Abstract
Background
Patients with postural tachycardia syndrome (POTS) have exaggerated orthostatic tachycardia often following a viral illness, suggesting autoimmunity may play a pathophysiological role in POTS. We tested the hypothesis that they harbor functional autoantibodies to adrenergic receptors (AR).
Methods and Results
Fourteen POTS patients (7 each from 2 institutions) and 10 healthy subjects were examined for α1AR autoantibody‐mediated contractility using a perfused rat cremaster arteriole assay. A receptor‐transfected cell‐based assay was used to detect the presence of β1AR and β2AR autoantibodies. Data were normalized and expressed as a percentage of baseline. The sera of all 14 POTS patients demonstrated significant arteriolar contractile activity (69±3% compared to 91±1% of baseline for healthy controls, P<0.001) when coexisting β2AR dilative activity was blocked; and this was suppressed by α1AR blockade with prazosin. POTS sera acted as a partial α1AR antagonist significantly shifting phenylephrine contractility curves to the right. All POTS sera increased β1AR activation (130±3% of baseline, P<0.01) and a subset had increased β2AR activity versus healthy subjects. POTS sera shifted isoproterenol cAMP response curves to the left, consistent with enhanced β1AR and β2AR agonist activity. Autoantibody‐positive POTS sera demonstrated specific binding to β1AR, β2AR, and α1AR in transfected cells.
Conclusions
POTS patients have elevated α1AR autoantibodies exerting a partial peripheral antagonist effect resulting in a compensatory sympathoneural activation of α1AR for vasoconstriction and concurrent βAR‐mediated tachycardia. Coexisting β1AR and β2AR agonistic autoantibodies facilitate this tachycardia. These findings may explain the increased standing plasma norepinephrine and excessive tachycardia observed in many POTS patients.
Keywords: adrenergic receptor, autoantibody, autonomic function, postural tachycardia syndrome
Introduction
Postural tachycardia syndrome (POTS) occurs most commonly in young women of child‐bearing age, less frequently in males or at older ages,(2013)–(2009) and affects 500 000 in the United States alone.(1999) The characteristic hemodynamic finding is an excessive increase in standing heart rate.(2006)–(2011) In contrast to patients with orthostatic hypotension, blood pressure may rise with upright posture. This suggests an overshoot of the normal arterial compensatory vasoconstriction associated with orthostasis. Plasma norepinephrine levels are often elevated in recumbency and rise significantly with upright posture.(2005)–(2002) The etiology of POTS is unknown although several pathophysiological mechanisms have been proposed.(2006) Some subjects report a “viral‐like” illness 2 to 6 months prior to the onset of symptoms, thus supporting the hypothesis that an autoimmune‐mediated molecular mimicry might be present. Interest generally has focused on the abnormal circulating catecholamines and abnormal cardiovascular conditioning. Current therapeutic approaches using β‐blockade(2011) can lead to partial alleviation of the tachycardia but are frequently associated with additional side effects.
We previously reported that patients with “idiopathic” orthostatic hypotension (OH) have a strong association with activating autoantibodies to several autonomic G‐protein coupled receptors (GPCR) including the β1‐ and β2‐adrenergic receptors (β1AR and β2AR) and M2 and M3 muscarinic receptors (M2R and M3R).(2012)–(2012) These autoantibodies primarily target the second extracellular loop (ECL2) of the respective receptors and mediate physiologic effects.(2000)–(2007) Subjects with marked idiopathic OH predominantly harbor the vasodilatory β2AR and M3R autoantibodies, while the co‐presence of M2R and/or β1AR autoantibodies respectively appear to decrease or increase the heart rate. These autoantibodies appear to act alone as allosteric agonists yet also possess partial antagonist capability.(2012)
We hypothesized subjects with POTS manifest autoantibodies to the pressor α1‐adrenergic receptor (α1AR) and could partially block the effectiveness of the normal α1AR endogenous ligand norepinephrine central to the homeostatic response to upright posture. This impaired vasoconstrictor response would increase baroreceptor activation, increase central sympathetic nervous activity, and normalize vasoconstriction and blood pressure. The relatively unprotected β1AR cardiac chronotropic receptors would respond to this increased sympathoneural output and circulating norepinephrine with an exaggerated tachycardia. The presence of β1/2AR‐activating autoantibodies potentially would enhance this tachycardia.
