Abstract
Purpose
To report the outcomes of current vitreoretinal surgical management of proliferative sickle retinopathy and to compare current methods to previous studies.
Design
A retrospective, interventional case series.
Methods
Fifteen eyes of 14 patients with proliferative sickle retinopathy were managed with vitreoretinal surgery over a 12-year period at a single institution.
Results
Nine patients had a sickle cell-hemoglobin C (SC) profile, 1 was sickle cell-beta+ thalassemia (S beta+-thal), and 4 were sickle cell trait (AS). All 15 eyes underwent pars plana vitrectomy (PPV): 6 for vitreous hemorrhage (VH), 1 for epiretinal membrane (ERM), and an additional 8 for tractional retinal detachment (RD) and/or rhegmatogenous RD. In addition, an encircling scleral buckle (SB) was used in 2 cases. 20-gauge PPV was used in 7 cases, 23-gauge in 3, and 25-gauge in 5. All 7 eyes with VH or ERM had improved vision postoperatively. Four (50%) of the 8 patients with traction and/or rhegmatogenous RD developed recurrent detachments and required a second operation. All retinas were attached at last follow-up, and visual acuity was 20/400 or better in all eyes. No cases of anterior segment ischemia were encountered.
Conclusions
Anterior segment ischemia is no longer a common occurrence in eyes undergoing surgery for proliferative sickle retinopathy. Although PPV has replaced the use of SB in many situations, an encircling SB may still be used in this population when necessary. Surgery for VH and ERM generally results in favorable outcomes, but eyes undergoing surgery for traction/rhegmatogenous RD carry a more guarded prognosis.
Introduction
Proliferative sickle retinopathy is characterized by the development of peripheral retinal neovascularization, vitreous hemorrhage, and tractional or combined tractional-rhegmatogenous retinal detachment. Natural history studies demonstrate a high rate of autoinfarction of neovascular complexes in the absence of treatment, but selected cases require vitreoretinal surgical management to improve or stabilize vision.1–3
The earliest surgical series for proliferative sickle retinopathy was published prior to the era of vitrectomy and reported a high rate of anterior segment ischemia, prompting some authors to recommend preoperative exchange transfusion and avoidance of an encircling scleral buckle.4 Subsequent reports demonstrated both improved outcomes and a lower rate of complications in this disease, despite the use of encircling scleral buckles in some cases.5–9
The present study describes the indications, treatments, and outcomes for surgically managed cases of proliferative sickle retinopathy at the Bascom Palmer Eye Institute over the past 12 years, and offers updated treatment recommendations compared to a previous study from this same institution.8
Methods
This study was a retrospective, interventional case series of all patients undergoing vitreoretinal surgery from 2001–2013 with an ICD-9 diagnosis code of sickle cell disease (282.60–282.68) or proliferative retinopathy not elsewhere classified (362.29). Data collected included demographics, sickle cell status, clinical indication for surgery, best-corrected visual acuity (BCVA), lens status, operative procedure(s), postoperative outcome, and duration of follow-up. Indications for surgical management included vitreous hemorrhage (VH), epiretinal membrane (ERM), traction retinal detachment (RD), rhegmatogenous RD, or combined traction/rhegmatogenous RD. The operating surgeon selected the surgical approach for each individual case: there was no defined management protocol in this study. The institutional review board at the University of Miami approved the study protocol, and HIPAA compliance was maintained.
Results
One hundred and sixty-six records were identified, but only 15 eyes of 14 patients were operated for a vitreoretinal condition associated with proliferative sickle retinopathy. One case was excluded because the patient had a penetrating keratoplasty and corneal ulcer prior to his vitreoretinal complication, and it was felt that these pre-existing conditions would complicate the analysis of his vitreoretinal management. Three patients had diabetes mellitus, and 1 of these 3 patients had early changes of non-proliferative diabetic retinopathy (this patient underwent surgery for ERM only). The other 2 patients showed no signs of diabetic retinopathy. All patients had at least 2 months of follow-up. There were 8 male and 6 female patients with a mean age of 48 years (range: 23–69) during the study period. Six right eyes and 9 left eyes had surgery. Fourteen of 15 eyes were phakic at the time of primary surgery. All cases were performed under local anesthesia. Hemoglobin subtypes were as follows: sickle cell-hemoglobin C (SC, 9), sickle cell-beta+ thalassemia (S beta+-thal, 1), and sickle cell trait (AS, 4). The mean postoperative follow-up was 26.7 months (range: 2–140 months, Tables 1 and 2).
