Abstract
The decline in testosterone levels in men during normal aging increases risks of dysfunction and disease in androgen-responsive tissues, including brain. The use of testosterone therapy has the potential to increase the risks for developing prostate cancer and or accelerating its progression. To overcome this limitation, novel compounds termed “selective androgen receptor modulators” (SARMs) have been developed that lack significant androgen action in prostate but exert agonist effects in select androgen-responsive tissues. The efficacy of SARMs in brain is largely unknown. In this study, we investigate the SARM RAD140 in cultured rat neurons and male rat brain for its ability to provide neuroprotection, an important neural action of endogenous androgens that is relevant to neural health and resilience to neurodegenerative diseases. In cultured hippocampal neurons, RAD140 was as effective as testosterone in reducing cell death induced by apoptotic insults. Mechanistically, RAD140 neuroprotection was dependent upon MAPK signaling, as evidenced by elevation of ERK phosphorylation and inhibition of protection by the MAPK kinase inhibitor U0126. Importantly, RAD140 was also neuroprotective in vivo using the rat kainate lesion model. In experiments with gonadectomized, adult male rats, RAD140 was shown to exhibit peripheral tissue-specific androgen action that largely spared prostate, neural efficacy as demonstrated by activation of androgenic gene regulation effects, and neuroprotection of hippocampal neurons against cell death caused by systemic administration of the excitotoxin kainate. These novel findings demonstrate initial preclinical efficacy of a SARM in neuroprotective actions relevant to Alzheimer's disease and related neurodegenerative diseases.
The normal age-related decline in testosterone in men can increase the risks for dysfunction and disease in several androgen-responsive tissues throughout the body (1, 2). In brain, low testosterone is an established factor for the development of Alzheimer's disease (AD). Circulating (3, 4) and brain (5, 6) levels of testosterone are lower in men with AD, and this androgen depletion occurs prior to clinical (7) and neuropathological (5, 6) diagnoses of the disease, suggesting that low testosterone contributes to AD pathogenesis. In transgenic mouse models of AD, depletion of endogenous androgens by surgical (8) or chemical (9) castration accelerates development of AD-like pathology whereas elevation of endogenous testosterone above normal levels significantly impedes pathology development (10).
Androgens induce numerous beneficial neural effects relevant to a protective role against AD, including reduction of the AD-related protein β-amyloid (Aβ) (8, 11, 12) and promotion of synapse formation (13, 14), neurogenesis (15, 16), and specific aspects of cognition (1, 17). An androgen action particularly important to neurodegenerative diseases is neuroprotection. Testosterone can increase neuron survival in several cell culture and animal models of injury (18). Although testosterone neuroprotective actions are largely androgen receptor (AR) dependent, testosterone is metabolized to several steroids that can act through other mechanisms (18). For example, testosterone is metabolized to dihydrotestosterone (DHT) by the enzymatic actions of 5α-reductase (19). Because DHT is a more potent androgen than testosterone at AR, the conversion of testosterone to DHT results in more robust androgen signaling in tissues like prostate (20, 21). DHT is metabolized to other steroids, including 5α-androstane-3 α,17βdiol, which can reduce some forms of neural injury (22). Testosterone is also converted by the enzyme aromatase to 17β-estradiol (E2), which signals through estrogen receptors (ER) (23). In some paradigms, testosterone neuroprotection is dependent upon conversion to E2 (24–26). Thus, neural benefits of testosterone can be mediated largely by AR, ER, or a combination of both. For example, both AR (12) and ER (27, 28) are implicated in testosterone reduction of Aβ levels, whereas AR, but not ER, mediates testosterone increases in spine density. These and other experimental data (29) predict that androgen-based hormone therapy may be an effective approach for the prevention of AD and related neurodegenerative disorders in aging men.
One significant limitation of androgen therapy is the potential for increased risk of developing prostate cancer and or accelerated growth of existing prostate tumors. To overcome this problem, new classes of synthetic testosterone-like compounds, called “selective androgen receptor modulators” (SARMs), have been developed (30, 31). SARMs are ligands for AR that exert limited effects in prostate and other reproductive tissues but have potent androgenic actions in muscle and bone (32, 33). Although SARMs such as 7α-methyl-19-nortestosterone undergo enzymatic aromatization to yield metabolites that bind to ER (34), most of the currently available SARMs are poor substrates for aromatase and interact specifically with AR (35). The possible utility of SARMs for therapeutic use in AD and other neural disorders has only recently begun to be investigated.
Clinical utility of SARMs for neural disorders requires that they mimic androgen actions in brain. Although efficacy of SARMs for peripheral tissues such as muscle is well established, the extent to which SARMs exert protective androgen effects in brain is unclear. To begin addressing this issue, we evaluated the neuroprotective efficacy of the SARM RAD140 using in vitro and in vivo paradigms previously demonstrated to be androgen responsive. RAD140 is a novel SARM with high affinity and specificity for AR, is orally available, and exhibits potent anabolic effects in rodents and nonhuman primates (36). We determined the effects of RAD140 against toxic insults in both primary neuron cultures and the rat kainate lesion, an animal model of hippocampal neuron loss relevant to neurodegenerative diseases (37), which has previously been established to respond to androgen neuroprotection (38). These data represent the first preclinical report investigating the neuroprotective actions of SARMs.
Materials and Methods
Reagents
Testosterone, DHT (Steraloids), and RAD140 and RAD192 (Radius Health Inc) were solubilized in 100% ethanol and then diluted into culture medium (cell culture experiments; final ethanol concentration < 0.001%) or in 0.5% methyl cellulose at 1 mg/mL (in vivo experiments). U0126 (EMD Millipore Chemicals) and zVAD-fmk (Sigma-Aldrich) were dissolved in dimethylsulfoxide and 100% ethanol, respectively, and then diluted into culture medium. Additional reagents include Aβ peptide Aβ1–42 (Tocris Bioscience), apoptosis activator II (AAII) (EMD Millipore Chemicals), and hydrogen peroxide (H2O2) (Sigma-Aldrich).