Methods
Patient Selection
The present study was performed in 2 phases. In Phase I, 7 POTS patients and 8 healthy subjects were recruited for study at the Oklahoma University (OU) Health Science Center and Oklahoma City Veteran's Affairs Medical Center. In this group, we tested our hypothesis for the presence of autoantibodies to the α1AR. In Phase II, we retested our hypothesis using blinded serum samples from 7 POTS patients and an additional 2 healthy subjects studied at the Vanderbilt University (VU) Autonomic Dysfunction Center (Nashville, TN). These protocols were approved by the Biomedical Ethics Committees at the Oklahoma University Health Science Center and the Human Research Protection Program at the Vanderbilt University Medical Center, and each subject gave signed, informed consent.
Patients met criteria for POTS(2013)–(2013) developing symptoms of orthostatic intolerance accompanied by an increased heart rate rise ≥30 min−1 within 10 minutes of standing in the absence of significant hypotension (a fall in blood pressure [BP] of >20/10 mm Hg). All patients had a ≥6 month history of symptoms, the absence of another chronic debilitating disorder, were ≥18 years of age, free of medications that could impair autonomic tone, and were not taking fludrocortisone for at least 5 days prior to testing. Five of the 14 POTS subjects and 2 of the 10 “healthy controls” recalled a respiratory infection in the 6 months prior to onset of their symptoms or inclusion in the study for the healthy controls.
Baseline Standing Challenges
The standing challenge was performed as previously described.(2013) Heart rate, systolic BP (SBP), diastolic BP (DBP), and plasma catecholamines were measured after overnight rest (VU criteria) with the patient in the supine position and again after standing 10 (OU) to 30 (VU) minutes (as tolerated). Hemodynamic measures were assessed using an automated oscillometric monitor (Dinamap, Critikon Corp). For catecholamine measurements an indwelling cannula was inserted at least 20 minutes prior to starting the study.
Sample Preparation
In Phase I, sera from OU subjects were purified to extract IgG (NAb Protein A/G Spin Kit, Pierce). This IgG was used to demonstrate that the effects were related to the autoantibodies and not to other serum components (eg, catecholamines). In Phase II, sera available for the VU subjects were not sufficient to extract IgG. Throughout the manuscript, we will use the term “serum” to describe these samples. Blood for catecholamines was collected and assayed as described.(2007)
β1AR and β2AR Assays
Serum‐mediated β1AR and β2AR activation of cAMP production in transfected Chinese hamster ovary (CHO) cells (with either β1AR or β2AR stimulation) was measured using the cAMP Hunter eXpress GPCR Assay kit (DiscoveRx, Fremont, CA) as described.(2012) cAMP standard, negative (buffer), and positive (isoproterenol) controls were included in each assay. All samples were tested in triplicate. The cAMP values are expressed as percentage of buffer baseline to normalize the individual data.
M3R Assay
IgG activation of M3R was measured in the OU samples using the PathHunter eXpress β‐arrestin GPCR Assay kit (DiscoveRx) as described.(2012) Negative (buffer) and positive (acetylcholine) controls were included in each assay. The β‐arrestin recruitment levels were expressed as a percentage of buffer baseline to normalize the individual data.
Isolated Cremaster Arteriole Assay
The vasoconstrictor and vasodilator effect of the subjects' sera via GPCR activation on resistance vessels were examined using an isolated rat cremaster arteriole assay as described.(2012),(2000) After equilibration and development of steady‐state myogenic tone, the arterioles were perfused with the respective serum or IgG, and generally diluted 1:50 with Krebs's buffer. The dosage response curves demonstrated that this dilution provided optimal and consistent data. The specific β2AR blocker ICI‐118551 (5 μmol/L) was then added to eliminate β2AR‐mediated vasodilation. The selective α1AR blocker prazosin (10 μmol/L) was subsequently added to the perfusate to eliminate α1AR‐mediated vasoconstriction, and the effects on vessel diameter were recorded. The buffer baseline diameters were normalized to 100% and subsequent sera/IgG‐induced contractility was reported as a percentage change from baseline. This procedure was approved by the Oklahoma University Health Sciences Center Institutional Animal Care and Use Committee.
Immunofluorescence Staining
β1AR/β2AR‐transfected CHO cells (DiscoveRx) and α1AR‐transfected Chem‐1 cells (Millipore) were cultured on glass cover slips in 6‐well plates for 24 hours. The cells were fixed with 4% paraformaldehyde. After blocking, cells were incubated with healthy control sera, POTS sera (1:100) with and without preabsorption with an excess of the target ECL2 receptor peptide, or rabbit polyclonal antibodies to the respective receptors (positive controls) for 1 hour, followed by incubation with fluorescein isothiocyanate (FITC)‐labeled anti‐human or anti‐rabbit IgG antibodies respectively (Jackson ImmunoResearch Laboratories). Nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI). Fluorescence images were obtained using a fluorescence microscope (Olympus).
