Abstract
The description of two novel human defects in the last ten years has uncovered new aspects of thyroid hormone physiology with regard to cellular-membrane transport and intracellular metabolism. Mutations in the X-linked monocarboxylate transporter 8 (MCT8) gene result in an invalidating neurodevelopmental phenotype in males and pathognomonic thyroid functions tests with high T3, low rT3, low or low normal T4, and normal or slightly high TSH. Recessive mutations in the selenocysteine insertion sequence binding protein 2 (SBP2) gene present a variable clinical phenotype depending on the severity of the defect and its consequences on the selenoprotein hierarchy. Most characteristic is the thyroid phenotype of low serum T3, high T4, high rT3, and slightly elevated TSH levels. Herein we review all known cases of MCT8 and SBP2 deficiency and describe each disease in terms of the clinical, biochemical, genetic, and therapeutic aspects.
Keywords: thyroid hormone cell-membrane transporter, MCT8, Allan-Herndon-Dudley syndrome, deiodinase, selenoprotein, SBP2
Introduction and thyroid physiology
Thyroid hormone (TH) is essential for human development, growth and metabolism. The effects of TH deficiency and excess during development can be profound and permanent, especially with regards to the nervous system (1). The feedback regulatory system involving the hypothalamus-pituitary-thyroid axis maintains the circulating TH available to tissues. Intracellular concentrations of T3 are regulated by iodothyronine deiodinases (Ds), which provide a mechanism for local regulation of TH supply. These selenoenzymes convert the hormone precursor thyroxine (T4) through outer-ring deiodination (5′-deiodination) by D1 and D2 to form the active 3,3′,5-triiodothyronine (T3), or, inactivate T4 and T3 through inner-ring deiodination (5-deiodination) by D3, to form 3,3′,5′-triiodothyronine (reverse T3; rT3) and 3,3′-diiodothyronine (T2), respectively (2). Several classes of cell-membrane transporters mediate the influx and efflux of T3 and T4 into the cells (3).
1. TH cell-membrane transport defect
1A. TH cell-membrane transporters
Several types of TH transporters located in cellular membranes have been recognized, including the Na+/taurocholate cotransporting polypeptide (4), the Na+-independent organic anion transporting polypeptide (OATP) family (5), the heterodimeric L-type amino acid transporters (LAT1, LAT2) (6), and the monocarboxylate transporter (MCT) family (7). Among them, OATP1C1 (8), MCT8 (9) and MCT10 (10) have narrower substrate specificities, indicating their relatively important role in TH bioavailability.
OATP1C1, with preferential transport of T4, is highly enriched in brain capillaries (8, 11, 12), which may act as a bridge between the circulating T4 and astrocytes, where T4 is deiodinated to active T3. Accordingly, the unique role of OATP1C1 in T4 transport in the brain is supported by the finding of central nervous system specific hypothyroidism in Oatp1C1 knockout (KO) mice (13). However, no human with OATP1C1 deficiency has been identified.
MCT8 is an active and specific TH cell-membrane transporter expressed in many tissues (9), and its deficiency causes a severe complex phenotype in humans (14, 15). The human (h) MCT8 gene is located on chromosome Xq13.2 and consists of six exons. It encodes two variant proteins of 613 and 539 amino acids translated from two putative in-frame start sites. There is a high degree of homology in amino acid sequences of MCT8 among different species. However, the non-primate MCT8 gene lacks the upstream translation start site (16). Currently, the functional importance of the additional 75 amino acids located in the amino-end of hMCT8 remains unknown. The predicted structure contains 12 hydrophobic transmembrane domains (TMDs) with intracellular amino- and carboxyl-ends (17). In this review, the numbering of hMCT8 amino acids starts from the upstream translation start site. Both rat MCT8 (rMct8) and hMCT8 markedly stimulate the uptake of TH, but fail to influence the transport of other molecules such as aromatic amino acids. MCT8 is widely distributed in tissues, including brain, liver, kidney, heart, thyroid and placenta (9, 18, 19).
MCT10, initially characterized as a T-type amino acid transporter, has the highest homology to MCT8 within the MCT family. It was demonstrated as an alternative TH transporter with increased affinity for T3 (10). In both humans and rodents, MCT10 is widely expressed in tissues such as skeletal muscle, kidney, liver and intestine (20, 21). Mutations in the MCT10 gene have not been identified.
1B. Patients with MCT8 deficiency
MCT8 gene defects were first reported in 2004. All affected males display severe neurodevelopmental deficits and pathognomonic thyroid tests including high serum T3, low rT3, low normal to reduced T4, and normal or slightly elevated TSH (14, 15). MCT8 gene mutations were also found to be responsible for the Allan-Herndon-Dudley syndrome (ADHS), an X-linked mental retardation syndrome (XLMR) initially described in 1944 (22) that is now synonymous with MCT8 defect. To date, more than 100 families of all races and diverse ethnic origins harboring more than 70 different mutations have been described.
The defect has 100% penetrance in males. There is, however, one case of a female patient with typical features of MCT8 deficiency attributed to the disruption of MCT8 by a de novo translocation and unfavorable nonrandom X-inactivation (23). MCT8 gene mutations are distributed throughout the coding region and form apparent clusters in the TMDs, which are highly conserved across species. Mutations range from single nucleotide substitutions to large deletions involving one or more exons.
Pathological consequences of the reported mutations have been confirmed by functional analysis using fibroblasts from affected individuals and mammalian cells transfected with mutant MCT8 cDNA. Cultured skin fibroblasts from affected subjects showed a significant reduction of T4 and T3 uptake while D2 enzymatic activity was higher, compared to those from normal individuals (24). Functional studies in mammalian cells transfected with different MCT8 mutants alone and in combination with D3 for the measurement of T3 uptake and metabolism, respectively, revealed that most mutations result in a complete loss of TH transport function, primarily T3 (25). Notable exceptions are the mutants S194F, L434W, L492P, F501del, and L598P that showed significant residual transport capacity, consistent with their milder clinical manifestations in patients (24, 26, 27). Among them, the F501del and L492P mutants have a minimal reduction of T3 uptake, however a relatively greater decrease in T3 efflux was observed (24, 26).