Primary neuron culture
Timed-pregnant female Sprague Dawley rats (Harlan Laboratories, Inc.) were euthanized via CO2 inhalation, and embryonic day 17–18 pups were collected for preparation of neuronal cultures. All animal procedures were conducted under a protocol that was approved by the University of Southern California Institutional Animal Care and Use Committee and in accordance with National Institute of Health standards. Primary rat hippocampal cultures (∼95% neuronal as determined by immunoreactivity with the neuron-specific antibody NeuN) were prepared with some modifications of a previously described protocol (39). Dissociated hippocampal neurons (n ≥ 6 pups per preparation) were plated onto poly-l-lysine-coated multiwell plates at a final density of 2.5 × 104 cells/cm2 for cell-viability assays. Each culture preparation used 12–14 pups to ensure a mix of male and female pups to control for sex differences. Cultures were maintained at 37°C in a humidified incubator supplemented with 5% CO2. All experiments were started after 1–2 days in vitro. All in vitro experiments were repeated in at least 3 independent culture preparations.
Cell-viability assay
Cell viability was determined at the end of the treatment period by counts of the number of viable neurons as determined by staining with the vital dye calcein acetoxymethyl ester (Invitrogen), as previously described (40). In brief, cultures were incubated for 5 minutes with 2 μM calcein acetoxymethyl ester and then examined using an inverted fluorescent IX70 Olympus microscope. The number of healthy, positively stained cells was counted in 4 separate fields (in a predetermined, regular pattern) per well, 3 wells per condition in each experiment (n ≥ 3 independent culture preparations). Counts of viable neurons in vehicle-treated controls ranged from 250–300 per well.
Western blots
Lysates were collected from treated cultures using a reducing sample buffer (62.5 mM Tris-HCl, 1% sodium dodecyl sulfate, 2.5% glycerol, 0.5% 2-β-mercaptoethanol), boiled for 5 minutes, and centrifuged at 13 000 × g for 10 minutes. The supernatants were analyzed by immunoblotting using a standard protocol previously described (39) with 1 μg/mL anti-phosphoERK1/2 (EMD Millipore Chemicals) primary antibody and corresponding horseradish peroxidase-conjugated secondary antibody (1:5000) and detected using enhanced luminescence (Amersham). Band densities were measured using Image J software (version 1.45s) and the relative percent intensity of the phospho-ERK bands were plotted on a graph after normalizing to total ERK levels.
In vivo hormone treatments and kainate lesions
Male Sprague Dawley rats (n = 8 per group) were purchased gonadectomized (GDX) and sham-GDX at 3 months of age (Harlan Laboratories, Inc.). All animals were housed individually with ad libitum access to food and water under a 12 hour light, 12 hour-dark cycle. Animals underwent GDX 14 days prior to the start of treatment, allowing for the depletion of endogenous hormones. For testosterone (T) treatment, GDX male rats were implanted with a 30-mm length SILASTIC capsule (1.47 mm inner diameter × 1.96 mm outer diameter; Dow Corning) packed with dry T to a length of 20 mm and capped on both ends with 5 mm of silicone glue. Vehicle-treated animals were implanted with an empty capsule with the same dimensions. For SARM treatment, GDX rats were administered 1 mg/kg RAD140 suspended in 0.5% methyl cellulose (1 mg/mL) by daily oral gavage for 2 weeks. This dose was chosen based on previous reports for RAD140 efficacy (36). Vehicle-treated animals were gavaged with a similar weight/volume of 0.5% methyl cellulose. On day 13 of the 2-week hormone treatment period, kainate (10 mg/kg; Enzo Life Sciences) or sterile water control was injected ip. Kainate was dissolved immediately prior to use in sterile water and lightly heated to fully solubilize. On day 14, SARM-treated rats were administered 1 mg/mL of RAD140 suspended in safflower oil by sc injection because oral gavage is difficult following kainate lesion.
At the end of the treatment period, animals were euthanized by CO2 inhalation. Brains were removed and hemisected: one half was immersion fixed for 48 hours in cold 4% paraformaldehyde/Sorenson's phosphate buffer, and the other half was snap frozen on dry ice and then stored at −80°C for use in RT-PCR analyses. The prostate, seminal vesicles, and levator ani were dissected according to standard procedures (41) and weighed.
Seizure assessment
Kainate induces seizures that are characterized in part by stereotypic behaviors (42). To assess seizures, animals were continuously monitored for 3 hours following kainate injection for both seizure latency and severity. Latency is defined as the period from the kainate injection to the appearance of the first “wet dog shake,” a stereotypic seizure-related behavior. Seizure severity was behaviorally assessed according to the Racine scale: 0 = no seizure activity; 1 = occasional wet dog shake; 2 = head nodding and facial clonus; 3 = unilateral forelimb clonus; 4 = bilateral clonus with rearing, but without falling; 5 = seizures accompanied by rearing and falling (43). One sham-GDX animal that reached stage 5 was immediately euthanized and excluded from analysis.
RT-PCR
For RNA extractions, rat hypothalami were homogenized using TRIzol reagent (Invitrogen Corp.) and processed for total RNA extraction as per manufacturer's protocol. Purified RNA (1–2 μg) was used for reverse transcription using the iScript cDNA synthesis system (Bio-Rad Laboratories), and the resulting cDNA was used for real-time quantitative PCR. Quantitative PCR was carried out using Bio-Rad CFX Connect (Bio-Rad). The amplification efficiency was estimated from the standard curve for each gene. Relative quantification of mRNA levels from various treated samples was determined by the ΔΔCt method (44) with β-actin as the normalizing control. The PCR products were also analyzed qualitatively on a 1% agarose gel. The following primer pair was used: ERα, forward: 5′-CATCGATAAGAACCGGAG-3′, reverse: 5′-AAGGTTGGCAGCTCTCAT-3′; β-actin, forward: 5′-AGCCATGTACGTAGCCATCC-3′, reverse: 5′-CTCTCAGCTGTGGTGGTGAA-3′.
