Abstract
Uterine leiomyomas (fibroids) are a major public health problem. Current medical treatments with GnRH analogs do not provide long-term benefit. Thus, permanent shrinkage or inhibition of fibroid growth via medical means remains a challenge. The AKT pathway is a major growth and survival pathway for fibroids. We propose that AKT inhibition results in a transient regulation of specific mechanisms that ultimately drive cells into cellular senescence or cell death. In this study, we investigated specific mechanisms of AKT inhibition that resulted in senescence. We observed that administration of MK-2206, an allosteric AKT inhibitor, increased levels of reactive oxygen species, up-regulated the microRNA miR-182 and several senescence-associated genes (including p16, p53, p21, and β-galactosidase), and drove leiomyoma cells into stress-induced premature senescence (SIPS). Moreover, induction of SIPS was mediated by HMGA2, which colocalized to senescence-associated heterochromatin foci. This study provides a conceivable molecular mechanism of SIPS by AKT inhibition in fibroids.
Uterine leiomyomas (fibroids) are smooth-muscle tumors of the myometrium that occur in 77% of women in the United States (1, 2). They cause significant morbidity from profuse menstrual bleeding and pelvic discomfort as well as reproductive dysfunction and account for more than 200 000 hysterectomies each year. Treatment by GnRH agonists and antagonists can reduce/shrink tumor size, but fibroids return to original size after termination of the therapy (2, 3). Furthermore, the side effects of antihormonal treatment are not ideal for long-time usage, and therefore only preoperative treatment is recommended (4). Long-term therapies aimed at permanent shrinkage or inhibition of fibroid growth are currently not available in the United States. Thus, nonhormonal, noninvasive therapeutic modalities are an attractive alternative.
We have previously shown that the AKT pathway is activated in approximately 30% of fibroids as determined by immunohistochemistry (5). This is most likely an underestimation due to the insensitivity of immunohistochemistry staining because more sensitive Western blot analysis reveal that all leiomyoma tissues tested have remarkably higher phosphorylated (Ser473)-AKT levels than matched myometrial tissues, establishing AKT activation as a potential molecular signature of leiomyomas (5, 6, 8). Although the AKT pathway is known to promote proliferation and survival in many tissues (9), the underlying molecular mechanisms are cell specific and complex involving the interaction of numerous proteins, genes, and pathways. Development of therapeutic modalities by inactivation of the AKT pathway may provide a valuable nonhormonal treatment option for this common and understudied disease that affects millions of reproductive aged women in the United States and worldwide. Inhibition of mammalian target of rapamycin (mTOR), a downstream of AKT pathway, has been reported previously (10). MK-2206 is an allosteric AKT inhibitor and is currently in clinical trials for some solid tumors. Preclinical, MK-2206 is effective in reducing xenograft growth in models of breast, prostate, nonsmall cell lung, and ovarian cancers and, more recently, uterine leiomyomas (11–13). In phase 1 trials, this inhibitor was shown to have antitumor properties, while causing minor side effects, including skin rash, nausea, pruritus, hyperglycemia, and diarrhea (14). Currently, phase I and II trials are being conducted with MK-2206 in solid tumors and blood cancers (www.clinicaltrials.gov).
The AKT pathway is tightly regulated in a normal cell depending on whether the cells undergo proliferation vs cellular senescence (15). Recent studies revealed that AKT inhibition results in stress-induced premature senescence (SIPS) in some cell types (16, 17). Naturally occurring cellular senescence is common in fibroids (18). Furthermore, because the AKT pathway is commonly activated in fibroids (6–8) and it is key for survival of leiomyomas (11), we hypothesized that inhibition of AKT can lead to cellular senescence in leiomyoma.
In this study, we investigated the mechanisms by which AKT inhibition induces senescence in fibroid cells in vitro. We observed that administration of MK-2206, an AKT inhibitor, increased levels of reactive oxygen species (ROS), up-regulated miR-182, HMGA2, and several senescence-associated genes, and promoted SIPS in leiomyoma cells.
Materials and Methods
Tissue collection and cell culture
Leiomyoma tissues were collected from premenopausal women undergoing hysterectomy or myomectomy at Northwestern University Prentice Women's Hospital (Chicago IL) according to an IRB-approved protocol. Women included in the study were not taking hormonal contraceptives or GnRH antagonist or agonist (GnRHa) at least 3 months prior to tissue collection. Consent was granted by all women included in the study. Tumor sections adjacent to the peripheral zone were collected (19) and minced into small pieces and digested with collagenase A and DNAase (Sigma Aldrich) for 6 hours on a 37°C tissue shaker. The digested material was filtered to obtain single-cell suspension. The primary cells were cultured in smooth muscle cell basal medium (SmBM; Clonetics, Lonza Group) no longer than 10 days. All experiments involving primary leiomyoma cells were repeated with cells from at least 3 different patient tissues. More than 10 leiomyoma tissues were collected for this study. The human myometrial cell line myo-hTert and the leiomyoma cell line DD-HLM cells were kindly provided by C. Mendelson (University of Texas Southwestern) and A.Al Hendy (Meharry Medical College), respectively. Cells were maintained in advanced DMEM/F12 (1:1) (Invitrogen) medium supplemented with 10% fetal bovine serum (USA Scientific).
