Abstract
Rhizobia colonize their legume hosts by different modes of entry while initiating symbiotic nitrogen fixation. Most legumes are invaded via growing root hairs by the root hair-curl mechanism, which involves epidermal cell responses. However, invasion of a number of tropical legumes happens through fissures at lateral root bases by cortical, intercellular crack entry. In the semiaquatic Sesbania rostrata, the bacteria entered via root hair curls under nonflooding conditions. Upon flooding, root hair growth was prevented, invasion on accessible root hairs was inhibited, and intercellular invasion was recruited. The plant hormone ethylene was involved in these processes. The occurrence of both invasion pathways on the same host plant enabled a comparison to be made of the structural requirements for the perception of nodulation factors, which were more stringent for the epidermal root hair invasion than for the cortical intercellular invasion at lateral root bases.
Keywords: ethylene, host invasion, nodulation factor, symbiosis, flooding-adapted growth
Leguminous plants can engage in a symbiotic interaction with rhizobia (nitrogen-fixing bacterial symbionts), resulting in the formation of specialized root organs, the nodules. In the central nodule tissue, internalized bacteria fix dinitrogen to be used by the host. Many legumes are of agronomic importance as major food and feed crops, whereas others have a great potential as green manure. The legume–rhizobia interaction is initiated by a complex signal exchange during which recognition of bacterial nodulation (Nod) factors switches on the nodulation program in the plant. Nod factors are lipochitooligosaccharides that carry different substitutions. Recently, plant genes have been characterized that encode components of the Nod factor receptor/perception complexes (1–5).
In legume symbiosis, bacterial invasion can follow different routes, the best known of which is via root hairs. Rhizobia induce the curling of growing root hairs, are entrapped in the curl, and enter the root hair by local hydrolysis of cell walls and invagination of the plasma membrane. Tip growth toward the base of the root hair results in an intracellular infection thread that proceeds through the cortical cells to reach the nodule primordia, where bacteria are released inside plant cells. The process takes place in the zone of developing root hairs (zone I, ref. 6), located just above the root meristem. Root hair invasion is used in pea, bean, soybean, vetch, and alfalfa, and in the model legumes Medicago truncatula and Lotus japonicus (7, 8).
Another mode of entry, via intercellular invasion at lateral root bases, has been observed in many tropical legumes. The bacteria enter via cracks formed by the protrusion of lateral roots and colonize large intercellular spaces called infection pockets. The mechanism for deeper invasion varies. In Sesbania rostrata and in Neptunia sp., infection pockets narrow down to form intercellular infection threads, and subsequently intracellular infection threads intrude into the nodule primordium (9–12). In Aeschynomene, Stylosanthes, and Arachis, invasion progresses by means of cell collapse, and bacteria enter the cells of the nodule primordia by direct uptake from the infection pockets (13–16).
The process of intercellular invasion has been examined in most detail in the S. rostrata–Azorhizobium caulinodans interaction, which has become a model for the study of this type of entry (12, 17). On S. rostrata, nodules arise not only on the root but also on the stem, at positions of adventitious root primordia (12). These so-called stem nodules are actually adventitious root nodules, and their development is morphologically equivalent to the development of lateral root base nodules. The hormone ethylene, which controls the physiology of aquatic plants (18), is required for lateral root base nodulation, where it plays a role in the Nod factor-induced cell death that is needed for infection pocket formation (19). This ethylene dependency for nodule initiation is special. Indeed, root hair curl invasion has been found to be either inhibited by or insensitive to ethylene (20). In M. truncatula, ethylene modulates the calcium spiking that precedes root hair deformation (21). In vetch, ethylene affects preinfection thread formation, thus blocking invasion (22).
Both root hair and intercellular invasion are Nod factor-dependent processes (23–25). In Medicago sp., pea, and vetch, the Nod factor structure requirements for entry in root hairs are more stringent than those for induction of cortical cell division (26–28), suggesting the occurrence of very specific entry receptors. Genes coding for putative entry receptors of M. truncatula, pea, and L. japonicus have recently been identified (3–5). Interestingly, during intercellular invasion in S. rostrata, bacteria that produce Nod factors without decoration were still active, and functional nodules were still formed, be it at very low frequency (23). Although these observations suggest that intercellular invasion may need less stringent structural Nod factor requirements, no conclusions can be reached because root hair invasion and intercellular invasion took place on different host plants.
The fact that different entry routes exist to invade the host and to form functional nodules has attracted the attention of many legume biologists. Because of its occurrence in the less evolved groups of legumes, intercellular invasion was proposed to be the more ancient mechanism. On the other hand, legumes that allow intercellular invasion bear only few root hairs, suggesting that intercellular invasion might be an adaptive behavior (29).
We show that under nonaquatic conditions, S. rostrata roots, in contrast to hydroponic roots, have plenty of root hairs through which infection proceeds. Root hair infection was inhibited by ethylene and required more stringent Nod factor features than intercellular invasion. Ethylene also affected root architecture and the formation of new root hairs. Other aquatic legumes, such as Neptunia sp., also prefer the root hair curling process under nonaquatic conditions. Thus, intercellular invasion is seemingly an adaptation to waterlogging, and the root hair curling process is the default pathway taken by rhizobia to enter legume plants.