Immunohistochemistry
Fixed brains were sectioned exhaustively in the horizontal plane at 40 μm using a vibratome (Leica Microsystems) and stored at 4°C in PBS with 0.03% sodium azide until use. Every eighth section was immunostained with NeuN antibody (1:250; Millipore) using standard avidin-biotinylated enzyme complex immunohistochemistry with Vectastain Elite ABC kit (Vector Laboratories), as previously described (38). Stained sections were mounted on slides, allowed to dry overnight, and then coverslipped with Krystalon (EMD Chemicals) without further dehydration. A second set of tissue was mounted and dried and underwent a routine thionin stain for comparison and imaging.
The number of NeuN immunoreactive cells in the CA2/3 region of the hippocampus was estimated by 2-dimensional cell counts using random sampling based on the optical dissector technique, which has been previously used to estimate the number of total cells in the hippocampus (38, 45). Briefly, an Olympus BX50 microscope equipped with a motorized stage and computer guided CASTGrid software (Olympus) was used for unbiased sampling. In every eighth section of hippocampus (8–12 sections per brain), the CA2/CA3 region of the hippocampus was outlined, and a randomly oriented counting frame (476 μm2) with an X-Y step of 150 μm × 150 μm was used for cell counts. Only cells with positively stained nuclei were counted, not dead or dying cells or cells that were on the upper or lower edge of the section the cytoplasm of which was stained but appeared without a nucleus. To control for variability in the number of sections analyzed, the total number of NeuN-immunoreactive nuclei in each animal was divided by the number of sections assessed and then expressed as a percentage of neurons counted in the sham-GDX, nonlesioned group. Of the 24 rats that received kainate and survived the kainate-induced seizures, 2 were excluded from analyses because they showed extensive loss of CA1 neurons indicating hypoxic injury, which is known to occur in a subset of lesioned animals (46).
Statistical analyses
Raw data were statistically assessed using ANOVA followed by between-group comparisons using Fisher's least significant different test. Significance was indicated by P ≤ .05.
Results
RAD140 protects cultured neurons against Aβ in a dose-dependent manner
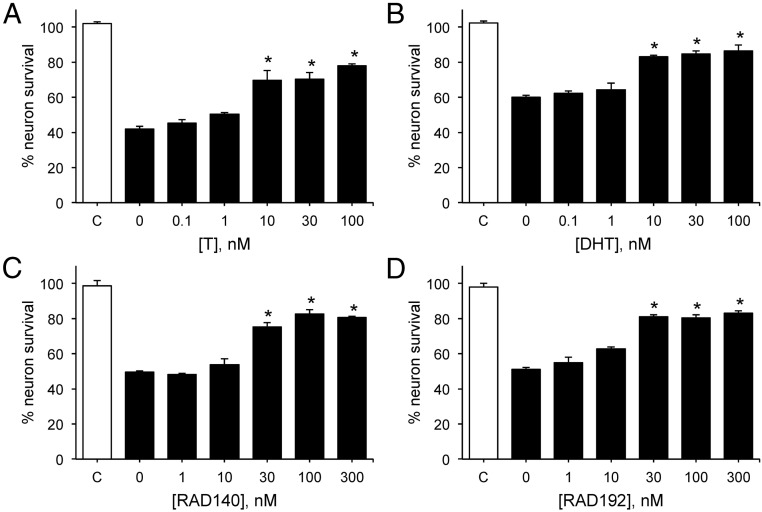
As an initial step to evaluate the neuroprotective potential of RAD140, we compared RAD140 with the endogenous androgens T and DHT for their relative abilities to reduce neuron death induced by aggregated Aβ1–42, the peptide implicated in AD neurodegeneration. We found that 24 hours' exposure to Aβ decreased the number of viable neurons by approximately 50%, as compared to vehicle treatment. Consistent with previous observations (47), treatment with T and DHT beginning 1 hour prior to Aβ significantly reduced cell death (Figure 1, A and B). In comparison, treatment of cultures with increasing doses of RAD140 (Figure 1C) or the related SARM RAD192 (Figure 1D) provided similar levels of neuroprotection. The minimum effective concentration of both SARMs was 30 nM whereas T and DHT yielded significant protection at 10 nM.
Figure 1.
RAD140 increases neuron viability against Aβ in a concentration-dependent manner. Neuron survival was measured in cultures pretreated with 0–100 nM T (A) and DHT (B); 0–300 nM RAD140 (C) and RAD192 (D) for 1 hour, followed by 24-hour exposure to 50 μM Aβ1–42 (solid bars). Cell viability data show the mean (± SEM) cell counts of viable cells expressed as percentage of vehicle-treated control group (C, open bar). *, P < .0001 relative to vehicle + AAII condition (0, solid bar); n ≥ 3.
