Park et al., 2002; Przedborski et al., 1996; Schulz et al., 1995; Watabe et al., 2008). Tyrosine nitration promotes α-synuclein oligomerization and aggregation (Giasson et al., 2000; Hodara et al., 2004; Paxinou et al., 2001; Souza et al., 2000), and exposure to parkinsonian mimetics has been shown to mediate α-synuclein nitration (Przedborski et al., 2001). Interestingly, transgenic mice overexpressing α-synuclein Y39C mutant variant show age-dependent progressive neuronal degeneration (Zhou et al., 2008). Inhibition of Parkin’s ubiquitin E3 ligase activity by S-nitros(yl)ation has also been found in PD brains, (Chung et al., 2004).
3.1.3. Huntington’s Disease (HD)
HD is a genetic autosomal dominant neurodegenerative disorder caused by highly polymorphic CAG trinucleotide repeat expansion in the exon-1 of the huntingtin (Htt) gene (IT15), yielding proteins containing polyglutamine repeats that become misfolded aggregates and resist degradation. Alleles of Htt harboring between 36–40 CAGs may or may not develop HD symptoms. However, individuals with alleles containing more than 40 CAG repeats will develop symptoms. HD neuropathological changes are predominantly detected in the striatum, although marked alterations are also observed in other areas of the brain such as cerebellar cortex, thalamus and cerebellum (Finkbeiner, 2011; Oliveira, 2010; Zuccato et al., 2010). Several biomarkers of oxidative stress including lipid peroxidation, nucleic acid and protein oxidation byproducts, are increased in HD brains (Browne et al., 2006; Browne et al., 1997; Chen et al., 2007; Hersch et al., 2006; Klepac et al., 2007; Polidori et al., 1999; Sorolla et al., 2008; Sorolla et al., 2010). However, there are some studies that have failed to detect any sign of oxidative damage in HD brains (Alam et al., 2000). Despite its ubiquitous expression, mutant HTT (mtHtt) selectively affects medium spiny striatal neurons, and oxidative stress together with mitochondrial dysfunction have been implicated in the pathology of HD (Bano et al., 2011). Oxidative DNA damage has been reported in the caudate, parietal cortex, and peripherally in the serum and leukocytes of patients diagnosed with HD (Long et al., 2012; Weir et al., 2011).
Transgenic mice expressing exon 1 of the human Htt gene with an expanded CAG repeat develop a progressive neurologic disorder and present increased levels of oxidative DNA-damage (Bogdanov et al., 2001). Striatal cells expressing mtHtt show higher basal levels of mitochondrial-generated ROS and mitochondrial DNA damage (Siddiqui et al., 2012). Impaired respiratory chain complexes and tricarboxylic acid cycle enzyme activities have also been found in the brain of HD patients (Costa et al., 2012; Mochel et al., 2011; Shirendeb et al., 2011). Succinate dehydrogenase (SDH) or complex II subunits Fp (FAD) and Ip (iron–sulphur cluster) are found reduced in HD brains and in striatal neurons overexpressing the N-terminal fragment of mtHtt (Figure 3). Accordingly, the administration of inhibitors such as malonate and 3-nitropropionic acid (3-NP) induces both the biochemical and clinical alterations in vivo that resemble those in HD. The iron–sulphur containing dehydratase, aconitase, is one of the most affected tricarboxylic acid cycle enzymes in HD (Costa et al., 2012). 3-NP toxicity is enhanced by dopamine (Benchoua et al., 2008; Charvin et al., 2005). In addition, 3-NP induces a decrease in ATP levels and the activation of N-methyl-d-aspartate (NMDA) receptors, which mediate ROS formation (Liot et al., 2009). Although mitochondrial dysfunction has been largely proposed as a major mechanism for ROS formation, a recent report demonstrates that aggregation of a polyglutamine Htt fragment directly causes ROS formation (Hands et al., 2011). mtHtt itself is oxidized in cysteine residues proximal to the N-terminal domain promoting oligomerization and delayed clearance (Fox et al., 2011). Interestingly, antioxidants seem to exert a deleterious effect in polyglutamine models by inhibition of autophagy (Underwood et al., 2010).
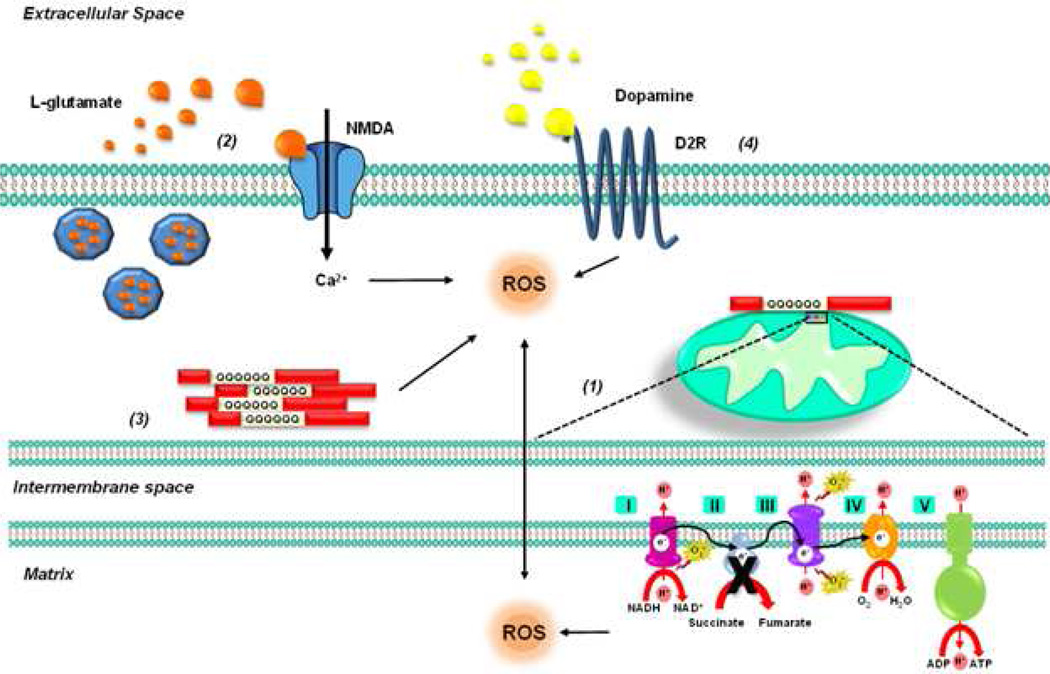
Figure 3.
HD is caused by highly polymorphic CAG trinucleotide repeat expansions in the exon-1 of the Htt gene, yielding proteins containing polyglutamine repeats that become misfolded aggregates and resist degradation. (1) Levels of SDH or complex II subunits Fp (FAD) and Ip (iron–sulphur cluster) are found reduced in HD brains and in striatal neurons overexpressing the N-terminal fragment of mtHtt. Accordingly, complex II inhibitors such as malonate and 3-NP induce both biochemical and clinical alterations in vivo that resemble those in HD. (2) 3-NP induces a decrease in ATP levels, a reduction in the uptake of the excitatory amino acid glutamate and the activation of NMDA receptors, which mediate ROS formation. (3) A recent report demonstrates that aggregation of polyglutamine Htt fragments directly causes ROS formation. (4) 3-NP toxicity is enhanced by dopamine, which mediates and exacerbates ROS production.
3.1.4. Amyotrophic Lateral Sclerosis (ALS)
ALS is characterized by the progressive degeneration of motor neurons in the motor cortex and lower motor neurons connecting the spinal cord and brain stem to muscle fibers. ALS typically develops between 50 and 60 years of age as a relentless progressive neuromuscular failure leading to muscle denervation and atrophy. Only ~10% of ALS cases have a clear inherited genetic component, while the majority of ALS cases are sporadic with no family history of disease, and the gene-environmental factors involved remain poorly defined. Approximately 10–20% of familial ALS cases are caused by a variety of dominant mutations in the copper-zinc SOD (CuZnSOD) gene (SOD1). Over 110 mutations in 153 amino acids spread throughout all five exons as well as a small number in untranslated regions of SOD1 have been described to date. Other mutations leading to ALS include genes such as the “fused in sarcoma/translated in liposarcoma” (FUS/TLS), the TAR DNA-binding protein (TDP-43), the charged multivesicular body protein 2B (CHMP2B), the vesicle-associated membrane protein (synaptobrevin-associated protein) B (VAPB), and angiogenin. Increased oxidative stress biomarkers are found in ALS postmortem tissues, such as brain and spinal cord as well as in cerebrospinal fluid (Abe et al., 1997; Beal et al., 1997; Bogdanov et al., 2000; Ferrante et al., 1997a; Ihara et al., 2005; Shaw et al., 1995{Abe, 1995 #431; Shibata et al., 2001; Simpson et al., 2004; Smith et al., 1998), and in vivo models (Andrus et al., 1998; Casoni et al., 2005; Ferrante et al., 1997b; Liu et al., 1998; Poon et al., 2005). In fact, recent studies suggest that SOD1 could be pathogenic in sporadic ALS through non-heritable modifications, such as posttranslational modifications including hyperoxidation of wild type SOD1. Hyperoxidized SOD1 recapitulates mutant SOD1-like properties (Guareschi et al., 2012). However, it is now widely accepted that loss of SOD1 dismutase activity is not sufficient to cause ALS and that mutant SOD1 promotes release of toxic factors. This is demonstrated by two specific findings: 1) SOD1 knockout does not develop ALS, and 2) some ALS related mutations such as G75R and G93R do not alter dismutase activity (Barber et al., 2010).
SOD1 mutants induce Nox-dependent ROS production in microglia and neuronal death. More specifically both Nox2 and Nox1 knockdown increase survival in SOD1 mutant transgenic mice (Li et al., 2011b). Dysregulation of proteins involved in Fe+2 influx and sensing of intracellular Fe+2, with a concomitant accumulation of Fe+2 iron in ventral motor neurons and increased mitochondrial Fe+2 load in neurons and glial cells, have been shown in mouse overexpressing human SOD1 (G37R) mutant. Similarly, aberrant coordination of Cu2+ by mutant SOD1 has also been demonstrated to mediate oxidative stress (Kishigami et al., 2010).
3.2. Brain Ischemia and Excitotoxicity
It is estimated that every year 15 million people suffer from an acute cerebrovascular stroke, taking the life of 5.5 million and accounting for 5 million permanently disabled patients. Ischemic stroke is characterized by a significant reduction in regional cerebral blood flow causing deprivation of O2 and glucose resulting in brain damage. Focal ischemia is a reduction in the blood flow to a very specific brain region (for example, middle cerebral artery embolic occlusion is a frequently used experimental model), whereas global ischemia occurs when cerebral blood flow (CBF) is reduced throughout most parts of the brain (like it takes place in cardiac arrest). There are significant differences in the mode of cell death between global and focal cerebral ischemia. Brief periods of global cerebral ischemia cause delayed neuronal death with distinct morphological features characteristic of apoptosis, though the apoptotic machinery might contribute significantly to cell death progression. In focal cerebral ischemia, most of cells in the ischemic core undergo necrosis, while cell death within the ischemic penumbra region is considered to be largely dependent on the activation of apoptotic signaling (Chen et al., 2011; Nakka et al., 2008; Niizuma et al., 2009).
During reperfusion, when O2 is replenished, pro-oxidant enzymes and mitochondria utilize O2 as a substrate to generate ROS (Figure 4). Multiple markers of oxidative damage are increased immediately after ischemic stroke, and remain elevated for several days (Seet et al., 2011). Mitochondria have been reported to act as major sources of ROS in ischemia and the inhibition of the mitochondrial complex I inhibits both hypoxic- (ischemic) and reperfusion-mediated oxidative damage (Niatsetskaya et al., 2012). Current data provide evidence suggesting Nox2 as the most important NADPH oxidase mediating cerebral injury (Kahles et al., 2013; Lu et al., 2012). Nox2 has also been shown to contribute to ischemic/reperfusion injury in hippocampal cells in vitro, and neuronal cell death and microglial activation in vivo (Chen et al., 2009a; Kahles et al., 2013). Nox1 contributes to ischemic injury in mice (Kahles et al., 2010). However, a comparative study between Nox1, 2 and 4 demonstrated that Nox4 is the major contributor to oxidative damage and neuronal cell death after both transient and permanent ischemia (Kleinschnitz et al., 2010). Interestingly, neither the knockout of Nox2 nor the inhibition of Nox activity prevents perinatal brain injury in newborn mice (Doverhag et al., 2008).
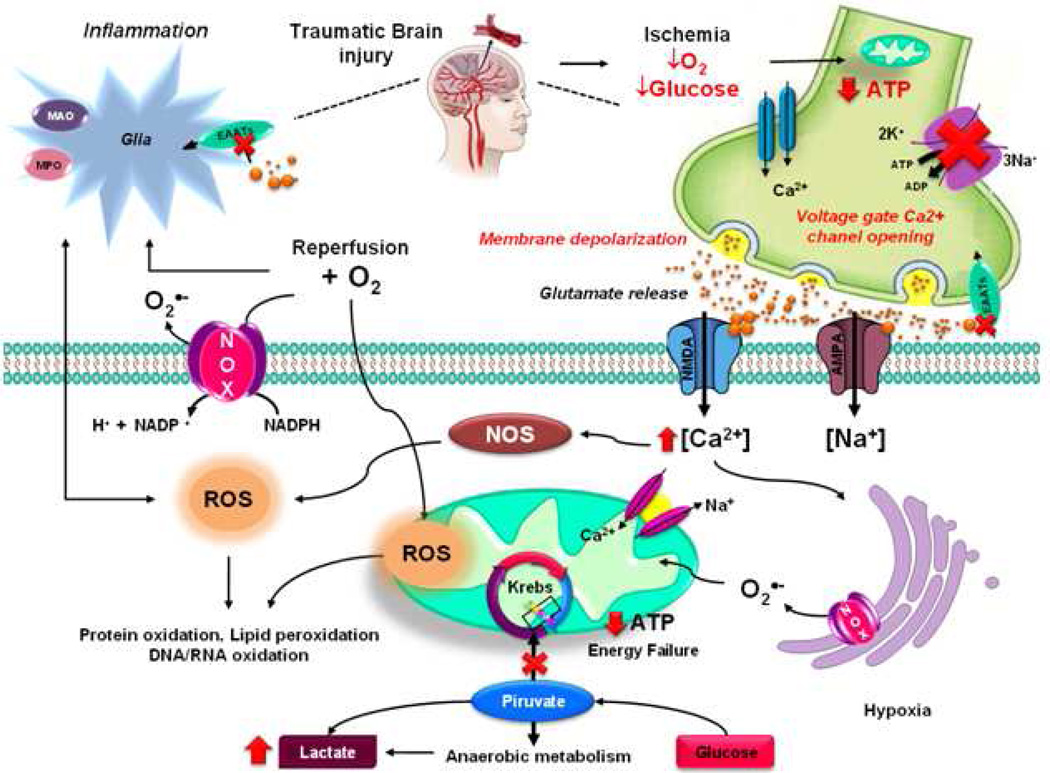
Figure 4. Oxidative stress during Brain Ischemia, Excitotoxicity and Traumatic Brain Injury (TBI).