Materials and Methods
Plant and Bacterial Growth Conditions. Seedlings of S. rostrata Brem were germinated (30) and grown in tubes and Leonard jars (31). l-α-(2-aminoethoxyvinyl)-glycine (AVG), 1-aminocyclopropane-1-carboxylate (ACC), and Ag2SO4 were added to the lower container of Leonard jars or in tubes at final concentrations of 7 μM, 20 μM, and 10 μM, respectively. The tubes and Leonard jars were protected from light. The components were added 2 days before inoculation, except when otherwise indicated.
The strains ORS571, ORS571(pRG960SD-32) (32), ORS571(pBBR5-hem-gfp5-S65T) (W. D'Haeze and M.H., unpublished data), ORS571(4.2K), ORS571(1.2), ORS571(1.11Z-ΩK), and ORS571 (1.31U-ΩK) (23) were grown and inoculated as described (31).
Neptunia plena seeds were germinated according to James et al. (9). The plants were grown in tubes and Leonard jars according to the same protocols as those for S. rostrata. Rhizobium sp. DUS239 was cultivated as described (9). To construct a strain that is detectable by GFP analysis, the hem-gfp5 cassette was cut from the plasmid pBBR5-hem-gfp5-S65T by XhoI–PstI digestion and introduced into the tetracycline-resistant plasmid pBBR1MCS-3 by standard procedures (33), creating the plasmid pBBR3-hem-gfp5-S65T. The plasmid was introduced into DUS239 by triparental mating (33).
Microscopic Analysis. Staining for β-glucuronidase (GUS) was as described (34). For semithin sectioning, the tissues were embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany), sectioned, and stained with ruthenium red or toluidine blue (35, 36). GFP analysis was performed according to Van de Velde et al. (35). Roots and root hairs were stained with methylene blue (23).
Results
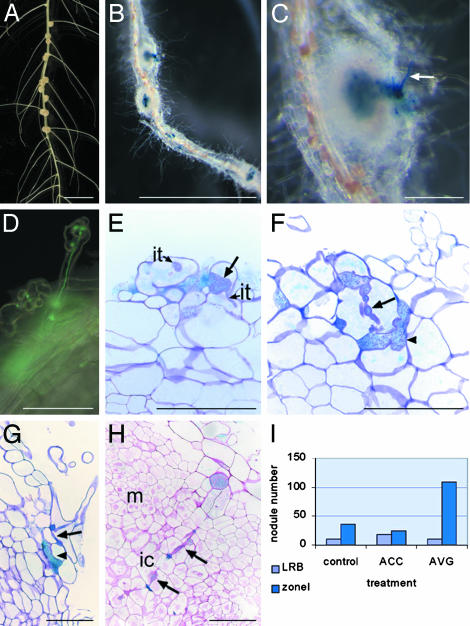
S. rostrata Roots, Grown in Vermiculite, Are Invaded via Root Hairs. On hydroponic roots of S. rostrata, nodules arose at the bases of lateral roots, and bacteria invaded intercellularly between cortical cells (Fig. 1A). However, when plants were grown on vermiculite in Leonard jars, nodules formed along the well-aerated roots and not preferentially at the lateral root bases (Fig. 1B). Upon inoculation with the A. caulinodans strains ORS571(pRG960SD-32) and ORS571 (pBBR5-hem-gfp5-S65T), which can be visualized by GUS staining or fluorescence microscopy, respectively, curled root hairs with infection threads were detected (Fig. 1 C and D). Because this response took place in zone I of emerging root hairs (data not shown), the resulting nodules will be referred to as zone I nodules. Analysis of semithin sections revealed that the bacteria entered the root hairs by sometimes very broad infection threads that narrowed down toward the base of the root hairs (Fig. 1E). Upon leaving the root hair, the infection proceeded through the cortex intracellularly (Fig. 1F) or intercellularly (Fig. 1G), occasionally accompanied by small infection pocket-like structures (Fig. 1 F and G). Progression into the nodule primordium happened by intracellular infection threads (Fig. 1H).
Fig. 1.
Features of S. rostrata nodulation. (A) Nodulated hydroponic S. rostrata root, 7 days postinoculation (DPI). Nodules are present at the lateral root bases. (B) GUS staining of a vermiculite-grown root nodulated by ORS571(pRG960SD-32). Nodules are distributed over the root (zone I nodules) 4 DPI. (C) Enlargement of B. Arrow indicates GUS-stained root hair. (D) Root hair with an infection thread containing A. caulinodans carrying a GFP marker. (E–G) Toluidine blue-stained sections through vermiculite-grown roots, 2 DPI with ORS571(pRG960SD-32), and stained for GUS. Arrows indicate initiating broad infection thread (E), an intracellular cortical infection thread (F), and an intercellular infection thread (G); arrowheads mark small infection pocket-like structures (F and G). (H) Ruthenium red-stained section through a developing zone I nodule, 4 DPI with ORS571(pRG960SD-32), and stained for GUS. Arrows indicate intracellular invasion track. (I)Influence of ethylene on lateral root base and on zone I nodulation on vermiculite-grown roots. The histogram presents the number of nodules derived from zone I or from lateral root base infection, 7 DPI, on roots grown in vermiculite (control), on vermiculite-grown roots to which ACC has been added 2 days before inoculation (ACC), and to which AVG has been added 2 days before inoculation (AVG). ic, infection center; it, infection thread; LRB, lateral root base nodule; m, meristem; zoneI, zone I nodules. (Bars = 1 cm in A and B,1mmin C, and 100 μmin D–H.)