RAD140 protects cultured neurons against apoptotic insults
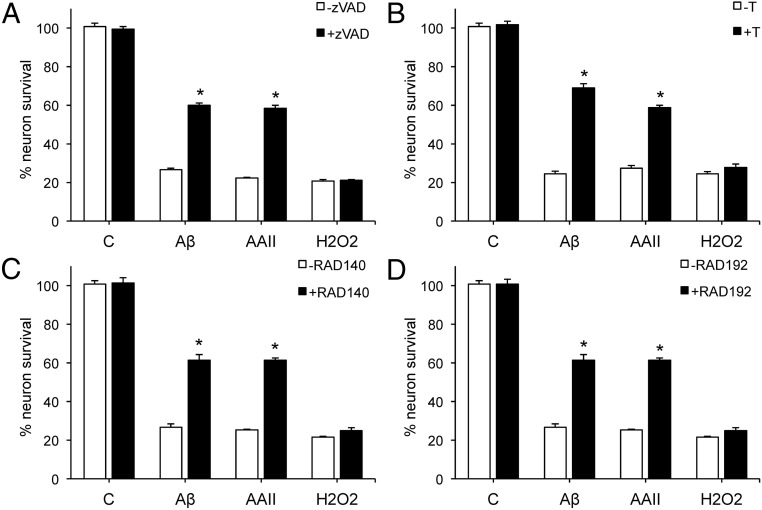
We previously showed that androgen neuroprotection is limited to insults that involve apoptosis (48). To investigate whether the SARMs mimic this established androgen-protective pathway, we assessed their abilities to reduce cell death induced by 3 insults: Aβ, apoptosis activator II (AAII), and hydrogen peroxide (H2O2). To confirm our prior observations that Aβ and AAII, but not H2O2, induce cell death by caspase-dependent apoptosis in our culture system, we evaluated the ability of the caspase inhibitor zVAD to attenuate cell death. Exposure of cultures to 50 μM zVAD-fmk for 2 hours prior to insult exposure significantly attenuated cell death due to 50 μM Aβ and 3 μM AAII but did not significantly affect cell loss caused by 25 μM H2O2 (Figure 2A). Next, we compared the pattern of protection against the 3 insults by T and the 2 SARMs. Cultures were pretreated for 1 hour with 10 nM testosterone, 100 nM RAD140, or 100 nM RAD192, and then exposed for 24 hours to Aβ, AAII, or H2O2. T (Figure 2B), RAD140 (Figure 2C), and RAD192 (Figure 2D) shared similar protective profiles of significantly protecting against neuronal death induced by Aβ and AAII, but not H2O2.
Figure 2.
RAD140 is neuroprotective against apoptotic insults. Cultures were treated with 50 μM zVAD-fmk (A), 10 nM T (B), 100 nM RAD140 (C), or 100 nM RAD192 (D) for 1 hour, followed by exposure to 50 μM Aβ1–42, 3 μM AAII, or 25 μM H2O2 for 24 hours, and processed for cell viability. Data show mean (± SEM) cell viability expressed as percentage of vehicle-treated control (Veh, open bar). *, P < .001 relative to the corresponding vehicle-treated condition (gray bars); n ≥ 3.
MAPK signaling is involved in SARM-mediated neuroprotection
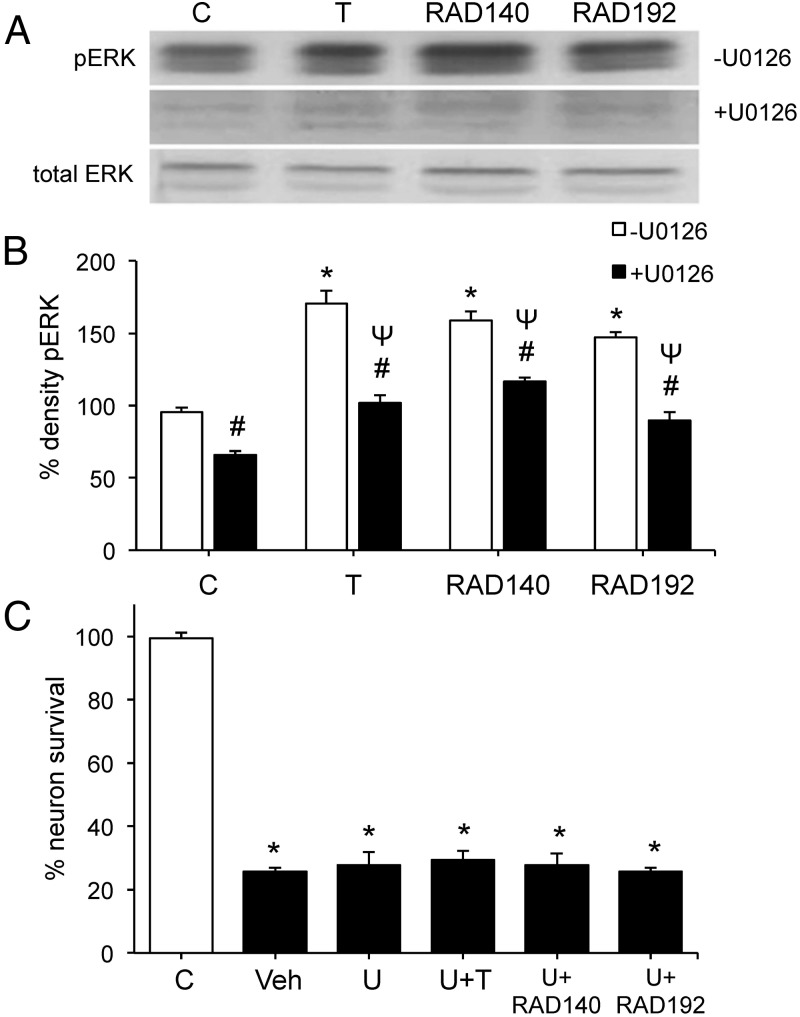
Androgen-mediated neuroprotection against apoptosis is dependent upon activation of a MAPK/ERK signaling pathway (40). To evaluate the role of MAPK/ERK signaling in mediating the observed neuroprotective effects of SARMs, we first determined whether RAD140 and RAD192 activate MAPK/ERK signaling. Neuronal cultures were exposed for 15 minutes to 10 nM T, 100 nM RAD140, or 100 nM RAD192 in the presence or absence of pretreatment with 10 μM U0126, a MEK inhibitor that blocks MAPK/ERK signaling (49). Western blots with phospho-specific and pan-ERK antibodies show that T, RAD140, and RAD192 induce a significant increase in levels of phosphorylated but not total ERK (Figure 3, A and B). Both the basal levels of ERK phosphorylation and the androgen-mediated increases were significantly attenuated by U0126 (Figure 3, A and B).
Figure 3.