TBI produces a direct tissue damage and impaired regulation of cerebral blood flow (CBF) causing deprivation of O2 and glucose resulting in brain damage by Ischemia. Ischemic injury is mediated by depletion in energy stores impairing ATP-dependent processes. Malfunction of the Na+/K+-ATPase leads to the disruption of ionic gradients of K+, Na+, Cl−, and Ca+2. This leads to plasma membrane depolarization, reversal of excitatory amino acid uptake and Ca+2-dependent exocytosis. The presynaptic cell releases excitatory amino acid (glutamate) that activates NMDA and AMPA receptors, which permit Ca+2 entry/overload in the postsynaptic cell, which is partially buffered by mitochondrial 2Na+/Ca2+ exchanger. Mitochondrial Ca+2 overload leads to a loss of mitochondrial membrane potential (ΔΨm) and an increase in ROS generation. Energy failure caused by mitochondrial dysfunction leads to a metabolic switch towards anaerobic metabolism leading to increased lactate production. The elevated cytosolic Ca+2 levels also activate nNOS and increases •NO. During reperfusion, when O2 is replenished, pro-oxidant enzymes, such as Nox, and mitochondria utilize O2 as a substrate to generate additional ROS. Inflammation also contributes to oxidative damage and release of excitatory amino acids.
Ischemia/reperfusion injury depletes energy stores impairing ATP-dependent processes. Malfunction of the Na+/K+-ATPase leads to disruption of ionic gradients of potassium (K+), sodium (Na+), chloride (Cl−), and calcium (Ca+2). These alterations in ionic homeostasis induce plasma membrane depolarization, Ca+2-dependent exocytosis, and reversal of excitatory amino acid uptake. The release of excitatory amino acids (glutamate) and the activation of ionotropic NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors promote Ca+2 overload and cell death progression by a variety of signaling mechanisms involving mitochondrial dysfunction (Figure 4). ROS formation upon ischemic conditions (O2-glucose deprivation) has been shown to be specific for neuronal populations in CA1 region of the hippocampus, and this was associated with NMDA receptor and NOS activation (Fekete et al., 2008). In cortical neurons, glutamate excitotoxicity and ROS formation are also mediated by Nox4 (Ha et al., 2010).
3.3. Traumatic Brain Injury (TBI)
TBI is a pathological condition resulting from occupational activity (sports or accidents) in civil population and is the leading cause of death and disability for people under the age of 45. The annual burden associated with TBI is estimated at over $60 billion dollars. In the United States, it has been estimated that more than 1.7 million individuals annually suffer a TBI event that results in the development of complex neurological deficits caused by both primary and secondary injuries. Primary injury events encompass the mechanical damage that occurs at the time of trauma as a result of shearing, tearing or stretching, while the secondary injury develops progressively as a result from chronic biochemical, metabolic and cellular effects. Secondary injury is associated with progressive development of a number of neurological deficits. Brain traumatic insults are classified as: 1) focal, caused by direct impact with solid object with subsequent pathologies developing locally in the vicinity of the site of impact, or 2) diffuse, caused by forces acting inside the entire volume of brain parenchyma and resulting from head acceleration-deceleration. An emerging field of research are military brain injuries, in particular blast-induced TBI, whose pathobiology has characteristics not seen in other types of TBI (Cernak et al., 2010; Tyler, 2012).
Glutamate excitotoxicity and ischemia have been shown to participate in oxidative stress upon TBI (Figure 4). Direct tissue damage and impaired regulation of CBF and metabolism lead to ATP depletion, ionic gradient homeostasis and release of excitatory neurotransmitters (Cheng et al., 2012). Oxidative stress is known to play an important role in the pathology of TBI (Ansari et al., 2008; Tyurin et al., 2000), and both mitochondria and Nox enzymes have been reported to mediate ROS formation (Singh et al., 2006). A biphasic generation of ROS by Nox2 has been reported in TBI in vivo models (Zhang et al., 2012b). In addition, iNOS inhibition reduces neuronal damage induced by brain contusion (Gahm et al., 2006).
Characterization of oxidative stress in blast TBI models is a relatively unexplored area of research. Recently, Abdul-Muneer and colleagues performed an extensive characterization of ROS/NOS in the rat model of primary blast mild TBI (Abdul-Muneer et al., 2013). They found that after exposure to a moderate intensity (130 kPa peak overpressure) single blast, the oxidative damage of the cerebrovascular barrier interface (the blood–brain barrier, BBB) was manifested by induction of Nox1 and inducible nitric oxide synthase (iNOS), and corresponding elevated levels of the signatures of oxidative and nitrosative damage, 4-hydroxynonenal (4-HNE) and 3-nitrotyrosine (3-NT). Similarly, the effects of blast exposure with moderate intensity (120 kPa) in rats resulted in reduced neurological function immediately following exposure to blast. Quantitative immunostaining revealed the temporal course of brain oxidative and nitrosative stress following the injury. An increase in the levels of 4-HNE and 3-NT were observed at 3 hours, and returned to base levels at 24 hr post exposure (Readnower et al., 2010).
4. Gene delivery approaches to target the Central Nervous System
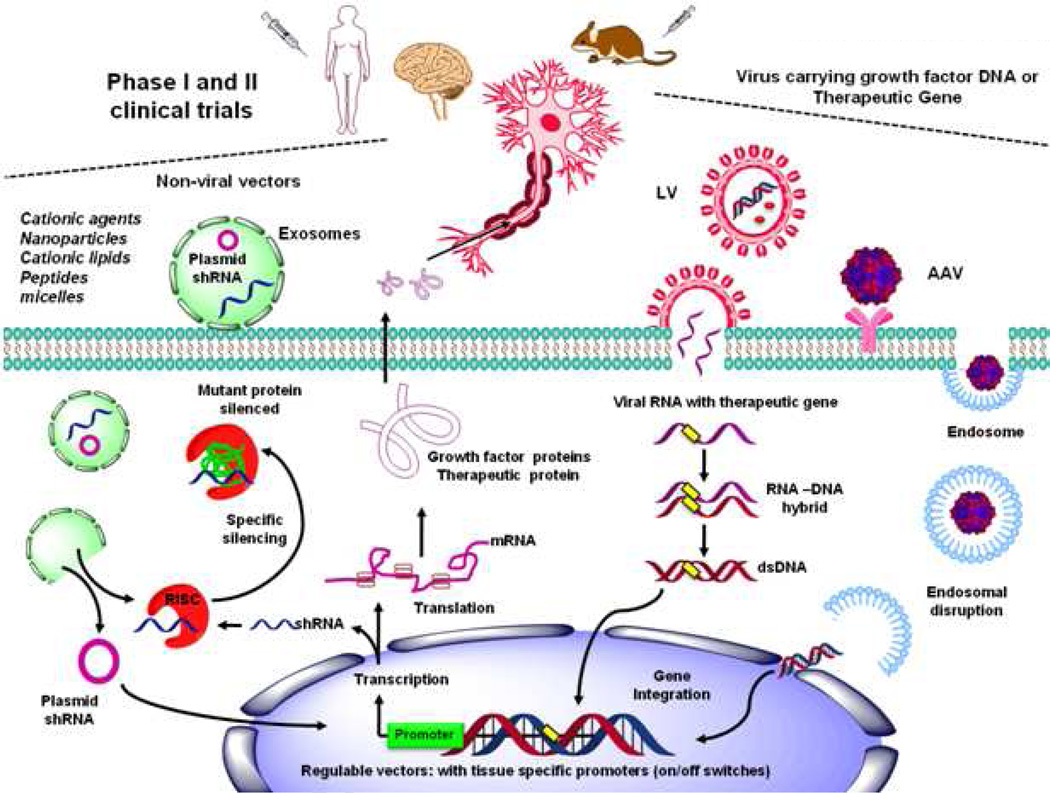
Gene therapy is the process of delivering genes to cells. Most gene therapy studies employ viral vectors because they are highly efficient and can support lifetime protein expression in the brain (Figure 5). Virions can carry and protect viral genetic information, and can be designed to possess determinants on their surface to specify which cells to infect, minimizing immunostimulatory potential within the host. An ideal therapeutic viral vector will: 1) show specific tropism and highly efficient transduction of target tissues with minimal off-target transduction; 2) express the transgene for a length of time and at a level as to exert maximal therapeutic impact; and 3) show minimal side effects such as vector related pathologies or host immune responses (Manfredsson et al., 2012).
Figure 5. Target delivery of therapeutic genes in neuronal populations.
Adenovirus, lentivirus (LV) and adeno-associated viral (AAV) have been used as viral vectors for gene transfer. Viral vectors carrying therapeutic genes (1) can be bonded to cell surface receptors to selectively allow entry of DNA into neurons by receptor-mediated endocytosis, which then becomes encapsulated in endosomes, from which they must escape to inject the corresponding new gene into the nucleus to promote the expression of the therapeutic protein of interest. In addition (2), viruses can inject the viral RNA, which by reverse transcription produces a dsDNA that could be integrated in host genome. Although viruses offer excellent gene expression efficiency, non-viral gene therapy offers the potential to target specific cells, being less immunogenic and non-integrating into the host genome. An example are exosomes that have been re-engineered for targeted gene therapy, their small size and flexibility allows them to cross biological membranes, while the “cargo” is protected from degradation by their bi-lipid structure. Major advances in gene-therapy research for neurological disorders have been achieved in recent years by overexpressing pro-survival growth factors or targeting endogenous mutant or wild type genes associated with disease pathogenesis.
Viral vectors such as adenovirus, lentivirus (LV) and adeno-associated viral (AAV) have been designed for gene transfer (Figure 5). Adenoviruses display a rather promiscuous tropism and have significant immunological issues, thus, they have not been employed clinically for neurological diseases (Manfredsson et al., 2012). LV vectors have excellent safety and efficacy in rodent and primate models of neurodegenerative diseases. However, because they insert their genetic material into the host genome they present an oncogenic risk. Major advantages of LV vectors are their ability to transduce non-dividing cells including differentiated neurons, and that large genes can be inserted and permanently incorporated into the host cell (Dreyer, 2011).
Recombinant AAV (rAAV) are non-pathogenic, have low immunogenicity and high efficiency transducing brain cells. rAAV can in fact support transgene expression in post-mitotic cells for the lifetime of an individual. One of the major limitations of rAAV vectors is that they only support a genomic/gene carrying capacity of ~6 kb. AAVs can integrate into the host genome with lower frequency than LV. AAV safely integrate at the AAVS1 site found at chromosome 19q13.4 qtr mapped to the first exon of the myosin binding subunit 85 of protein phosphatase 1. However, rAAV vectors have the potential to lose their site-specific integration and can instead integrate randomly introducing point mutations (Manfredsson et al., 2012). Recombinant AAV vectors form stable episomal concatemers, and in postmitotic cells, episomal AAV genomes can provide long-term (>1 yr) transgene expression. AAV vectors have been used in animal models and clinical trials showing more specificity at transducing certain brain areas compared to LV (Ramaswamy et al., 2012). Twelve different serotypes of AAV have been discovered with distinct affinities for different cell types depending on the proteins displayed on the surface of their capsids that recognize different cell-surface receptors. Cells in the brain are transduced by AAV serotypes 1, 2 and 4–9. Most studies in the brain have been carried out with AAV-2 and 5. While AAV5 conveys a more wide-spread infection of neurons and astrocytes, AAV2 is more neuronal-specific. AAV2 transduces neurons by binding to the heparan sulfate proteoglycan receptor and using bFGF (basic Fibroblast Growth Factor) receptor as a co-receptor. Distinct rAAV have been created in which the genome of the AAV2 serotype is packaged in the capsid protein of other AAV serotypes that better bind to receptors on neurons, glia and ependymal cells (Manfredsson et al., 2012). Interestingly, AAV9 has been shown to produce global expression in the brain and spinal cord neurons after a peripheral, systemic route of administration to neonatal mice, while in adult mice the transduction is mainly observed in glial cells, which reduces its use as a therapeutic alternative in aging-related diseases (Dayton et al., 2012).
The design, production, and efficiency of viral vectors have been improved remarkably, leading to safer transduction, long-term and robust transgene expression. Most clinical vector-based gene-therapy trials have shown success regarding vector safety. However, clinical efficacy attributed to the gene transfer has been lacking in many cases. Despite a number of successful phase I therapy clinical trials, only few have reached phase II. One potential reason is that experimental therapeutics is often not applied until later stages of the disease, when it can no longer provide a benefit. Other reasons might be associated with the lack of a clear understanding of the underlying molecular mechanisms associated with neuronal cell death and the neuropathology of specific neurological diseases, including the lack of animal models that fully recapitulate the etiology of the disease states, and not with our current ability to efficiently and safely deliver gene targeting (Manfredsson et al., 2010).
An increased focus in gene-therapy research has been directed to the development of clinical regulatable vectors (on/off switches), tissue specific (tyrosine hydroxylase promoter for catecholaminergic cells), or conditional promoters (hypoxia [HRE] or antioxidant response elements [ARE]) in order to address safety concerns, tissue specificity and responsiveness to appropriate local factors that are related to disease. To date, none of the vectors in clinical use have any direct way to reverse or control their transgene product in the event continued protein expression should become problematic (Manfredsson et al., 2012).
Viruses offer excellent gene expression efficiency. However, non-viral gene therapy offers the potential to target specific cells, being less immunogenic and non-integrating into the host genome. Delivery agents utilized in non-viral gene therapy include complexes consisting of DNA/RNA and carriers such as cationic agents, modified silica nanoparticles, cationic lipids and peptides, polymeric micelles and receptor targeting peptides or proteins (Figure 5). Similar to viral therapy, tissue-specific promoters have been used to direct non-viral transgene expression to neurons following gene delivery to the brain (Rogers et al., 2012). Non-viral delivery systems can target specific cell populations by the incorporation of receptor binding agents, ligands (glycosylated molecules), peptides, proteins or antibodies. The pathway followed by receptor mediated gene delivery is known to involve endocytosis, endosomal escape and nuclear entry prior to transcription. Although the BBB precludes the entry of therapeutic molecules from the circulation to the brain, the use of endogenous receptor-mediated transport systems or retrograde machinery that viruses “hijack”, allows the delivery of non-viral agents across the BBB and their delivery to neurons (Rogers et al., 2012).
Exosomes are extracellular vesicles produced constitutively by most cell types that contain mRNAs and non-coding micro RNAs (miRNAs) as well as proteins, and are naturally used as a mechanism of horizontal gene transfer. Exosomes have been re-engineered for targeted gene therapy. Because they are comprised of non-synthetic and non-viral components, exosomes are in principle ideal vectors for gene therapy delivery. The small size and flexibility of exosomes allows them to cross biological membranes, while the RNA and protein cargo is protected from degradation by their bi-lipid structure (Figure 5). A recent study demonstrated the ability of modified systemic intravenous delivery of murine exosomes to deliver short interfering RNA (siRNA) in the brain with the aid of a rabies virus glycoprotein peptide, without major effects in peripheral organs or adverse immune responses (Alvarez-Erviti et al., 2011; Lee et al., 2012b).