Table 1.
Demographics and Clinical Features of Patients Undergoing Vitrectomy for Complications of Proliferative Sickle Retinopathy
| Case | Gender | Age (Years) | Sickle Type | Diabetes | Eye | Preoperative Visual Acuity |
|---|---|---|---|---|---|---|
| 1 | Male | 67 | SC | No | Right | HM |
| 2 | Male | 59 | AS | No | Right | LP |
| 3 | Female | 59 | SC | No | Left | 20/200 |
| 4 | Male | 39 | AS | No | Left | CF 5 Feet |
| 5 | Female | 61 | S B+ Thal | Yes, without NPDR | Left | CF 3 Feet |
| 6 | Female | 42 | SC | Yes, without NPDR | Left | HM |
| 7 | Female | 69 | SC | Yes, with NPDR | Right | 20/200 |
| 8 | Female | 54 | AS | No | Both | HM (Right) 20/40 (Left) |
| 9 | Male | 23 | SC | No | Left | 20/200 |
| 10 | Male | 28 | SC | No | Left | 20/200 |
| 11 | Male | 33 | SC | No | Left | 20/400 |
| 12 | Male | 49 | SC | No | Right | 20/80 |
| 13 | Female | 53 | AS | No | Left | HM |
| 14 | Male | 29 | SC | No | Right | HM |
AS: sickle cell trait, CF: counting fingers, HM: hand motions, LP: light perception, NPDR: non-proliferative diabetic retinopathy, S B+Thal: sickle cell-Beta+ Thalassemia disease, SC: sickle cell – hemoglobin C disease
Table 2.
Primary Surgical Procedures and Outcomes for Complications of Proliferative Sickle Retinopathy
| Case | Indication | Surgery | Valved Cannula | Last Recorded Visual Acuity | Follow-up |
|---|---|---|---|---|---|
| 1 | VH | 20G PPV, EL | No | 20/40 | 3 months |
| 2 | VH | 20G PPV | No | 20/20 | 40 months |
| 3 | VH, ERM | 20G PPV, MP, EL, Air | No | 20/30 | 63 months |
| 4 | VH | 20G PPV, MP, EL, SF6 | No | 20/20 | 4 months |
| 5 | VH | Phaco/IOL, 23G PPV, EL, SF6 | No | 20/40 | 39 months |
| 6 | VH | 20G PPV, Cryotherapy | No | 20/30 | 140 months |
| 7 | ERM | 20G PPV, MP, EL | No | 20/60 | 50 months |
| 8R | RRD | SB, 23G PPV, EL, C3F8 | Yes | 20/60+2 | 11 months |
| 8L | RRD | 25G PPV, EL, C3F8 | Yes | 20/200 | 2 months |
| 9 | VH/TRD/RRD | SB, 20G PPV, MP, EL, SO | No | 20/400 | 24 months |
| 10 | TRD | 23G PPV, MP, EL, SO | Yes | 20/80 | 9 months |
| 11 | TRD, ERM | 25 G PPV, MP, EL, C3F8 | Yes | 20/300 | 2 months |
| 12 | TRD/RRD | 25G PPV, MP, EL, SO | Yes | 20/400 | 7 months |
| 13 | VH/TRD/RRD | Phaco/IOL, 25G PPV, EL, SF6 | Yes | 20/400 | 3 months |
| 14 | VH/TRD/RRD | 25G PPV EL, C3F8 | Yes | 20/100 | 3 months |
C3F8: perfluoropropane gas, EL: endolaser, ERM: epiretinal membrane, G: gauge, IOL: intraocular lens, MP: membrane peel, Phaco: phacoemulsification, PPV: pars plana vitrectomy, RRD: rhegmatogenous retinal detachment, SF6: sulfur hexafluoride gas, SO: silicone oil, TRD: tractional retinal detachment, VH: vitreous hemorrhage
Six vitreoretinal procedures were performed for the primary indication of VH, and 1 operation was performed for ERM. Of these 7 eyes, 6 underwent 20-gauge pars plana vitrectomy (PPV), and one underwent 23-gauge PPV. Each of these cases was performed with non-valved cannulas. One patient (Case 1) developed a postoperative hyphema and elevated intraocular pressure that was successfully managed with topical steroid and ocular antihypertensive medications. The preoperative BCVA of the patients ranged from 20/200-light perception, and all 7 eyes had significantly improved BCVA at last follow-up, ranging from 20/20 – 20/60 (Table 2).