Quantitative analyses of MAPK signaling in SARM neuroprotection. A, Pretreatment of cultures with vehicle (top panel) or 10 μM U0126 (middle panel) for 2 hours was followed by exposure to 10 nM T, 100 nM RAD140, or 100 nM RAD192 for 15 minutes, and then examined by Western blot using phosphorylated (top and middle panel) and total (bottom panel) ERK-1/2 antibodies. B, The graph shows the percent phospho-ERK (pERK) expressed as a ratio between phosphorylated to total ERK-1 (44 kDa), normalized to the vehicle-treated control condition. C, Following a 2-hour pretreatment with vehicle or 10 μM of MEK inhibitor U0126 (U), cultures were exposed to 10 nM T, 100 nM RAD140, or 100 nM RAD192 for 1 hour and then treated with 50 μM Aβ1–42 for 24 hours. Cell viability data show mean (± SEM) counts of viable neurons plotted as percentage vehicle-treated control (C, open bar). *, P < .0001 relative to vehicle control (C, open bar); #, P ≤ .001 between each vehicle and the corresponding U0126 treatments (open bar vs solid bars); Ψ, P < .01 relative to U0126-treated control (C, solid bar); n ≥ 3. Veh, vehicle.
We then examined the effect of U0126 inhibition of MAPK/ERK signaling on the extent of neuroprotection by SARMs against Aβ-induced cell death. Pretreatment with 10 μM U0126 completely blocked androgen protection by T, RAD140, and RAD192 (Figure 3C). U0126 alone had no effect on either basal cell viability or the magnitude of Aβ toxicity (Figure 3C).
RAD140 has tissue-specific effects in vivo
Based on the cell culture observations, we investigated the neuroprotective potential of RAD140 in vivo. Young adult male rats were randomly assigned to sham-GDX, GDX, GDX+T, and GDX+RAD140 groups that were exposed to the neurotoxin kainate 2 weeks after the initiation of androgen treatments. Following the lesion, seminal vesicles, prostate, and levator ani were removed and weighed to confirm the reported tissue-selective androgen effects of RAD140 (36). GDX resulted in significantly reduced weight of all 3 androgen-responsive tissues, but tissue weights were restored to sham-GDX levels in the GDX+T group (Figure 4, A–C). RAD140 treatment resulted in a nonsignificant increase in the weights of seminal vesicles and prostate, which were still significantly lower than weights observed in the sham-GDX and GDX+T groups. On the other hand, RAD140 significantly increased the weight of the levator ani, an androgen-responsive muscle, to levels similar to those observed in the sham-GDX and GDX+T groups (Figure 4, A–C).
Figure 4.
RAD140 induces tissue-specific androgenic effects. Data show mean (± SEM) tissue weights of (A) seminal vesicles, (B) prostate, and (C) levator ani muscle from male rats in sham-GDX (open bar), GDX (solid bar), GDX+T (gray bar), and GDX+RAD140 (gray bar) conditions. D, Relative mRNA levels of ERα in the hypothalamus were determined across groups by real-time PCR. Data show expression relative to the Sham condition. *, P < .001 vs sham; Ψ, P < .0001 vs T; n ≥ 7 per condition.
We next investigated whether RAD140 exerts androgenic effects in brain. To accomplish this, we examined androgen regulation of ERα mRNA expression in the hypothalamus, a brain region largely unaffected by kainate. Consistent with prior observations (50), we observed that GDX resulted in increased ERα mRNA expression in comparison to sham-GDX animals. Both GDX+T and GDX+RAD140 groups exhibited significantly decreased ERα mRNA expression relative to the GDX animals (Figure 4D).
RAD140 is neuroprotective against kainate-induced hippocampal neuron loss
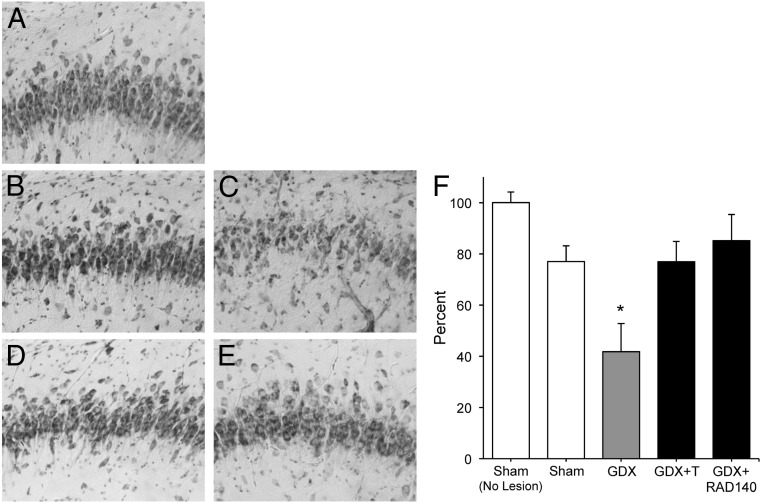
To investigate the neuroprotective effects of RAD140 in vivo, the extent of kainate-induced neuron death was assessed by counts of surviving cells immunostained with the neuron-specific antibody NeuN in the CA2/3 region of hippocampus. Relative to vehicle treatment, kainate induced approximately 20% cell loss in the sham-GDX animals (Figure 5, A and B). Neuron survival was significantly reduced in the GDX group, an effect that was prevented by T treatment (Figure 5, C and D; F (3, 29) = 6.93, P < .001). Notably, RAD140 was as effective as T in protecting GDX rats from kainate-induced neuron loss (Figure 5, E and F).
Figure 5.
RAD140 reduces neuronal cell death cause by kainate. The extent of neuronal cell death in the CA2/3 region of the hippocampus following kainate lesion was determined qualitatively and quantitatively across groups. Images show representative thionin-stained sections of CA3 hippocampus from (A) nonlesioned sham-GDX rats and the following kainate-lesioned groups: B, Sham-GDX; C, GDX; D, GDX+T; E, GDX+RAD140. F, Neuron survival was quantified by counts of NeuN-immunoreactive cells. Data show mean (± SEM) counts expressed as a percentage of values from nonlesioned sham animals. *, P < .05 compared with nonlesioned sham-GDX; n ≥ 7 per condition.