4.1. Current strategies used for gene therapy in neurological diseases
Major advances in gene-therapy research for neurological disorders have been achieved in recent years. By overexpressing pro-survival growth factors or targeting endogenous mutant or wild type genes associated with disease pathogenesis (Figure 5), different research groups have demonstrated the feasibility of gene-therapy approaches against neurological diseases. We next summarize the research in this area in order to highlight some examples and routes of administration.
4.1.1. Growth factors
One of the major successful areas of research in gene therapy has been the use of pro-survival growth factors. Gene delivery of Brain-Derived Neurotrophic Factor (BDNF) using a LV vector decreases learning and memory impairment in AD animal models (Nagahara et al., 2009). Similarly, AAV2/1 delivery of basic Fibroblast Growth Factor (bFGF) restores hippocampal functions in an APP+PSEN1 bigenic mice model of AD (Kiyota et al., 2011). Nerve growth factor (NGF) is known to enhance the function and survival of basal forebrain cholinergic neurons that are vulnerable in AD. A clinical trial is ongoing using AAV2-mediated overexpression of NGF for the treatment of AD (Mandel, 2010).
Anterograde transport of AAV vectors from the putamen to the substantia nigra is widely used as therapeutic approach in PD. LV delivery of Glial-cell-line-Derived Neurotrophic Factor (GDNF) prevents nigrostriatal degeneration induced by MPTP (Kordower et al., 2000). An AAV2 vector encoding human Neurturin (NTN) injected into the striatum and/or substantia nigra decreased neurodegeneration in primate and rodent models of PD (Gasmi et al., 2007; Kordower et al., 2006). Preliminary data disclosed from ongoing clinical trials using bilateral intraputaminal AAV2-NTN injection, reports significant improvement in patients at 18 months post-administration, but NTN expression does not seem to reach the substantia nigra (Bartus et al., 2011; Berry et al., 2011; Marks et al., 2010).
Similarly, the overexpression of BDNF using AAV reduces motor impairment and neuronal damage induced by quinolinic acid as a model for HD (Kells et al., 2008). The adenoviral delivery of Ciliary Neurotrophic Factor (CNTF) also reduces neuronal damage induced by 3-NP (Mittoux et al., 2002). However, opposite effects have been observed when LV or AAV have been used to deliver CNTF to HD transgenic mice (Denovan-Wright et al., 2008; Zala et al., 2004). AAV delivery of GDNF protects rats against 3-NP toxicity and also reduces degeneration in the N171-82Q transgenic mouse model of HD (McBride et al., 2003; McBride et al., 2006). AAV2-NRTN also exerts protective effects against 3-NP in the N171-82Q transgenic mouse model (Ramaswamy et al., 2012).
The rapid disease progression of ALS is a major concern for therapeutic intervention. Different injection pathways have been used to deliver transgenes to spinal motor neurons including peripheral delivery via intramuscular or intraneural routes of administration, or systemic delivery via intravascular or intrathecal administration (Federici et al., 2012). Injection of an AAV2 vector encoding human Insulin Growth Factor-1 (IGF-1) into the ventral gray matter in the lumbar region slows disease onset and increases the survival of SOD1G93A mice (Lepore et al., 2007). Viral vector delivery of IGF-1 or Vascular Endothelial Growth Factor (VEGF) to the CNS through bilateral injection into the deep cerebellar nuclei also significantly improves lifespan in SOD1G93A mice (Dodge et al., 2008); these results were confirmed in presymptomatic SOD1G93A rats (Franz et al., 2009). AAV4-mediated delivery of IGF-1 or VEGF into the ventricular system and spinal cord central canal delays motor decline and death in SOD1G93A mice (Dodge et al., 2010). Intramuscular delivery of IGF, GDNF or VEGF using viral vectors delays the disease progression in SOD1G93A mice (Azzouz et al., 2004; Kaspar et al., 2003; Wang et al., 2002). AAV-mediated delivery of the Cytokine Granulocyte-colony Stimulating Factor (G-CSF) to the spinal cord improves the motor function and also delays the disease progression in SOD1G93A mice (Henriques et al., 2011).
Direct vector administration into the cerebrospinal fluid within the ventricular or perivascular systems, or into the brain parenchyma via the striatum has been used in stroke animal models (Gabriel et al., 2007). Gene therapy studies using viral delivery of bFGF-2 have also demonstrated a protective effect of this approach against cerebral stroke (Leker et al., 2007; Watanabe et al., 2004). A number of studies also report that viral delivery of GDNF protects against brain ischemia (Hermann et al., 2001; Iwai et al., 2001; Tsai et al., 2000; Zhang et al., 2002). AAV-VEGF transduction prior to cerebral ischemia also exerts a protective effect (Bellomo et al., 2003; Shen et al., 2006).
Non-viral gene therapy has also been explored to mediate transgene overexpression of growth factors. Cationic carriers conjugated to neurotensin can carry DNA plasmids into dopaminergic neurons (Hernandez-Baltazar et al., 2012) and have been used as a potential therapeutic approach in PD (Gonzalez-Barrios et al., 2006; Martinez-Fong et al., 2012). Use of transferring receptor (TrfR) antibodies conjugated to immunoliposomes, have been proven to be a viable route to deliver DNA plasmid encoding GDNF encapsulated in pegylated liposomes to reverse TH depletion in vivo and to protect against experimental PD (Zhang et al., 2009; Zhang et al., 2004). Liposome-mediated NGF cDNA transfer also increases the number of surviving cholinergic neurons in a TBI model (Zou et al., 1999).
4.1.2. Targeting of endogenous/mutant genes
Neurodegenerative diseases are in many cases associated with specific gene mutations. In these cases, the modification of endogenous levels of wild type or mutant genes might represent a more direct approach for gene therapy. LV-delivered of siRNA against BACE1 (APP beta secretase 1) decreases amyloid plaque levels and neurodegeneration in APP transgenic mice (Singer et al., 2005). Overexpression of ApoE2, whose allele is associated with a decreased risk for developing AD, reduces Aβ burden in transgenic mice models of AD (Dodart et al., 2005). Viral delivery of parkin to the substantia nigra protects against α-synuclein mediated neurodegeneration (Lo Bianco et al., 2004; Yamada et al., 2005), while effective knockdown of α-synuclein in rats using viral delivery of shRNA ameliorates motor dysfunction in rats overexpressing human α-synuclein (Khodr et al., 2011). AAV2 encoding a rotenone-insensitive genetic variant of the mitochondrial complex I NADH–quinone oxidoreductase (NDI1) has also been shown to protect against MPTP toxicity (Barber-Singh et al., 2009).
The identification of the huntingtin gene allows for the identification carriers well before symptom onset. Because neuronal dysfunction precedes cell death in HD, genetic testing and disease state evaluation could allow early therapeutic intervention to be initiated prior to onset of symptoms to prevent neuronal cell death (Ramaswamy et al., 2012). Normal huntingtin protein deletion is lethal and thus, RNA interference approaches to treat HD should preferably target knockdown of mHtt. A number of studies using siRNA, shRNA or miRNA to down-regulate the production of the mHtt protein have shown effectiveness in transgenic HD mouse models (Ramaswamy et al., 2012). Similar to HD, familial ALS arises through a toxic gain-of-function of mutant SOD1. Knockdown of mutant SOD1 via viral vectors leads to increase lifespan in the G93ASOD1 ALS mice (Federici et al., 2012; Ralph et al., 2005; Raoul et al., 2005).
4.1.3. Other genetic targets
Aβ deposition and inflammation are major components in AD pathology. LV-mediated expression of the lysosomal cysteine protease cathepsin B in APP transgenic mice reduced preexisting Aβ deposits (Mueller-Steiner et al., 2006). LV-mediated overexpression of neprilysin (NEP), the dominant Aβ peptide-degrading enzyme in the brain, also reduces Aβ peptide levels and ameliorates neurodegeneration in AD mouse models (El-Amouri et al., 2008; Marr et al., 2003; Spencer et al., 2008). AAV2/1 delivery of anti-inflammatory interleukin-10 was shown to ameliorate cognitive dysfunction in an APP+PSEN1 bigenic mice model of AD (Kiyota et al., 2012).
Dopamine deficiency is the hallmark feature in PD and gene therapy studies have aimed to correct this by overexpression of enzymes involved in dopamine synthesis. An AAV2 vector encoding human aromatic-l-amino decarboxylase (AADC), the enzyme responsible for dopamine synthesis, reduces motor deficits in MPTP-treated primates and these results have led to a phase I clinical trial (Bankiewicz et al., 2006; Christine et al., 2009; Muramatsu et al., 2010). Because the restoration of AADC still requires L-dopa supplementation, other approaches have aimed to transduce enzymes involved in its synthesis. Triple transduction of TH, AADC and GTP cyclohydrolase 1 (GCH1) in rats using AAV vectors reduces 6-OHDA-induced neurodegeneration (Shen et al., 2000). A LV that delivers GCH1, TH and AADC in patients with PD is currently in use (Azzouz et al., 2002). Another explored strategy is increasing the expression of glutamic acid decarboxylase (GAD) (that mediates GABA synthesis) to inhibit the synchronous oscillatory activity of the globus pallidus and subthalamic nucleus observed in PD patients. AAV2-GAD decreases neurodegeneration induced by 6-OHDA and MPTP (Emborg et al., 2007; Lee et al., 2005; Luo et al., 2002). These results led to phase I/II trials in PD patients that demonstrated a significant improvement of PD patients (Kaplitt et al., 2007; LeWitt et al., 2011). Importantly, the safety and tolerability analysis of unilateral subthalamic injection of the AAV2-GAD65/67 in 12 patients reported no adverse effects related to the procedure and no change in the patients’ immunity against AAV or GAD were reported after 12 months.
Neuronal cell death in neurodegenerative diseases and upon brain ischemia and TBI insults is mediated by apoptotic signaling. Delivery of anti-apoptotic Bcl-2 or Bcl-xl proteins using rAAV also reduces degeneration in transgenic ALS mice (Azzouz et al., 2000; Yamashita et al., 2002). In TBI, gene therapy research uses different routes of delivery. Extravascular delivery of viral vectors via direct injection into the injured brain conveys precise focus positioning. However, the area transfected is limited, repeated injections are not allowed, and the injected volume has to be minimized to prevent extrusion and distortion of the surrounding brain tissues. In contrast, intraventricular injection of gene vectors has a smaller invasion area and wider tissue distribution through the cerebrospinal fluid circulation. Intravascular delivery is more acceptable in clinical use, but delivered viral vectors do not permeate the BBB. A recombinant adenovirus vector expressing Bcl-2 has also been used as a therapeutic approach in a TBI experimental model (Yang et al., 2006). Similarly, AAV delivery of the anti-apoptotic protein Bcl-w improves the neuronal function in cerebral ischemia (Sun et al., 2003)
5. Catalytic antioxidant defenses against neuronal cell death
Cells have evolved intrinsic antioxidant mechanisms to maintain a tight homeostatic control of the ROS/RNS generated under physiological conditions, and to detoxify their excessive accumulation in pathological conditions. Under physiological conditions a balance exists among the activities of enzymatic antioxidant defenses, the intracellular levels of non-enzymatic antioxidants, and the production of ROS/RNS as signaling molecules essential for proper cellular function. In addition to their ability to scavenge ROS/RNS, antioxidants systems modulate cell signaling pathways by redox dependent mechanisms (Figure 6).
Figure 6. Catalytic antioxidant systems.
Cells have intrinsic antioxidant mechanisms to maintain a tight homeostatic control of ROS/RNS generated under physiological conditions, and also detoxify their excessive accumulation in pathological conditions. SODs dismutate O2•− to O2 and H2O2. (1) MnSOD is localized in the mitochondrial matrix, CuZnSOD in the IMS, peroxysomes, nucleous or cytosol, and EcSOD in the extracellular space (2). (3) Catalase catalyzes the decomposition of H2O2 to water and oxygen and is primarily localized in the peroxisomes. (4) Gpx are selenoproteins that reduce peroxides using GSH as substrate. Gpx isozymes encoded by different genes vary in their cellular location and substrate specificity. For example, Gpx1 preferably scavenges H2O2, whileGpx4 hydrolyses lipid hydroperoxides. GR, which reduces GSSG back to GSH, requires NADPH as electron donor reductant, and G6PD is indispensable for the regeneration of NADPH from NADP+ in then cytosol. (5) Prxs are ubiquitous thiol peroxidases that scavenge peroxides. Catalysis of H2O2 is mediated by the reaction of their active site cysteine residue with H2O2 with a rate comparabale to that of catalases. The catalytic activity of Prxs requires reducing equivalents provided by the Trx/TrxR system. (6) In addition, Trxs can directly bind to proteins modifying their function.
Based on the evidence highlighted above regarding the role of oxidative stress in neuronal cell death associated with a number of neurological diseases (neurodegeneration, brain ischemia and TBI), a number of clinical trials have aimed at using antioxidant supplementation in the diet as therapeutic approach. However, the clinical trial results so far have been mostly negative. Indeed, most antioxidants tested have been chosen based on their easy availability rather than because they have proved to be the best antioxidant candidates. Not only their efficiency to scavenge ROS/RNS, but many other factors such as absorbability, metabolism, ability to penetrate the BBB and distribution should also be considered to identify potential antioxidant candidates. Other possibility for the lack of benefit of antioxidants might be that the clinical trials have not lasted long enough or have not started early enough to prevent disease progression, our failure to understand their basic mechanisms of action in relationship to human diseases, and to conduct preclinical studies to identify concentration and time parameters relevant to the clinical setting of each pathology. In addition, it is clear that multiple pathogenetic factors other than ROS/RNS participate in neuronal cell death in distinct neurological disorders suggesting that an integrated approach might prove to be more successful (Giustarini et al., 2009; Kamat et al., 2008; Shen et al., 2010).
Another possibility that has not been taken into account is that experimental evidence demonstrates that ROS/RNS formation, oxidative stress and redox signaling involved in neuronal cell death show a high degree of selectivity regarding the ROS/RNS implicated and their subcellular compartmentalization. With the technological advance in the design of viral vectors leading to safer transduction as well as selective, long-term and robust transgene expression, together with the progress in using gene therapy in clinical trials, we consider that the targeted expression of specific catalytic antioxidants might be a more selective therapeutic approach to counteract oxidative stress, compared to dietary supplementation of non-selective antioxidants. We next summarize the in vivo experimental evidence demonstrating the potential of selective regulation of endogenous catalytic antioxidant enzymes to counteract oxidative damage and neuronal cell death.