On hydroponic roots, the bacteria entered the plant solely by intercellular invasion at lateral root bases (data not shown). On vermiculite-grown roots, both types of invasion occurred, but with a clear preference for zone I nodulation and intracellular root hair invasion (Fig. 1I, control).
Opposite Requirement for Ethylene During Intercellular and Intracellular Invasion. Previously, we have found that ethylene is required for the intercellular invasion at lateral root bases of S. rostrata (19). On the other hand, ethylene has been shown to have no or a negative effect on the root hair invasion process (20). Is ethylene dependency of lateral root base nodulation in S. rostrata linked to the plant or the process? To address this question, we investigated the role of ethylene in root hair invasion (Fig. 1I).
Two days before inoculation of vermiculite-grown S. rostrata roots, 7 μM AVG, an inhibitor of ethylene synthesis, was added, and nodules were counted 1 week after inoculation. The number of zone I nodules had clearly increased. Similar results were obtained with Ag2SO4, an inhibitor of ethylene action (data not shown). In contrast, the amount of zone I nodules decreased when ACC, an ethylene precursor, was added to the medium (Fig. 1I). These results show an opposite ethylene dependence for the intercellular invasion and the root hair invasion, the latter being inhibited by ethylene.
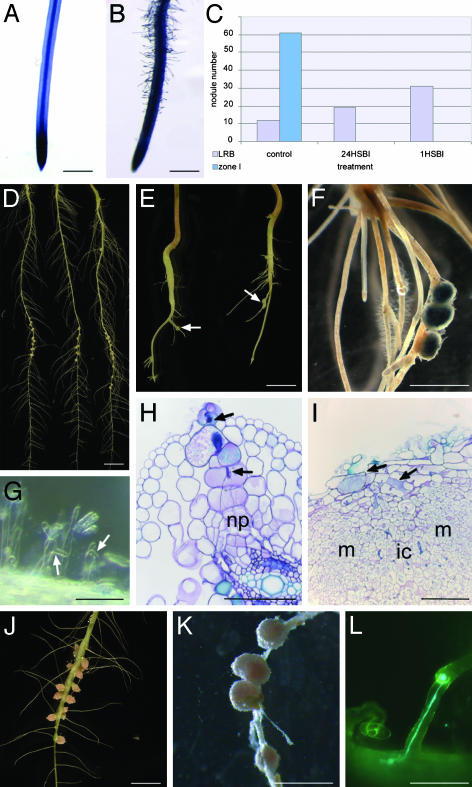
Submergence Blocks Root Hair Invasion. In S. rostrata, root hair distribution and susceptibility to the symbiotic bacteria depend on root growth conditions and are controlled by ethylene. Hydroponic roots hardly had root hairs, the tips were naked (Fig. 2A), and root hairs present at the upper, oldest part of the root did not curl upon inoculation (19). On the contrary, when grown in vermiculite, roots were completely covered with root hairs, starting from zone I just above the tip (Fig. 2B). When vermiculite-grown roots were submerged for 24 h or 1 h before inoculation, the root hair invasion was completely blocked, and no zone I nodules were detected 7 DPI (Fig. 2C).
Fig. 2.
Comparison of root morphology and nodulation traits between hydroponically and vermiculite-grown S. rostrata and N. plena plants. (A and B) Methylene blue-stained root tip of hydroponically and vermiculite-grown S. rostrata root, respectively. No root hairs are detected on the hydroponically grown root tip, whereas the vermiculite-grown root contains plenty. (C) Influence of submersion on lateral root base and zone I nodule numbers of vermiculite-grown S. rostrata roots. The nodule number is derived from lateral root base or zone I nodulation, 7 DPI on vermiculite-grown roots (control), on vermiculite-grown roots that were submerged 24 h before inoculation (24HSBI), and 1 h before inoculation (1HSBI). (D) Nodulated hydroponic roots, 7 DPI. Nodules are located at lateral root bases. (E) Nodulated hydroponic root, grown in the presence of AVG, 7 DPI. Nodules are distributed over the root (arrows). (F) Hydroponic root grown in the presence of AVG, infected with ORS571(pRG960SD-32), and stained for GUS. (G) Root hair deformation and curling on hydroponically grown roots in the presence of AVG, 2 DPI. Arrows mark curled and deformed root hairs. (H and I) Toluidine blue-stained sections of developing nodules on hydroponic roots grown in the presence of AVG. Arrows indicate intracellular infection thread (H) and small infection pockets (I). (J) Hydroponically grown nodulated N. plena roots, 7 DPI. Nodules are present at lateral root bases. (K) Vermiculite-grown nodulated N. plena root. Nodules are distributed over the root. (L) Curled N. plena root hair containing an infection thread visualized by GFP-containing Rhizobium sp. DUS239. Abbreviations, see Fig. 1 legend; np, nodule primordium. (Bars = 1 cm in A, B, D–F, J, and K and 100 μm in G–I and L.)