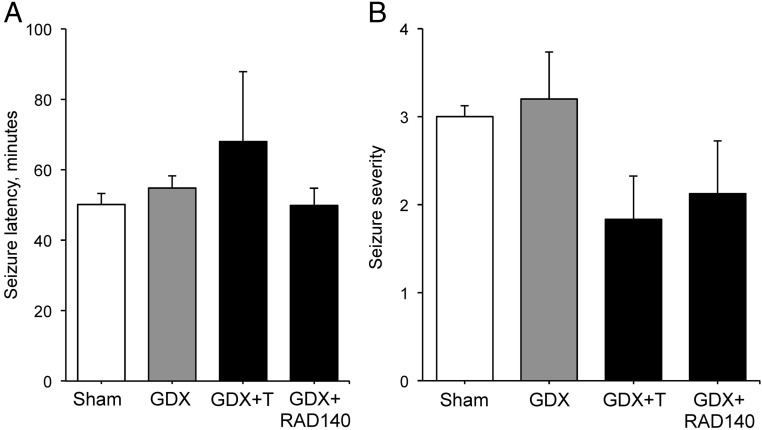
Because kainate induces seizures, we also assessed the effects of the hormonal manipulations on both the latency to seizure onset and maximum seizure severity. The intensity of seizure behavior is known to affect the degree of hippocampal neuron loss. All kainate-treated animals achieved at least level 1 seizure within the 3-hour observation period but none of the vehicle-injected animals showed any seizure-related behaviors. Among the lesioned animals, there was no significant difference across groups in seizure latency (Figure 6A; F (3, 24) = 0.75 P = .53). Similarly, seizure severity did not significantly vary by treatment group (F (3, 26) = 1.36 P = .28), although there was a trend toward reduced severity in the GDX+T and GDX+RAD140 groups (Figure 6B).
Figure 6.
Kainate-induced seizures are not affected by androgen status Behavioral features of kainate-induced seizures were monitored and quantified for a 3-hour period following the lesion. Data show mean values (± SEM) of (A) latency to seizure onset and (B) seizure severity across groups (n ≥ 7 per condition).
Discussion
In this study, we report the first findings of neuroprotective actions by SARMs in both cell culture and in vivo. Our results show in primary neuron cultures that the SARM RAD140 increases cell viability against Aβ toxicity in a concentration-dependent manner. The neuroprotective effects of RAD140 are specific to apoptotic insults and dependent upon a MAPK-signaling pathway. In GDX rats treated with RAD140, RAD140 induces androgenic responses in muscle and brain, but not in reproductive tissues. Moreover, RAD140 treatment significantly protects hippocampal neurons from kainate lesion.
SARMs are steroidal and nonsteroidal ligands for AR capable of activating androgen signaling in a tissue-specific manner (35, 51). Many factors contribute to the tissue specificity of SARMs. For example, unlike testosterone, nonsteroidal SARMs are not substrates for aromatase and 5α-reductase and thus do not yield potent estrogen and androgen metabolites (35, 52). Tissue specificity of SARMs can also be related to tissue-specific expression of AR coregulators and protein-protein interactions associated with SARM binding that can differ across tissues (51, 53). The promise of selective androgen treatment has been realized in animal models, with SARMs shown to promote muscle and bone health in the absence of prostate growth (33, 52, 54, 55). These advances have encouraged evaluation of SARMs in clinical trials for disorders including cachexia (clinical trial NCT00467844) (55) and osteoporosis (51). The data presented here are among the first highlighting the potential efficacy of SARMs for neural endpoints. Our finding that RAD140 can exert androgenic actions in brain at a dose that retains peripheral tissue selectivity is consistent with prior observations in rodents that the SARM ACP-105 can ameliorate cognitive deficits associated with apolipoprotein E (56) and irradiation (57).
Androgens exert numerous beneficial actions in brain by several distinct mechanisms (18). Many, but not all, neural androgen actions involve AR activation, which triggers a wide range of rapid cell-signaling pathways as well as classic genomic regulation (58). Because SARMs can interact with AR differently than endogenous androgens, the efficacy of specific SARMs in activating defined androgenic pathways is a key consideration in pursuing translational goals. In terms of androgen neuroprotection, our prior work has defined a mechanism that is both dependent upon MAPK/ERK signaling pathway (40) and limited in protective efficacy to apoptosis (48). Our findings with RAD140 and the related compound RAD192 demonstrate that both SARMs mimic this established mechanism of neuroprotection in cultured neurons: they activate MAPK/ERK signaling as evidenced by ERK phosphorylation, their neuroprotection is blocked by pharmacologic inhibition of MAPK signaling, and they protect against 2 apoptotic insults but not a nonapoptotic insult. MAPK/ERK signaling is also known to contribute to androgen protection in non-neural cells (59, 60). In adult male rats depleted of endogenous androgens by GDX, RAD140 matched the neuroprotection observed with T against kainate, a neurotoxin known to kill hippocampal neurons via apoptosis (61). Because neither T nor RAD140 significantly affected kainate-induced seizure behavior, a variable that can alter lesion severity (38), these observations suggest a direct mechanism of androgen neuroprotection consistent with our prior observations (39).
Still unclear is the extent to which RAD140 mimics activation of other beneficial androgen pathways in brain. MAPK/ERK signaling is involved in several important functions in the brain including neurogenesis, differentiation, synaptic plasticity, memory formation, and cell survival (62–65). However, additional signaling pathways likely contribute to androgen actions in brain. in addition to MAPK/ERK, androgens also rapidly activate cAMP response element binding protein signaling in neurons by a protein kinase C-dependent mechanism (66). In addition to rapid cell-signaling pathways, many neural androgen effects involve classic genomic responses. For example, androgen regulation of gene expression is implicated in protecting against AD pathology by reducing Aβ accumulation. Specifically, androgens reduce the expression of the proamyloidogenic enzyme β-secretase (10) and increase expression of the Aβ degrading enzyme neprilysin by an AR-dependent genomic mechanism (12). Androgen-induced neurogenesis is also AR dependent in male rats (67) and, at least in songbirds, involves genomic mechanisms including up-regulation of matrix metalloproteinases (68). Similarly, androgen-mediated increases in dendritic spines in mouse hippocampus has been linked to up-regulation of brain-derived neurotrophic factor and postsynaptic density protein 95 (69). It is important to note that several neural actions of testosterone, including its effects on sexual behavior and some aspects of neuroprotection, involve its conversion to E2 (24, 70). Because RAD140 and related SARMs are not aromatase substrates and are not known to signal through estrogen pathways, there are limitations in their abilities to fully mimic T actions in brain.