Statistical Analysis
Data are expressed as mean±SEM. Appropriateness of assumption of normal distributions for the continuous variables was confirmed by D'Agostino–Pearson omnibus normality test; consequently, paired and unpaired Student's t tests, as appropriate, were used for the comparison of continuous variables. Fisher's exact tests were used for dichotomous variables. Statistical significance was set at P<0.05.
Results
Clinical Data
Demographic data are included in Table. The POTS patients were older than the healthy control subjects (39±13 years vs 27±6 years, P=0.013). This difference existed only from the older patients recruited at the OU VAMC. The POTS subjects from VU were not significantly different from the control subjects (P=0.38). There was no significant difference in gender between the POTS and control groups (Fisher's exact test, P=0.21). Five of the 14 POTS subjects reported some form of intercurrent viral‐like illness in the 6 months preceding onset of their postural tachycardia‐induced symptoms, while 2 of the 10 healthy controls recalled some form of infectious‐related symptoms prior to their inclusion in the study (Fisher's exact test of association between POTS and viral‐like illness, P=0.65). The POTS patients had a significantly higher increase in heart rate with upright posture than healthy subjects (P<0.001). The change in BP with upright posture was not significantly different between POTS patients and healthy subjects. Serum catecholamines were increased in POTS compared to their controls (P<0.05).
Table 1.
Demographics and Postural Signs and Catecholamines of the POTS and Healthy Control Subjects
| POTS n=14 | Control n=10 | P Value | |
|---|---|---|---|
| Female, n (%) | 10 (71) | 4 (40) | 0.21 |
| Age, y | 39±13 | 27±6 | 0.013 |
| History of infection, n (%) | 5 (36) | 2 (20) | 0.65 |
| Supine | |||
| Heart rate, min−1 | 76±13 | 73±13 | |
| SBP, mm Hg | 117±14 | 119±9 | |
| DBP, mm Hg | 76±13 | 69±10 | |
| Norepinephrine, pg/mL* | 215±66 | 223±159 | |
| Epinephrine, pg/mL* | 19±13 | 18±20 | |
| Standing | |||
| Heart rate, min−1 | 120±22 | 81±15 | |
| SBP, mm Hg | 123±12 | 118±13 | |
| DBP, mm Hg | 83±13 | 75±10 | |
| Norepinephrine, pg/mL | 873±302 | 553±263 | |
| Epinephrine, pg/mL | 58±32 | 35±31 | |
| Change (∆) from supine to standing | |||
| Heart rate, min−1 | 44±16 | 7±5 | <0.001 |
| SBP, mm Hg | 6±7 | 1±7 | 0.10 |
| DBP, mm Hg | 8±12 | 5±9 | 0.51 |
| Norepinephrine, pg/mL | 658±296 | 330±207 | 0.0065 |
| Epinephrine, pg/mL | 40±21 | 17±23 | 0.0185 |
Data are presented as mean±SD. Comparisons between the POTS and control groups were performed using unpaired t test or Fisher's exact test. DBP indicates diastolic blood pressure; POTS, postural tachycardia syndrome; SBP, systolic blood pressure.
SI conversion factor: norepinephrine (pg/mL×0.005911=nmol/L).
Epinephrine (pg/mL×5.458=pmol/L).
β1/2AR Activity
Since β2AR vasodilatory activity, if present, would affect the cremaster vasoconstrictor bioassay, we first determined if any samples contained this autoantibody. The ability of the sera to stimulate cAMP in the β2AR‐transfected cells in each case was expressed as a percentage of that observed for buffer alone. The activity in 4 of the 7 OU POTS subjects (Figure 1A) was higher than observed in the control subjects. Similarly, sera from 3 of 7 VU POTS patients had elevated β2AR activity. The elevated β2AR activity observed in POTS was significantly higher than the β2AR activity observed in the control subjects (123±6% vs 94±5% of baseline, P<0.01). This elevated activity was eliminated following selective β2AR blockade with ICI‐118551 (101±2% of baseline, P<0.01 vs POTS) and not significantly different from the control subjects (P=0.2) (Figure 1B). There was an increase in β1AR‐stimulated cAMP production in all of the POTS compared to the control subjects (130±3% vs 99±2% of baseline, P<0.001) (Figure 1C and 1D); and this activity was blocked by the nonselective βAR blocker propranolol (99±1% of baseline, P<0.001 vs POTS) and not significantly different from the control subjects (P=0.1) (Figure 1D). Only 1 of 7 OU POTS subjects had an increase in M3R activity (Figure 1E) and there was no significant increase in this autoantibody (Figure 1F). There was a significant dose effect on activation of β2AR for both sera and IgG from POTS subjects (Figures 2A and 2B). A similar dose effect on β1AR activation for these samples was observed (Figures 2C and 2D).