Eight eyes underwent vitrectomy for traction RD and/or rhegmatogenous RD. Of these detachments, 7 (88%) involved the macula. The preoperative BCVA ranged from 20/40 to HM (hand motions). An encircling scleral buckle (SB) was employed in 2 cases in order to provide additional support for peripheral traction: a #41 band was used in one case, and a #240 band was used in the second case. 20-gauge PPV was used in 1 case, 23-gauge in 2 cases, and 25-gauge in 5 cases. Valved cannulas were employed in all cases except for the 20-gauge PPV. Silicone oil was used in 3 cases, and gas was used in 5 cases. Final BCVA was improved in 5 of these 8 cases (63%), with cataract limiting visual potential in 1 of the remaining 3 cases. All eyes achieved anatomic success at last follow-up, and all eyes achieved 20/400 or better vision (Figures 1 and 2, Table 2).
Figure 1.
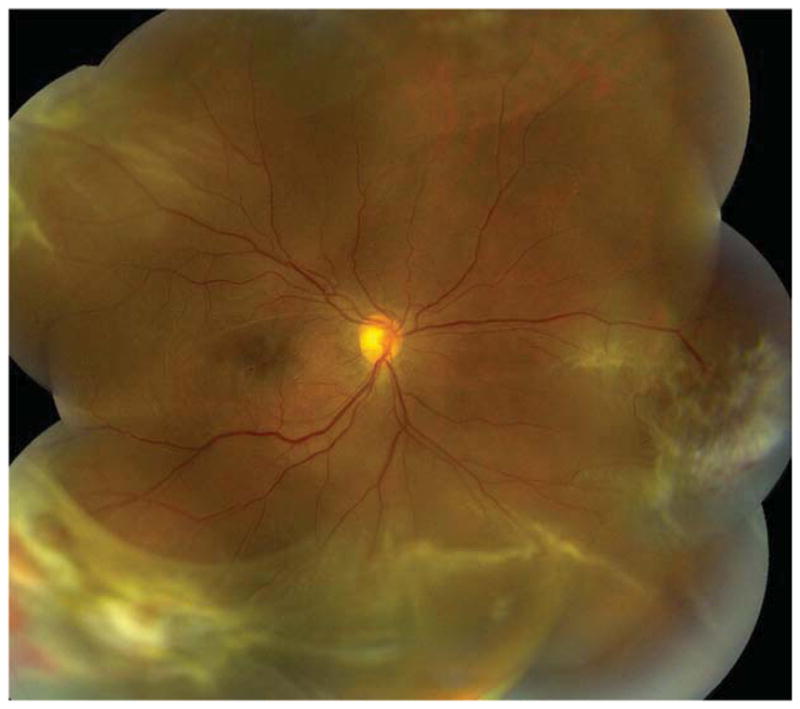
Case 12: A 49-year-old male with sickle cell-hemoglobin C disease had a progressive combined tractional/rhegmatogenous retinal detachment (RD) involving the macula. Best corrected visual acuity (BCVA) was 20/80-2. He underwent 25-gauge pars plana vitrectomy (PPV), endolaser to the area of neovascularization and margin of RD, membrane peel, retinectomy, and silicone oil tamponade. Four months later, he developed a recurrent RD with proliferative vitreoretinopathy, and he underwent a second 25-gauge PPV, membrane peel, and silicone oil tamponade. Three months following the second procedure, his retina is attached, and BCVA is 20/400.