Because submergence is known to cause entrapment of ethylene, we examined the putative role of ethylene in prevention of root hair growth and root hair response to nodulating bacteria. When seedlings were transferred to tubes containing 7 μM AVG, the main root and lateral roots were much smaller than those grown without AVG (Fig. 2 D and E), and lateral roots were partially covered with root hairs (Fig. 2F). Inoculation resulted in deformation and curling of the root hairs (Fig. 2G); the nodules were preferentially distributed over the lateral roots (Fig. 2 E and F). Semithin sections through young developing nodules revealed infection threads inside root hairs and a subsequent intracellular track toward the nodule primordia (Fig. 2H). At later stages, also small intercellular infection pockets were observed (Fig. 2I), similarly as for nodule formation in vermiculite-grown roots.
The Versatile Invasion Is Typical for Water-Adapted Legumes. Are these versatile invasion properties typical for S. rostrata or do other semiaquatic legumes have the same behavior? N. plena is an aquatic legume that is invaded under hydroponic conditions in a manner very similar to that of S. rostrata (data not shown, ref. 9). N. plena seedlings were grown either in tubes or in Leonard jars. As for S. rostrata, the root hair distribution differed between the two physiological conditions (9). Upon inoculation with the microsymbiont Rhizobium sp. DUS239, nodules arose at lateral root bases of the hydroponic roots and were distributed all over the vermiculite-grown roots (Fig. 2 J and K). To follow bacterial invasion, the plasmid pBBR3-hem-gfp5-S65T was introduced into Rhizobium sp. DUS239 (see Materials and Methods), and vermiculite-grown, inoculated roots were analyzed by fluorescent microscopy. As shown in Fig. 2L, root hairs with infection threads were observed.
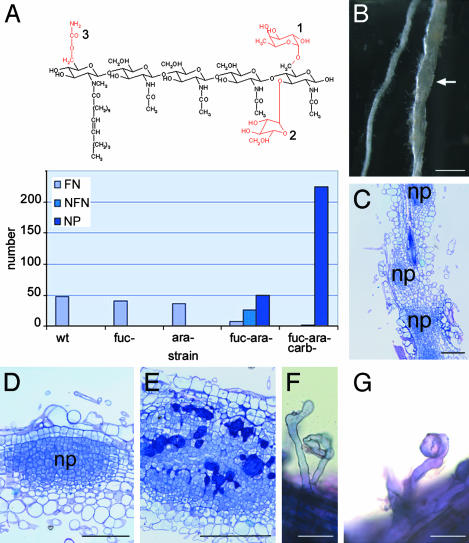
Nod Factor Structure Requirement Is More Stringent for Root Hair Invasion Than for Intercellular Invasion. A. caulinodans produces mainly pentameric Nod factors with a common fatty acid, an N-methyl, and a 6-O-carbamoyl group at the nonreducing terminal GlcNAc residue and with a d-arabinosyl, an l-fucosyl, or both at the reducing-end GlcNAc (Fig. 3A and ref. 37). By using bacterial mutants that produce well characterized sets of altered Nod factors, none of the Nod factor substituents was shown to be strictly required for lateral root base nodulation, although their synergistic presence determined the nodulation frequency (23). Hence, we investigated the Nod factor structure requirements for the root hair invasion of S. rostrata. Roots grown in vermiculite were inoculated with ORS571(4.2K), ORS571(1.2), ORS571(1.11Z-ΩK), or ORS571(1.31U-ΩK), which are strains that produce Nod factors without a fucosyl group, without an arabinosyl group, without reducing-end glycosylations, or devoid of reducing-end glycosylations and carbamoylation, respectively. For each strain, the total number of nodules (primordia) derived from zone I invasion in 12 plants was counted at 7 DPI (Fig. 3A). Inoculation with bacteria producing nonarabinosylated, nonfucosylated, or nonarabinosylated and nonfucosylated Nod factors yielded 40, 36, and 32 nodules, respectively, barely fewer than during wild-type infection (47 nodules). Only one nonfunctional nodule (with white to slightly pink central tissue; data not shown) was counted on plants inoculated with strain ORS571(1.31U-ΩK), which produces nonglycosylated and noncarbamoylated Nod factors. These plants were yellow, indicating a lack of nitrogen fixation. In the infection with ORS571(1.11Z-ΩK), 25 of 32 nodules were small with a white-to-pinkish central tissue. In the experiments with ORS571(1.11Z-ΩK) and ORS571(1.31U-ΩK), many small nodule-like structures were observed; in the latter case, these were so abundant that the root had a swollen appearance (Fig. 3B). Sectioning through such a swollen root showed multiple nodule primordia (Fig. 3C), consisting of densely packed dividing cells and located in the outer cortex (Fig. 3D). Sometimes these primordia were invaded intercellularly (Fig. 3E). To see at which level the interaction was blocked, vermiculite-grown roots were infected with ORS571(pBBR5-hem-gfp5-S65T), and 45 roots were screened for infection thread formation. Although the root hairs were deformed (Fig. 3F) and curled (Fig. 3G), neither infection threads nor colonization of the curls was observed.
Fig. 3.