1B.1. Clinical features
Most affected subjects are referred to medical investigation during infancy or early childhood due to severe neurodevelopmental abnormalities. Truncal hypotonia and feeding problems appear in the first 6 months of life. Hypotonia, accompanied by motor, speech, and mental delays, persist into adulthood while the spastic quadriplegia and joint contractures develop over time. Most affected subjects are unable to sit, stand, or walk, and are unable to speak. However, patients harboring S194F, L434W, L492P, F501del, and L598P mutations, have been reported to acquire the ability to walk with ataxic gait and/or develop some dysarthric speech (22, 24, 26), correlated to the in vitro data on these mutations.
Dystonia and involuntary movements commonly occur with the progress of the disease. Characteristic paroxysms of kinesigenic dyskinesias provoked by somatosensory stimuli have been reported in several patients (28, 29). In addition, true seizures occur in 25% of the patients. Hyperreflexia, clonus, Babinski sign are often present, while nystagmus is less common (14).
Intrauterine growth is normal in most affected subjects. Weight gain lags and muscle mass is diminished with generalized muscle weakness characterized by poor head control. Failure to thrive is a prominent and common feature, which may require placement of gastric feeding tube in some cases. Possible reasons for low weight and muscle wasting in MCT8 deficient patients are the difficulty to swallow due to the neurological deficit, and the increased metabolism due to the thyrotoxic state caused by the effect of high serum T3 on peripheral tissues. Linear growth seems to proceed normally, however, length measurement of adult patients is inaccurate due to the presence of scoliosis and contractures (22).
Some somatic features have been noted to be characteristic, though nonspecific: an elongated and myopathic facies with ptosis, open mouth, and a tented upper lip, attributed to the prenatal and infantile hypotonia (22). Long, thick and cup-shaped ears and thick noses have been also reported.
Cognitive impairment in affected subjects is severe. However, they can communicate by smile, crying and other sounds. Hearing and vision are usually normal, except for one case of blindness (15). Death during childhood or teens is relatively common and is frequently caused by recurrent infections and aspiration pneumonia. In only few instances, survival beyond 70 years was observed (22).
Most female carriers of MCT8 gene mutations show random X-inactivation resulting in normal thyroid and neurodevelopmental function (23). However, an unfavorable nonrandom X-inactivation could alter the phenotype in these females, leading to classic clinical features including intellectual delay and MR (14, 22, 23, 30). However, considering that MR has diverse etiologies, the causative link between MCT8 gene mutations in heterozygotes and cognitive impairments remains to be proven (31).
1B.2. Laboratory findings
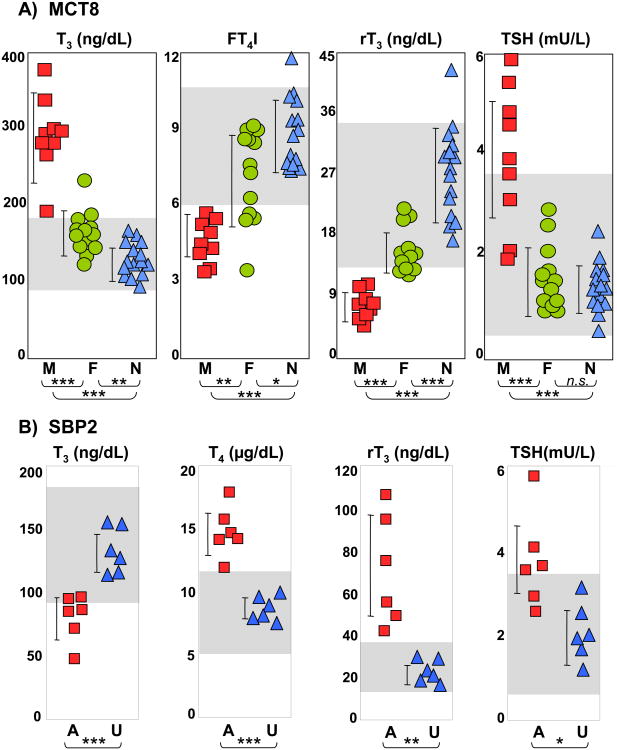
Thyroid function tests with high serum total and free T3, low rT3, low or low normal T4 and normal or slightly elevated TSH are pathognomonic. Heterozygous female carriers may show only mild thyroid test abnormalities with intermediate iodothyronine concentrations between affected males and unaffected relatives and normal TSH levels (14, 22, 31, 32) (Figure 1A). TSH response to TRH was tested in several individuals, and reported to be increased (30), decreased (28) or normal (33). However, administration of incremental doses of L-T3 showed reduced pituitary sensitivity to the hormone [(34) and unpublished data].
Figure 1.
Thyroid function tests in A) MCT8 deficiency (M – males, red squares; F – carrier females, green circles; N – unaffected family members, blue triangles); and B) SBP2 deficiency (A – affected, red squares; U – unaffected family members, blue triangles).
* p<0.05, ** p<0.01, *** p<0.001. Grey boxes indicate the normal range for each test. Note the characteristic abnormalities as described in the test.
Peripheral markers of hyperthyroidism, such as reduced cholesterol and increased SHBG, ammonium and lactic acid, are found in some patients with MCT8 gene mutations (28, 30, 34-36) and can be due to the effect of the high serum T3 levels on liver and skeletal muscle. Biopsies of muscle showed reduced activity of succinate dehydrogenase and cytochrome oxidase, and increased citrate synthase, which are mitochondrial enzymes that perform critical steps of the citric acid cycle and/or the electron transport chain [(37) and unpublished data]. It is unclear whether these alterations result from the abnormal TH status of muscle or represent a yet not understood effect of MCT8 on the mitochondria.
All affected patients have normal linear growth during childhood. Bone age has only been reported in few cases and found to be delayed (33, 38, 39), normal (38), or slightly advanced (30, 36). Of note, nutritional status and neurological deficits affect bone mass and thus confound the putative MCT8-dependent TH effect.
A common finding in early life is mild to severe delayed myelination or dysmyelination, detected by brain magnetic resonance imaging (MRI) (40-43). However, hypomyelination is transient in MCT8 deficiency and is not detected by MRI by 4 years of age. This is different from other leukodystrophies with permanent myelination defect (40). Mild cerebellar atrophy with normal anatomy has been also reported (34, 42, 43).