Figure 2.
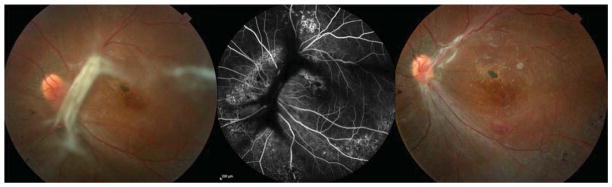
Case 11: A 33-year-old male with sickle cell-hemoglobin C disease developed a progressive tractional retinal detachment in the left eye. Best corrected visual acuity (BCVA) was 20/400. He underwent 25-gauge pars plana vitrectomy (PPV), endolaser to peripheral vitreoretinal proliferation, membrane peeling, and 14% C3F8 gas tamponade. BCVA 2 months after the operation was 20/300, with vision limited by macular atrophy and lamellar hole. Left: A tractional vitreoretinal membrane is involving the nasal and superior macula. There is an epiretinal membrane with lamellar hole. Middle: Fluorescein angiography revealed shadowing of fluorescence from the tractional membrane and areas of hyperfluorescence from multiple window defects, thought secondary to a previous inferior choroidal infarct. Right: Two months after PPV, the traction is released, and the retina is attached; however, BCVA is limited by pre-existing foveal atrophy.
The surgical objectives (clearance of vitreous hemorrhage, improvement of macular pucker, complete retinal reattachment) were achieved by a single operation in 9 (60%) of 15 eyes; 6 of 15 eyes underwent additional vitreoretinal surgery, including 4 for recurrent RD, 1 for silicone oil removal, and 1 for ERM peeling (Table 3). One case with an initial indication of VH developed a giant retinal tear following the initial vitrectomy, but was managed successfully with pneumatic retinopexy. In addition, 7 eyes underwent subsequent cataract extraction. Exchange transfusion was not performed for any patients and anterior segment ischemia did not occur in any study eyes postoperatively.
Table 3.
Additional Procedures after Initial Vitrectomy for Proliferative Sickle Retinopathy
| Case | Indication for 2nd Surgery | Second Surgery | Indication for 3rd Surgery | Third Surgery |
|---|---|---|---|---|
| 2 | Giant Retinal Tear | Pneumatic Retinopexy | Cataract | Phaco/IOL |
| 3 | Cataract | Phaco/IOL | None | |
| 5 | ERM | 23G PPV, MP | None | |
| 7 | Cataract | Phaco/IOL | None | |
| 8R | Cataract | Phaco/IOL | None | |
| 9 | Cataract, Retained SO, PVR, Recurrent RD | Phaco/IOL, 20G PPV, SO Removal, MP, EL, C3F8 | PVR, Recurrent RD | IOL removal, 20G PPV, MP, EL, SO |
| 10 | Cataract, Retained SO | Phaco/IOL, 25G PPV SO Removal, MP, EL | None | |
| 12 | PVR, Recurrent RD | 25G PPV, MP, EL SO | None | |
| 13 | PVR, Recurrent RD | 25G PPV, MP, PFO, EL, SO | None | |
| 14 | Cataract, PVR, Recurrent RD | Phaco/IOL, 25G PPV, MP, EL, SO | None |
C3F8: perfluoropropane gas, EL: endolaser, ERM: epiretinal membrane, G: gauge, IOL: intraocular lens, MP: membrane peel, PFO: perfluro-n-octane liquid, Phaco: phacoemulsification, PPV: pars plana vitrectomy, PVR: proliferative vitreoretinopathy, R: Right, RD: retinal detachment, SO: silicone oil
Features of Cases with Recurrent Detachment
Four eyes (Cases 9, 12, 13, 14) developed recurrent detachments following the initial vitrectomy (Table 3). Three patients had an SC hemoglobin profile, while 1 was AS. All 4 eyes featured both traction and rhegmatogenous components, and the macula was detached in all eyes. In 3 eyes, a vitreous hemorrhage was present. The initial BCVA ranged from 20/80 to HM. An encircling scleral buckle with a #240 band was used in 1 case, and a retinectomy was performed in another case. The choice of tamponade agent was silicone oil in 2 cases, perfluoropropane gas in 1 case, and sulfur hexafluoride gas in 1 case. Proliferative vitreoretinopathy was a factor for recurrent detachment in all 4 eyes. Recurrent vitreous hemorrhage occurred in 1 case and was successfully cleared at the time of the second operation. The time from initial to second surgery ranged from 2 to 11 months. Case 9 required a third operation and was left aphakic under silicone oil tamponade. At last follow-up, all 4 cases had retinal reattachment, and BCVA ranged from 20/100–20/400.