Treatment of cells
DD-HLM or primary leiomyoma cells were cultured on tissue culture plates, glass coverslips, or in 3-dimensional collagen pellets. Cells were treated with MK-2206 (generously provided by Merck Sharp & Dohme, Corp and the National Cancer Institute, National Institutes of Health), BEZ235 (SelleckChem), GDC0980 (SelleckChem), or doxorubicin (DOX; Sigma). Cells were also pulse treated once each hour for 5 hours with hydrogen peroxide (H2O2; 100 μM) and cultured for up to 48 hours. In some cases, N-acetyl-cysteine (NAC; Sigma) was added to the cells 30 minutes prior to cotreatment with MK-2206 for an additional 1 hour.
3-Dimensional leiomyoma cell cultures
Primary cells or immortalized cells were trypsinized from the plate and washed twice with PBS/solution. Cell count and viability were confirmed using 0.4% Trypan Blue solution. Cells were suspended into rat type IV collagen (1 mg/mL, BD Biosciences) at 106 per 40 μL. The 40-μL cell-collagen mixture was dropped onto the Millicell Hanging Cell Culture Insert (pore size 3.0 μm, Millipore Corp.) placed in 24-well plate, and incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. After 0.5 hour, cultured medium was added into both the insert and the well and the system was incubated overnight and then treated with MK-2206, BEZ235, DOX, or H2O2. Collagen-enclosed cells were sectioned consecutively (5 μm) on a freezing microtome and stored at −20°C for future use.
Small interfering RNA (siRNA) transfection
The siRNAs for HMGA2 were purchased from Invitrogen Life Technologies and have been tested in a previous study (20). Briefly, cells were placed in 6-well plate (2 × 105 per well) in media without antibiotics for 24 hours. After 70% confluence, cells were transfected with HMGA2-siRNA (60 pmol/well) or control small RNA (Block-iT fluorescent double-stranded random 22mer RNA from Invitrogen), using Lipofectamine 2000 according to the manufacturer's protocol. After transfection, cells were harvested and analyzed at the indicated times.
ROS assays
Intracellular ROS was detected using the O2--sensitive fluorescent probe dye dihydroethidium (DHE; Invitrogen). Briefly, cells (5 × 104) were seeded onto 6-well plate 1 day prior to detection. Cells were treated with NAC with or without MK-2206. DHE (10 μM) was then added for 20 minutes. Cells were washed in Hanks balanced salt solution, and the intracellular ROS levels represented by the percentage of cells with DHE staining were visualized under a Zeiss Axiovert fluorescent microscope.
Senescence-associated β-gal stain
Cells were seeded onto coverslips placed in 6-well plates overnight and then treated with the test compounds: MK2206 (2 μM), H2O2 (100 μM), or DOX (0.2 μg/mL). Cells were fixed with 2% formaldehyde + 0.2% glutaraldehyde in PBS at room temperature for 3–5 minutes. After washing in PBS, cells were stained with staining solution containing 1 mg/mL X-gal and incubated in a CO2-free incubator at 37°C for 16 hours. Blue cells were counted under microscope and statistically analyzed. Three randomly selected fields (1 × 1 mm2) of images were captured to count the senescence rate (%).
Immunofluorescence
Cells cultured on coverslips were washed in PBS and fixed with 4% paraformaldehyde for 10 minutes. Cells were then permeabilized in 0.2% Triton X-100 for 10 minutes, blocked in 5% normal goat serum for 30 minutes, and then incubated with specific primary antibodies including mouse antihuman phospho-H2AX (1:200; Millipore) or rabbit antihuman high-mobility group (HMG)A2 (1:100, BioCheck) at 37°C for 1 hour. Mouse or rabbit IgG was used as the negative control. After washing in PBS, cells were incubated with tetramethylrhodamine isothiocyanate-conjugated goat antimouse or goat antirabbit secondary antibody at room temperature for 1 hour. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the senescence-associated heterochromatin foci (SAHF) (21). Three randomly selected fields of fluorescent images were captured under a fluorescence microscope, and a total of 50 cells was counted in each sample to calculate the percentage of positive-staining cells. The percentage of phospho-H2AX-positive cells was calculated to demonstrate the level of DNA damage caused by different stimulation, and the percentage of SAHF-positive cells was calculated to indicate the senescent cells.
RNA extraction and quantitative real-time RT-PCR
Total RNA was extracted using the TRIzol (Invitrogen) or micro-RNA (miRNA) extraction kit (Ambion) according to the manufacturer's instructions. Total RNA (1 μg) or 50 ng small RNA were reverse transcribed to cDNA in a 20 μL volume using an Advantage RT for PCR Kit (Clontech) or miRNA kit (Ambion). β-Actin or U6 were used as internal controls for all PCR. Quantitative real-time PCR was performed with SYBR Green real-time PCR master mix (Bio-Rad Laboratories) using a MyiQ and iQ5 real-time PCR Detection System with sequence-specific primers. All PCRs were run for 40 cycles (95°C for 15 seconds, 60°C for 1 minute) after a 10-minute incubation at 95°C. The fold change in expression of each gene was calculated with the change in cycle threshold value method (ΔΔ Ct). The primers for tested genes are summarized in Supplemental Table 1 published on The Endocrine Society's Journals website at http://endo.endojournals.org.