The loss of androgens with normal aging can negatively impact androgen-responsive tissues, including the brain, and has been shown to be a significant risk factor for development of neurodegenerative disorders including AD (6, 7). The increased risk for prostate cancer makes T therapy a risky treatment option (71). Moreover, even in neurons, high doses of T can be harmful rather than beneficial (72, 73). In this regard, SARMs like RAD140 can be better alternatives to T because they are only partial agonists or antagonists to androgenic regulation of prostate while still having androgenic effects on other tissues such as muscle and brain. In addition, higher doses of SARMs as compared with T might still promote neuron viability and not induce apoptosis, making them a suitable therapeutic strategy against age-related disorders such as AD.
Acknowledgments
This study was supported by grants from the Alzheimer's Association (IIRG-10–174301) and National Institutes of Health (P50 AG05142) (to C.J.P.).
Disclosure Summary: A.J., A.C., V.A.M., R.V., and C.J.P. have nothing to disclose and no conflicts of interest with the information presented in this manuscript including any personal, financial, or other conflicts. G.H. is an employee and stockholder and C.P.M. is a stockholder of Radius Health, Inc.
Footnotes
- AAII
- apoptosis activator II
- Aβ
- β-amyloid
- AD
- Alzheimer's disease
- AR
- androgen receptor
- DHT
- dihydrotestosterone
- E2
- 17β-estradiol
- ER
- estrogen receptor
- GDX
- gonadectomized
- SARM
- selective AR modulator.
References
- 1. Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69:322–337 [DOI] [PubMed] [Google Scholar]
- 2. Morley JE. Testosterone replacement and the physiologic aspects of aging in men. Mayo Clin Proc. 2000;75(Suppl):S83–S87 [PubMed] [Google Scholar]
- 3. Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuro Endocrinol Lett. 2003;24:203–208 [PubMed] [Google Scholar]
- 4. Fuller SJ, Tan RS, Martins RN. Androgens in the etiology of Alzheimer's disease in aging men and possible therapeutic interventions. J Alzheimers Dis. 2007;12:129–142 [DOI] [PubMed] [Google Scholar]
- 5. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011;32:604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–1432 [DOI] [PubMed] [Google Scholar]
- 7. Moffat SD, Zonderman AB, Metter EJ, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193 [DOI] [PubMed] [Google Scholar]
- 8. Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006;26:13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosario ER, Carroll JC, Pike CJ. Evaluation of the effects of testosterone and luteinizing hormone on regulation of β-amyloid in male 3xTg-AD mice. Brain Res. 2012;1466:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAllister C, Long J, Bowers A, et al. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates β-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30:7326–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gouras GK, Xu H, Gross RS, et al. Testosterone reduces neuronal secretion of Alzheimer's β-amyloid peptides. Proc Natl Acad Sci USA. 2000;97:1202–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ. Androgens regulate neprilysin expression: role in reducing β-amyloid levels. J Neurochem. 2008;105:2477–2488 [DOI] [PubMed] [Google Scholar]
- 13. Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965 [DOI] [PubMed] [Google Scholar]
- 15. Kay JN, Hannigan P, Kelley DB. Trophic effects of androgen: development and hormonal regulation of neuron number in a sexually dimorphic vocal motor nucleus. J Neurobiol. 1999;40:375–385 [PubMed] [Google Scholar]
- 16. Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333 [DOI] [PubMed] [Google Scholar]
- 17. Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020 [DOI] [PubMed] [Google Scholar]
- 18. Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53:693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruchovsky N, Wilson JD. The conversion of testosterone to 5-α-androstan-17-β-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–2021 [PubMed] [Google Scholar]
- 20. Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure-activity relationships. Current Med Chem. 2000;7:211–247 [DOI] [PubMed] [Google Scholar]
- 21. Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5alpha-reductase. Endocrinology. 1999;140:4509–4515 [DOI] [PubMed] [Google Scholar]
- 22. Frye CA, McCormick CM. The neurosteroid, 3α-androstanediol, prevents inhibitory avoidance deficits and pyknotic cells in the granule layer of the dentate gyrus induced by adrenalectomy in rats. Brain Res. 2000;855:166–170 [DOI] [PubMed] [Google Scholar]
- 23. Gooren LJ, Toorians AW. Significance of oestrogens in male (patho)physiology. Ann Endocrinol(Paris). 2003;64:126–135 [PubMed] [Google Scholar]
- 24. Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47:318–329 [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578 [DOI] [PubMed] [Google Scholar]
- 26. Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25:3039–3046 [DOI] [PubMed] [Google Scholar]
- 27. Goodenough S, Engert S, Behl C. Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci Lett. 2000;296:49–52 [DOI] [PubMed] [Google Scholar]
- 28. Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones JO. Improving selective androgen receptor modulator discovery and preclinical evaluation. Expert Opin Drug Discov. 2009;4:981–993 [DOI] [PubMed] [Google Scholar]
- 31. Narayanan R, Mohler ML, Bohl CE, Miller DD, Dalton JT. Selective androgen receptor modulators in preclinical and clinical development. Nucl Recept Signal. 2008;6:e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosen J, Negro-Vilar A. Novel, non-steroidal, selective androgen receptor modulators (SARMs) with anabolic activity in bone and muscle and improved safety profile. J Musculoskelet Neuronal Interact. 2002;2:222–224 [PubMed] [Google Scholar]