Discussion
The current study of patients undergoing vitreoretinal surgery for proliferative sickle retinopathy reports contemporary surgical techniques and outcomes. Although exchange transfusions were not performed, and an encircling scleral buckle was used in 2 cases, no cases of anterior segment ischemia were encountered.
The first report of surgery for proliferative sickle retinopathy was published in 1971 by Ryan and Goldberg.4 They reported a high incidence of anterior segment ischemia in eyes undergoing standard scleral buckling for retinal detachment. They reasoned that the encircling procedure and surgically-induced inflammation interfered with blood flow in the ciliary vessels sufficiently to precipitate localized sickling and ischemia. Thus, their recommendations included preoperative exchange transfusion, local anesthesia with retrobulbar anesthetics without sympathomimetics, supplemental oxygen, avoidance of treatment or compression of long posterior ciliary vessels, careful intraocular pressure control, avoidance of excessive cryopexy, and avoidance of extraocular muscle removal intraoperatively, among other suggestions. Many of these recommendations reflected techniques of the day, before vitrectomy became available.
In 1982, Jampol and associates reported 19 eyes that underwent vitrectomy and/or scleral buckling procedures for complications of proliferative sickle retinopathy.6 Patients who had rhegmatogenous RD or VH underwent surgery by SB and PPV, respectively, with excellent outcomes. However, only 50% of eyes undergoing combined SB/PPV for a traction RD with or without a rhegmatogenous component had visual improvement following surgery. In addition, iatrogenic retinal breaks occurred in 5 of 15 patients with combined traction/rhegmatogenous RD. Most patients in this series underwent exchange transfusions prior to surgery; however, 5 patients with SC disease did not undergo exchange transfusions – one of these patients (who also received epinephrine in the retrobulbar anesthetic) developed anterior segment ischemia.
More recently, the risks of exchange transfusion forced renewed scrutiny of the need for transfusions. In 1988 Pulido reported on 11 eyes of 10 patients with SC disease who underwent PPV for the complications of proliferative sickle retinopathy.8 Nine eyes had traction RD with or without rhegmatogenous RD or VH, and 2 eyes had VH alone. BCVA improved in both patients with VH, and 8 of 9 patients with traction RD. An encircling scleral buckle was employed in 5 cases. No cases of anterior segment ischemia occurred, but an exchange transfusion was performed in 5 patients encountered earlier in the series - 2 undergoing PPV alone, and 3 undergoing SB in addition to PPV. The authors concluded that the risks of exchange transfusions outweighed the potential benefits, especially given that the complications of anterior segment ischemia could likely be avoided with attention to factors other than preoperative exchange transfusions, such as adequate hydration, oxygenation, and intraocular pressure control.
In 2009, Williamson treated 18 eyes of 17 patients with complications of proliferative sickle retinopathy with PPV- 8 eyes had VH alone, 3 had ERM, 2 had macular hole, 3 had rhegmatogenous RD with or without VH, and 2 had traction RD with or without VH.9 Iatrogenic breaks occurred in 7 (39%) of 18 eyes, and 15 (83%) of 18 eyes had improved postoperative vision. All 8 eyes with VH had improved vision following surgery, as did 3 eyes with rhegmatogenous RD. Of the 2 eyes with traction RD, 1 improved after surgery, and the other remained detached under silicone oil. All eyes underwent 20-gauge PPV, and no patients had preoperative exchange transfusions.