5.1. Superoxide dismutases (SODs)
The most important cellular defense against O2•− are SODs. In mammals, there are three isoforms specifically compartmentalized, which catalyze the dismutation of O2•− to O2 and H2O2 by cycling of the transition metal ion at the active site: MnSOD (SOD2) localized in the mitochondrial matrix (Sutton et al., 2003), CuZnSOD or SOD1 in the IMS, peroxysomes, nucleus or cytosol (Okado-Matsumoto et al., 2001), and the extracellular SOD (EcSOD or SOD3) anchored to the cells’ surface through an heparin-binding domain (Hjalmarsson et al., 1987). MnSOD contains one manganese atom at the active site (Bakthavatchalu et al., 2011), while CuZnSOD and EcSOD contain a metal cluster of copper and zinc atoms (Crapo et al., 1992; Oshikawa et al., 2010).
Genetic ablation of one copy of the MnSOD allele SOD2(+/−) in mutant human APP (hAPP) transgenic mice exacerbates the Aβ burden and significantly increases the levels of phosphorylated tau and oxidative stress (Esposito et al., 2006; Lee et al., 2012a; Li et al., 2004; Melov et al., 2007). Conversely, the overexpression of MnSOD in hAPP mice reduces oxidative stress and amyloid deposition (Dumont et al., 2009). Similar to MnSOD, CuZnSOD deficiency in hAPP accelerates the Aβ oligomerization, memory impairment and oxidative damage (Murakami et al., 2011).
In experimental PD models, the overexpression of CuZnSOD (Barkats et al., 2006; Przedborski et al., 1992) or MnSOD (Klivenyi et al., 1998) has been shown to decrease MPTP toxicity, while MnSOD or CuZnSOD deficiencies increase it (Andreassen et al., 2001; Zhang et al., 2000). However, contradictory results also report that overexpression of MnSOD or CuZnSOD does not prevent MPP+ or rotenone toxicity (Rodriguez-Rocha et al., 2013; Sanchez-Ramos et al., 1997). Adenoviral-mediated overexpression of MnSOD but not CuZnSOD protects against paraquat toxicity suggesting a specific role for mitochondrial ROS formation (Rodriguez-Rocha et al., 2013).
Ischemic brain damage and TBI, as well as 3-NP/malonate (HD models)-induced oxidative stress are also exacerbated in SOD2(+/−) mice (Andreassen et al., 2001; Fujimura et al., 1999; Kim et al., 2002a; Kim et al., 2002b; Lewen et al., 2001; Mehta et al., 2011; Murakami et al., 1998; Noshita et al., 2001), while in transgenic mice overexpressing MnSOD or CuZnSOD, mitochondrial dysfunction induced by TBI is reduced (Xiong et al., 2005). In contrast, while MnSOD deficiency decreases the lifespan of G93ASOD1 (pseudo-wildtype) ALS mice, it had no effect on H46R/H48QSOD1 (metal-deficient) mice, suggesting that different mechanisms other than mitochondrial O2•− formation mediate ALS caused by mutations in SOD1 (Andreassen et al., 2000; Muller et al., 2008).
5.2. Catalase
Catalase enzymatically decomposes H2O2 to H2O and O2 and is primarily localized in the peroxisomes. It contains four porphyrin heme (iron) groups that allow the enzyme to react with H2O2. A reduction in Aβ levels and oxidative stress is found in hAPP mice overexpressing catalase targeted to the mitochondria, using the first 25 amino acids of the ornithine transcarbamylase leader sequence (Mao et al., 2012). Similarly, mitocatalase also decreases MPTP-induced mitochondrial ROS accumulation and dopaminergic cell death (Perier et al., 2010). We have not been able to observe any effects of adenoviral-mediated overexpression of catalase or mitocatalase on MPP+-induced toxicity in human neuroblastoma cells (unpublished data).
5.3. Enzymes involved in GSH recycling and synthesis
Glutathione (L-γ-glutamyl-L-cysteinyl-glycine, GSH) is the most abundant non-protein thiol in mammalian cells acting as a major reducing agent and antioxidant defense maintaining a tight control of the redox status. GSH synthesis is initiated by the generation of γ-glutamylcysteine from glutamate and cysteine via glutamate-cysteine ligase (GCL), with the subsequent addition of glycine by the activity of GSH synthetase (GS) (Franco et al., 2009, 2012; Jones, 2006; Meister, 1995; Schafer et al., 2001; Sies, 1999). GCL is a heterodimer composed of a catalytic subunit (GCLC) and a modifier subunit (GCLM), and knockout of GCLM decreases the survival of G93ASOD1 ALS mice (Vargas et al., 2011).
Brain GSH concentration is approximately 2–3 mM. The cysteine availability is rate limiting for the synthesis of GSH. In neurons, cysteine uptake is mediated primarily by the Na+-dependent excitatory amino acid transporter (EAAT), also known as system XAG− (Aoyama et al., 2008; Dringen et al., 2003). EAAT3 (or EAAC1) can transport cysteine at a rate comparable to that of glutamate. Extracellular cysteine is unstable and has neurotoxic effects mediated by oxidative stress (Janaky et al., 2000). However, the CSF cysteine concentration is much higher than in plasma. A major extracellular source for cysteine is the dipeptide cystine. Cystine uptake occurs by a Na+-independent heteroexchange mechanism denominated Xc−, which is found in immature neurons, astrocytes, microglia, retinal Muller and Bergmann glial cells (Lewerenz et al., 2012). An inter-relationship exists between astrocytes and neurons where neurons rely on extracellular cysteine provided by GSH efflux from astrocytes via GAP junction hemichannels, multidrug resistance protein transporters or EAAT1 (GLAST) (Fernandez-Fernandez et al., 2012; Minich et al., 2006; Rana et al., 2006; Stridh et al., 2010; Stridh et al., 2008). Interestingly, while mice deficient in Xc− system have no alterations in brain GSH content, EAAT3-deficient mice have reduced GSH levels (Aoyama et al., 2006; Aoyama et al., 2008). EAAT3 knockout mice are more susceptible to ischemia-induced oxidative stress (Li et al., 2011a).
GSH reductase (GR), which reduces glutathione disulfide (GSSG) back to GSH, requires reduced NADPH) as the electron donor reductant, and glucose-6-phosphate dehydrogenase (G6PD) is indispensable for the regeneration of NADPH from NADP+(Ho et al., 2007). Mice overexpressing G6PD are more resistant to MPTP-induced PD-like neurodegeneration (Mejias et al., 2006).
5.4. Glutathione peroxidases (Gpx)
Gpx are selenoproteins that reduce peroxides. Gpx isozymes are encoded by different genes and vary in their cellular location and substrate specificity. While GPx1, found primarily in the cytoplasm, preferably scavenges H2O2, Gpx4 hydrolyses lipid hydroperoxides and can be found in both cytosolic and mitochondrial compartments. Gpx1 transgenic mice and LV-mediated expression of Gpx1 in nigral dopaminergic neurons in vivo significantly protect against 6-OHDA toxicity, but these results could not be reproduced in vitro using adenoviral vectors (Barkats et al., 2002; Bensadoun et al., 1998; Ridet et al., 2006). MPTP toxicity is increased in mice deficient in Gpx1 (Klivenyi et al., 2000; Zhang et al., 2000). Malonate-induced striatal cell loss is also decrease in transgenic Gpx1 mice (Zeevalk et al., 1997). A number of studies from independent research groups demonstrate that Gpx1 knockout exacerbates, while Gpx1 overexpression protects against ischemic injury (Crack et al., 2006; Keller et al., 2000; Sheldon et al., 2008; Taylor et al., 2005; Weisbrot-Lefkowitz et al., 1998; Wong et al., 2008), and increases the survival of G93A SOD1 mice (Cudkowicz et al., 2002). Contradictory results exist regarding the protective effect of Gpx1 overexpression in TBI (Potts et al., 2009; Tsuru-Aoyagi et al., 2009). Gpx4 deficiency increases lipid peroxidation and Aβ levels in hAPP mice (Chen et al., 2008).
5.5. Peroxiredoxins (Prxs)
Prxs are ubiquitous thiol peroxidases comprising up to 1% of soluble cellular proteins that scavenge peroxides in cells (Figure 6). The catalysis of H2O2 is initiated by the reaction of their active site cysteine residue with H2O2, and recent studies have demonstrated that the reaction rate for Prxs with H2O2 is comparable to that of catalase (Cox et al., 2010; Hall et al., 2009). Mammals have six Prxs, with Prx1, 2 and 6 found in the cytoplasm, Prx4 in the ER, Prx3 in the mitochondria, and Prx5 in various compartments within the cell, including peroxisomes and mitochondria. Prx3 transgenic mice are resistant to oxidative damage and mitochondrial dysfunction induced by paraquat in hAPP mice (Chen et al., 2012). Transgenic mice overexpressing Prx2 showed reduced neuronal injury and oxidative stress induced by ischemia (Gan et al., 2012). LV-mediated over-expression of Prxs2 protects against 6-OHDA toxicity in vivo. In addition, a comparable degree of protection was observed in vitro by overexpression of Prx1 and Prx4 (Chen et al., 2012).
5.6. Thioredoxins (Trxs)
Trxs are thiol-redox proteins with a conserved Cys-Gly-Pro-Cys catalytic site that reduce or bind to proteins modifying their function. The Trx redox system depends on thiol-disulfide exchange reactions at the active site. Thioredoxin reductase (TrxR), a homodimeric selenium containing flavoprotein, transfers reducing equivalents from NADPH to Trxs reducing them. While the Trx1/TrxR1 system is localized in the cytoplasm, the Trx2/TrxR2 system is mitochondrial. In addition, Trxs provide reducing equivalents for the peroxide scavenging activity of Prxs (see section above). Trx1 has also been shown to reduce and activate a number of transcription factors. Overexpression of Trx1 decreases the neuronal cell apoptosis induced by ischemia (Takagi et al., 1999; Zhou et al., 2009).
5.7. Glutaredoxins (Grxs)
Glutathionylation is a reversible post-translational oxidative modification observed upon oxidative stress involving the formation of a mixed disulfide bond between GSH and protein cysteines. Grxs are oxidoreductases that utilize the reducing power of GSH to catalyze protein deglutathionylation. However, Grxs can induce both glutahionylation/deglutathionylation depending on the GSH/GSSG ratio and the presence of thiyl radicals (GS•). Grx1 is localized in the cytosol and intermembrane space, whereas mitochondrial Grx2 is localized in the matrix (Beer et al., 2004; Mieyal et al., 2008). In vivo down-regulation of Grx1 using anti-sense oligonucleotides exacerbates the impairment of complex I by MPTP, while in vitro adenoviral delivery of Grx1 protects against paraquat and 6-OHDA toxicity (Kenchappa et al., 2003; Rodriguez-Rocha et al., 2012). Grx2 overexpression protects against MPP+-induced toxicity (Karunakaran et al., 2007).
5.8. Methionine sulfoxide reductases (Msrs)
Methionine (Met) is one of the most vulnerable amino acid residues to oxidation. The addition of an oxygen atom oxidizes Met to methionine sulfoxide (MetSO), and its further oxidation leads to methionine sulfone (MetSO2) formation. Two different stereoisomers of MetSO, L-Met-S-SO and L-Met-R-SO are found at physiological conditions and they are reverted by Msrs. In humans, there are two different Msrs, MsrA and MsrB. MsrA is specific to the S-stereoisomer, and human MsrA is localized in the mitochondria, whereas MsrB reduces the R- stereoisomer. Mammals have three MsrB proteins. MsrB1 is a selenoprotein that contains selenium (Se) in the catalytic cysteine residue. MsrB1 is primarily located in the cytosol and nucleus, while MsrB2 is located in the mitochondria. MsrB3A is located in the ER and MsrB3B is targeted to the mitochondria (Lee et al., 2011). In AD a high percentage of Aβ aggregates contain MetSO at position 35, and the oxidation of Met(35) to Met(35)SO decreases the extent of Aβ aggregation and toxicity, whereas the oxidation of Met(35) to MetSO2 yields a toxicity similar to that of unoxidized Aβ. In MsrA(−/−) mice, the toxicity between native Aβ and Aβ-Met(35)SO is similar suggesting that the lower toxicity of Aβ-Met(35)SO might be a result of MsrA-mediated reduction (Moskovitz et al., 2011).
5.9. Heme-oxygenase (HO)
HO is a microsomal enzyme that catalyzes the first rate-limiting step in the heme degradation playing an important role in Fe2+ recycling. HO cleaves the carbon bridge of heme, yielding equimolar quantities of carbon monoxide (CO), Fe2+ and biliverdin. HO activity results in decreased oxidative stress by removal of heme, a potent pro-oxidant. Although Fe2+ can give rise to •OH radicals, simultaneous up-regulation of ferritin and cytosolic Fe2+ efflux protect cells from oxidative stress. Biliverdin and bilirubin are also potent antioxidants, capable of attenuating oxidative injury by scavenging peroxyl radicals (Jozkowicz et al., 2007). HO-1 is an inducible isoform in response to stress, whereas HO-2 is a constitutive isoform that is expressed under homeostatic conditions. HO-1 transgenic mice are protected against cerebral ischemia (Hung et al., 2008). Adenoviral-mediated overexpression of HO-1 also reduces oxidative brain injury induced by cerebral ischemia (Chao et al., 2013), and dopaminergic cell death triggered by MPTP (Hung et al., 2008). However, another study reports that HO-1 deficiency does not affect MPTP toxicity (Innamorato et al., 2010). HO-2 knockout mice exhibit increased neuronal loss after TBI insult (Chang et al., 2003).
5.10. Transcriptional regulation of antioxidant defenses
Oxidative stress activates the transcription of a variety of antioxidant genes through cis-acting sequences known as antioxidant response elements (ARE) or electrophile-responsive elements (EpRE). Nuclear factor (erythroid-derived 2)-like 1 (Nrf1) and 2 (Nrf2), members of the Cap-N-Collar family of transcription factors bind ARE. Nrf2 regulates the expression of antioxidant proteins and proteins that mediate GSH synthesis or recycling. Nrf2 is kept in a cytosolic complex with the kelch-like ECH-associated protein 1 (Keap1), where it is subjected to proteasomal degradation. Oxidants or electrophiles induce a conformational change in Keap1 that allow the translocation of Nrf2 to the nucleus (Brigelius-Flohe et al., 2011). Accordingly, in vivo activation of Nrf2 has been linked to the protective effects of triterpenoids (MPTP-PD model) (Kaidery et al., 2013), sulforaphane (MPTP-PD model and TBI) (Hong et al., 2010; Jazwa et al., 2011), histone deacetylase inhibitors, and carbon monoxide (brain ischemia) (Wang et al., 2011; Wang et al., 2012). Nrf2 deficiency exacerbates dopaminergic degeneration induced by α-synuclein (Lastres-Becker et al., 2012), MPTP (Burton et al., 2006; Innamorato et al., 2010; Rojo et al., 2010), 6-OHDA (Burton et al., 2006), as well as 3-NP/malonate-induced striatal cell loss (Calkins et al., 2005), ischemic injury (Shah et al., 2007; Shih et al., 2005), and neuronal cell death in TBI (Hong et al., 2010; Jin et al., 2009). Interestingly, the selective overexpression of Nrf2 in astrocytes protects against MPTP-induced toxicity (Chen et al., 2009b) and increases survival of G93ASOD1 ALS mice (Vargas et al., 2008). Viral-mediated delivery of Nrf2 also improves cognitive function but not Aβ burden in an APP/PS1 AD mouse model (Kanninen et al., 2009).