- 33. Yarrow JF, Conover CF, McCoy SC, et al. 17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am J Physiol Endocrinol Metab. 2011;300:E650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LaMorte A, Kumar N, Bardin CW, Sundaram K. Aromatization of 7 α-methyl-19-nortestosterone by human placental microsomes in vitro. J Steroid Biochem Mol Biol. 1994;48:297–304 [DOI] [PubMed] [Google Scholar]
- 35. Bhasin S, Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care. 2009;12:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller CP, Shomali M, Lyttle CR, et al. Design, synthesis, and preclinical characterization of the selective androgen receptor modulator (SARM) RAD140. ACS Med Chem Lett. 2011;2:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005;31:3–16 [DOI] [PubMed] [Google Scholar]
- 38. Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578 [DOI] [PubMed] [Google Scholar]
- 39. Pike CJ. Estrogen modulates neuronal Bcl-xL expression and β-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem. 1999;72:1552–1563 [DOI] [PubMed] [Google Scholar]
- 40. Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651 [DOI] [PubMed] [Google Scholar]
- 41. Gray LE, Jr, Furr J, Ostby JS. Hershberger assay to investigate the effects of endocrine-disrupting compounds with androgenic or antiandrogenic activity in castrate-immature male rats. Curr Protoc Toxicol. 2005;Chapter 16:Unit 16.9 [DOI] [PubMed] [Google Scholar]
- 42. Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42:1–32 [DOI] [PubMed] [Google Scholar]
- 43. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294 [DOI] [PubMed] [Google Scholar]
- 44. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 45. West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497 [DOI] [PubMed] [Google Scholar]
- 46. Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403 [DOI] [PubMed] [Google Scholar]
- 47. Pike CJ. Testosterone attenuates β-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165 [DOI] [PubMed] [Google Scholar]
- 48. Nguyen TV, Jayaraman A, Quaglino A, Pike CJ. Androgens selectively protect against apoptosis in hippocampal neurones. J Neuroendocrinol. 2010;22:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu D, Gore AC. Changes in androgen receptor, estrogen receptor α, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58:306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Narayanan R, Coss CC, Yepuru M, Kearbey JD, Miller DD, Dalton JT. Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol Endocrinol. 2008;22:2448–2465 [DOI] [PubMed] [Google Scholar]
- 52. Yarrow JF, McCoy SC, Borst SE. Tissue selectivity and potential clinical applications of trenbolone (17β-hydroxyestra-4,9,11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids. 2010;75:377–389 [DOI] [PubMed] [Google Scholar]
- 53. Bohl CE, Miller DD, Chen J, Bell CE, Dalton JT. Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J Biol Chem. 2005;280:37747–37754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allan G, Sbriscia T, Linton O, et al. A selective androgen receptor modulator with minimal prostate hypertrophic activity restores lean body mass in aged orchidectomized male rats. J Steroid Biochem Mol Biol. 2008;110:207–213 [DOI] [PubMed] [Google Scholar]
- 55. Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Acevedo SE, McGinnis G, Raber J. Effects of 137Cs γ irradiation on cognitive performance and measures of anxiety in Apoe−/− and wild-type female mice. Radiat Res. 2008;170:422–428 [DOI] [PubMed] [Google Scholar]
- 57. Dayger C, Villasana L, Pfankuch T, Davis M, Raber J. Effects of the SARM ACP-105 on rotorod performance and cued fear conditioning in sham-irradiated and irradiated female mice. Brain Res. 2011;1381:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Falkenstein E, Wehling M. Nongenomically initiated steroid actions. Eur J Clin Invest. 2000;30(Suppl 3):51–54 [DOI] [PubMed] [Google Scholar]
- 59. Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329 [DOI] [PubMed] [Google Scholar]
- 60. Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730 [PubMed] [Google Scholar]
- 61. Pollard H, Charriaut-Marlangue C, Cantagrel S, et al. Kainate-induced apoptotic cell death in hippocampal neurons. Neuroscience. 1994;63:7–18 [DOI] [PubMed] [Google Scholar]
- 62. Bajetto A, Barbero S, Bonavia R, et al. Stromal cell-derived factor-1α induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001;77:1226–1236 [DOI] [PubMed] [Google Scholar]
- 63. Qui MS, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717 [DOI] [PubMed] [Google Scholar]
- 64. Krapivinsky G, Krapivinsky L, Manasian Y, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784 [DOI] [PubMed] [Google Scholar]
- 65. Alonso M, Viola H, Izquierdo I, Medina JH. Aversive experiences are associated with a rapid and transient activation of ERKs in the rat hippocampus. Neurobiol Learn Mem. 2002;77:119–124 [DOI] [PubMed] [Google Scholar]
- 66. Nguyen TV, Yao M, Pike CJ. Dihydrotestosterone activates CREB signaling in cultured hippocampal neurons. Brain Res. 2009;1298:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA. Androgens increase survival of adult born neurons in the dentate gyrus by an androgen receptor dependent mechanism in male rats. Endocrinology. 2013;154:3294–3304 [DOI] [PubMed] [Google Scholar]
- 68. Kim DH, Lilliehook C, Roides B, et al. Testosterone-induced matrix metalloproteinase activation is a checkpoint for neuronal addition to the adult songbird brain. J Neurosci. 2008;28:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76–84 [DOI] [PubMed] [Google Scholar]
- 70. Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav Neurosci. 1997;111:381–397 [PubMed] [Google Scholar]
- 71. Hijazi RA, Cunningham GR. Andropause: is androgen replacement therapy indicated for the aging male? Annu Rev Med. 2005;56:117–137 [DOI] [PubMed] [Google Scholar]
- 72. Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281:25492–25501 [DOI] [PubMed] [Google Scholar]
- 73. Caraci F, Pistarà V, Corsaro A, et al. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J Neurosci Res. 2011;89:592–600 [DOI] [PubMed] [Google Scholar]