Western blotting
Cultured cells were harvested and lysed (ie, in mammalian protein extraction reagent; Thermo Scientific) supplemented with protease and phosphatase inhibitors (Sigma) on ice. Total proteins (30 μg) were separated by SDS-PAGE and electrotransferred onto polyvinylidene fluoride membrane. The membrane was incubated with primary antibodies overnight at 4°C (Supplemental Table 2). Proteins of interest were detected with the appropriate horseradish peroxidase-conjugated secondary antibodies and developed using the ECL PLUS kit (Amersham Biosciences).
Statistical analysis
Continuous data were measured for means and SEs in triplicate experimental samples. The data including 3 or more groups were checked for the normality and then preceded to one-way ANOVA analysis. Student's t test was used for comparisons between 2 groups. Significance for noncontinuous data was calculated by X2 analysis. P < .05 was considered significant.
Results
Inhibition of AKT results in increased cellular senescence in uterine leiomyoma cells
The AKT pathway is activated in most uterine leiomyomas (6–8); however, the AKT-regulated mechanisms in leiomyoma cells are largely unknown. In our previous studies, we found that activation of AKT was essential for leiomyoma growth and survival (11). Evidence is emerging that AKT can protect against stress-induced premature senescence (SIPS) (16). We proposed that inhibiting the AKT pathway triggers multiple steps of stress responses that ultimately navigate cells into cellular senescence.
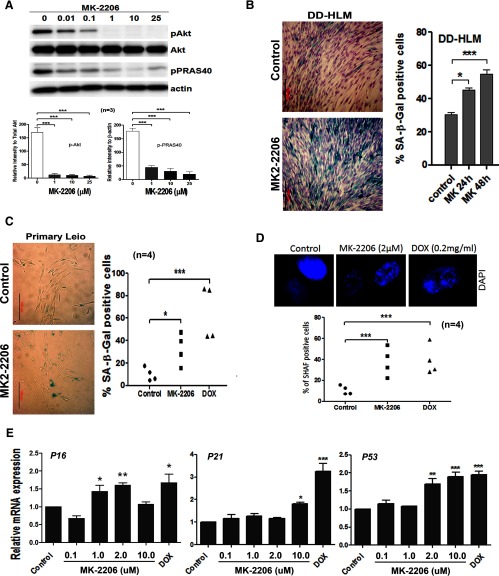
Primary leiomyoma cells (n = 4) were treated with increasing concentrations of MK-2206 for 48 hours, and protein levels of p(Ser473)-AKT and its downstream effector pPARS40 were measured by Western blot (Figure 1A). We found that with as little as 0.01 μM MK-2206 treatment, the levels of p(Ser473)-AKT and its effector pPARS40 were decreased; 1 μM and high doses of MK-2206 could completely inhibit pAKT and pPARS40 (Figure 1A).
Figure 1.
Inhibition of AKT induces senescence. A, Western blot analysis of pAKT and its downstream effector (pPRAS40) in primary leiomyoma cells treated from left to right: DMSO (0), MK-2206 0.01, 0.1, 1, 10, 25 μM. Relative expression levels of pAKT and pPRAS40 were shown below. B, Senescence detected by SA-β-Gal in DD-HLM (right) treated with MK-2206 in 24 and 48 hours (left). C, Senescence analysis in primary leiomyomas cells (n = 4) in control, MK-2206 (2 μM), and DOX (0.2 μg/mL) treatment. D, SAHF observed by florescent microscope (upper panel) and dotplot analysis of SAHF (bottom panel) in primary leiomyoma cells (n = 4) treated with MK-2206 or DOX. E, Leiomyoma cell lines were treated with increasing doses of MK-2206 and expression analysis of senescence-associated genes P16, P21, and P53 was measured by real time RT-PCR. The data shown are means ± SEM from 3 independent experiments (n = 3). *, P < .05; **, P < .01; ***, P < .001.
To investigate whether inhibition of AKT induced senescence, leiomyoma cells were treated with MK-2206 and subjected to β-galactosidase staining, a widely used biomarker for senescent cells. Upon treatment with MK-2206, β-galactosidase-stained cells were evident in both DD-HLM (Figure 1B) and primary (Figure 1C) leiomyoma cells. The percentage of β-galactosidase stained DD-HLM and primary leiomyoma cells with MK-2206 treatment were significantly higher than untreated cells (P < .05). DOX treatment was added as a positive control for inducing senescence. Similarly, the percentage of β-galactosidase-stained cells increased for the myometrial cell line, myo-hTert (Supplemental Figure 1B). Another inhibitor of the AKT pathway, GDC-0980, which is specifically a dual phosphatidylinositol-3 kinase and mTOR inhibitor (1 μM) increased β-galactosidase staining in DD-HLM cells (Supplemental Figure 1A), demonstrating that induction of senescence was not specific to MK-2206. Next, we examined and counted the percentage of senescence-associated heterochromatin foci (SAHF), which are characteristic structural changes that occur in primary leiomyoma cells undergoing senescence (22). They appear as punctate, focal staining within the nuclei stained with DAPI. Approximately 32% of cells exhibited SAHF when treated with MK-2206 compared with 8% in control cells (Figure 1D). DOX was used as a positive control for inducing SIPS. Finally, we examined the expression of genes associated with senescence using real-time RT-PCR. When leiomyoma cells were treated with MK-2206, the senescence-associated genes, P16, P21, and P53, were significantly up-regulated (Figure 1E). These data demonstrate that inhibition of AKT in leiomyoma cells induces senescence that resembles SIPS.