Nod factor structure requirements for root hair infection on S. rostrata. (A) Structure of the main Nod factor produced by A. caulinodans ORS571. Substitutions in red are the chemical groups that are removed in the different mutant strains used in the experiment. 1, fucosyl moiety; 2, arabinosyl moiety; and 3, carbamoyl moiety. The histogram shows the number of functional nodules (FN), nonfunctional nodules (NFN), and nodule primordia (NP) caused by zone I infection on vermiculite-grown roots, 7 DPI, infected with wild-type ORS571 or several mutants. Fuc-, ara-, and carbindicate that the mutant strain produced Nod factors lacking the fucosyl, arabinosyl, or carbamoyl moiety, respectively. Because of the variable nodulation efficiency of different plants, the results presented are the sum of lateral root base or zone I nodules formed on 12 plants. (B) Vermiculite-grown roots infected with ORS571(1.31U-ΩK), 7 DPI. Arrow indicates swollen root. (C) Toluidine blue-stained section through a swollen root reveals that the swollen root appearance is due to the presence of a sequence of nodule primordia. (D) Toluidine blue-stained sections through a nodule primordium grown after infection with ORS571(1.31U-ΩK). (E) Toluidine blue-stained section of a colonized nodule primordium grown after infection with ORS571(1.31U-ΩK). Bacteria are seen in large intercellular spaces between the primordial cells. (F and G) Methylene blue-stained deformed and curled root hairs, respectively, of vermiculite-grown S. rostrata roots after infection with ORS571(1.31U-ΩK). Abbreviations, see Fig. 2 legend. (Bars = 1 cm in B and 100 μm in C–G.)
Discussion
To study legume–rhizobium symbiosis, two model plants have been selected, L. japonicus and M. truncatula (25, 38, 39). However, not all nodulation-related questions can be addressed with these two systems. For instance, the specific adaptations related to nodulation under aquatic conditions, a trait that is of agronomic importance, requires appropriate study organisms.
During the symbiosis between the semiaquatic legume S. rostrata and its microsymbiont A. caulinodans, bacteria enter the plant intercellularly via cracks at lateral or adventitious root bases (10, 40). However, an old and nearly forgotten paper mentioned the presence on S. rostrata of curled root hairs that contained infection threads (41). We show that indeed both ways of invasion occur in S. rostrata, but under different conditions. On well aerated roots growing in Leonard jars in vermiculite, nodules do not arise at the lateral root bases, but are distributed mostly over the lateral roots. In contrast to hydroponically grown roots, which are bare, the well aerated roots are covered with root hairs. After inoculation with A. caulinodans, zone I root hairs, located near the root tip, are curled and contain infection threads to guide the bacteria to nodule primordium cells. These infection threads are often very broad and interrupted by an intercellular track at the borders between cells. At later stages of nodule development, also small intercellular infection pockets can be observed in the outer cortical regions. Probably, A. caulinodans can colonize cracks that appear once the nodule primordium expands from the inner cortex. It is improbable that these bacteria progress within the plant to cause the nodule organogenesis and infection. Indeed, mutant bacteria that produce unsubstituted Nod factors fail to induce functional nodules via root hairs in zone I, although they are able to proliferate in small intercellular pockets.
Because S. rostrata is capable of switching entry modes when grown on flooded and unflooded soils, we were curious to investigate the role of ethylene in the control of this versatility. In addition to being involved in various developmental processes, the plant hormone ethylene is also tightly related to flooding-adapted growth. Ethylene, being gaseous, diffuses at a more reduced rate in water than in air, leading to a quick accumulation upon flooding, and is a primary signal that activates water-adapted growth responses in Rumex palustris and deepwater rice (42, 43). In S. rostrata, ethylene affects nodule meristem maintenance (31) and is necessary for the intercellular invasion at lateral root bases of hydroponic roots (19). Ethylene also affects root growth and root hair distribution (this work). Hydroponically grown roots have no root hairs in zone I, whereas aeroponically grown roots have plenty. Ethylene might be partially responsible for this difference because adding AVG results in a partial restoration of root hair growth on the lateral roots of hydroponic seedlings. In Arabidopsis thaliana, ethylene is involved in both root hair initiation and elongation (44). In hydroponic S. rostrata roots, ethylene is necessary for root hair elongation in the axils of lateral roots (19) but inhibits root hair initiation (this work), illustrating again the specific physiology of flooding-adapted plants that cannot always be compared with terrestrial plants (19, 43).
Ethylene also affects the root hair invasion process in S. rostrata. When inhibitors of ethylene synthesis or perception are added to vermiculite-grown roots before inoculation with azorhizobia, the numbers of zone I nodules increase, whereas addition of ACC, the precursor of ethylene, reduces root hair invasion. Flooding of roots grown in Leonard jars and thus bearing susceptible zone I root hairs results in a 100% inhibition of root hair infections 1 h before inoculation with A. caulinodans. On the contrary, root hairs present on roots grown hydroponically in the presence of AVG were sensitive to curling and to root hair invasion. The difference between the two root hair situations is the presence of ethylene, the accumulation of which is responsible for the root hair curl inhibition upon flooding. Thus, root hair curl invasion in S. rostrata is sensitive to ethylene, similar to the situation described for M. truncatula and several other legumes (21).