6. Conclusions and Perspectives
Oxidative stress has been largely reported to trigger and/or regulate neuronal cell death in a number of neurological disorders such as neurodegenerative disorders, brain ischemia and TBI (Franklin, 2011). A large number of studies have reported the beneficial effects of antioxidant approaches on counteracting oxidative stress-induced neuronal cell death in different experimental models. However, clinical trials aimed at using antioxidant supplementation in diet as a therapeutic approach have been largely unsuccessful. Indeed, many factors can explain the lack of success of such clinical interventions including: 1) intrinsic characteristics of the antioxidant studied (absorption, metabolism, ability to penetrate the BBB, and distribution); 2) caveats in the design of clinical trials (duration, concentration and time of intervention); 3) a gap in our understanding of their basic mechanisms of action in relationship to human disease progression; and 4) the existence of multiple pathogenic factors other than ROS/RNS contributing to neuronal cell death (Giustarini et al., 2009; Kamat et al., 2008; Shen et al., 2010). However, with the advances in the design of viral vectors leading to safer transduction as well as selectivity, long-term and robust transgene expression, and the recent successes in recent gene therapy clinical trials, we consider that the targeted expression of specific catalytic antioxidants might be a more selective therapeutic approach to counteract oxidative stress. This is also supported by experimental evidence demonstrating that the ROS/RNS formation and redox signaling involved in neuronal death on neurodegenerative disorders, as well as the subcellular compartmentalization of oxidative stress, is specific and different to each neurological diseases. The experimental evidence supporting a role for oxidative stress in neuronal cell loss/damage associated with neurological diseases is strong. However, it is necessary to carefully consider the biomarkers of oxidative stress and methods employed for their evaluation in the clinic. Attention to confounding factors must be taken to objectively measure and evaluate biomarkers of oxidative stress as indicators of normal biological processes, pathogenic processes, or responses to therapeutic intervention.
We have summarized here the in vivo experimental evidence demonstrating the potential for selective regulation of endogenous catalytic antioxidant enzymes to counteract the oxidative damage and neuronal cell death observed in neurodegeneration and ischemia/TBI insults. We have included in this discussion both experimental studies in vivo using viral expression vectors and transgenic/knockout models. However, we need to consider that the experimental evidence gathered using transgenic/knockout animals has to be corroborated using viral delivery approaches. Long-term modification of gene expression might induce compensatory mechanisms during development that could not be observed during transgene overexpression in adulthood. The experimental work reviewed here aims to encourage future experimentation in this are with the potential to be translated in the design of clinical trials. The question now is…what are we waiting for?
Acknowledgements
This work was supported by the National Institutes of Health Grant P20RR17675 Centers of Biomedical Research Excellence (COBRE), the Interdisciplinary Grant from the Research Council, the Life Sciences Grant Program of the University of Nebraska-Lincoln, the Scientist Development Grant of the American Heart Association (12SDG12090015) and by CONACYT-Mexico (Grant #104316).
Abbreviations
- ΔΨm
mitochondrial membrane potential
- 3-NP
3-nitropropionic acid
- 6-OHDA
6-hydroxydopamine
- AADC
Aromatic-l-amino decarboxylase
- AAV
Adeno-associated virus
- Aβ
amyloid-β peptide
- AD
Alzheimer's disease
- AICD
amyloid precursor protein intracellular domain
- ALS
Amyotrophic Lateral Sclerosis
- APP
Amyloid precursor protein
- ARE
Antioxidant Response Element
- ApoE
apolipoprotein E
- BDNF
Brain-derived eurotrophic factor
- BBB
blood-brain barrier
- CBF
Cerebral blood flow
- CNTF
Ciliary neurotrophic factor
- COX
Cyclooxygenases
- CSF
Cerebrospinal fluid
- CuZnSOD
Copper-zinc superoxide dismutase
- DMT1
divalent metal transporter 1
- EpRE
Electrophile-responsive elements
- EAAT
Excitatory amino acid transporter
- EcSOD
Extracellular superoxide dismutase
- ETC
Electron transport chain
- bFGF
Fibroblast Growth Factor
- G-CSF
Granulocyte-colony stimulating factor
- G6PD
Glucose-6-phosphate dehydrogenase
- GAD
Glutamic acid decarboxylase
- GDNF
Glial-cell-line-derived neurotrophic factor
- GCL
Glutamate-cystein ligase
- GCLC
Glutamate-cystein ligase catalytic subunit
- GCLM
Glutamate-cystein ligase modifier subunit
- GSH
Glutathione
- GSSG
Glutathione disulfide
- GR
Glutathione reductase
- Gpx
Glutathione peroxidases
- Grx
Glutaredoxin
- GCH-1
GTP cyclohydrolase-1
- HD
Huntington’s disease
- HO
Heme-oxygenase
- IMS
Inner membrane space
- IGF-1
Insulin growth factor 1
- LRRK2
Leucine rich repeat kinase 2
- LV
Lentivirus
- MetSO
Methionine sulfoxide
- mtHtt
mutant Huntingtin
- Msrs
MetSO reductases
- MnSOD
Manganese superoxide dismutase
- MPOs
Myeloperoxidases
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NGF
Nerve growth factor
- Nox
NADPH oxidase
- •NO
Nitric oxide, (e) endotelial, (i) inducible, (n) neuronal
- NOS
Nitric oxide synthase
- O2
Oxygen
- O2•−
Superoxide anion
- •OH
Hydroxyl radical
- ONOO−
Peroxynitrite
- ONOOH
peroxynitrous acid
- PD
Parkinson's disease
- Prx
Peroxiredoxin
- PSEN
presenilin
- RAGE
Receptor for advanced glycation end products
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SNpc
Substantia nigra pars compacta
- SODs
Superoxide dismutases
- TBI
Traumatic brain injury
- TH
Tyrosine hydroxylase
- TOM
translocase of the outer membrane
- Trx
Thioredoxin
- TrxR
Thioredoxin reductase
- VEGF
Vascular endothelial growth factor
- VMAT2
Vesicular monoamine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors disclosure statement: Authors declare no conflict of interest
References
- Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free radical biology & medicine. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Pan LH, Watanabe M, Konno H, Kato T, Itoyama Y. Upregulation of protein-tyrosine nitration in the anterior horn cells of amyotrophic lateral sclerosis. Neurol Res. 1997;19:124–128. doi: 10.1080/01616412.1997.11740784. [DOI] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997a;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Halliwell B, Jenner P. No evidence for increased oxidative damage to lipids, proteins, or DNA in Huntington's disease. J Neurochem. 2000;75:840–846. doi: 10.1046/j.1471-4159.2000.0750840.x. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997b;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Dedeoglu A, Albers DW, Klivenyi P, Carlson EJ, Epstein CJ, Beal MF. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp Neurol. 2001;167:189–195. doi: 10.1006/exnr.2000.7525. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Klivenyi P, Klein AM, Shinobu LA, Epstein CJ, Beal MF. Partial deficiency of manganese superoxide dismutase exacerbates a transgenic mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2000;47:447–455. [PubMed] [Google Scholar]
- Andrus PK, Fleck TJ, Gurney ME, Hall ED. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1998;71:2041–2048. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Natl Acad Sci U S A. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P, Bueler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet. 2000;9:803–811. doi: 10.1093/hmg/9.5.803. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, Kingsman SM, Kingsman AJ, Mazarakis ND. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease. J Neurosci. 2002;22:10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Bakthavatchalu V, Dey S, Xu Y, Noel T, Jungsuwadee P, Holley AK, Dhar SK, Batinic-Haberle I, St Clair DK. Manganese superoxide dismutase is a mitochondrial fidelity protein that protects Polgamma against UV-induced inactivation. Oncogene. 2011 doi: 10.1038/onc.2011.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bano D, Zanetti F, Mende Y, Nicotera P. Neurodegenerative processes in Huntington's disease. Cell Death Dis. 2011;2:e228. doi: 10.1038/cddis.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber-Singh J, Seo BB, Nakamaru-Ogiso E, Lau YS, Matsuno-Yagi A, Yagi T. Neuroprotective effect of long-term NDI1 gene expression in a chronic mouse model of Parkinson disorder. Rejuvenation Res. 2009;12:259–267. doi: 10.1089/rej.2009.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Barkats M, Horellou P, Colin P, Millecamps S, Faucon-Biguet N, Mallet J. 1-methyl-4-phenylpyridinium neurotoxicity is attenuated by adenoviral gene transfer of human Cu/Zn superoxide dismutase. J Neurosci Res. 2006;83:233–242. doi: 10.1002/jnr.20696. [DOI] [PubMed] [Google Scholar]
- Barkats M, Millecamps S, Bilang-Bleuel A, Mallet J. Neuronal transfer of the human Cu/Zn superoxide dismutase gene increases the resistance of dopaminergic neurons to 6-hydroxydopamine. J Neurochem. 2002;82:101–109. doi: 10.1046/j.1471-4159.2002.00952.x. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, Johnson EM, Jr, Olanow CW, Mufson EJ, Kordower JH. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov Disord. 2011;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast A, Haenen GR. Ten misconceptions about antioxidants. Trends Pharmacol Sci. 2013;34:430–436. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- Belkacemi A, Ramassamy C. Time sequence of oxidative stress in the brain from transgenic mouse models of Alzheimer's disease related to the amyloid-beta cascade. Free Radic Biol Med. 2012;52:593–600. doi: 10.1016/j.freeradbiomed.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Bellomo M, Adamo EB, Deodato B, Catania MA, Mannucci C, Marini H, Marciano MC, Marini R, Sapienza S, Giacca M, Caputi AP, Squadrito F, Calapai G. Enhancement of expression of vascular endothelial growth factor after adeno-associated virus gene transfer is associated with improvement of brain ischemia injury in the gerbil. Pharmacol Res. 2003;48:309–317. doi: 10.1016/s1043-6618(03)00128-2. [DOI] [PubMed] [Google Scholar]
- Benchoua A, Trioulier Y, Diguet E, Malgorn C, Gaillard MC, Dufour N, Elalouf JM, Krajewski S, Hantraye P, Deglon N, Brouillet E. Dopamine determines the vulnerability of striatal neurons to the N-terminal fragment of mutant huntingtin through the regulation of mitochondrial complex II. Hum Mol Genet. 2008;17:1446–1456. doi: 10.1093/hmg/ddn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Bensadoun JC, Mirochnitchenko O, Inouye M, Aebischer P, Zurn AD. Attenuation of 6-OHDA-induced neurotoxicity in glutathione peroxidase transgenic mice. Eur J Neurosci. 1998;10:3231–3236. doi: 10.1046/j.1460-9568.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- Berry AL, Foltynie T. Gene therapy: a viable therapeutic strategy for Parkinson's disease? J Neurol. 2011;258:179–188. doi: 10.1007/s00415-010-5796-9. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Blumberg J. Use of biomarkers of oxidative stress in research studies. J Nutr. 2004;134:3188S–3189S. doi: 10.1093/jn/134.11.3188S. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Brown RH, Matson W, Smart R, Hayden D, O'Donnell H, Flint Beal M, Cudkowicz M. Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med. 2000;29:652–658. doi: 10.1016/s0891-5849(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF. Increased oxidative damage to DNA in a transgenic mouse model of Huntington's disease. J Neurochem. 2001;79:1246–1249. doi: 10.1046/j.1471-4159.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L, Marinova-Mutafchieva L, Sadeghian M, Davis JB, Medhurst AD, Dexter DT. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011;50:633–640. doi: 10.1016/j.freeradbiomed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Brown CM, Wright E, Colton CA, Sullivan PM, Laskowitz DT, Vitek MP. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med. 2002;32:1071–1075. doi: 10.1016/s0891-5849(02)00803-1. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23:153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Galvan V, Lange MB, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhang J, Sultana R, Bredesen DE. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2010;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casoni F, Basso M, Massignan T, Gianazza E, Cheroni C, Salmona M, Bendotti C, Bonetto V. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J Biol Chem. 2005;280:16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Wong RJ, Vreman HJ, Igarashi T, Galo E, Sharp FR, Stevenson DK, Noble-Haeusslein LJ. Heme oxygenase-2 protects against lipid peroxidation-mediated cell loss and impaired motor recovery after traumatic brain injury. J Neurosci. 2003;23:3689–3696. doi: 10.1523/JNEUROSCI.23-09-03689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao XD, Ma YH, Luo P, Cao L, Lau WB, Zhao BC, Han F, Liu W, Ning WD, Su N, Zhang L, Zhu J, Fei Z, Qu Y. Up-regulation of heme oxygenase-1 attenuates brain damage after cerebral ischemia via simultaneous inhibition of superoxide production and preservation of NO bioavailability. Exp Neurol. 2013;239:163–169. doi: 10.1016/j.expneurol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Charvin D, Vanhoutte P, Pages C, Borrelli E, Caboche J. Unraveling a role for dopamine in Huntington's disease: the dual role of reactive oxygen species and D2 receptor stimulation. Proc Natl Acad Sci U S A. 2005;102:12218–12223. doi: 10.1073/pnas.0502698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochem Biophys Res Commun. 2007;359:335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009a;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Na R, Gu M, Richardson A, Ran Q. Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer's disease. J Neurochem. 2008;107:197–207. doi: 10.1111/j.1471-4159.2008.05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yoo SE, Na R, Liu Y, Ran Q. Cognitive impairment and increased Abeta levels induced by paraquat exposure are attenuated by enhanced removal of mitochondrial H(2)O(2) Neurobiol Aging. 2012;33:432 e415–432 e426. doi: 10.1016/j.neurobiolaging.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009b;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol. 2012;167:699–719. doi: 10.1111/j.1476-5381.2012.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew KC, Ang ET, Tai YK, Tsang F, Lo SQ, Ong E, Ong WY, Shen HM, Lim KL, Dawson VL, Dawson TM, Soong TW. Enhanced autophagy from chronic toxicity of iron and mutant A53T alpha-synuclein: implications for neuronal cell death in Parkinson disease. J Biol Chem. 2011;286:33380–33389. doi: 10.1074/jbc.M111.268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, Jackson-Lewis V, Vila M, Vonsattel JP, Heinecke JW, Przedborski S. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J Neurosci. 2005;25:6594–6600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine CW. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicology Letters. 1995;82–83:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Ciani E, Severi S, Contestabile A, Bartesaghi R. Nitric oxide negatively regulates proliferation and promotes neuronal differentiation through N-Myc downregulation. J Cell Sci. 2004;117:4727–4737. doi: 10.1242/jcs.01348. [DOI] [PubMed] [Google Scholar]