ROS mediates MK-2206-induced SIPS in leiomyoma cells
Previously, we demonstrated that treatment of leiomyoma cells with MK-2206 caused damage to the mitochondria (11). Because mitochondrial damage can increase levels of ROS and ROS is also a well-known stress factor for DNA and mitochondrial damage for promoting SIPS, leiomyoma cells were treated with MK-2206, and intracellular ROS production was measured by DHE. In response to MK-2206 as well as the dual phosphatidylinositol 3-kinase/mTOR inhibitor, BEZ235, ROS levels increased in primary leiomyoma cells (Figure 2A).To demonstrate that ROS can directly induce SIPS, leiomyoma cells were treated with 100 μM H2O2 and, as a result, more than 50% of cells stained positively for β-galactosidase indicating SIPS (Figure 2B). Quantitation of β-galactosidase-positive cells showed increased percentage of cells staining blue with H2O2 at 8 hours and 24 hours. H2O2 also increased senescence-associated genes, GLB1, P16, and P53 (Figure 2C). Treatment of cells with the antioxidant NAC attenuated MK-2206-induced P16 expression, but not P53 expression (Figure 2D).
Figure 2.
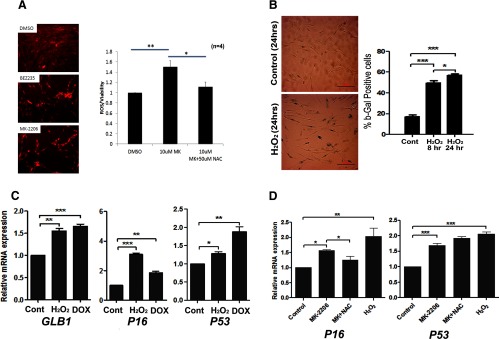
MK-2206 treatment increases ROS in primary and immortalized leiomyoma cells. A, Primary leiomyoma cells treated with vehicle (DMSO), BEZ235 (phosphatidylinositol 3-kinase/mTOR inhibitor) or MK-2206 (AKT inhibitor). Immunofluorescent staining of ROS, using DHE was done (left), and the relative levels of ROS were quantitatively measured (right). B, Primary leiomyoma cells were treated with H2O2 (100 μM) for 8 hours or 24 hours. Number of β-gal-positive staining cells was counted. C, Leiomyoma cell lines were treated with H2O2 (100 μM), and expression of senescence-associated genes GLB1, P16, and P53 was measured by real time RT-PCR. D, Leiomyoma cell lines were treated with MK-2206 (2 μM), MK-2206 (2 μM) with NAC, and H2O2 (100 μM), and expression of P16 and P53 was measured by real time RT-PCR. *, P < .05; **, P < .01; ***, P < .001.
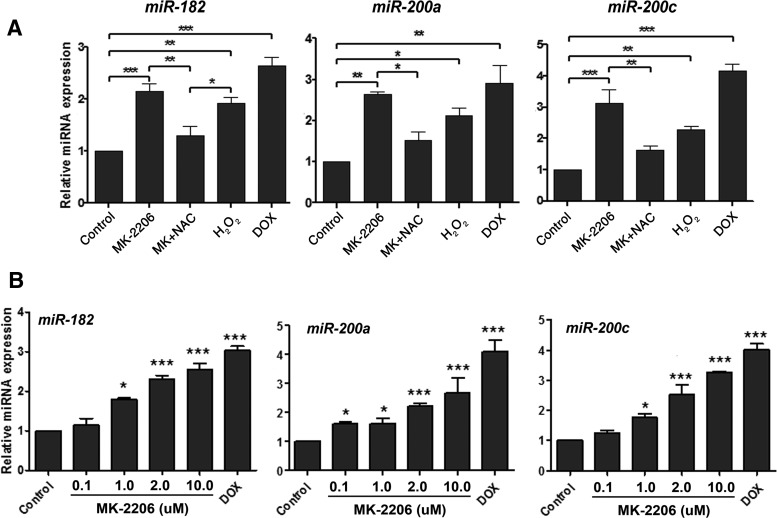
MK-2206-mediated ROS production regulates miRNAs expression
miRNAs play a key role in response to stress factors. Among them, miR-182 and miR-200s are critical molecules in stress-mediated cellular function, and they are consistently up-regulated by ROS (23, 24). MiR-182 plays a major role in DNA damage response through regulating several DNA damage response-related gene expressions, including BRCA1, FOXO3a, and HMGA2 (25, 26). MiR-200s regulate cellular senescence and cell proliferation (24, 27). The regulation of these 2 miRNAs by AKT, specifically, when AKT is inactivated, has never been studied. First, we examined miR-182 and miR-200a/c expression when DD-HLM cells were treated with H2O2. Consistent with results found in other cell types, miR-182 and miR-200a/c were induced in DD-HLM cells with H2O2 treatment (Figure 3A). Similarly, miR-182 and miR-200a/c expression increased in leiomyoma cells treated with MK-2206 in a dose-dependent manner (Figure 3B). The addition of NAC to MK-2206-treated cells blunted the expression of the 3 miRNAs, suggesting that ROS is mediating this increase (Figure 3A). In addition to miR-182 and miR-200a/c, other stress-related miRNAs were examined. MiR-182 and its family members, miR-183 and miR-96, were increased in response to MK-2206. Interestingly, miR-29b was significantly down-regulated in leiomyoma cells treated with H2O2 (Supplemental Figure 2).