Figure 1.
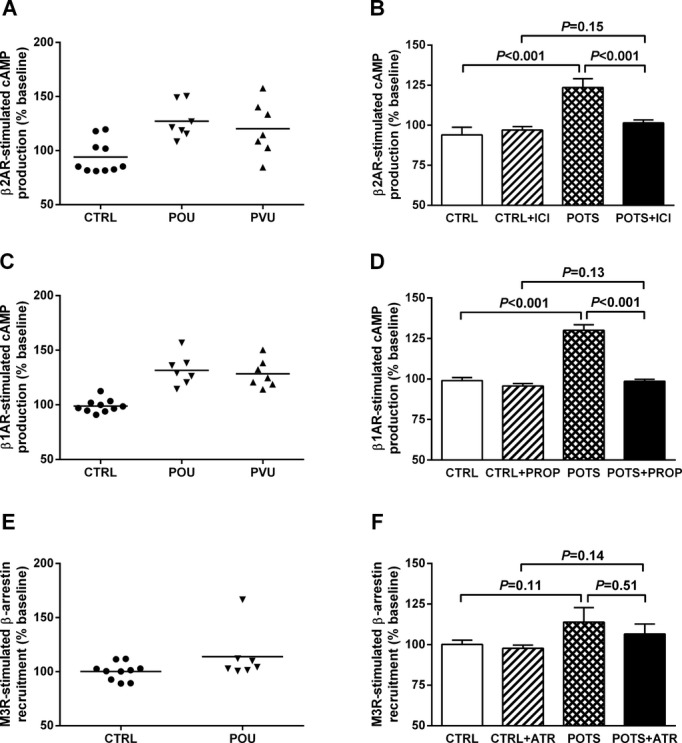
Effects of serum/IgG from POTS patients and healthy control subjects on activation of β2AR, β1AR, and M3R in receptor‐transfected CHO cells. Serum IgG (100 μg/mL) from 7 OU POTS (POU), serum (1:50) from 7 VU POTS (PVU), and serum/IgG from 10 control (CTRL) subjects were measured for activation of cAMP in cultured CHO cells expressing β2AR (A) or β1AR (C). The cAMP values are expressed as a percentage of buffer baseline. Samples from each respective institution were run simultaneously to eliminate inter‐assay variation. There was a significant increase in β2AR (B) and β1AR (D) activity in both POTS groups compared to control values. The addition of the specific β2 blocker ICI‐118551 (ICI) or β‐blocker propranolol (PROP) during the incubation period significantly suppressed the values to control levels. IgG from the 7 OU POTS and 10 control subjects were also measured for M3R activation in cultured CHO cells expressing M3R (E). The M3R‐mediated β‐arrestin recruitment levels are expressed as a percentage of buffer baseline. These samples failed to demonstrate significant M3R activation (F). AR indicates adrenergic receptors; ATR, atropine; CHO, Chinese hamster ovary; OU, Oklahoma University; POTS, postural tachycardia syndrome; VU, Vanderbilt University.
Figure 2.
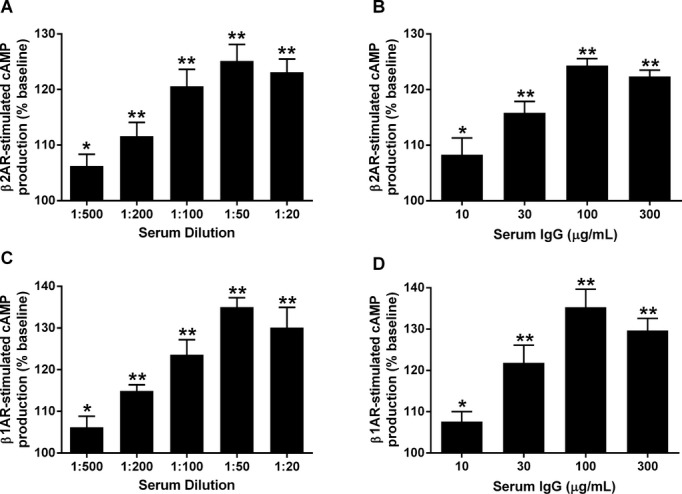
Dose effects of serum and IgG from 3 POTS patients on β2AR and β1AR activation of cAMP production in receptor‐transfected CHO cells. Values are expressed as a percentage of buffer baseline. The serum (A and C) and IgG (B and D) demonstrated similar dosage effects. *P<0.05, **P<0.01 vs buffer baseline. AR indicates adrenergic receptors; CHO, Chinese hamster ovary; POTS, postural tachycardia syndrome.