Intercellular invasion often takes place on plants without root hairs (29), but in S. rostrata the situation is more complex and variable (29). In this water stress-adapted plant, ethylene is the main switch that determines how the invasion will happen because ethylene prevents root hair growth, inhibits the invasion of accessible root hairs upon submersion, and is necessary for infection pocket formation, which is an essential trait of intercellular invasion (ref. 19 and this work).
How about other flooding-adapted legumes? On hydroponically grown N. plena roots, nodules arose at lateral root bases, and infection took place intercellularly as in S. rostrata. On the other hand, in Leonard jars nodules were distributed all over the lateral roots, and infection threads were detected within curled root hairs. In the literature on aquatic legumes, we found no further direct support for our observations, mainly because most articles do not focus on infection. However, between the lines, several authors hint at a versatile infection behavior on aquatic legumes, suggesting that the intercellular invasion was recruited to allow infection in situations where root hair invasion is inhibited (13, 15, 45).
The observation that S. rostrata and N. plena, two semiaquatic legumes belonging to different subfamilies, display a similar versatility in entry mode is interesting in view of the interpretation of the evolutionary aspect of infection. S. rostrata belongs to the Hologalegina tribe of the Papilionoideae family and N. plena to the family of the Mimosoideae (46). It has often been postulated that intercellular invasion would be more ancestral than root hair invasion simply because it looks more primitive and has more characteristics in common with pathogen invasion, from which it could have been derived. On the other hand, Sprent (29) suggested, on the basis of evolutionary tree interpretations, that root hair invasion would be ancestral. The observation that root hair invasion indeed happens on N. plena (under nonflooding conditions) demonstrates that it is more spread among the legume members than previously thought. Moreover, we have shown that the intercellular infection bypasses the root hair invasion inhibition by flooding, suggesting that root hair invasion is the default program.
Because in S. rostrata the two types of invasion occur, the respective Nod factor structure requirements can be compared. The bacterial Nod factors activate the nodulation program of the legume host. They trigger various responses, such as root hair deformation, root hair swelling, gene expression, and cortical cell division. For nodulation of vetch, pea, and M. truncatula, the structural Nod factor requirements for infection thread formation are very stringent, whereas they are less stringent for root hair swelling, root hair deformation, and cell division (26–28). Concerning the intercellular invasion, A. caulinodans mutants that produce Nod factors stripped of carbamoyl and glycosyl substitutions can still induce functional nodules, but the nodulation efficiency drops considerably. Thus, intercellular invasion at lateral root bases, although Nod factor dependent, does not require the recognition of a very strict structure (23). By using the same set of mutant strains, root hair invasion was found to present higher structural demands than did intercellular invasion. Whereas removal of one of the reducing-end glycosylations does not diminish the nodulation efficiency, removal of both glycosylations decreases the number of functional nodules and causes the formation of numerous small white nodules and nodule primordia. The effect is most prominent after infection with a mutant that produces Nod factors that lack the reducing-end glycosylations as well as the carbamoyl group at the nonreducing end. Functional nodules are no longer observed; only a few nonfunctional white nodules and a huge number of small nodule primordia are present, giving the roots a swollen appearance. Microscopic analysis showed that root hair deformation, swelling, and, to a lesser extent, curling were still detectable, but no infection threads were found. In a very few cases, bacteria are found in intercellular infection pocket-like structures and in intercellular infection threads. These bacteria do not enter via root hairs but most probably via cracks that inevitably appear when the primordia grow out from the root cortex. They are incapable of invading the plant cells and transforming into bacteroids.
Interestingly, aerated roots of S. rostrata react to an infection-deficient strain by allowing many more nodule initiation events. The number of nodule primordia increases according to the level of infection deficiency. A similar event has been observed by Ardourel et al. (26). (Auto)regulation of nodulation is complex and takes place at several levels, such as control of primordium formation and control of infection site formation (47–50). The latter probably involves ethylene, which negatively regulates the persistence of infection, interfering with the Nod factor signal transduction (19, 51). Thus, infection in susceptible zone I would trigger ethylene production, which negatively modulates infection initiation. Because of inability of the mutant strains to efficiently invade the plant, the infection-related autoregulation system is not switched on, leading to an excess of nodulation events. Whereas the S. meliloti strains, which cannot invade the root hairs of M. truncatula, induce only some cell divisions in the cortex (26), the corresponding A. caulinodans strains induce dense nodule primordia in S. rostrata. Probably, the Nod factors produced by the bacteria within the intercellular spaces maintain the cortical cell divisions. The lack of locally high ethylene levels may be the reason why the intercellular bacteria are not able to further progress in the plant.
Root hair infection and, especially, infection thread formation are often inhibited by ethylene, and this ethylene inhibition is probably active in the epidermis (20). Under aquatic conditions, where ethylene accumulates, this step has to be circumvented; instead, an infection route was recruited in which ethylene is necessary. Although intercellular invasion may look more simple, physiological demands, such as ethylene concentrations, make it as complex as root hair invasion. The differences in Nod factor structure requirements for root hair invasion at the level of the epidermis and intercellular invasion at the level of the cortex provide an interesting challenge to compare epidermal and cortical responses at the gene expression level.