Figure 3.
AKT inhibition and ROS regulate redox-sensitive miRNA expression. A, DD-HLM cells were treated with MK-2206 (2 μM), MK-2206 (2 μM) with NAC, and H2O2 (100 μM), and expression of miR-182 and miR-200a/c was measured by real time RT-PCR. DOX was used as positive control. B, DD-HLM cells were treated with increasing concentrations of MK-2206, and expression of miR-182 and miR-200 was measured by real-time RT-PCR. *, P < .05; **, P < .01; ***, P < .001.
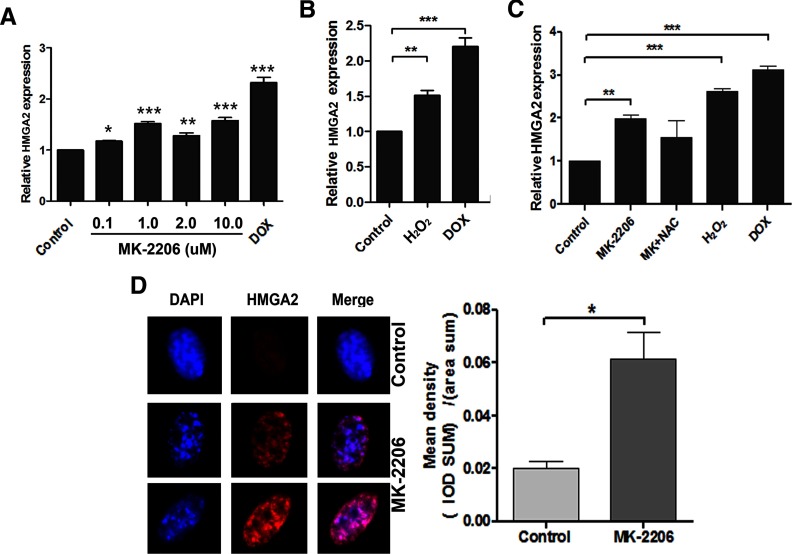
MK-2206-induced SIPS up-regulates HMGA2, which localizes to SAHF
HMGA2 is an oncogene and is overexpressed in a small portion of leiomyomas due to t (12; 14) translocation (28). In leiomyomas, HMGA2 is thought to promote cell proliferation (28) and prevents senescence through inhibition of genes associated with senescence (29). However, it has been shown in other cells that undergo oncogene stress that HMGA2 is up-regulated and coordinates with other senescence-associated factors such as P16 for cellular senescence (22). To investigate the role of HMGA2 in SIPS induced by AKT inhibition, HMGA2 expression was examined in leiomyoma cells treated with MK-2206. As shown in Figure 4A, HMGA2 expression was significantly increased in a dose-dependent manner when AKT was inactivated by MK-2206. Consistent with MK-2206, H2O2 treatment also increased HMGA2 expression (Figure 4B). Cotreatment with NAC and MK-2206 slightly reduced HMGA2 expression compared with MK-2206 (Figure 4C). These findings suggest that ROS may be involved but not the major mediator of MK-2206 up-regulation of HMGA2.
Figure 4.
MK-2206 and ROS induce HMGA2 expression in leiomyoma cells. A–C, DD-HLM cells were treated with various doses of MK-2206 (A), H2O2 (100 μM), NAC, and DOX (0.2 μg/mL). HMGA2 expression was measured by real time RT-PCR. D, Immunofluorescent staining for HMGA2 of leiomyoma cells in control and MK-2206-treated cells was done. Cells were costained with DAPI. Immunointensity of HMGA2 expression was measured and calculated. *, P < .05; **, P < .01; ***, P < .001.
To determine the localization of HMGA2 in senescent cells, immunofluorescent staining for HMGA2 was performed in leiomyoma cells treated with MK-2206. In the untreated cells, HMGA2 levels were low and found evenly distributed throughout the entire nuclei (Figure 4D). In contrast, leiomyoma cells treated with MK-2206 showed punctate localized staining of DAPI in the nuclei also termed as “senescence-associated heterochromatin foci” (SAHF). Furthermore, there was higher immunoreactivity for HMGA2 mainly localized in these punctate foci (Figure 4D).