1C. Mechanisms of MCT8 deficiency
Considerable insight into the pathophysiology of the MCT8 deficiency has been possible through the study of the Mct8-deficient (Mct8KO) mice (44, 45), as they replicate the serum TH abnormalities found in humans. Mct8KO mice showed variable availability of the circulating hormones to specific tissues, depending on the redundant presence of TH cell-membrane transporters. The expression of TH transporters other than Mct8 in liver results in a high intracellular T3 concentration, which increases D1 expression and enzymatic activity in Mct8KO mice. This hyperthyroid state is confirmed by decreased serum cholesterol and increased serum alkaline phosphatase. On the contrary, the absence of Mct8 in brain resulted in reduced T3 content together with the increased D2 activity in astrocytes and decreased D3 activity in neurons, thus indicating a relative hypothyroid state. The activation of D1 and D2 stimulated by opposite states of intracellular TH availability leads to an additive consumptive effect on T4 and excess T3 generation. Studies on the double Mct8 and D1 or D2 knock out mice demonstrated that D1 is responsible for maintaining the high serum T3 level in Mct8 defect, whereas D2 mainly functions intracellular to compensate for local hypothyroidism (46). The low serum T4 in Mct8 deficiency is not only attributed to the consumption through deiodination but also to reduced secretion from the thyroid gland and increased renal losses (47, 48). The modestly increased serum TSH in MCT8 defect is related to central resistance to T3, particularly at hypothalamic level (48).
However, the lack of a neurological phenotype in Mct8KO mice limits their use as a model of the psychomotor manifestations in humans. A possible reason for this difference is the sufficient compensation of alternative transporters, such as LAT2 and OATP1C1, in mouse brain compared to humans (12, 19). Thus, mice models with combined deficiencies of Mct8 and other TH transporters could manifest an obvious neurological phenotype. Recently, mice deficient in both Mct8 and Oatp1C1 have been generated (49). In contrast to the single respective KOs, the Mct8/Oatp1C1 KO mice exhibited a more severe hypothyroid state of the brain and manifested coordination and locomotor deficits. However, it is uncertain if these mice can serve as a model for human MCT8 deficiency, considering that they have an additional defect that will confound the interpretations. The zebrafish is also emerging as an encouraging model to study the role of MCT8 in brain development (50).
1D. Treatment
Treatment options for patients with MCT8 gene mutations remain limited. Coexistence of TH excess or deprivation in different tissues complicates the management of the disease. Tissues expressing other cell-membrane transporters than MCT8 respond to the high circulating T3 level, resulting in a hyperthyroid state, while tissues dependent on MCT8 for TH entry into cells, are hypothyroid.
The administration of supraphyiological doses of L-T4, alone or in combination with T3, was able to suppress serum TSH levels in two cases, however, no neurological improvement was observed and the existing hypermetabolic state was further exaggerated (34, 51). Several patients were treated with the combination of L-T4 and propylthiouracil (PTU), as a specific inhibitor of D1. Although this treatment partially corrected the TH abnormalities without thyrotoxic side effects, it did not improve the psychomotor deficit (26, 35, 38). Considering that PTU has been reported to cause liver toxicity particularly in children, it is to be used with caution and liver enzymes checked periodically.
The use of thyromimetic compounds that are independent of MCT8 for cellular entry has been tested. One such analogue, diiodothyropropionic acid (DITPA), has been shown to be transported into the brain and correct the TH deficit without causing hepatic thyrotoxic effect in Mct8KO mice (52). Based on this data, DITPA was also given to four children with MCT8 deficiency (38). Its administration in doses of 1-2mg/kg/d almost completely normalized the thyroid tests and reduced the hypermetabolic state. However, no significant improvements of psychomotor function were observed, although some subjective benefits were observed. A TH metabolite, TETRAC (3, 3′, 5, 5′-tetraiodothyroacetic acid), was demonstrated recently to mimic TH effect during brain development in Mct8 deficient mice (53). It is possible that for any thyromimetic treatment to be effective in neurodevelopment, it will have to be initiated early, perinatally or in utero. Thus, studies concerning earlier initiation and long-term therapy of these compounds remain to be performed.
Supportive measurements including intensive physical, mental, and occupational therapies may be beneficial, as patients undergoing these therapies have shown some psychomotor progress (38). Other measures to be considered are the use of braces to prevent mal-position contractures. Aspiration should also be prevented, as it is a source of serious complications in these subjects. Dystonia could be improved with anticholinergics, L-DOPA, carbamazepine and lioresol. Drooling might be reduced with glycopyrolate or scopolamine. Seizures are treated with standard anticonvulsants. Most heterozygous female carriers of MCT8 mutations are not necessarily treated. However, when pregnant, prenatal diagnosis for male fetuses should be assessed early for MCT8 mutations by direct sequencing. In a recent report, two carrier females who were pregnant with unaffected fetuses were treated with L-T4 in the 2nd half of pregnancy (54). It is unclear if this had any effect, either beneficial or detrimental. Of note, unaffected males and heterozygous females born to untreated carrier mothers are normal.
Practice points
- TH cell-membrane transport defect caused by X-linked MCT8 gene mutations manifests a characteristic thyroid phenotype with high serum T3, low rT3, low normal to reduced T4, and normal or slightly elevated TSH concentrations.
- Males with MCT8 deficiency manifest severe neurodevelopmental abnormalities: truncal hypotonia, feeding problems, dystonia, generalized muscle weakness, poor head control, no speech, inability to walk, stand or sit unsupported.
- The hypermetabolic state and weight loss can improve with high L-T4 combined with PTU treatment, and with administration of the thyromimetic compound DITPA. However, no significant neurodevelopmental improvement was observed with either therapy.
Research agenda
- To find treatment options for the psychomotor deficit, considerations are beinggiven to initiation of high L-T4 and PTU, or treatment with thyroid analogues, at birth or even during pregnancy.
- Delivery of a normal MCT8 gene into the central nervous system by means of adenoviral associated vectors is being tested in Mct8KO mice
2. Thyroid hormone metabolism defect
2A. Intracellular TH metabolism and selenoproteins
The three selenoprotein iodothyronine deiodinases D1, D2 and D3 are responsible for the Intracellular metabolism of TH as summarized in the introduction. They are differentially expressed in tissues and in response to local environments, consequently fine-tuning intracellular availability of TH in a cell-specific manner (2).