Plants have adapted to live in situations of flooding or partial flooding by versatile mechanisms. Whereas S. rostrata root nodules develop and function on submerged roots, there is a switch from indeterminate to determinate nodule type (31) and from root hair infection to intercellular invasion. A hope remains to understand the legume symbiosis in such detail that the process can be introduced into important nonlegume crops, such as rice (52). Because rice is also a flooding-adapted plant, intercellular invasion at lateral root bases could well be the preferred mode to be used to let nitrogen-fixing bacteria enter.
Acknowledgments
We thank Christa Verplancke and Annick De Keyser for technical assistance and Martine De Cock for help in preparing the manuscript. This work was supported by the Interuniversity Poles of Attraction Program-Belgian Science Policy (P5/13) and by the Fund for Scientific Research-Flanders (Project no. G027601 and Krediet aan Navorsers 1.5.088.99N and 1.5.192.01N). W.C. is indebted to the Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen for a predoctoral fellowship.
Abbreviations: ACC, 1-aminocyclopropane-1-carboxylate; AVG, l-α-(2-aminoethoxyvinyl)-glycine; DPI, days postinoculation; GUS, β-glucuronidase; Nod factor, nodulation factor.
References
- 1.Endre, G., Kereszt, A., Kevei, Z., Mihacea, S., Kató, P. & Kiss, G. B. (2002) Nature 417, 962-966. [DOI] [PubMed] [Google Scholar]
- 2.Stracke, S., Kistner, C., Yoshida, S., Mulder, L., Sato, S., Kaneko, T., Tabata, S., Sandal, N., Stougaard, J., Szczyglowski, K. & Parniske, M. (2002) Nature 417, 959-962. [DOI] [PubMed] [Google Scholar]
- 3.Limpens, E., Franken, C., Smit, P., Willemse, J., Bisseling, T. & Geurts, R. (2003) Science 302, 630-633. [DOI] [PubMed] [Google Scholar]
- 4.Madsen, E. B., Madsen, L. H., Radutoiu, S., Olbryt, M., Rakwalska, M., Szczyglowski, K., Sato, S., Kaneko, T., Tabata, S., Sandal, N. & Stougaard, J. (2003) Nature 425, 637-640. [DOI] [PubMed] [Google Scholar]
- 5.Radutoiu, S., Madsen, L. H., Madsen, E. B., Felle, H. H., Umehara, Y., Grønlund, M., Sato, S., Nakamura, Y., Tabata, S., Sandal, N. & Stougaard, J. (2003) Nature 425, 585-592. [DOI] [PubMed] [Google Scholar]
- 6.Mathesius, U., Weinman, J. J., Rolfe, B. G. & Djordjevic, M. A. (2000) Mol. Plant–Microbe Interact. 13, 170-182. [DOI] [PubMed] [Google Scholar]
- 7.Vance, C. P., Johnson, L. E. B., Stade, S. & Groat, R. G. (1982) Can. J. Bot. 60, 505-518. [Google Scholar]
- 8.Wood, S. M. & Newcomb, W. (1989) Can. J. Bot. 67, 3108-3122. [Google Scholar]
- 9.James, E. K., Minchin, F. R. & Sprent, J. I. (1992) Ann. Bot. 69, 181-187. [Google Scholar]
- 10.Ndoye, I., de Billy, F., Vasse, J., Dreyfus, B. & Truchet, G. (1994) J. Bacteriol. 176, 1060-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subba-Rao, N. S., Mateos, P. F., Baker, D., Pankratz, H. S., Palma, J., Dazzo, F. B. & Sprent, J. I. (1995) Planta 196, 311-320. [Google Scholar]
- 12.Goormachtig, S., Mergaert, P., Van Montagu, M. & Holsters, M. (1998) in Plant—Microbe Interactions, eds. Biswas, B. B. & Das, H. K. (Plenum, New York), Vol. 29, pp. 117-164. [Google Scholar]
- 13.Chandler, M. R., Date, R. A. & Roughley, R. J. (1982) J. Exp. Bot. 33, 47-57. [Google Scholar]
- 14.Alazard, D. & Duhoux, E. (1990) J. Exp. Bot. 41, 1199-1206. [Google Scholar]
- 15.Loureiro, M. F., James, E. K., Sprent, J. I. & Franco, A. A. (1995) New Phytol. 130, 531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boogerd, R. C. & van Rossum, D. (1997) FEMS Microbiol. Lett. 21, 5-27. [Google Scholar]
- 17.Lievens, S., Goormachtig, S. & Holsters, M. (2001) Nucleic Acids Res. 17, 3459-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson, M. B. (1985) Annu. Rev. Plant Physiol. 36, 145-174. [Google Scholar]
- 19.D'Haeze, W., De Rycke, R., Mathis, R., Goormachtig, S., Pagnotta, S., Verplancke, C., Capoen, W. & Holsters, M. (2003) Proc. Natl. Acad. Sci. USA 100, 11789-11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guinel, F. C. & Geil, R. D. (2002) Can. J. Bot. 80, 695-720. [Google Scholar]
- 21.Oldroyd, G. E. D., Mitra, R. M., Wais, R. J. & Long, S. R. (2001) Plant J. 28, 191-199. [DOI] [PubMed] [Google Scholar]
- 22.van Spronsen, P. C., van Brussel, A. A. N. & Kijne, J. W. (1995) Eur. J. Cell Biol. 68, 463-469. [PubMed] [Google Scholar]
- 23.D'Haeze, W., Mergaert, P., Promé, J.-C. & Holsters, M. (2000) J. Biol. Chem. 275, 15676-15684. [DOI] [PubMed] [Google Scholar]
- 24.Cullimore, J. V., Ranjeva, R. & Bono, J.-J. (2001) Trends Plant Sci. 6, 24-30. [DOI] [PubMed] [Google Scholar]
- 25.Oldroyd, G. E. D. (2001) Ann. Bot. 87, 709-718. [Google Scholar]
- 26.Ardourel, M., Demont, N., Debellé, F., Maillet, F., de Billy, F., Promé, J.-C., Dénarié, J. & Truchet, G. (1994) Plant Cell 6, 1357-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geurts, R., Heidstra, R., Hadri, A.-E., Downie, J. A., Franssen, H., van Kammen, A. & Bisseling, T. (1997) Plant Physiol. 115, 351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker, S. A. & Downie, J. A. (2000) Mol. Plant–Microbe Interact. 13, 754-762. [DOI] [PubMed] [Google Scholar]
- 29.Sprent, J. I. (2002) Nodulation in Legumes (Royal Botanical Gardens, Kew, U.K.).