We therefore proposed that HMGA2 up-regulation and localization to SAHF were required for MK-2206-induced SIPS in leiomyoma when AKT is inactivated. In order to determine whether HMGA2 plays a functional role in SIPS, HMGA2 was knocked down using siRNA, and cells were treated with MK-2206 or H2O2. HMGA2 knockdown was efficient even in response to MK-2206, H2O2, or DOX, which up-regulates its levels (Figure 5C). In response to HMGA2 knockdown, the SAHF bodies, as visualized by the punctate staining pattern in the nuclei, which form in response to MK-2206, H2O2, or DOX, were no longer evident (Figure 5A). Quantitation of the percentage of heterochromatin-positive cells revealed a significant decrease when HMGA2 was silenced (Figure 5B) (P < .05). These observations strongly suggest that HMGA2 plays an active role in promoting SAHF formation and ultimately promotes senescence in response to AKT inhibition.
Figure 5.
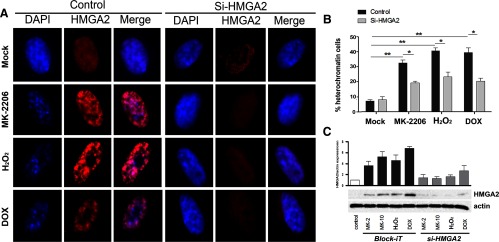
AKT inhibition or ROS-induced senescence is mediated by HMGA2 up-regulation and colocalization to SAHF. Leiomyoma cells were transiently transfected with siRNA to HMGA2 or a scrambled control siRNA. Cells were then treated with MK-2206 (2 μM), H2O2 (100 μM), NAC, and DOX (0.2 μg/mL). A, Immunofluorescent staining of HMGA2 was done and visualized. Cells were costained with DAPI. B, Percentage of cells with heterochromatin foci was calculated. C, Levels of HMGA2 at the protein level were measured by Western blot analysis to verify silencing efficacy (n = 3). *, P < .05; **, P < .01; ***, P < .001.
Discussion
Leiomyomas require prosurvival mechanisms to adapt to an unfavorable hypoxic microenvironment (30), and emerging evidence points to AKT as a major survival pathway for leiomyomas (9, 11). Not only is the AKT pathway activated in most leiomyomas, we demonstrated that inhibition of this pathway leads to decreased growth of leiomyoma tumors and promotes cell death (11). Inhibiting the AKT pathway results in a dynamic and transient regulation of specific pathways that ultimately navigate cells into various downstream fates, including decreased proliferation and cellular senescence. It has been shown in other cell types that AKT can protect against senescence that is induced by RAS (16, 17). We have demonstrated in this study that inhibition of the AKT pathway in leiomyoma cells induces senescence that is reminiscent of SIPS. Several key senescence-associated genes (P16, P21, and P53) were up-regulated when treated with AKT inhibitor MK-2206. P16 was induced with a very low dose of MK-2206, whereas P21 and P53 up-regulation required higher doses of MK-2206 (Figure 1E). We further demonstrated that AKT inhibition results in multiple cellular and molecular alterations, including increased ROS production, which induces redox-sensitive miRNA expression, senescence-associated gene expression, and increase of SAHF, a hallmark of SIPS.
Senescence is permanent growth arrest that occurs in response to either aging, called “aging replication senescence” (ARS), or to stress, termed “stress induced premature senescence” (SIPS). The underlying causes for natural occurring SIPS in leiomyomas are largely unknown. One study proposed that cellular senescence in leiomyoma occurs in order to balance cell proliferation and stress, which are mediated by cell cycle regulators, including HMGA2 and p53(31). Induction of SIPS with AKT inhibitors may uncover a potential therapeutic modality in leiomyoma.
Global miRNA profiling analyses reveal that miR-182 is one of a few miRNAs that are induced in cells responding to stress (32) and, in particular, for ROS-induced senescence in fibroblasts and other cell types (23, 24). Here, we demonstrated that miR-182 and miR-200a/c were up-regulated with MK-2206 and H2O2 in leiomyoma cells. Studies are underway to determine which downstream targets of miR-182 and miR-200c are responsible for the senescent phenotype. Based on our previous study showing that miR-182 up-regulates expression of HMGA2 in ovarian cancer cells (25), increased miR-182 expression in leiomyoma cells may be a major regulator of HMGA2 expression.