- Collins AR, Cadet J, Moller L, Poulsen HE, Vina J. Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells? Arch Biochem Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Colton CA, Brown CM, Cook D, Needham LK, Xu Q, Czapiga M, Saunders AM, Schmechel DE, Rasheed K, Vitek MP. APOE and the regulation of microglial nitric oxide production: a link between genetic risk and oxidative stress. Neurobiol Aging. 2002;23:777–785. doi: 10.1016/s0197-4580(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, Van Nostrand WE, Weinberg JB, Dawson H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Costa V, Scorrano L. Shaping the role of mitochondria in the pathogenesis of Huntington's disease. EMBO J. 2012;31:1853–1864. doi: 10.1038/emboj.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM, Ali U, Mansell A, Hertzog PJ. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 2006;37:1533–1538. doi: 10.1161/01.STR.0000221708.17159.64. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristovao AC, Choi DH, Baltazar G, Beal MF, Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristovao AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, Kim YS. NADPH oxidase 1 mediates alpha-synucleinopathy in Parkinson's disease. J Neurosci. 2012;32:14465–14477. doi: 10.1523/JNEUROSCI.2246-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz ME, Pastusza KA, Sapp PC, Mathews RK, Leahy J, Pasinelli P, Francis JW, Jiang D, Andersen JK, Brown RH., Jr Survival in transgenic ALS mice does not vary with CNS glutathione peroxidase activity. Neurology. 2002;59:729–734. doi: 10.1212/wnl.59.5.729. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- Dayton RD, Wang DB, Klein RL. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther. 2012;12:757–766. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci U S A. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright EM, Attis M, Rodriguez-Lebron E, Mandel RJ. Sustained striatal ciliary neurotrophic factor expression negatively affects behavior and gene expression in normal and R6/1 mice. J Neurosci Res. 2008;86:1748–1757. doi: 10.1002/jnr.21636. [DOI] [PubMed] [Google Scholar]
- Devasagayam TP, Boloor KK, Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Sian J, Rose S, Hindmarsh JG, Mann VM, Cooper JM, Wells FR, Daniel SE, Lees AJ, Schapira AH, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, Paul SM. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2005;102:1211–1216. doi: 10.1073/pnas.0409072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, Roskelley EM, Treleaven CM, Rizo L, Martin H, Kim SH, Kaspar R, Taksir TV, Griffiths DA, Cheng SH, Shihabuddin LS, Kaspar BK. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16:1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, Rao M, Eagle A, Matthews JC, Taksir TV, Cheng SH, Shihabuddin LS, Kaspar BK. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doverhag C, Keller M, Karlsson A, Hedtjarn M, Nilsson U, Kapeller E, Sarkozy G, Klimaschewski L, Humpel C, Hagberg H, Simbruner G, Gressens P, Savman K. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiol Dis. 2008;31:133–144. doi: 10.1016/j.nbd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in parkinsonism. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. Lentiviral vector-mediated gene transfer and RNA silencing technology in neuronal dysfunctions. Mol Biotechnol. 2011;47:169–187. doi: 10.1007/s12033-010-9334-x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MW. A review of approaches to the analysis of 3-nitrotyrosine. Amino Acids. 2003;25:351–361. doi: 10.1007/s00726-003-0022-z. [DOI] [PubMed] [Google Scholar]
- Dusek P, Jankovic J, Le W. Iron dysregulation in movement disorders. Neurobiol Dis. 2012;46:1–18. doi: 10.1016/j.nbd.2011.12.054. [DOI] [PubMed] [Google Scholar]
- El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am J Pathol. 2008;172:1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Carbon M, Holden JE, During MJ, Ma Y, Tang C, Moirano J, Fitzsimons H, Roitberg BZ, Tuccar E, Roberts A, Kaplitt MG, Eidelberg D. Subthalamic glutamic acid decarboxylase gene therapy: changes in motor function and cortical metabolism. J Cereb Blood Flow Metab. 2007;27:501–509. doi: 10.1038/sj.jcbfm.9600364. [DOI] [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puolivali J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici T, Boulis NM. Gene therapy for amyotrophic lateral sclerosis. Neurobiol Dis. 2012;48:236–242. doi: 10.1016/j.nbd.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Fekete A, Vizi ES, Kovacs KJ, Lendvai B, Zelles T. Layer-specific differences in reactive oxygen species levels after oxygen-glucose deprivation in acute hippocampal slices. Free Radic Biol Med. 2008;44:1010–1022. doi: 10.1016/j.freeradbiomed.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012;443:3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr, Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997a;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, Gurney ME, Beal MF. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997b;42:326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. Huntington's Disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- Fox JH, Connor T, Stiles M, Kama J, Lu Z, Dorsey K, Lieberman G, Sapp E, Cherny RA, Banks M, Volitakis I, DiFiglia M, Berezovska O, Bush AI, Hersch SM. Cysteine oxidation within N-terminal mutant huntingtin promotes oligomerization and delays clearance of soluble protein. J Biol Chem. 2011;286:18320–18330. doi: 10.1074/jbc.M110.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. Glutathione Efflux and Cell Death. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JL. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid Redox Signal. 2011;14:1437–1448. doi: 10.1089/ars.2010.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CK, Federici T, Yang J, Backus C, Oh SS, Teng Q, Carlton E, Bishop KM, Gasmi M, Bartus RT, Feldman EL, Boulis NM. Intraspinal cord delivery of IGF-I mediated by adeno-associated virus 2 is neuroprotective in a rat model of familial ALS. Neurobiol Dis. 2009;33:473–481. doi: 10.1016/j.nbd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, Chan PH. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- Gabriel RA, Yang GY. Gene therapy in cerebrovascular diseases. Curr Gene Ther. 2007;7:421–433. doi: 10.2174/156652307782793496. [DOI] [PubMed] [Google Scholar]
- Gahm C, Holmin S, Wiklund PN, Brundin L, Mathiesen T. Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J Neurotrauma. 2006;23:1343–1354. doi: 10.1089/neu.2006.23.1343. [DOI] [PubMed] [Google Scholar]
- Gan Y, Ji X, Hu X, Luo Y, Zhang L, Li P, Liu X, Yan F, Vosler P, Gao Y, Stetler RA, Chen J. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal. 2012;17:719–732. doi: 10.1089/ars.2011.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011a;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson's disease. Environ Health Perspect. 2011b;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson's disease. Neurobiol Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gedik CM, Collins A, Escodd Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Schapira AH, Taanman JW. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS One. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Guzman JN, Estep CM, Ilijic E, Kondapalli J, Sanchez-Padilla J, Surmeier DJ. Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson's disease. Nat Neurosci. 2012;15:1414–1421. doi: 10.1038/nn.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Barrios JA, Lindahl M, Bannon MJ, Anaya-Martinez V, Flores G, Navarro-Quiroga I, Trudeau LE, Aceves J, Martinez-Arguelles DB, Garcia-Villegas R, Jimenez I, Segovia J, Martinez-Fong D. Neurotensin polyplex as an efficient carrier for delivering the human GDNF gene into nigral dopamine neurons of hemiparkinsonian rats. Mol Ther. 2006;14:857–865. doi: 10.1016/j.ymthe.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence of neuronal oxidative damage in Alzheimer's disease. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, Heinecke JW. Neuronal expression of myeloperoxidase is increased in Alzheimer's disease. J Neurochem. 2004;90:724–733. doi: 10.1111/j.1471-4159.2004.02527.x. [DOI] [PubMed] [Google Scholar]
- Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, Trotti D, Pasinelli P. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci U S A. 2012;109:5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JS, Lee JE, Lee JR, Lee CS, Maeng JS, Bae YS, Kwon KS, Park SS. Nox4-dependent H2O2 production contributes to chronic glutamate toxicity in primary cortical neurons. Exp Cell Res. 2010;316:1651–1661. doi: 10.1016/j.yexcr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins--structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hands S, Sajjad MU, Newton MJ, Wyttenbach A. In vitro and in vivo aggregation of a fragment of huntingtin protein directly causes free radical production. J Biol Chem. 2011;286:44512–44520. doi: 10.1074/jbc.M111.307587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson Petersen CA, AN, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The Amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, Beal MF. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu LJ, Rockenstein E, Takenouchi T, Mallory M, Masliah E. alpha-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem. 2002;277:11465–11472. doi: 10.1074/jbc.M111428200. [DOI] [PubMed] [Google Scholar]
- He Y, Imam SZ, Dong Z, Jankovic J, Ali SF, Appel SH, Le W. Role of nitric oxide in rotenone-induced nigro-striatal injury. J Neurochem. 2003;86:1338–1345. doi: 10.1046/j.1471-4159.2003.01938.x. [DOI] [PubMed] [Google Scholar]
- Heeman B, Van den Haute C, Aelvoet SA, Valsecchi F, Rodenburg RJ, Reumers V, Debyser Z, Callewaert G, Koopman WJ, Willems PH, Baekelandt V. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci. 2011;124:1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, Schneider A. CNS-targeted viral delivery of G-CSF in an animal model for ALS: improved efficacy and preservation of the neuromuscular unit. Mol Ther. 2011;19:284–292. doi: 10.1038/mt.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M. Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiol Dis. 2001;8:655–666. doi: 10.1006/nbdi.2001.0399. [DOI] [PubMed] [Google Scholar]
- Hernandez-Baltazar D, Martinez-Fong D, Trudeau LE. Optimizing NTS-polyplex as a tool for gene transfer to cultured dopamine neurons. PLoS One. 2012;7:e51341. doi: 10.1371/journal.pone.0051341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2'dG. Neurology. 2006;66:250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Brandel JP, Galle P, Javoy-Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson's disease: an X-ray microanalysis. J Neurochem. 1991;56:446–451. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K, Marklund SL, Engstrom A, Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987;84:6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HY, Cheng ML, Chiu DT. Glucose-6-phosphate dehydrogenase--from oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007;12:109–118. doi: 10.1179/135100007X200209. [DOI] [PubMed] [Google Scholar]
- Hochstrasser H, Tomiuk J, Walter U, Behnke S, Spiegel J, Kruger R, Becker G, Riess O, Berg D. Functional relevance of ceruloplasmin mutations in Parkinson's disease. FASEB J. 2005;19:1851–1853. doi: 10.1096/fj.04-3486fje. [DOI] [PubMed] [Google Scholar]
- Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Yan W, Chen S, Sun CR, Zhang JM. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol Sin. 2010;31:1421–1430. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SY, Liou HC, Kang KH, Wu RM, Wen CC, Fu WM. Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol. 2008;74:1564–1575. doi: 10.1124/mol.108.048611. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Nobukuni K, Takata H, Hayabara T. Oxidative stress and metal content in blood and cerebrospinal fluid of amyotrophic lateral sclerosis patients with and without a Cu, Zn-superoxide dismutase mutation. Neurol Res. 2005;27:105–108. doi: 10.1179/016164105X18430. [DOI] [PubMed] [Google Scholar]
- Im JY, Kim D, Paik SG, Han PL. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic Biol Med. 2006;41:960–972. doi: 10.1016/j.freeradbiomed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Innamorato NG, Jazwa A, Rojo AI, Garcia C, Fernandez-Ruiz J, Grochot-Przeczek A, Stachurska A, Jozkowicz A, Dulak J, Cuadrado A. Different susceptibility to the Parkinson's toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS One. 2010;5:e11838. doi: 10.1371/journal.pone.0011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Abe K, Kitagawa H, Hayashi T. Gene therapy with adenovirus-mediated glial cell line-derived neurotrophic factor and neural stem cells activation after ischemic brain injury. Hum Cell. 2001;14:27–38. [PubMed] [Google Scholar]
- James SA, Volitakis I, Adlard PA, Duce JA, Masters CL, Cherny RA, Bush AI. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic Biol Med. 2012;52:298–302. doi: 10.1016/j.freeradbiomed.2011.10.446. [DOI] [PubMed] [Google Scholar]
- Janaky R, Varga V, Hermann A, Saransaari P, Oja SS. Mechanisms of Lcysteine neurotoxicity. Neurochem Res. 2000;25:1397–1405. doi: 10.1023/a:1007616817499. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 up-regulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic Biol Med. 2005;38:1604–1613. doi: 10.1016/j.freeradbiomed.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14:2347–2360. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- Jenner P, Dexter DT, Sian J, Schapira AH, Marsden CD. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol. 1992;32(Suppl):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z, Feng J. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun. 2012;3:668. doi: 10.1038/ncomms1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- Jin L, Wang J, Zhao L, Jin H, Fei G, Zhang Y, Zeng M, Zhong C. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson's disease. Brain. 2011;134:50–58. doi: 10.1093/brain/awq319. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y, Tang K. Role of Nrf2 in protection against traumatic brain injury in mice. J Neurotrauma. 2009;26:131–139. doi: 10.1089/neu.2008.0655. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9:2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal. 2013;18:1400–1417. doi: 10.1089/ars.2012.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis. 2010;40:185–192. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, Sporn MB, Beal MF, Gazaryan IG, Thomas B. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson's disease. Antioxid Redox Signal. 2013;18:139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivendi SV, Kotamraju S, Cunningham S, Shang T, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem J. 2003;371:151–164. doi: 10.1042/BJ20021525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Gil SJ, Koh HC. Paraquat induces alternation of the dopamine catabolic pathways and glutathione levels in the substantia nigra of mice. Toxicol Lett. 2009;188:148–152. doi: 10.1016/j.toxlet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yla-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Saeed U, Ramakrishnan S, Koumar RC, Ravindranath V. Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res. 2007;1185:8–17. doi: 10.1016/j.brainres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kaur A, Van PT, Busch CR, Robinson CK, Pan M, Pang WL, Reiss DJ, DiRuggiero J, Baliga NS. Coordination of frontline defense mechanisms under severe oxidative stress. Mol Syst Biol. 2010;6:393. doi: 10.1038/msb.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2000;20:1467–1473. doi: 10.1097/00004647-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Kells AP, Henry RA, Connor B. AAV-BDNF mediated attenuation of quinolinic acid-induced neuropathology and motor function impairment. Gene Ther. 2008;15:966–977. doi: 10.1038/gt.2008.23. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- Khodr CE, Sapru MK, Pedapati J, Han Y, West NC, Kells AP, Bankiewicz KS, Bohn MC. An alpha-synuclein AAV gene silencing vector ameliorates a behavioral deficit in a rat model of Parkinson's disease, but displays toxicity in dopamine neurons. Brain Res. 2011;1395:94–107. doi: 10.1016/j.brainres.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Chan PH. Involvement of superoxide in excitotoxicity and DNA fragmentation in striatal vulnerability in mice after treatment with the mitochondrial toxin, 3-nitropropionic acid. J Cereb Blood Flow Metab. 2002a;22:798–809. doi: 10.1097/00004647-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002b;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishigami H, Nagano S, Bush AI, Sakoda S. Monomerized Cu, Zn-superoxide dismutase induces oxidative stress through aberrant Cu binding. Free Radic Biol Med. 2010;48:945–952. doi: 10.1016/j.freeradbiomed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Jacobsen MT, Xiong H, Ikezu T. FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer's disease and has therapeutic implications for neurocognitive disorders. Proc Natl Acad Sci U S A. 2011;108:E1339–E1348. doi: 10.1073/pnas.1102349108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac N, Relja M, Klepac R, Hecimovic S, Babic T, Trkulja V. Oxidative stress parameters in plasma of Huntington's disease patients, asymptomatic Huntington's disease gene carriers and healthy subjects : a cross-sectional study. J Neurol. 2007;254:1676–1683. doi: 10.1007/s00415-007-0611-y. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, St Clair D, Wermer M, Yen HC, Oberley T, Yang L, Flint Beal M. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol Dis. 1998;5:253–258. doi: 10.1006/nbdi.1998.0191. [DOI] [PubMed] [Google Scholar]