- 30.Goormachtig, S., Valerio-Lepiniec, M., Szczyglowski, K., Van Montagu, M., Holsters, M. & de Bruijn, F. J. (1995) Mol. Plant–Microbe Interact. 8, 816-824. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-López, M., Goormachtig, S., Gao, M., D'Haeze, W., Van Montagu, M. & Holsters, M. (1998) Proc. Natl. Acad. Sci. USA 95, 12724-12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Eede, G., Deblaere, R., Goethals, K., Van Montagu, M. & Holsters, M. (1992) Mol. Plant–Microbe Interact. 5, 228-234. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 2nd Ed.
- 34.D'Haeze, W., Gao, M., De Rycke, R., Van Montagu, M., Engler, G. & Holsters, M. (1998) Mol. Plant–Microbe Interact. 11, 999-1008. [Google Scholar]
- 35.Van de Velde, W., Mergeay, J., Holsters, M. & Goormachtig, S. (2003) Plant Sci. 165, 1281-1288. [Google Scholar]
- 36.Goormachtig, S., Van Montagu, M. & Holsters, M. (1998) Mol. Plant–Microbe Interact. 11, 237-241. [DOI] [PubMed] [Google Scholar]
- 37.Mergaert, P., Van Montagu, M., Promé, J.-C. & Holsters, M. (1993) Proc. Natl. Acad. Sci. USA 90, 1551-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stougaard, J. (2001) Curr. Opin. Plant Biol. 4, 328-335. [DOI] [PubMed] [Google Scholar]
- 39.Limpens, E. & Bisseling, T. (2003) Curr. Opin. Plant Biol. 6, 343-350. [DOI] [PubMed] [Google Scholar]
- 40.Duhoux, E. (1984) Can. J. Bot. 62, 982-994. [Google Scholar]
- 41.Olsson, J. E. & Rolfe, B. G. (1985) J. Plant Physiol. 121, 199-210. [Google Scholar]
- 42.Lorbiecke, R. & Sauter, M. (1999) Plant Physiol. 119, 21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voesenek, L. A. C. J., Benschop, J. J., Bou, J., Cox, M. C. H., Groeneveld, H. W., Millenaar, F. F., Vreeburg, R. A. M. & Peeters, A. J. M. (2003) Ann. Bot. 91, 205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiefelbein, J. W. (2000) Plant Physiol. 124, 1525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James, E. K. & Sprent, J. I. (1999) New Phytol. 142, 219-231. [Google Scholar]
- 46.Doyle, J. J. & Lucknow, M. A. (2003) Plant Physiol. 131, 900-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krusell, L., Madsen, L. H., Sato, S., Aubert, G., Genua, A., Szczyglowski, K., Duc, G., Kaneko, T., Tabata, S., de Bruijn, F., et al. (2002) Nature 420, 422-426. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura, R., Hayashi, M., Wu, G.-J., Kouchi, H., Imaizumi-Anraku, H., Murakami, Y., Kawasaki, S., Akao, S., Ohmori, M., Nagasawa, M., et al. (2002) Nature 420, 426-429. [DOI] [PubMed] [Google Scholar]
- 49.Searle, I. R., Men, A. E., Laniya, T. S., Buzas, D. M., Iturbe-Ormaetxe, I., Carroll, B. J. & Gresshoff, P. M. (2003) Science 299, 109-112. [DOI] [PubMed] [Google Scholar]
- 50.Penmetsa, R. V., Frugoli, J. A., Smith, L. S., Long, S. R. & Cook, D. R. (2003) Plant Physiol. 131, 998-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penmetsa, R. V. & Cook, D. R. (1997) Science 275, 527-530. [DOI] [PubMed] [Google Scholar]
- 52.James, E. K., Gyaneshwar, P., Mathan, N., Barraquio, W. L., Reddy, P. M., Iannetta, P. P. M., Olivares, F. L. & Ladha, J. K. (2002) Mol. Plant–Microbe Interact. 15, 894-906. [DOI] [PubMed] [Google Scholar]