α1AR Contractile Activity
Since β2AR activity was present in a significant number of the POTS subjects, we analyzed the subject sera in the cremaster artery vasoconstriction bioassay in the presence of the selective β2AR blocker ICI‐118551. IgG from each of the 7 OU POTS patients (Figure 3A) and sera from the 7 VU POTS (Figure 3B) caused a >10% decrease in the cremaster artery diameter when incubated with a standardized IgG concentration of 100 μg/mL or 1:50 serum. Prazosin blocked this contractility almost completely in each group. There was a dosage‐dependent vasoconstrictor effect for both IgG (Figure 3C) and sera (Figure 3D). Therefore, we combined the 2 groups (Figure 4). The POTS subjects had a significant difference in contractility (76±3% of baseline vs control 94±1%, P<0.001) in the absence of β2AR blockade with ICI‐118551. When assayed in the presence of ICI‐118551, the POTS had even greater contractility (69±3% of baseline vs control 91±1, P<0.001), which was reversed by prazosin (Figure 4).
Figure 3.
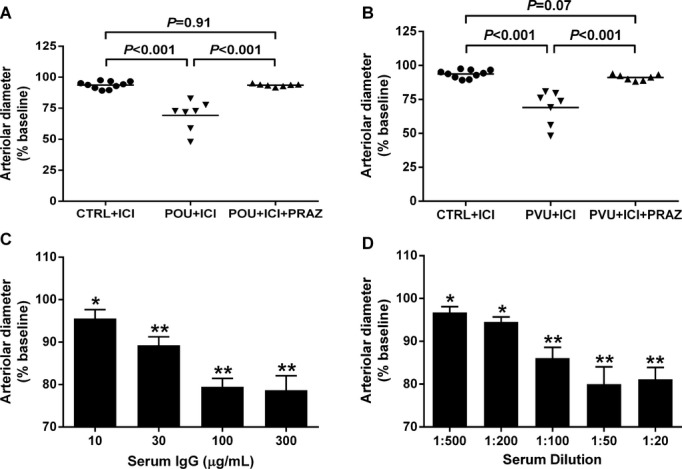
Effects of serum/IgG from POTS patients and healthy control subjects on basal spontaneous myogenic tone of rat cremaster arterioles. Values are expressed as % decrease in arteriolar diameter from baseline. IgG (100 μg/mL) from the 7 OU POTS patients (A) and serum (1:50) from the 7 VU POTS patients (B) caused significant vasoconstriction compared to serum/IgG from the 10 control subjects. This assay was performed after blocking β2AR activity by addition of ICI‐118551 (5 μmol/L). The addition of the α1AR blocker prazosin (PRAZ, 10 μmol/L) markedly suppressed the contractility to control levels. There was a significant dosage‐dependent vasoconstrictive effect for both IgG (C) and serum (D) from the POTS patients. *P<0.05, **P<0.01 vs baseline, n=3. AR indicates adrenergic receptors; OU, Oklahoma University; POTS, postural tachycardia syndrome; VU, Vanderbilt University.
Figure 4.
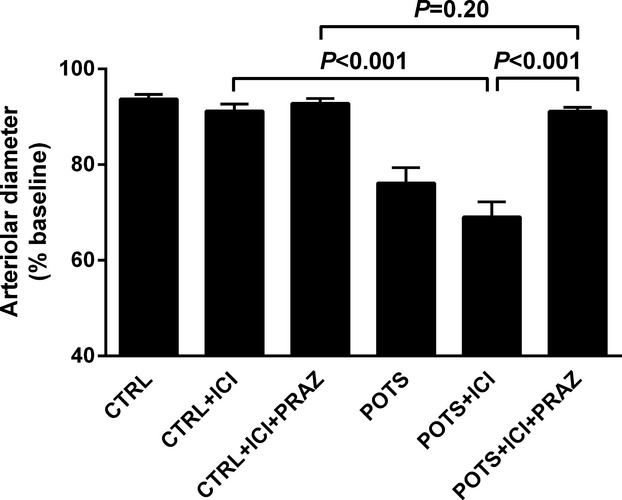
Vasoconstrictive effect for the combined POTS groups. The vasoconstriction for the combined POTS groups was significantly greater than in control subjects. This constriction was enhanced with withdrawal of β2AR‐mediated vasodilatation with ICI‐118551. Prazosin reversed this to baseline. No significant vasoconstriction was observed for the control subjects. AR indicates adrenergic receptors; POTS, postural tachycardia syndrome; PRAZ, prazosin.