In the current study, the most common indication for surgery was traction RD or combined traction/rhegmatogenous RD (Table 4). There were some patients with VH only or ERM, cases that might engender less extensive surgical maneuvers and less stimulus for anterior segment ischemia. The complication rate was higher for eyes with traction RD and/or rhegmatogenous RD, as 50% required an additional vitreoretinal procedure for management of recurrent RD. All eyes achieved anatomic success at last follow-up. The visual outcomes for the proliferative sickle retinopathy eyes with traction +/− rhegmatogenous RD approximate those of diabetic eyes with traction RD involving the macula, where visual acuity may improve in 59–70% and worsen in 15–34% following vitrectomy.10,11
Table 4.
Comparison of Rhegmatogenous and Traction Retinal Detachment Outcomes in Published Proliferative Sickle Retinopathy Case Series
| Authors/Year | Institution | Surgical Indication | Procedures | SB | Last Measured VA ≥ 20/400 | Anterior Segment Ischemia |
|---|---|---|---|---|---|---|
| Ryan, Goldberg 1971 | Wilmer/UIC | RRD | 7 | 5 | 71% | 4 |
| TRD +/− RRD | 3 | 3 | 0% | 2 | ||
| Jampol et al 1982 | UIC | RRD | 4 | 4 | 100% | 0 |
| TRD +/− RRD | 10 | 10 | 50% | 1 | ||
| Pulido et al 1988 | BPEI | RRD | 0 | 0 | No cases | 0 |
| TRD +/− RRD | 9 | 6 | 78% | 0 | ||
| Williamson et al 2009 | St. Thomas’ Hospital | RRD | 3 | 0 | 100% | 0 |
| TRD +/− RRD | 2 | 0 | 100% | 0 | ||
| Current Study 2013 | BPEI | RRD | 2 | 0 | 100% | 0 |
| TRD +/− RRD | 6 | 2 | 100% | 0 |
BPEI: Bascom Palmer Eye Institute, University of Miami, RRD: rhegmatogenous retinal detachment, SB: scleral buckle, TRD: tractional retinal detachment, UIC: University of Illinois Eye and Ear Infirmary, VA: visual acuity, Wilmer: Wilmer Institute, Johns Hopkins University
There are two important limitations to the current series. First, due to the study’s retrospective nature, the choice of vitreoretinal management technique did not follow a standardized protocol. Second, because proliferative sickle retinopathy is an uncommon condition, the sample size is limited in this study despite its being a collaboration of several surgeons at a large university practice.
Since the initial report 42 years ago, it is evident that vitreoretinal surgical techniques have changed substantially. Scleral buckles are no longer placed high and broad, and the introduction of PPV and, even more recently, the preponderance of small incision techniques have resulted in less dependence on scleral buckling and shorter operating times. Wide-field viewing systems have enabled better visualization for peripheral dissection, and valved cannulas have improved intraocular pressure management during surgery. Together, these changes in vitreoretinal techniques may result in less inflammation-induced ischemia in this vulnerable population.
Based on the experience gained from the current study, patients with proliferative sickle retinopathy may not always be aware of their hemoglobin subtype, often because they do not have appropriate access to a primary care physician. One patient who underwent PPV for VH reported a history of sickle trait, but also stated that she had experienced 5 systemic pain crisis episodes in the past, events that would be atypical for a patient with sickle trait alone. In this patient, the funduscopic evaluation revealed sea fans in the peripheral retina. Because the patient did not currently have a primary care physician, the sickle cell prep was performed and was confirmed to be positive. A subsequent hemoglobin electrophoresis revealed the SC genotype, rather than AS. This case illustrates the importance of confirming the patient’s reported sickle status with hemoglobin electrophoresis, as the results may impact future ocular and systemic management.