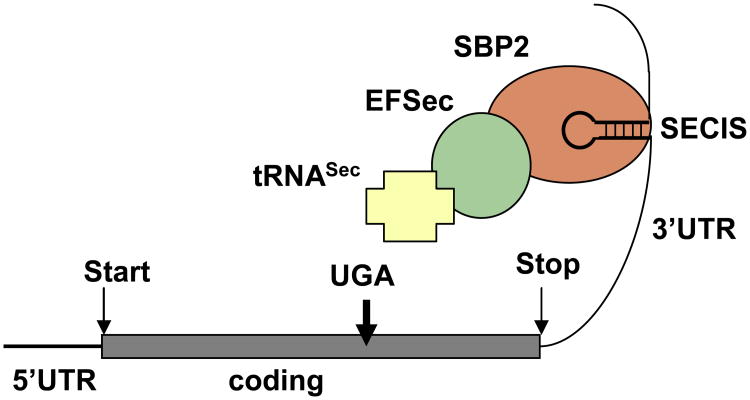
At least 25 selenoproteins have been identified to form the human selenoproteome (55). As for other selenoproteins, the enzymatic activity of the deiodinases relies on the rare amino acid selenocysteine (Sec), present in their active center. Sec is encoded through a unique mode of translation by a UGA codon, which under most circumstances serves as a signal to stop protein synthesis. A conserved cis-acting element, Sec insertion sequence (SECIS), in the 3′-untraslated region of the selenoproteins is recognized by SECIS-binding protein 2 (SECISBP2; in short SBP2), which recruits the elongation factor (EFSec), the specific selenocysteine transfer RNA (tRNASec) and additional factors for insertion of Sec at this particular UGA codon (Figure 2) (56). Acquired defects in Ds activity are observed during severe illness and starvation, however, genetic defects in the Ds have not been reported in humans (2). To date, the only known inherited TH metabolism defect in humans is caused by mutations in SBP2 gene (57).
Figure 2.
Schematic representation of Sec incorporation. For detailed explanation see text. Reproduced with permission from Dumitrescu AM and Refetoff S. Biochim Biophys Acta. 2012 Aug 16. [Epub ahead of print]
2B. Patients with SBP2 deficiency
The first subjects with mutations in the SBP2 gene were first reported in 2005 (57). Affected individuals presented with transient growth retardation and characteristic thyroid tests abnormalities, high serum T4, low T3, high rT3 and normal or slightly elevated serum TSH (Figure 1B). Since the initial report, a total of eight families with SBP2 deficiency have been identified. The overall phenotype is more complex than initially observed (57-61). The inheritance is autosomal recessive and the ethnic origins of the affected individuals are Bedouin from Saudi Arabia, African, Irish, Brazilian, English, Turkish and Japanese. The human SBP2 gene is located on chromosome 9, has 17 exons and encodes a protein of 854 amino acids (62). The C-terminal half of the protein is required for all known SBP2 functions, including SECIS binding, Sec incorporation and ribosome binding (63). The N-terminal domain contains a nuclear localization signal and the C-terminal domain also contains a nuclear export signal, thus enabling SBP2 to shuttle between nucleus and cytoplasm (64). SBP2 is widely expressed (62). Fourteen different mutations have been identified in the eight families, summarized in Table 1.
Table 1. Mutations in the SBP2 gene.
| Family | SBP2 gene | Protein | Comments on putative defect | No of affected | Defect | Ref |
|---|---|---|---|---|---|---|
| 1 | c.1619 G>A | R540Q | hypomorphic allele | 3 | homozygous | (57) |
| 2 | c.1312 A>T | K438X | missing C terminus | 1 | compound heterozygous | (57) |
| IVS8ds+29 G>A | fs | abnormal splicing | ||||
| 3 | c.382 C>T | R128X | smaller isoforms* | 1 | homozygous | (58) |
| 4 | c.358 C>T | R120X | smaller isoforms* | 1 | compound heterozygous | (59) |
| c.2308 C>T | R770X | disrupted C-terminus | ||||
| 5 | c.668delT | F223 fs 255X | truncation and smaller isorforms* | 1 | compound heterozygous | (60) |
| intron 6 -155 delC | fs | abnormal splicing, missing C-terminus | ||||
| 6 | c.2071 T>C | C691R | increased proteasomal degradation | 1 | compound heterozygous | (60) |
| intronic SNP | fs | transcripts lacking exons 2-4, or 3-4 | ||||
| 7 | c.1529_1541dup CCAGCGCCCCACT | M515 fs 563X | missing C terminus | 1 | compound heterozygous | (61) |
| c.235 C>T | Q79X | smaller isoforms* | ||||
| 8 | c.2344 C>T | Q782X | missing C terminus | 1 | compound heterozygous | (65) |
| c.2045-2048 delAACA | K682 fs 683X | missing C terminus |
generated from downstream ATGs; fs – frame shift.
Modified from Dumitrescu AM and Refetoff S. Biochim Biophys Acta. 2012 Aug 16. [Epub ahead of print], with permission.
2B.1. Clinical features
The inheritance of SBP2 defects is autosomal biallelic (homozygous or compound heterozygous) thus males and females are expected to be equally affected. In the eight families known to date there are eight males and two affected females, but the number is too small to conclude at this point that there is gender preponderance. Except for one adult individual, all other probands ranged in age from 2 to 14.5 years. The extent of manifestations varies among the known cases. Delayed growth and bone maturation are common abnormalities that brought the subjects to medical attention. Delayed motor and intellectual milestones were recognized in five cases (59-61, 65). The affected individuals started walking and speaking at 1.8-3 years of age. Speech therapy or special education for children with mental retardation was required for some of the patients.
Progressive congenital myopathy occurred in two patients (59, 60). Hypotonia and weakness developed early in life and motor development was delayed. For the only adult subject, walking and running remained problematic during adolescence, with genu valgus and external rotation of the hip requiring orthotic footwear (60). Similarly, a 12-yr-old girl, had difficulty walking, with asymmetry of the legs, hyporeflexia, kyphoscoliosis, limited flexion of the neck and hip girdle weakness. Impaired motor coordination, waddling gait, and Gower's sign were also observed (59). T1-weighted MRI in three patients showed connective or fatty tissue infiltration in the axial and adductor muscle at the mid-thigh level (59-61).