Cremaster Artery Phenylephrine Dose Response
Phenylephrine was used to construct cremaster contractility α1AR dose response curves (Figure 5A). Sera from 3 POTS patients were used to examine the effect of the α1AR autoantibodies on phenylephrine‐induced arteriolar contractility. In the presence of a 1:200 dilution of the POTS patient's serum, there was a significant shift of the curve to the right showing a decrease in the contractile response to phenylephrine. This was not observed using control sera.
Figure 5.
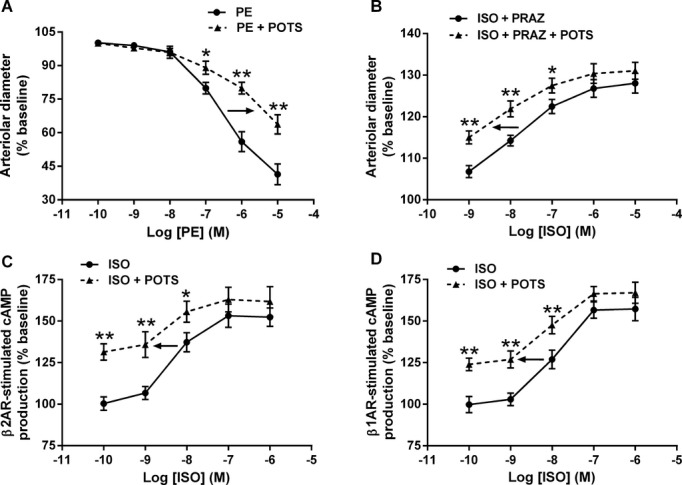
Allosteric effects of POTS serum/IgG on phenylephrine and isoproterenol dose response. A, Serum (1:200) from 3 POTS subjects with α1AR activating activity shifted the phenylephrine (PE) dosage response curve to the right in a cremaster vasoconstriction assay. Control serum failed to alter the curve. B, Serum (1:200) from 3 POTS subjects was incubated with prazosin (10 μmol/L, to block coexisting α1AR pressor activity) and used to construct an isoproterenol (ISO) vasodilator response curve in the cremaster arteriole assay. In contrast to the α1AR autoantibody dosage response, the β2AR activation curve was shifted significantly to the left compatible with a positive allosteric effect. C, Likewise, IgG (50 μg/mL) from 3 β2AR antibody‐positive POTS subjects significantly shifted to the left an isoproterenol activation curve of cAMP production in β2AR‐transfected CHO cells. D, IgG (50 μg/mL) from 3 POTS subjects containing β1AR autoantibodies produced a similar leftward shift of an isoproterenol activation curve in β1AR‐transfected CHO cells. *P<0.05, **P<0.01. AR indicates adrenergic receptors; CHO, Chinese hamster ovary; PRAZ, prazosin; POTS, postural tachycardia syndrome.
Isoproterenol Dose Response
The impact of POTS sera on isoproterenol‐induced β2AR‐mediated vasodilatation was examined using the cremaster artery contractility assay (Figure 5B). Contractility curve was shifted significantly to the left in the presence of POTS sera compared to control. The effect of POTS IgG was also examined using β2AR‐ and β1AR‐transfected CHO cells. The POTS IgG‐augmented cAMP production was shifted significantly to the left, demonstrating positive allosteric effects for both β2AR (Figure 5C) and β1AR (Figure 5D) with these autoantibodies.
Immunofluorescence Staining
We used receptor‐transfected cells to demonstrate specific autoantibody binding to the receptors. Sera from a POTS subject who demonstrated activating autoantibodies to β1AR, β2AR, and α1AR, sera from a healthy control subject and rabbit polyclonal antibodies to each receptor as positive controls were separately incubated with these cells without and with an excess of their respective ECL2 target peptide. These images are shown in Figure 6. There was significant binding to each of the receptors from the POTS sera, which was completely blocked by preabsorption with the specific ECL2 peptides. No significant binding to the cells from the control sera was observed.
Figure 6.