Proliferative sickle retinopathy remains a challenging ocular condition, and anterior segment ischemia is a possibility even with modern surgical techniques and in the absence of scleral buckling or muscle detachment.12 Although the value of prophylactic laser treatment was not addressed in the current study, this question has been investigated previously.13–20 Exchange transfusions do not seem to be necessary, but prior recommendations to maintain adequate perioperative hydration and oxygenation, and to avoid vasoconstrictive agents such as epinephrine in both the retrobulbar anesthetic and irrigating solution, are still potentially important. When managing elevated intraocular pressure, one should refrain from using carbonic anhydrase inhibitors.21 Encircling scleral buckles may be applied safely, but avoiding unnecessarily high buckles is still recommended. Given the severity of disease occurring in younger patients, eyes requiring surgery are often phakic, and unlike the more posterior pathology found in proliferative diabetic retinopathy, the traction points in proliferative sickle retinopathy that require dissection are often encountered anteriorly in areas of ischemic retina. Together, these factors contribute to the high rate of iatrogenic tears reported in previous studies, as well as a lower rate of single operation success. For those patients undergoing a second surgery for traction/rhegmatogenous RD, useful vision may still be achieved.
Acknowledgments
Funding/Support: This study was supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, and Department of Defense (DOD-Grant#W81XWH-09-1-0675). RC was supported by a merit award from the Heed Ophthalmic Foundation and Society of Heed Fellows.
Biography
 Royce Chen, MD, is a second-year fellow in vitreoretinal surgery at the Bascom Palmer Eye Institute. He completed his ophthalmology residency at Columbia University in New York City, where he served as Chief resident. Dr. Chen is a Heed Fellow and has an interest in retinal imaging and the vitreoretinal management of complex retinal detachments and macular holes. He has accepted a position as Assistant Professor in the Department of Ophthalmology at Columbia University.
Royce Chen, MD, is a second-year fellow in vitreoretinal surgery at the Bascom Palmer Eye Institute. He completed his ophthalmology residency at Columbia University in New York City, where he served as Chief resident. Dr. Chen is a Heed Fellow and has an interest in retinal imaging and the vitreoretinal management of complex retinal detachments and macular holes. He has accepted a position as Assistant Professor in the Department of Ophthalmology at Columbia University.
Footnotes
Author Contributions: design and conduct of study (RC, HF, WL), management, analysis, and interpretation of data (RC, HF, WL), preparation, review, or approval of manuscript (RC, HF, WL, DP, RI, JD, WS).
Financial Disclosures: HF is a consultant for Santen Pharmaceutical Co., Ltd. (Osaka, Japan) and Vindico Medical Education (Thorofare, NJ). JD is a consultant for Santen Pharmaceutical Co., Ltd. (Osaka, Japan), Xoma Corporation (Berkeley, CA), and Clearside Biomedical (Alpharetta, GA). The remaining authors (RC, WL, DP, RI, and WS) indicate no financial support or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg MF. Natural history of untreated proliferative sickle retinopathy. Arch Ophthalmol. 1971;85(4):428–37. doi: 10.1001/archopht.1971.00990050430006. [DOI] [PubMed] [Google Scholar]
- 2.Downes SM, Hambleton IR, Chuang EL, Lois N, Serjeant GR, Bird AC. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology. 2005;112(11):1869–75. doi: 10.1016/j.ophtha.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Condon PI, Serjeant GR. Behaviour of untreated proliferative sickle retinopathy. Br J Ophthalmol. 1980;64(6):404–11. doi: 10.1136/bjo.64.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan SJ, Goldberg MF. Anterior segment ischemia following scleral buckling in sickle cell hemoglobinopathy. Am J Ophthalmol. 1971;72(1):35–50. doi: 10.1016/0002-9394(71)91588-1. [DOI] [PubMed] [Google Scholar]