- Ko L, Mehta ND, Farrer M, Easson C, Hussey J, Yen S, Hardy J, Yen SH. Sensitization of neuronal cells to oxidative stress with mutated human alpha-synuclein. J Neurochem. 2000;75:2546–2554. doi: 10.1046/j.1471-4159.2000.0752546.x. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kubota C, Torii S, Hou N, Saito N, Yoshimoto Y, Imai H, Takeuchi T. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem. 2010;285:667–674. doi: 10.1074/jbc.M109.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rabano A, Kirik D, Cuadrado A. alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum Mol Genet. 2012;21:3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Peroxynitrite- and nitrite-induced oxidation of dopamine: implications for nitric oxide in dopaminergic cell loss. J Neurochem. 1999;73:2546–2554. doi: 10.1046/j.1471-4159.1999.0732546.x. [DOI] [PubMed] [Google Scholar]
- Lee B, Lee H, Nam YR, Oh JH, Cho YH, Chang JW. Enhanced expression of glutamate decarboxylase 65 improves symptoms of rat parkinsonian models. Gene Ther. 2005;12:1215–1222. doi: 10.1038/sj.gt.3302520. [DOI] [PubMed] [Google Scholar]
- Lee BC, Gladyshev VN. The biological significance of methionine sulfoxide stereochemistry. Free Radic Biol Med. 2011;50:221–227. doi: 10.1016/j.freeradbiomed.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Andersen JK. Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? J Neurochem. 2010;112:332–339. doi: 10.1111/j.1471-4159.2009.06470.x. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Lee HP, Pancholi N, Esposito L, Previll LA, Wang X, Zhu X, Smith MA, Lee HG. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS One. 2012a;7:e28033. doi: 10.1371/journal.pone.0028033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012b;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–161. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, Rothstein JD. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–265. doi: 10.1016/j.brainres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE, Palmiter RD, Brandt U, Drose S, Wittig I, Willem M, Haass C, Reichert AS, Muller WE. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal. 2012;16:1421–1433. doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson's disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen A, Fujimura M, Sugawara T, Matz P, Copin JC, Chan PH. Oxidative stress-dependent release of mitochondrial cytochrome c after traumatic brain injury. J Cereb Blood Flow Metab. 2001;21:914–920. doi: 10.1097/00004647-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Maher P, Methner A. Regulation of xCT expression and system x (c) (−) function in neuronal cells. Amino Acids. 2012;42:171–179. doi: 10.1007/s00726-011-0862-x. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Van Meter L, Sapan CV, During MJ, Kaplitt MG, Feigin A. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011a;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Spencer NY, Pantazis NJ, Engelhardt JF. Alsin and SOD1(G93A) proteins regulate endosomal reactive oxygen species production by glial cells and proinflammatory pathways responsible for neurotoxicity. J Biol Chem. 2011b;286:40151–40162. doi: 10.1074/jbc.M111.279711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AK, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–124. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Nguyen JL, Hulleman JD, Li L, Rochet JC. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson's disease. J Neurochem. 2008;105:2435–2453. doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- Liu HQ, Zhu XZ, Weng EQ. Intracellular dopamine oxidation mediates rotenone-induced apoptosis in PC12 cells. Acta Pharmacol Sin. 2005;26:17–26. doi: 10.1111/j.1745-7254.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Berchner-Pfannschmidt U, Moller U, Brecht M, Wotzlaw C, Acker H, Jungermann K, Kietzmann T. A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc Natl Acad Sci U S A. 2004;101:4302–4307. doi: 10.1073/pnas.0400265101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Althaus JS, Ellerbrock BR, Becker DA, Gurney ME. Enhanced oxygen radical production in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann Neurol. 1998;44:763–770. doi: 10.1002/ana.410440510. [DOI] [PubMed] [Google Scholar]
- Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci U S A. 2011;108:12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JD, Matson WR, Juhl AR, Leavitt BR, Paulsen JS. 8OHdG as a marker for Huntington disease progression. Neurobiol Dis. 2012;46:625–634. doi: 10.1016/j.nbd.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002a;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson's disease. Hum Mol Genet. 2002b;11:2395–2407. doi: 10.1093/hmg/11.20.2395. [DOI] [PubMed] [Google Scholar]
- Lu Q, Wainwright MS, Harris VA, Aggarwal S, Hou Y, Rau T, Poulsen DJ, Black SM. Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures. Free Radic Biol Med. 2012;53:1139–1151. doi: 10.1016/j.freeradbiomed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Kaplitt MG, Fitzsimons HL, Zuzga DS, Liu Y, Oshinsky ML, During MJ. Subthalamic GAD gene therapy in a Parkinson's disease rat model. Science. 2002;298:425–429. doi: 10.1126/science.1074549. [DOI] [PubMed] [Google Scholar]
- Luo S, Wehr NB. Protein carbonylation: avoiding pitfalls in the 2,4- dinitrophenylhydrazine assay. Redox Rep. 2009;14:159–166. doi: 10.1179/135100009X392601. [DOI] [PubMed] [Google Scholar]
- Magalhães CR, Socodato RES, Paes-de-Carvalho R. Nitric oxide regulates the proliferation of chick embryo retina cells by a cyclic GMP-independent mechanism. International Journal of Developmental Neuroscience. 2006;24:53–60. doi: 10.1016/j.ijdevneu.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Maiti P, Lomakin A, Benedek GB, Bitan G. Despite its role in assembly, methionine 35 is not necessary for amyloid beta-protein toxicity. J Neurochem. 2010;113:1252–1262. doi: 10.1111/j.1471-4159.2010.06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, Reynolds WF. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J Biol Chem. 2009;284:3158–3169. doi: 10.1074/jbc.M807731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C, Perluigi M, Cini C, De Marco C, Giuffrida Stella AM, Calabrese V. Heme oxygenase and cyclooxygenase in the central nervous system: a functional interplay. J Neurosci Res. 2006;84:1385–1391. doi: 10.1002/jnr.21049. [DOI] [PubMed] [Google Scholar]
- Mandel RJ. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer's disease. Curr Opin Mol Ther. 2010;12:240–247. [PubMed] [Google Scholar]
- Manfredsson FP, Bloom DC, Mandel RJ. Regulated protein expression for in vivo gene therapy for neurological disorders: progress, strategies, and issues. Neurobiol Dis. 2012;48:212–221. doi: 10.1016/j.nbd.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Mandel RJ. Development of gene therapy for neurological disorders. Discov Med. 2010;9:204–211. [PubMed] [Google Scholar]
- Mao P, Manczak M, Calkins MJ, Truong Q, Reddy TP, Reddy AP, Shirendeb U, Lo HH, Rabinovitch PS, Reddy PH. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid beta production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Hum Mol Genet. 2012;21:2973–2990. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani S, Ventriglia M, Simonelli I, Donno S, Bucossi S, Vernieri F, Melgari JM, Pasqualetti P, Rossini PM, Squitti R. Fe and Cu do not differ in Parkinson's disease: A replication study plus meta-analysis. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, Verma IM, Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FL, Williamson SJ, Paleologou KE, Hewitt R, El-Agnaf OM, Allsop D. Fe(II)-induced DNA damage in alpha-synuclein-transfected human dopaminergic BE(2)-M17 neuroblastoma cells: detection by the Comet assay. J Neurochem. 2003;87:620–630. doi: 10.1046/j.1471-4159.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Fong D, Bannon MJ, Trudeau LE, Gonzalez-Barrios JA, Arango-Rodriguez ML, Hernandez-Chan NG, Reyes-Corona D, Armendariz-Borunda J, Navarro-Quiroga I. NTS-Polyplex: a potential nanocarrier for neurotrophic therapy of Parkinson's disease. Nanomedicine. 2012;8:1052–1069. doi: 10.1016/j.nano.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JL, During MJ, Wuu J, Chen EY, Leurgans SE, Kordower JH. Structural and functional neuroprotection in a rat model of Huntington's disease by viral gene transfer of GDNF. Exp Neurol. 2003;181:213–223. doi: 10.1016/s0014-4886(03)00044-x. [DOI] [PubMed] [Google Scholar]
- McBride JL, Ramaswamy S, Gasmi M, Bartus RT, Herzog CD, Brandon EP, Zhou L, Pitzer MR, Berry-Kravis EM, Kordower JH. Viral delivery of glial cell line-derived neurotrophic factor improves behavior and protects striatal neurons in a mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2006;103:9345–9350. doi: 10.1073/pnas.0508875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Lin Y, Chen W, Yu F, Cao L, He Q, Chan PH, Li PA. Manganese Superoxide Dismutase Deficiency Exacerbates Ischemic Brain Damage Under Hyperglycemic Conditions by Altering Autophagy. Transl Stroke Res. 2011;2:42–50. doi: 10.1007/s12975-010-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutathione biosynthesis and its inhibition. Methods Enzymol. 1995;252:26–30. doi: 10.1016/0076-6879(95)52005-8. [DOI] [PubMed] [Google Scholar]
- Mejias R, Villadiego J, Pintado CO, Vime PJ, Gao L, Toledo-Aral JJ, Echevarria M, Lopez-Barneo J. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci. 2006;26:4500–4508. doi: 10.1523/JNEUROSCI.0122-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni G, Vasak M. Redox activity of alpha-synuclein-Cu is silenced by Zn(7)-metallothionein-3. Free Radic Biol Med. 2011;50:1471–1479. doi: 10.1016/j.freeradbiomed.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena R, Edwards P, Perez-Olvera O, Wischik CM. Monitoring pathological assembly of tau and beta-amyloid proteins in Alzheimer's disease. Acta Neuropathol. 1995;89:50–56. doi: 10.1007/BF00294259. [DOI] [PubMed] [Google Scholar]
- Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, Mutus B. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem. 2008;283:18513–18521. doi: 10.1074/jbc.M800440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Gupta RC, Yu Y, Zaja-Milatovic S, Aschner M. Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicol Appl Pharmacol. 2011;256:219–226. doi: 10.1016/j.taap.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2006;97:373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- Mittoux V, Ouary S, Monville C, Lisovoski F, Poyot T, Conde F, Escartin C, Robichon R, Brouillet E, Peschanski M, Hantraye P. Corticostriatopallidal neuroprotection by adenovirus-mediated ciliary neurotrophic factor gene transfer in a rat model of progressive striatal degeneration. J Neurosci. 2002;22:4478–4486. doi: 10.1523/JNEUROSCI.22-11-04478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel F, Haller RG. Energy deficit in Huntington disease: why it matters. J Clin Invest. 2011;121:493–499. doi: 10.1172/JCI45691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Maiti P, Lopes DH, Oien DB, Attar A, Liu T, Mittal S, Hayes J, Bitan G. Induction of methionine-sulfoxide reductases protects neurons from amyloid beta-protein insults in vitro and in vivo. Biochemistry. 2011;50:10687–10697. doi: 10.1021/bi201426b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Jernigan A, Borchelt D, Richardson A, Van Remmen H. MnSOD deficiency has a differential effect on disease progression in two different ALS mutant mouse models. Muscle Nerve. 2008;38:1173–1183. doi: 10.1002/mus.21049. [DOI] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Murata N, Noda Y, Tahara S, Kaneko T, Kinoshita N, Hatsuta H, Murayama S, Barnham KJ, Irie K, Shirasawa T, Shimizu T. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of Alzheimer disease. J Biol Chem. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, Watanabe E, Ozawa K, Nakano I. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Mol Ther. 2010;18:1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E. Hydroxyl radical formation resulting from the interaction of nitric oxide and hydrogen peroxide. Biochim Biophys Acta. 1998;1380:55–63. doi: 10.1016/s0304-4165(97)00125-6. [DOI] [PubMed] [Google Scholar]
- Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, Ten VS. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J Neurosci. 2012;32:3235–3244. doi: 10.1523/JNEUROSCI.6303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Yu M, Wang C, Xu Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J Neurochem. 2012;122:650–658. doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Fujimura M, Morita-Fujimura Y, Chan PH. Manganese Superoxide Dismutase Affects Cytochrome c Release and Caspase-9 Activation After Transient Focal Cerebral Ischemia in Mice. J Cereb Blood Flow Metab. 2001;21:557–567. doi: 10.1097/00004647-200105000-00010. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- Olivares-Corichi IM, Ceballos G, Ortega-Camarillo C, Guzman-Grenfell AM, Hicks JJ. Reactive oxygen species (ROS) induce chemical and structural changes on human insulin in vitro, including alterations in its immunoreactivity. Front Biosci. 2005;10:838–843. doi: 10.2741/1577. [DOI] [PubMed] [Google Scholar]
- Oliveira JM. Nature and cause of mitochondrial dysfunction in Huntington's disease: focusing on huntingtin and the striatum. J Neurochem. 2010;114:1–12. doi: 10.1111/j.1471-4159.2010.06741.x. [DOI] [PubMed] [Google Scholar]
- Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SR, Shin HJ, Lee JH, Chang CS, Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J. 1999;340(Pt 3):821–828. [PMC free article] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Park SU, Ferrer JV, Javitch JA, Kuhn DM. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NJ, Chen MJ, Russo-Neustadt AA. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. European Journal of Pharmacology. 2010;633:1–9. doi: 10.1016/j.ejphar.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson's disease accelerate age-related neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bove J, Dehay B, Jackson-Lewis V, Rabinovitch PS, Przedborski S, Vila M. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann Neurol. 2010;68:184–192. doi: 10.1002/ana.22034. [DOI] [PubMed] [Google Scholar]
- Perry TL, Godin DV, Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. J Neurosci. 2010;30:14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidori MC, Mecocci P, Browne SE, Senin U, Beal MF. Oxidative damage to mitochondrial DNA in Huntington's disease parietal cortex. Neurosci Lett. 1999;272:53–56. doi: 10.1016/s0304-3940(99)00578-9. [DOI] [PubMed] [Google Scholar]