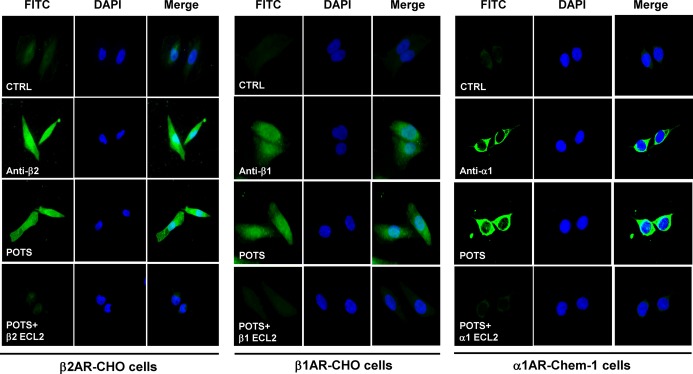
Immunofluorescence staining of β2AR, β1AR and α1AR in transfected cells with POTS serum. CHO cells expressing human β2AR/β1AR and Chem‐1 cells expressing human α1AR were stained with POTS serum, rabbit polyclonal antibodies to β2AR, β1AR, or α1AR (positive control), or control serum and FITC (green)‐labeled secondary antibodies. Nuclei were counterstained with DAPI (blue). Both POTS serum and the positive control antibodies demonstrated strong reactivity, while the control serum did not show any significant binding. POTS serum binding was abolished by preabsorption with the second extracellular loop (ECL2) peptide of the respective receptors. All images were obtained at ×40 magnification. AR indicates adrenergic receptors; DAPI, 4′,6‐diamidino‐2‐phenylindole; FITC, fluorescein isothiocyanate; POTS, postural tachycardia syndrome.
Discussion
These data demonstrate that sera from POTS patients induce predominantly pressor responses blocked primarily by the α1AR‐specific antagonist prazosin. These contractile responses were observed with sera/IgG from each of the POTS patients providing strong evidence that this effect was mediated by circulating autoantibodies to their respective target receptors and not from stray ligands in the sample (eg, norepinephrine). Specifically, autoantibodies directed toward the α1AR were responsible for the in vitro contractile effects on the cremaster resistance arterioles. These data were demonstrated in 2 separate cohorts, including one using blinded samples from a different center. These 2 groups, accumulated from different referral bases at separate institutions, had virtually indistinguishable immunological data. A significant difference in age was observed between the overall POTS and control groups despite considerable overlap. It is unlikely this affected the interpretation of the data because the subject group from VU was not different in age from the controls. The autoantibody activity for both the OU and VU groups were identical despite the slight differences in mean age. Autoantibodies may express variable allosteric effects on GPCR, in some circumstances acting as agonists and in other circumstances as inverse agonists or as partial antagonists.(2012) They may alter the effect of the orthosteric “natural” ligand on the receptor. The α1AR autoantibody‐containing sera from POTS patients shifted the respective orthosteric ligand dosage response curve to the right. This provides support for the hypothesis that autoantibody‐mediated α1AR partial antagonism is responsible for the POTS pathophysiology.
The observation that β1AR‐activating autoantibodies were present in all of the POTS subjects and β2AR in one half of the subjects is important inasmuch as these autoantibodies significantly facilitated isoproterenol β1AR and β2AR activation of cellular cAMP production. These would enhance the chronotropic effects of norepinephrine on the heart. Both β2AR and M3R autoantibodies possess vasodilatory properties. It is likely the presence of β2AR autoantibodies account for the initial borderline or blunted α1AR in vitro contractile responses observed in several POTS patients when initially studied without concurrent β2AR blockade. Because the primary α1AR cremaster artery assay involves measurement of contractility, concomitant vasodilators can cause artifacts. Currently, there are no available activity assays for the α1AR such as were used for the β1AR, β2AR, and M3R. The variable co‐presence of vasodilatory and vasoconstrictive autoantibodies supports a spectrum of autoantibody expression varying from idiopathic OH(2012)–(2012) to POTS, and likely includes patients with inappropriate sinus tachycardia.(2013)
There is prior clinical evidence that receptor blockade is present in POTS patients. Circulating plasma norepinephrine levels can be elevated in POTS patients both while supine and upright. Circulating catecholamines however may not reflect the ligand changes at the receptor level. A mild resting tachycardia is frequently observed in POTS,(2007) and an exaggerated heart rate is a hallmark. Increased venous pooling(1988)–(2002) and decreased plasma volume(2005) are consistent with increased norepinephrine effects.(2002),(2002) Others have demonstrated defects in norepinephrine activity at the receptor level.(2000) Wang et al recently reported 10 subjects with POTS demonstrated autoantibodies to proteins associated with lipid rafts purified from normal subjects.(2013) These unidentified targets would include GPCR such as α1AR and β1/2AR identified specifically in the present study. We have demonstrated by immunofluorescence that these autoantibodies bind to specific receptors and are inhibited by their respective ECL2 target peptides.