Our data showed that HMGA2 expression increases in response to MK-2206 as well as H2O2. The increased expression of HMGA2 during senescence seems paradoxical because HMGA2 is a known oncogene and promotes fibroid growth (28). About 7.5%-10% of leiomyomas overexpress HMGA2 due to nonrandom chromosomal translocation (5, 6, 33–35), and this overexpression of HMGA2 has been implicated in leiomyoma tumor formation and tumor growth (28, 31, 36). In most leiomyomas without the chromosomal translocation, HMGA2 is not expressed unless the endogenous gene is induced (37). It has been shown that activity of the AKT pathway determines the fate of HMGA2 for cell proliferation or senescence in some cell types (7). This regulation mechanism seems to be true in leiomyoma, because AKT inhibition induces HMGA2 up-regulation. Furthermore, HMGA2 colocalizes to the nucleus into SAHF bodies (22), which may attenuate oncogenic and mitogenic functions of HMGA2. These foci appear as compacted DNA and feature protein modifications typical of transcriptionally inactive heterochromatin. Silencing HMGA2 prohibits cells from forming these foci structures and further supports the role of HMGA2 in SIPS. Yu et al (7) showed that p16INK4A is negatively regulated by the AKT pathway in that activated AKT suppressed p16INK4A expression. In this study, we noted that P16INK4A was up-regulated in primary and immortalized fibroid cells when treated with either MK-2206 or H2O2. Because senescent cells require P16INK4A for HMGA2-mediated SAHF formation (22), increases of HMGA2 and P16INK4A by AKT inhibition may be necessary for SIPS.
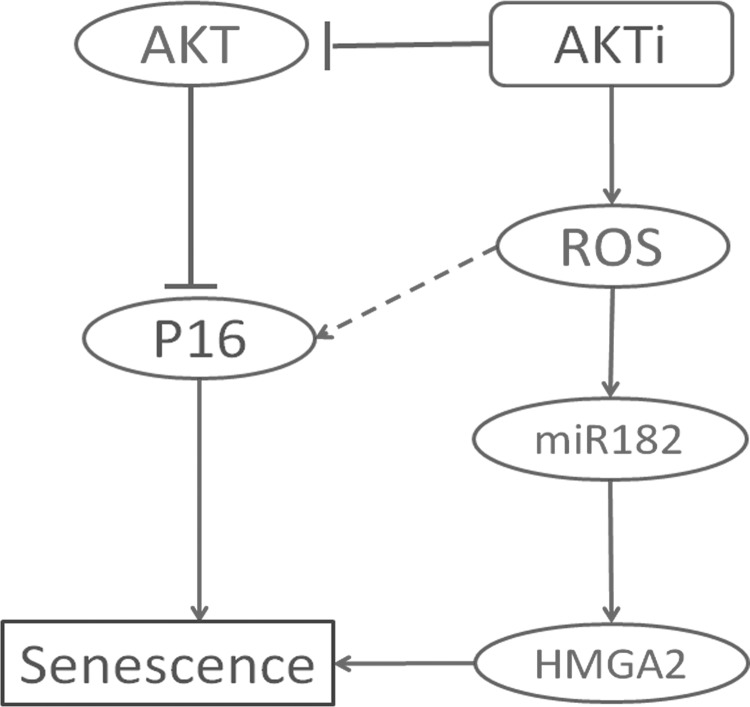
In summary, we observed multiple cellular and molecular changes induced by AKT inhibition that could be involved in driving leiomyoma cells into SIPS. Our working model, depicted in Figure 6, is that in leiomyomas, inhibition of the AKT pathway causes increased production of ROS and miR-182 expression, which thereby increases HMGA2 expression, which then localizes to the heterochromatin foci and forms a complex that involves p16INK4A. This complex prevents HMGA2 from acting as an oncogene but rather, promotes senescence, as well as structural changes that involve the formation of heterochromatin foci (SAHF). Our findings significantly further our understanding of the mechanisms of leiomyoma survival and open a new venue for a valuable therapeutic strategy in treating symptomatic leiomyomas.
Figure 6.
Proposed mechanism of cellular senescence upon AKT inhibition. AKT inhibitor MK-2206 inactivates AKT and promotes ROS production and miR-182 overexpression. This, in turn, enhances HMGA2 expression. AKT inactivation will also release the negative regulation of P16 expression. Increased HMGA2 and P16 expression triggers the SIPS.
Acknowledgments
We thank Dr Bushra Ayub for her technical assistance.
This work is supported by start-up funds (to J.J.W.) and National Institutes of Health Grant P01HD057877 (to J.J.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DAPI
- 4′,6-diamidino-2-phenylindole
- DHE
- dihydroethidium
- DOX
- doxorubicin
- HMG
- high-mobility group
- miRNA
- micro-RNA
- mTOR
- mammalian target of rapamycin
- NAC
- N-acetyl-cysteine
- ROS
- reactive oxygen species
- SAHF
- senescence-associated heterochromatin foci
- SIPS
- stress-induced premature senescence
- siRNA
- small interfering RNA.