Sensorineural hearing loss has been documented in four cases (59-61). Increased fat mass was observed in three cases (59, 60); two of them were markedly insulin sensitive, in particular a 2-yr-old boy who developed nonketotic hypoglycemia with low insulin levels and required supplemental parenteral nutrition. A diagnosis of early eosinophilic colitis is another unique feature of this boy (60).
Several distinct manifestations, including primary infertility, skin photosensitivity, and severe Raynaud disease were only seen in the adult patient with SBP2 deficiency (60). Although pubertal development was normal in this patient, he developed unilateral testicular torsion requiring orchiectomy and fixation of the remaining testis at age 15 (60).
The consequences of SBP2 defects could still be underestimated since other pathologies linked to oxidative damage such as neoplasia, neurodegeneration and premature ageing, may manifest with time.
2B.2 Laboratory Findings
Thyroid testing in some of these patients was prompted by delayed growth and delayed bone age. All affected subjects were found to have characteristic serum thyroid test abnormalities, with high total and free T4, low T3, high rT3 and slightly elevated serum TSH. In TSH suppression studies, higher doses and serum concentrations of T4, but not T3, were required to reduce TSH levels in affected children compared with normal siblings, suggesting an impairment of T4 to T3 conversion (57). None of the subjects had an enlarged thyroid gland confirmed by ultrasound examination. Bone age delay detected by X-ray was documented in all subjects.
In agreement with the role of SBP2 for the synthesis of selenoproteins, its deficiency affects multiple selenoproteins. Baseline and cAMP-stimulated D2 enzymatic activity was reduced in cultured skin fibroblasts from affected individuals, compared to those from unaffected family members. However, baseline and cAMP-stimulated D2 mRNA levels were similar between the two groups, supporting a post-transcriptional defect in the synthesis of the active D2 enzyme in the affected (57). Selenoprotein P (SePP) and glutathione peroxidase (Gpx) activities in serum and/or fibroblasts were also decreased in affected individuals, as were the circulating levels of selenium (Se) reflecting a global deficiency in selenoprotein synthesis (57, 60).
In patients manifesting a more severe phenotype, defects of other selenoproteins than D2, SePP and Gpx were detected. Selenoprotein N1 (SEPN1) deficiency results in clinical symptoms resembling SEPN1-related myopathies characterized by axial and adductor muscle hypotrophy as well as spinal rigidity (59, 60). Lack of testis-enriched selenoproteins, led to failure of the latter stages of spermatogenesis and azoospermia. Cutaneous deficits of antioxidant selenoenzymes caused increased cellular reactive oxygen species, and the decreased selenoproteins in peripheral blood cells resulted in immune deficits. Other selenoproteins with unknown functions, such as SELH, SELT, SELW, SELI, were also found to be affected (60). The consequences of these deficiencies remain to be uncovered.
2C. Mechanisms of SBP2 deficiency
SBP2 is indispensable for selenoprotein synthesis, in light of the fact that its immunodepletion abolished Sec incorporation (66). Some of the selenoproteins are considered to have essential functions, as removal of tRNASec in mouse causes embryonic death (67). Accordingly, complete loss of SBP2 function is predicted to be lethal. This hypothesis was recently demonstrated by a constitutional SBP2 KO mouse model (68). Thus, the survival of reported patients with SBP2 deficiency indicates preservation of partial SBP2 activity with distinct effects on the complex hierarchy in selenoprotein synthesis.
A relatively mild phenotype limited to growth retardation and thyroid metabolism defect was observed in the first three cases (57, 58). The mutant R540Q SBP2 behaved as a hypomorphic allele when studied in vitro using the corresponding R531Q mutation of the rat Sbp2. The mutant showed selective decrease in affinity for a subset of SECIS elements, resulting in the selective loss of some selenoproteins such as GP×1 and D1 (69). This supports the hierarchy of selenoprotein synthesis and explains in part the thyroid function abnormalities.
The thyroid phenotype of subjects with SBP2 deficiency is consistent with a defect in TH metabolism due to the deficiency in deiodinases. However, targeted disruption in mice of D1, D2 or D3 alone, or both D1 and D2, only partially replicate the thyroid abnormalities observed in SBP2 deficient humans (70-73). Thus, a putative, partial and uneven involvement of the three deiodinases might explain the noted difference in the thyroid tests abnormalities. Of note, delayed growth and bone maturation, and impaired auditory functions are also associated with deficiencies of the deiodinases (72, 74, 75).
In another family, compound heterozygous mutations with one truncated allele K438X missing the C-terminus, and another IVS8ds+29 G>A mutation causing alternative splicing, allowed 24% normal transcript of SBP2 (57). In the case of the homozygous R128X mutation, smaller SBP2 isoforms containing the intact C-terminus functional domains were translated from downstream ATGs (58).
More severe and complex phenotype due to an extensive impairment in SBP2 function was reported in another three patients (59, 60). In the patient with compound heterozygous mutations (R77X/R120X) (59), the mutant R770X truncates the C-terminal functional domain in all isoforms synthesized from this allele and is a putative null allele. The R120X mutant likely generates smaller functional SBP2 isoforms, similar to the R128X mutant, however, the overall amount of SBP2 function in this individual would be lesser than that in the homozygous R128X subject, because of to the concomitant deleterious effect of the R770X mutation. Two other individuals were reported to have absent expression of the full length SBP2, and increased proteasomal degradation was demonstrated for the C691R mutation, explaining the relatively more severe phenotype in these patients (60).
A mouse model of SBP2 defect was recently generated (68). However, neither heterozygous deletion, nor tissue-specific KO of Sbp2 replicate the phenotype observed in SBP2 deficient individuals, limiting their role in understanding the pathophysiology of the defect. Compared with the mouse model heterozygous for tRNASec deletion (67), heterozygous Sbp2 mutants have only minimal changes in selenoprotein expression, indicating significant residual Sbp2 function. Neuron-specific Sbp2KO mice exhibit remarkable motor abnormalities with an approximately three-week survival, while hepatocyte-specific Sbp2KO mice appear normal, suggesting that cerebral selenoproteins may be have particular importance for neurologic function.