- Poon HF, Hensley K, Thongboonkerd V, Merchant ML, Lynn BC, Pierce WM, Klein JB, Calabrese V, Butterfield DA. Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;39:453–462. doi: 10.1016/j.freeradbiomed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Potts MB, Rola R, Claus CP, Ferriero DM, Fike JR, Noble-Haeusslein LJ. Glutathione peroxidase overexpression does not rescue impaired neurogenesis in the injured immature brain. J Neurosci Res. 2009;87:1848–1857. doi: 10.1002/jnr.21996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S, Souza JM, Jackson-Lewis V, Lee VM, Ischiropoulos H. Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Neurochem. 2001;76:637–640. doi: 10.1046/j.1471-4159.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Averill D, Beffert U, Bastianetto S, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Davignon J, Quirion R, Poirier J. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer's disease is related to the apolipoprotein E genotype. Free Radic Biol Med. 1999;27:544–553. doi: 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Kordower JH. Gene therapy for Huntington's disease. Neurobiol Dis. 2012;48:243–254. doi: 10.1016/j.nbd.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Rana S, Dringen R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Structural characterization of copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson's disease. Proc Natl Acad Sci U S A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010;88:3530–3539. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Bensadoun JC, Deglon N, Aebischer P, Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: neuroprotection in murine models of Parkinson's disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rocha H, Garcia-Garcia A, Pickett C, Sumin L, Jones J, Chen H, Webb B, Choi J, Zhou Y, Zimmerman MC, Franco R. Compartmentalized oxidative stress in dopaminergic cell death induced by pesticides and complex I inhibitors: Distinct roles of superoxide anion and superoxide dismutases. Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rocha H, Garcia Garcia A, Zavala-Flores L, Li S, Madayiputhiya N, Franco R. Glutaredoxin 1 protects dopaminergic cells by increased protein glutathionylation in experimental Parkinson's disease. Antioxid Redox Signal. 2012;17:1676–1693. doi: 10.1089/ars.2011.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ML, Rush RA. Non-viral gene therapy for neurological diseases, with an emphasis on targeted gene delivery. J Control Release. 2012;157:183–189. doi: 10.1016/j.jconrel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- Rose F, Hodak M, Bernholc J. Mechanism of copper(II)-induced misfolding of Parkinson's disease protein. Sci Rep. 2011;1:11. doi: 10.1038/srep00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nunez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Song S, Facca A, Basit A, Epstein CJ. Transgenic murine dopaminergic neurons expressing human Cu/Zn superoxide dismutase exhibit increased density in culture, but no resistance to methylphenylpyridinium-induced degeneration. J Neurochem. 1997;68:58–67. doi: 10.1046/j.1471-4159.1997.68010058.x. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schönfeld P, Dymkowska D, Wojtczak L. Acyl-CoA-induced generation of reactive oxygen species in mitochondrial preparations is due to the presence of peroxisomes. Free Radical Biology and Medicine. 2009;47:503–509. doi: 10.1016/j.freeradbiomed.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Matthews RT, Muqit MM, Browne SE, Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- Seet RC, Lee CY, Chan BP, Sharma VK, Teoh HL, Venketasubramanian N, Lim EC, Chong WL, Looi WF, Huang SH, Ong BK, Halliwell B. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke. 2011;42:2326–2329. doi: 10.1161/STROKEAHA.111.618835. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Dore S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ince PG, Falkous G, Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–695. doi: 10.1002/ana.410380424. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Christen S, Ferriero DM. Genetic and pharmacologic manipulation of oxidative stress after neonatal hypoxia-ischemia. Int J Dev Neurosci. 2008;26:87–92. doi: 10.1016/j.ijdevneu.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, Young WL, Yang GY. Adeno-associated viral-vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- Shen L, Ji HF. Insights into the disappointing clinical trials of antioxidants in neurodegenerative diseases. J Alzheimers Dis. 2010;19:1141–1142. doi: 10.3233/JAD-2010-1307. [DOI] [PubMed] [Google Scholar]
- Shen Y, Muramatsu SI, Ikeguchi K, Fujimoto KI, Fan DS, Ogawa M, Mizukami H, Urabe M, Kume A, Nagatsu I, Urano F, Suzuki T, Ichinose H, Nagatsu T, Monahan J, Nakano I, Ozawa K. Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson's disease. Hum Gene Ther. 2000;11:1509–1519. doi: 10.1089/10430340050083243. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson's disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZH, Nie G, Duan XL, Rouault T, Wu WS, Ning B, Zhang N, Chang YZ, Zhao BL. Neuroprotective mechanism of mitochondrial ferritin on 6-hydroxydopamine-induced dopaminergic cell damage: implication for neuroprotection in Parkinson's disease. Antioxid Redox Signal. 2010;13:783–796. doi: 10.1089/ars.2009.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Hakuno F, Yamanaka D, Okajima H, Fukushima T, Hasegawa T, Ogata T, Toyoshima Y, Chida K, Kimura K, Sakoda H, Takenaka A, Asano T, Takahashi S. Paraquat-induced oxidative stress represses phosphatidylinositol 3-kinase activities leading to impaired glucose uptake in 3T3-L1 adipocytes. J Biol Chem. 2010;285:20915–20925. doi: 10.1074/jbc.M110.126482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N, Nagai R, Uchida K, Horiuchi S, Yamada S, Hirano A, Kawaguchi M, Yamamoto T, Sasaki S, Kobayashi M. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res. 2001;917:97–104. doi: 10.1016/s0006-8993(01)02926-2. [DOI] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Adachi Y, Mitsushita J, Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K, Miyazaki H, Kamata T. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J Biol Chem. 2010;285:4481–4488. doi: 10.1074/jbc.M109.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage. Hum Mol Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem. 2011;118:939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Rivera-Sanchez S, Castro MD, Acevedo-Torres K, Rane A, Torres-Ramos CA, Nicholls DG, Andersen JK, Ayala-Torres S. Mitochondrial DNA damage Is associated with reduced mitochondrial bioenergetics in Huntington's disease. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62:1758–1765. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of posttraumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Henry YK, Mattson MP, Appel SH. Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann Neurol. 1998;44:696–699. doi: 10.1002/ana.410440419. [DOI] [PubMed] [Google Scholar]
- Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med. 2008;45:667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Sorolla MA, Rodriguez-Colman MJ, Tamarit J, Ortega Z, Lucas JJ, Ferrer I, Ros J, Cabiscol E. Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free Radic Biol Med. 2010;49:612–621. doi: 10.1016/j.freeradbiomed.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- Spencer B, Marr RA, Rockenstein E, Crews L, Adame A, Potkar R, Patrick C, Gage FH, Verma IM, Masliah E. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 2008;9:109. doi: 10.1186/1471-2202-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem. 2011;286:8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Stridh MH, Correa F, Nodin C, Weber SG, Blomstrand F, Nilsson M, Sandberg M. Enhanced glutathione efflux from astrocytes in culture by low extracellular Ca2+ and curcumin. Neurochem Res. 2010;35:1231–1238. doi: 10.1007/s11064-010-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridh MH, Tranberg M, Weber SG, Blomstrand F, Sandberg M. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem. 2008;283:10347–10356. doi: 10.1074/jbc.M704153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5:525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Clark KR, Peel A, Mao XO, Chang Q, Simon RP, Greenberg DA. Adeno-associated virus-mediated delivery of BCL-w gene improves outcome after transient focal cerebral ischemia. Gene Ther. 2003;10:115–122. doi: 10.1038/sj.gt.3301868. [DOI] [PubMed] [Google Scholar]
- Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Carini M, Butterfield DA. Protein carbonylation. Antioxid Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci U S A. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chock PB, Stadtman ER. Oxidized messenger RNA induces translation errors. Proc Natl Acad Sci U S A. 2007;104:66–71. doi: 10.1073/pnas.0609737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Ali U, Iannello RC, Hertzog P, Crack PJ. Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J Neurochem. 2005;92:283–293. doi: 10.1111/j.1471-4159.2004.02863.x. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TH, Chen SL, Chiang YH, Lin SZ, Ma HI, Kuo SW, Tsao YP. Recombinant adeno-associated virus vector expressing glial cell line-derived neurotrophic factor reduces ischemia-induced damage. Exp Neurol. 2000;166:266–275. doi: 10.1006/exnr.2000.7505. [DOI] [PubMed] [Google Scholar]
- Tsikas D, Caidahl K. Recent methodological advances in the mass spectrometric analysis of free and protein-associated 3-nitrotyrosine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814:1–9. doi: 10.1016/j.jchromb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tsuru-Aoyagi K, Potts MB, Trivedi A, Pfankuch T, Raber J, Wendland M, Claus CP, Koh SE, Ferriero D, Noble-Haeusslein LJ. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol. 2009;65:540–549. doi: 10.1002/ana.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson's disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med. 2001;30:1163–1170. doi: 10.1016/s0891-5849(01)00513-5. [DOI] [PubMed] [Google Scholar]
- Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci. 2012;13:867–878. doi: 10.1038/nrn3383. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- Underwood BR, Imarisio S, Fleming A, Rose C, Krishna G, Heard P, Quick M, Korolchuk VI, Renna M, Sarkar S, Garcia-Arencibia M, O'Kane CJ, Murphy MP, Rubinsztein DC. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum Mol Genet. 2010;19:3413–3429. doi: 10.1093/hmg/ddq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Johnson JA. Decreased glutathione accelerates neurological deficit and mitochondrial pathology in familial ALS-linked hSOD1(G93A) mice model. Neurobiol Dis. 2011;43:543–551. doi: 10.1016/j.nbd.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini G, Colasanti M, Persichini T, Fioravanti E, Ascenzi P, Palomba L, Cantoni O, Musci G. Beta-amyloid inhibits NOS activity by subtracting NADPH availability. FASEB J. 2002;16:1970–1972. doi: 10.1096/fj.02-0186fje. [DOI] [PubMed] [Google Scholar]
- Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhu X, Kim Y, Li J, Huang S, Saleem S, Li RC, Xu Y, Dore S, Cao W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2012;52:928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu L, Zhang L, Peng Y, Zhou F. Redox reactions of the alpha-synuclein-Cu(2+) complex and their effects on neuronal cell viability. Biochemistry. 2010a;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM, Miller DS, Hong JS. MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005;19:1134–1136. doi: 10.1096/fj.04-2457fje. [DOI] [PubMed] [Google Scholar]
- Wang X, Moualla D, Wright JA, Brown DR. Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity. J Neurochem. 2010b;113:704–714. doi: 10.1111/j.1471-4159.2010.06638.x. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SHSY5Y cells. Mol Pharmacol. 2008;74:933–940. doi: 10.1124/mol.108.048546. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Okuda Y, Nonoguchi N, Zhao MZ, Kajimoto Y, Furutama D, Yukawa H, Shibata MA, Otsuki Y, Kuroiwa T, Miyatake S. Postischemic intraventricular administration of FGF-2 expressing adenoviral vectors improves neurologic outcome and reduces infarct volume after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:1205–1213. doi: 10.1097/01.WCB.0000136525.75839.41. [DOI] [PubMed] [Google Scholar]
- Wei T, Chen C, Hou J, Xin W, Mori A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim Biophys Acta. 2000;1498:72–79. doi: 10.1016/s0167-4889(00)00078-1. [DOI] [PubMed] [Google Scholar]
- Weir DW, Sturrock A, Leavitt BR. Development of biomarkers for Huntington's disease. Lancet Neurol. 2011;10:573–590. doi: 10.1016/S1474-4422(11)70070-9. [DOI] [PubMed] [Google Scholar]
- Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res. 1998;53:333–338. doi: 10.1016/s0169-328x(97)00313-6. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Bozinovski S, Hertzog PJ, Hickey MJ, Crack PJ. Absence of glutathione peroxidase-1 exacerbates cerebral ischemia-reperfusion injury by reducing post-ischemic microvascular perfusion. J Neurochem. 2008;107:241–252. doi: 10.1111/j.1471-4159.2008.05605.x. [DOI] [PubMed] [Google Scholar]
- Wright JA, Wang X, Brown DR. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB J. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci. 2008a;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Basha MR, Zawia NH. The environment, epigenetics and amyloidogenesis. J Mol Neurosci. 2008b;34:1–7. doi: 10.1007/s12031-007-0009-4. [DOI] [PubMed] [Google Scholar]
- Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc Natl Acad Sci U S A. 2013;110:7904–7909. doi: 10.1073/pnas.1217938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1–42- and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS. Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J Neurochem. 2005;95:732–744. doi: 10.1111/j.1471-4159.2005.03412.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mizuno Y, Mochizuki H. Parkin gene therapy for alphasynucleinopathy: a rat model of Parkinson's disease. Hum Gene Ther. 2005;16:262–270. doi: 10.1089/hum.2005.16.262. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Mita S, Kato S, Okado H, Ohama E, Uchino M. Effect on motor neuron survival in mutant SOD1 (G93A) transgenic mice by Bcl-2 expression using retrograde axonal transport of adenoviral vectors. Neurosci Lett. 2002;328:289–293. doi: 10.1016/s0304-3940(02)00454-8. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yang XF, Zheng XS, Liu WG, Feng JF. Bcl-2 gene therapy for apoptosis following traumatic brain injury. Chin J Traumatol. 2006;9:276–281. [PubMed] [Google Scholar]
- Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease. J Neurosci. 2011;31:2313–2320. doi: 10.1523/JNEUROSCI.4717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Wood NW. Cell death pathways in Parkinson's disease: role of mitochondria. Antioxid Redox Signal. 2009;11:2135–2149. doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem. 2009;284:31972–31981. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Bensadoun JC, Pereira de Almeida L, Leavitt BR, Gutekunst CA, Aebischer P, Hayden MR, Deglon N. Long-term lentiviral-mediated expression of ciliary neurotrophic factor in the striatum of Huntington's disease transgenic mice. Exp Neurol. 2004;185:26–35. doi: 10.1016/j.expneurol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Albers DS, Mirochnitchenko O, Nicklas WJ, Sonsalla PK. Energy stress-induced dopamine loss in glutathione peroxidase-overexpressing transgenic mice and in glutathione-depleted mesencephalic cultures. J Neurochem. 1997;68:426–429. doi: 10.1046/j.1471-4159.1997.68010426.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Rodriguez C, Spaulding J, Aw TY, Feng J. Age-dependent and tissue-related glutathione redox status in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2012a;28:655–666. doi: 10.3233/JAD-2011-111244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Graham DG, Montine TJ, Ho YS. Enhanced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J Neuropathol Exp Neurol. 2000;59:53–61. doi: 10.1093/jnen/59.1.53. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012b;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhang WR, Sato K, Iwai M, Nagano I, Manabe Y, Abe K. Therapeutic time window of adenovirus-mediated GDNF gene transfer after transient middle cerebral artery occlusion in rat. Brain Res. 2002;947:140–145. doi: 10.1016/s0006-8993(02)02923-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Near complete rescue of experimental Parkinson's disease with intravenous, non-viral GDNF gene therapy. Pharm Res. 2009;26:1059–1063. doi: 10.1007/s11095-008-9815-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlachetzki F, Zhang YF, Boado RJ, Pardridge WM. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum Gene Ther. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- Zhou F, Gomi M, Fujimoto M, Hayase M, Marumo T, Masutani H, Yodoi J, Hashimoto N, Nozaki K, Takagi Y. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res. 2009;1272:62–70. doi: 10.1016/j.brainres.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Zhou W, Milder JB, Freed CR. Transgenic mice overexpressing tyrosine-to-cysteine mutant human alpha-synuclein: a progressive neurodegenerative model of diffuse Lewy body disease. J Biol Chem. 2008;283:9863–9870. doi: 10.1074/jbc.M710232200. [DOI] [PubMed] [Google Scholar]
- Zou LL, Huang L, Hayes RL, Black C, Qiu YH, Perez-Polo JR, Le W, Clifton GL, Yang K. Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Ther. 1999;6:994–1005. doi: 10.1038/sj.gt.3300936. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]