References
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107 [DOI] [PubMed] [Google Scholar]
- 2. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369:1344–1355 [DOI] [PubMed] [Google Scholar]
- 3. Friedman AJ, Daly M, Juneau-Norcross M, Gleason R, Rein MS, LeBoff M. Long-term medical therapy for leiomyomata uteri: a prospective, randomized study of leuprolide acetate depot plus either oestrogen-progestin or progestin ‘add-back’ for 2 years. Hum Reprod. 1994;9:1618–1625 [DOI] [PubMed] [Google Scholar]
- 4. American College of Obstetricians and Gynecologists ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112:387–400 [DOI] [PubMed] [Google Scholar]
- 5. Peng L, Wen Y, Han Y, et al. Expression of insulin-like growth factors (IGFs) and IGF signaling: molecular complexity in uterine leiomyomas. Fertil Steril. 2009;91:2664–2675 [DOI] [PubMed] [Google Scholar]
- 6. Kovács KA, Lengyel F, Környei JL, et al. Differential expression of Akt/protein kinase B, Bcl-2 and Bax proteins in human leiomyoma and myometrium. J Steroid Biochem Mol Biol. 2003;87:233–240 [DOI] [PubMed] [Google Scholar]
- 7. Yu KR, Park SB, Jung JW, et al. HMGA2 regulates the in vitro aging and proliferation of human umbilical cord blood-derived stromal cells through the mTOR/p70S6K signaling pathway. Stem Cell Res. 2013;10:156–165 [DOI] [PubMed] [Google Scholar]
- 8. Varghese BV, Koohestani F, McWilliams M, et al. Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci USA. 2013;110:2187–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927 [DOI] [PubMed] [Google Scholar]
- 10. Crabtree JS, Jelinsky SA, Harris HA, et al. Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res. 2009;69:6171–6178 [DOI] [PubMed] [Google Scholar]
- 11. Sefton EC, Qiang W, Serna V, et al. 3 MK-2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth. Endocrinology. 2013;154:4046–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meng J, Dai B, Fang B, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PloS One. 2010;5:e14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967 [DOI] [PubMed] [Google Scholar]
- 14. Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695 [DOI] [PubMed] [Google Scholar]
- 15. Bononi A, Agnoletto C, De Marchi E, et al. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011;2011:329098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy AL, Morton JP, Manoharan I, et al. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868 [DOI] [PubMed] [Google Scholar]
- 18. Laser J, Lee P, Wei JJ. Cellular senescence in usual type uterine leiomyoma. Fertil Steril. 2010;93:2020–2026 [DOI] [PubMed] [Google Scholar]
- 19. Wei JJ, Zhang XM, Chiriboga L, Yee H, Perle MA, Mittal K. Spatial differences in biologic activity of large uterine leiomyomata. Fertil Steril. 2006;85:179–187 [DOI] [PubMed] [Google Scholar]
- 20. Wu J, Liu Z, Shao C, et al. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 2011;71:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tu Z, Aird KM, Bitler BG, et al. Oncogenic Ras Regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Dev Cell. 2011;21:1077–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narita M, Narita M, Krizhanovsky V, et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–514 [DOI] [PubMed] [Google Scholar]
- 23. Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev. 2009;130:731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magenta A, Cencioni C, Fasanaro P, et al. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18:1628–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Z, Liu J, Segura MF, et al. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol. 2012;228:204–215 [DOI] [PubMed] [Google Scholar]
- 26. Moskwa P, Buffa FM, Pan Y, et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell. 2011;41:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zavadil J, Ye H, Liu Z, et al. 2010 Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PloS one. 5:e12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hodge JC, Kim TM, Dreyfuss JM, et al. Expression profiling of uterine leiomyomata cytogenetic subgroups reveals distinct signatures in matched myometrium: transcriptional profilingof the t(12;14) and evidence in support of predisposing genetic heterogeneity. Hum Mol Genet. 2012;21:2312–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Markowski DN, Helmke BM, Belge G, et al. HMGA2 and p14Arf: major roles in cellular senescence of fibroids and therapeutic implications. Anticancer Res. 2011;31:753–761 [PubMed] [Google Scholar]
- 30. Mayer A, Höckel M, Wree A, Leo C, Horn LC, Vaupel P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008;68:4719–4726 [DOI] [PubMed] [Google Scholar]
- 31. Markowski DN, von Ahsen I, Nezhad MH, Wosniok W, Helmke BM, Bullerdiek J. HMGA2 and the p19Arf-TP53-CDKN1A axis: a delicate balance in the growth of uterine leiomyomas. Genes Chromosomes Cancer. 2010;49:661–668 [DOI] [PubMed] [Google Scholar]
- 32. Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533 [DOI] [PubMed] [Google Scholar]
- 33. Mine N, Kurose K, Nagai H, et al. Gene fusion involving HMGIC is a frequent aberration in uterine leiomyomas. J Hum Genet. 2001;46:408–412 [DOI] [PubMed] [Google Scholar]
- 34. Martin Chaves EB, Brum IS, Stoll J, Capp E, Corleta Hv. Insulin-like growth factor 1 receptor mRNA expression and autophosphorylation in human myometrium and leiomyoma. Gynecol Obstet Invest. 2004;57:210–213 [DOI] [PubMed] [Google Scholar]
- 35. Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86:5593–5599 [DOI] [PubMed] [Google Scholar]
- 36. Peng Y, Laser J, Shi G, et al. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res. 2008;6:663–673 [DOI] [PubMed] [Google Scholar]
- 37. Markowski DN, Winter N, Meyer F, et al. p14Arf acts as an antagonist of HMGA2 in senescence of mesenchymal stem cells-implications for benign tumorigenesis. Genes Chromosomes Cancer. 2011;50:489–498 [DOI] [PubMed] [Google Scholar]