2D. Treatment
Several treatments have been tested with the goal to normalize the thyroid hormone abnormalities and improve growth and bone maturation. Initially, Se supplementation in both organic and inorganic forms was administrated in three affected siblings, but the restoration of the TH metabolism dysfunction or of the growth retardation were not achieved (76). L-T3 administration was subsequently attempted in four patients and provided some beneficial effects (58-61), with improved linear growth and/or bone maturation. However, normalization of FT3 level associated with improved speech and neurodevelopment were seen only in one patient (60). In addition, the benefit of growth hormone treatment was limited to longitudinal growth (61). Other clinical features of SBP2 defects are treated symptomatically.
Practice points SBP2
- TH metabolism defect caused by SBP2 deficiency manifests a characteristic thyroid phenotype with high total and free T4, low T3, high rT3 and slightly elevated serum TSH
- Mild cases present bone age and growth delay, while cases with severe SBP2 deficiency present delayed motor and intellectual milestones, progressive congenital myopathy, primary infertility, skin photosensitivity, sensorineural hearing loss, immune deficits.
- Treatment with physiological doses of L-T3 may accelerate growth and bone maturation but does not correct other manifestations of the defect.
Research agenda SBP2
- Generation and study of additional mouse models of Sbp2 deficiency to understandthe pathophysiology of this defect and to assess for other possible phenotypes.
- Identification of additional patients with SBP2 deficiency
Summary
The only known inherited TH cell-membrane transport defects are caused by mutations in the X-linked MCT8 gene. Males present a characteristic thyroid phenotype and severe complex neurodevelopmental delay. Studies on Mct8 deficient mice have uncovered important mechanisms underlying the thyroid phenotype, however they do not manifest the neurological phenotype. The characteristic serum thyroid tests abnormalities are used in differentiating MCT8 defects from other conditions with similar manifestations. Carrier females are asymptomatic and the presence of a MCT8 defect is not suspected until the birth of an affected male. Aspiration pneumonia is the most common cause of death in affected males. Postnatal treatment with high doses of L-T4 and PTU, or with the thyromimetic compound DIPTA, ameliorates the hypermetabolic state, but does not improve the neurological manifestations.
Inherited defects of intracellular TH metabolism known to date are caused by SBP2 deficiency. Only eight families have been identified. Clinical manifestations, showing characteristic thyroid tests abnormalities, require biallelic SBP2 gene defects. The phenotype is variable and it depends on the degree of functional impairment of the mutant protein, with delayed growth seen in all patients. Severe cases manifest the consequences of deficiency in multiple selenoproteins. All subjects have some residual SBP2 function, as complete SBP2 deficiency is putatively lethal. Treatment with Se is not effective in correcting the phenotype, though physiological doses of L-T3 accelerate growth but do not correct other manifestations of the defect.
Acknowledgments
Supported in part by grant DK091016, from the National Institutes of Health to AMD and Award from China Scholarship Council to JF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jiao Fu, Email: fujiaoxyz@uchicago.edu, Department of Medicine, University of Chicago Medical Center, 5841 S. Maryland Avenue MC3090, Room M369, Chicago IL 60637, Phone 773-702-9273, Fax 773-702-6040; Address in China: Department of Endocrinology, The First Affiliated Hospital of Xi'an Jiaotong University School of Medicine, Xi'an 710061, The People's Republic of China.
Alexandra M. Dumitrescu, Email: alexd@uchicago.edu, Department of Medicine, University of Chicago Medical Center, 5841 S. Maryland Avenue MC3090, Room M369, Chicago IL 60637, Phone 773-702-6577, Fax 773-702-6040.
Bibliography
- 1.de Escobar GM, Obregon MJ, Del Rey FE. Role of thyroid hormone during early brain development. European Journal of Endocrinology. 2004;151(Suppl 3):U25–U37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- 2*.Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine reviews. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 3.Friesema ECH, Jansen J, Milici C, Visser TJ. Thyroid hormone transporters. Vitamins & Hormones. 2005;70:137–67. doi: 10.1016/S0083-6729(05)70005-4. [DOI] [PubMed] [Google Scholar]
- 4.Friesema ECH, Docter R, Moerings EPCM, et al. Identification of thyroid hormone transporters. Biochemical and Biophysical Research Communications. 1999;254(2):497–501. doi: 10.1006/bbrc.1998.9974. [DOI] [PubMed] [Google Scholar]
- 5.Hagenbuch B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Practice & Research Clinical Endocrinology & Metabolism. 2007;21(2):209–21. doi: 10.1016/j.beem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Friesema ECH, Docter R, Moerings EPCM, et al. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology. 2001;142(10):4339–48. doi: 10.1210/endo.142.10.8418. [DOI] [PubMed] [Google Scholar]
- 7.Visser WE, Friesema ECH, Jansen J, Visser TJ. Thyroid hormone transport by monocarboxylate transporters. Best Practice & Research Clinical Endocrinology & Metabolism. 2007;21(2):223–36. doi: 10.1016/j.beem.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Pizzagalli F, Hagenbuch B, Stieger B, et al. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Molecular Endocrinology. 2002;16(10):2283–96. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- 9.Friesema ECH, Ganguly S, Abdalla A, et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. Journal of Biological Chemistry. 2003;278(41):40128–35. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 10.Friesema ECH, Jansen J, Jachtenberg J, et al. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Molecular Endocrinology. 2008;22(6):1357–69. doi: 10.1210/me.2007-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiyama D, Kusuhara H, Taniguchi H, et al. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier. Journal of Biological Chemistry. 2003;278(44):43489–95. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- 12.Roberts LM, Woodford K, Zhou M, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149(12):6251–61. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- 13.Mayerl S, Visser TJ, Darras VM, et al. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153(3):1528–37. doi: 10.1210/en.2011-1633. [DOI] [PubMed] [Google Scholar]