- 5.Brazier DJ, Gregor ZJ, Blach RK, Porter JB, Huehns ER. Retinal detachment in patients with proliferative sickle cell retinopathy. Trans Ophthalmol Soc U K. 1986;105 (Pt 1):100–5. [PubMed] [Google Scholar]
- 6.Jampol LM, Green JL, Jr, Goldberg MF, Peyman GA. An update on vitrectomy surgery and retinal detachment repair in sickle cell disease. Arch Ophthalmol. 1982;100(4):591–3. doi: 10.1001/archopht.1982.01030030593008. [DOI] [PubMed] [Google Scholar]
- 7.Morgan CM, D’Amico DJ. Vitrectomy surgery in proliferative sickle retinopathy. Am J Ophthalmol. 1987;104(2):133–8. doi: 10.1016/0002-9394(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 8.Pulido JS, Flynn HW, Jr, Clarkson JG, Blankenship GW. Pars plana vitrectomy in the management of complications of proliferative sickle retinopathy. Arch Ophthalmol. 1988;106(11):1553–7. doi: 10.1001/archopht.1988.01060140721042. [DOI] [PubMed] [Google Scholar]
- 9.Williamson TH, Rajput R, Laidlaw DA, Mokete B. Vitreoretinal management of the complications of sickle cell retinopathy by observation or pars plana vitrectomy. Eye. 2009;23(6):1314–20. doi: 10.1038/eye.2008.296. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction retinal detachment of the macula. Arch Ophthalmol. 1987;105(4):497–502. doi: 10.1001/archopht.1987.01060040067035. [DOI] [PubMed] [Google Scholar]
- 11.Yang CM, Su PY, Yeh PT, Chen MS. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: clinical manifestations and surgical outcome. Can J Ophthalmol. 2008;43(2):192–8. doi: 10.3129/i08-007. [DOI] [PubMed] [Google Scholar]
- 12.Leen JS, Ratnakaram R, Del Priore LV, Bhagat N, Zarbin MA. Anterior segment ischemia after vitrectomy in sickle cell disease. Retina. 2002;22(2):216–9. doi: 10.1097/00006982-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hanscom TA. Indirect treatment of peripheral retinal neovascularization. Am J Ophthalmol. 1982;93(1):88–91. doi: 10.1016/0002-9394(82)90704-8. [DOI] [PubMed] [Google Scholar]
- 14.Rednam KR, Jampol LM, Goldberg MF. Scatter retinal photocoagulation for proliferative sickle cell retinopathy. Am J Ophthalmol. 1982;93(5):594–9. doi: 10.1016/s0002-9394(14)77374-x. [DOI] [PubMed] [Google Scholar]
- 15.Cruess AF, Stephens RF, Magargal LE, Brown GC. Peripheral circumferential retinal scatter photocoagulation for treatment of proliferative sickle retinopathy. Ophthalmology. 1983;90(3):272–8. doi: 10.1016/s0161-6420(83)34577-2. [DOI] [PubMed] [Google Scholar]
- 16.Kimmel AS, Magargal LE, Stephens RF, Cruess AF. Peripheral circumferential retinal scatter photocoagulation for the treatment of proliferative sickle retinopathy. An update. Ophthalmology. 1986;93(11):1429–34. doi: 10.1016/s0161-6420(86)33549-8. [DOI] [PubMed] [Google Scholar]
- 17.Kimmel AS, Magargal LE, Tasman WS. Proliferative sickle retinopathy and neovascularization of the disc: regression following treatment with peripheral retinal scatter laser photocoagulation. Ophthalmic Surg. 1986;17(1):20–2. [PubMed] [Google Scholar]
- 18.Farber MD, Jampol LM, Fox P, et al. A randomized clinical trial of scatter photocoagulation of proliferative sickle cell retinopathy. Arch Ophthalmol. 1991;109(3):363–7. doi: 10.1001/archopht.1991.01080030065040. [DOI] [PubMed] [Google Scholar]
- 19.Jampol LM, Farber M, Rabb MF, Serjeant G. An update on techniques of photocoagulation treatment of proliferative sickle cell retinopathy. Eye. 1991;5 (Pt 2):260–3. doi: 10.1038/eye.1991.41. [DOI] [PubMed] [Google Scholar]
- 20.Sayag D, Binaghi M, Souied EH, et al. Retinal photocoagulation for proliferative sickle cell retinopathy: a prospective clinical trial with new sea fan classification. Eur J Ophthalmol. 2008;18(2):248–54. doi: 10.1177/112067210801800213. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg MF. The diagnosis and treatment of sickled erythrocytes in human hyphemas. Trans Am Ophthalmol Soc. 1978;76:481–501. [PMC free article] [PubMed] [Google Scholar]