- 14*.Dumitrescu AM, Liao XH, Best TB, et al. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. The American Journal of Human Genetics. 2004;74(1):168–75. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Friesema ECH, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. The Lancet. 2004;364(9443):1435–7. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 16.Jansen J, Friesema ECH, Milici C, Visser TJ. Thyroid hormone transporters in health and disease. Thyroid. 2005;15(8):757–68. doi: 10.1089/thy.2005.15.757. [DOI] [PubMed] [Google Scholar]
- 17.Lafrenière RG, Carrel L, Willard HF. A novel transmembrane transporter encoded by the XPCT gene in Xq13. 2. Human Molecular Genetics. 1994;3(7):1133–9. doi: 10.1093/hmg/3.7.1133. [DOI] [PubMed] [Google Scholar]
- 18.Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochemical Journal. 1998;329(Pt 2):321. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirth EK, Roth S, Blechschmidt C, et al. Neuronal 3′, 3, 5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan–Herndon–Dudley syndrome. The Journal of Neuroscience. 2009;29(30):9439–49. doi: 10.1523/JNEUROSCI.6055-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai Y, Chairoungdua A, Matsuo H, et al. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. Journal of Biological Chemistry. 2001;276(20):17221–8. doi: 10.1074/jbc.M009462200. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug metabolism and pharmacokinetics. 2008;23(1):22–44. doi: 10.2133/dmpk.23.22. [DOI] [PubMed] [Google Scholar]
- 22*.Schwartz CE, May MM, Carpenter NJ, et al. Allan-Herndon-Dudley Syndrome and the Monocarboxylate Transporter 8 (MCT8) Gene. Am J Hum Genet. 2005;77(1):41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frints SGM, Lenzner S, Bauters M, et al. MCT8 mutation analysis and identification of the first female with Allan–Herndon–Dudley syndrome due to loss of MCT8 expression. European Journal of Human Genetics. 2008;16(9):1029–37. doi: 10.1038/ejhg.2008.66. [DOI] [PubMed] [Google Scholar]
- 24.Visser WE, Jansen J, Friesema EC, et al. Novel pathogenic mechanism suggested by ex vivo analysis of MCT8 (SLC16A2) mutations. Hum Mutat. 2008;30:29–38. doi: 10.1002/humu.20808. [DOI] [PubMed] [Google Scholar]
- 25.Friesema EC, Visser WE, Visser TJ. Genetics and phenomics of thyroid hormone transport by MCT8. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Edward Visser W, Vrijmoeth P, Visser FE, et al. Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clinical Endocrinology. 2012 doi: 10.1111/cen.12023. [DOI] [PubMed] [Google Scholar]
- 27.Jansen J, Friesema ECH, Kester MHA, et al. Genotype-phenotype relationship in patients with mutations in thyroid hormone transporter MCT8. Endocrinology. 2008;149(5):2184–90. doi: 10.1210/en.2007-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boccone L, Mariotti S, Dessi V, et al. Allan-Herndon-Dudley syndrome (AHDS) caused by a novel SLC16A2 gene mutation showing severe neurologic features and unexpectedly low TRH-stimulated serum TSH. Eur J Med Genet. 2010;53(6):392–5. doi: 10.1016/j.ejmg.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Brockmann K, Dumitrescu AM, Best TT, et al. X–linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. Journal of neurology. 2005;252(6):663–6. doi: 10.1007/s00415-005-0713-3. [DOI] [PubMed] [Google Scholar]
- 30.Herzovich V, Vaiani E, Marino R, et al. Unexpected Peripheral Markers of Thyroid Function in a Patient with a Novel Mutation of the MCT8 Thyroid Hormone Transporter Gene. Horm Res. 2006;67(1):1–6. doi: 10.1159/000095805. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab. 2007;21(2):307–21. doi: 10.1016/j.beem.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadimitriou A, Dumitrescu AM, Papavasiliou A, et al. A novel monocarboxylate transporter 8 gene mutation as a cause of severe neonatal hypotonia and developmental delay. Pediatrics. 2008;121(1):e199–202. doi: 10.1542/peds.2007-1247. [DOI] [PubMed] [Google Scholar]
- 33.Namba N, Etani Y, Kitaoka T, et al. Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr. 2007;167(7):785–91. doi: 10.1007/s00431-007-0589-6. [DOI] [PubMed] [Google Scholar]
- 34.Biebermann H, Ambrugger P, Tarnow P, et al. Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. European journal of endocrinology. 2005;153(3):359–66. doi: 10.1530/eje.1.01980. [DOI] [PubMed] [Google Scholar]
- 35.Wemeau JL, Pigeyre M, Proust-Lemoine E, et al. Beneficial effects of propylthiouracil plus L-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab. 2008;93(6):2084–8. doi: 10.1210/jc.2007-2719. [DOI] [PubMed] [Google Scholar]
- 36.Menezes Filho HC, Marui S, Manna TD, et al. Novel mutation in MCT8 gene in a Brazilian boy with thyroid hormone resistance and severe neurologic abnormalities. Arquivos Brasileiros de Endocrinologia & Metabologia. 2011;55(1):60–6. doi: 10.1590/s0004-27302011000100008. [DOI] [PubMed] [Google Scholar]
- 37.Crushell E, Reardon W. Elevated TSH levels in a mentally retarded boy. Eur J Pediatr. 2009;169(5):573–5. doi: 10.1007/s00431-009-1075-0. [DOI] [PubMed] [Google Scholar]
- 38.Verge CF, Konrad D, Cohen M, et al. Diiodothyropropionic Acid (DITPA) in the Treatment of MCT8 Deficiency. Journal of Clinical Endocrinology & Metabolism. 2012 doi: 10.1210/jc.2012-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs O, Pfarr N, Pohlenz J, Schmidt H. Elevated serum triiodothyronine and intellectual and motor disability with paroxysmal dyskinesia caused by a monocarboxylate transporter 8 gene mutation. Dev Med Child Neurol. 2009;51(3):240–4. doi: 10.1111/j.1469-8749.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 40.Vaurs-Barriere C, Deville M, Sarret C, et al. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann Neurol. 2009;65(1):114–8. doi: 10.1002/ana.21579. [DOI] [PubMed] [Google Scholar]
- 41.Gika AD, Siddiqui A, Hulse AJ, et al. White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med & Child Neurol. 2010;52(5):475–82. doi: 10.1111/j.1469-8749.2009.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holden KR, Zuniga OF, May MM, et al. X-linked MCT8 gene mutations: characterization of the pediatric neurologic phenotype. J Child Neurol. 2005;20(10):852–7. doi: 10.1177/08830738050200101601. [DOI] [PubMed] [Google Scholar]
- 43.Kakinuma H, Itoh M, Takahashi H. A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T4 levels in cerebrospinal fluid. J Pediatr. 2005;147(4):552–4. doi: 10.1016/j.jpeds.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 44*.Dumitrescu AM, Liao XH, Weiss RE, et al. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147(9):4036–43. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- 45*.Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. Journal of Clinical Investigation. 2007;117(3):627. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao XH, Di Cosmo C, Dumitrescu AM, et al. Distinct Roles of Deiodinases on the Phenotype of Mct8 Defect: A Comparison of Eight Different Mouse Genotypes. Endocrinology. 2011;152(3):1180–91. doi: 10.1210/en.2010-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Cosmo C, Liao XH, Dumitrescu AM, et al. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120(9):3377–88. doi: 10.1172/JCI42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trajkovic-Arsic M, Muller J, Darras VM, et al. Impact of Monocarboxylate Transporter-8 Deficiency on the Hypothalamus-Pituitary-Thyroid Axis in Mice. Endocrinology. 2010;151(10):5053–62. doi: 10.1210/en.2010-0593. [DOI] [PubMed] [Google Scholar]
- 49.Mayerl S, Visser T, Bauer R, et al. Consequences of brain-specific thyroid hormone deprivation in MCT8/OATP1C1 double knockout mice. status: published. 2012 [Google Scholar]
- 50.Vatine GD, Zada D, Lerer-Goldshtein T, et al. Zebrafish as a model for monocarboxyl transporter 8-deficiency. Journal of Biological Chemistry. 2013;288(1):169–80. doi: 10.1074/jbc.M112.413831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zung A, Visser TJ, Uitterlinden AG, et al. A child with a deletion in the monocarboxylate transporter 8 gene: 7-year follow-up and effects of thyroid hormone treatment. European Journal of Endocrinology. 2011;165(5):823–30. doi: 10.1530/EJE-11-0358. [DOI] [PubMed] [Google Scholar]
- 52.Di Cosmo C, Liao XH, Dumitrescu AM, et al. A thyroid hormone analogue with reduced dependence on the monocarboxylate transporter 8 (MCT8) for tissue transport. Endocrinology. 2009;150(9):4450–8. doi: 10.1210/en.2009-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horn S, Kersseboom S, Mayerl S, et al. Tetrac Can Replace Thyroid Hormone During Brain Development in Mouse Mutants Deficient in the Thyroid Hormone Transporter Mct8. Endocrinology. 2013;154(2):968–79. doi: 10.1210/en.2012-1628. [DOI] [PubMed] [Google Scholar]
- 54.Ramos HE, Morandini M, Carre A, et al. Pregnancy in women heterozygous for MCT8 mutations: risk of maternal hypothyroxinemia and fetal care. Eur J Endocrinol. 2011;164(2):309–14. doi: 10.1530/EJE-10-0679. [DOI] [PubMed] [Google Scholar]
- 55.Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 56*.Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annual review of nutrition. 2003;23(1):17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 57*.Dumitrescu AM, Liao XH, Abdullah MS, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nature genetics. 2005;37(11):1247–52. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 58.Di Cosmo C, McLellan N, Liao XH, et al. Clinical and molecular characterization of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R128X) Journal of Clinical Endocrinology & Metabolism. 2009;94(10):4003–9. doi: 10.1210/jc.2009-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azevedo MF, Barra GB, Naves LA, et al. Selenoprotein-related disease in a young girl caused by nonsense mutations in the SBP2 gene. Journal of Clinical Endocrinology & Metabolism. 2010;95(8):4066–71. doi: 10.1210/jc.2009-2611. [DOI] [PubMed] [Google Scholar]
- 60*.Schoenmakers E, Agostini M, Mitchell C, et al. Mutations in the selenocysteine insertion sequence–binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. The Journal of clinical investigation. 2010;120(12):4220. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamajima T, Mushimoto Y, Kobayashi H, et al. Novel compound heterozygous mutations in the SBP2 gene: characteristic clinical manifestations and the implications of GH and triiodothyronine in longitudinal bone growth and maturation. European journal of endocrinology. 2012;166(4):757–64. doi: 10.1530/EJE-11-0812. [DOI] [PubMed] [Google Scholar]
- 62.Lescure A, Allmang C, Yamada K, et al. cDNA cloning, expression pattern and RNA binding analysis of human selenocysteine insertion sequence (SECIS) binding protein 2. Gene. 2002;291(1):279–85. doi: 10.1016/s0378-1119(02)00629-7. [DOI] [PubMed] [Google Scholar]
- 63.Copeland PR. Regulation of gene expression by stop codon recoding: selenocysteine. Gene. 2003;312:17–25. doi: 10.1016/s0378-1119(03)00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Papp LV, Lu J, Striebel F, et al. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Molecular and cellular biology. 2006;26(13):4895–910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silers L, Gonc N, Kandemir N, et al. Selenoprotein Deficiency Syndrome Caused by Novel Heterozygous Mutations in the SBP2 Gene Endocrine Abstracts. 2012 [Google Scholar]
- 66.Copeland PR, Fletcher JE, Carlson BA, et al. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. The EMBO journal. 2000;19(2):306–14. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bösl MR, Takaku K, Oshima M, et al. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proceedings of the National Academy of Sciences. 1997;94(11):5531–4. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seeher S, Wirth E, Mahdi Y, et al. SECISBP2 syndrome: Mouse models for an atypical form of resistance to thyroid hormone. Endocrine Abstracts. 2012 [Google Scholar]
- 69.Bubenik JL, Driscoll DM. Altered RNA binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in selenocysteine insertion sequence-binding protein 2. Journal of Biological Chemistry. 2007;282(48):34653–62. doi: 10.1074/jbc.M707059200. [DOI] [PubMed] [Google Scholar]
- 70.Schneider MJ, Fiering SN, Thai B, et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147(1):580–9. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 71.Schneider MJ, Fiering SN, Pallud SE, et al. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Molecular Endocrinology. 2001;15(12):2137–48. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 72.Hernandez A, Martinez ME, Fiering S, et al. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. Journal of Clinical Investigation. 2006;116(2):476. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochimica et Biophysica Acta (BBA)-General Subjects. 2012 doi: 10.1016/j.bbagen.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3474–9. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952–60. doi: 10.1210/en.2008-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schomburg L, Dumitrescu AM, Liao XH, et al. Selenium supplementation fails to correct the selenoprotein synthesis defect in subjects with SBP2 gene mutations. Thyroid. 2009;19(3):277–81. doi: 10.1089/thy.2008.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]