The cause(s) leading to autoantibody production in these subjects is currently unknown. Five of our 14 POTS subjects recalled some possible “infection” in the 6 months prior to their symptoms. However 2 of our 10 control subjects recalled similar “infections” and so these can only be considered anecdotal at this point. Li et al demonstrated that molecular mimicry could account for the presence of autoantibodies to the β1AR in rats immunized with cardiac myosin.(2006) Since the ECL of these receptors are in the ectodomain, it is conceivable that enzymatic activity, oxidative activity or a viral element might cause them to be shed and enter the circulation as a peptide that would subsequently be incorrectly processed as a “foreign” peptide. These origins at present must be considered speculative and worthy of future investigation.
As depicted in Figure 7, in the presence of the α1AR autoantibodies, the normal α1AR response to upright posture would be blunted at the level of the peripheral vascular receptor. An inadequate homeostatic BP response to upright posture is sensed by the baroreceptors leading to a heightened sympathetic nervous system outflow. This exaggerated response would lead to increased norepinephrine release at the sympathetic nerve ending and at the systemic level. This would lead to a normalization of the BP with orthostasis. However the cardiac effect of the sympathetic activation would be a direct and relatively unopposed chronotropic action on the β1AR in the sinus node. β1AR‐activating autoantibodies were present in all of the POTS and these autoantibodies facilitated β1AR agonist activation of cAMP in vitro. The variable presence of β2AR autoantibodies would also contribute to this allosteric enhancement of cardiac βAR responsiveness to its natural ligand norepinephrine and would be expected to exaggerate the tachycardia.(2006) It is likely this facilitation of endogenous β1AR and β2AR ligand activity may produce adverse significant ramifications in other diseases such as cardiomyopathies that co‐harbor and/or express these autoantibodies.
Figure 7.
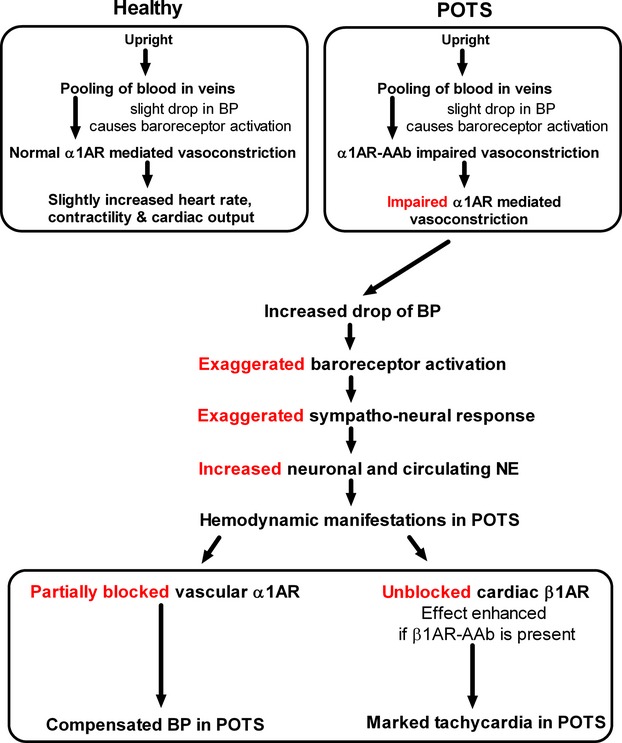
Diagram depicting the proposed effects of activating autoantibodies (AAb) on the pathophysiology of POTS. These conclusions are supported by data demonstrating the consistent presence of autoantibodies to the α1AR, the β1AR, and variably the β2AR. The differing allosteric impact on their respective receptors will lead to an exaggerated tachycardia characteristic of this common syndrome. AR indicates adrenergic receptors; BP, blood pressure; NE, norepinephrine; POTS, postural tachycardia syndrome.
In conclusion, these data support the addition of POTS to a growing number of cardiovascular entities with an autoimmune pathophysiology. A pharmacological approach to management would ideally block autoantibody activity and leave the receptors unblocked. While this might be feasible with development of specific epitope decoy targets, the use of currently available agents is complicated by their impact on the specific receptor(s) needed for a normal response to orthostasis.
Sources of Funding
This study was supported in part by funding from NIH grant R01 HL056267 (Cunningham, Kem), R01 HL102387 (Raj), U54 NS065736, UL1 TR000445 (Vanderbilt CTSA), VA Merit Review grant (Kem, Yu), American Heart Association Postdoctoral Fellowship (Li), Talley Research Award of the Harold Hamm Diabetes Center at the University of Oklahoma (Yu), Heart Rhythm Institute, OUHSC, and individual grant support from Will and Helen Webster. Liles was supported by the Oklahoma NARCH Student Development Program funded by NIH grant GM092238/U26IHS300412 through the Oklahoma Native American Research Centers for Health (ONARCH VI).
Disclosures
None.
References
- Raj SR. Postural tachycardia syndrome (pots). Circulation. 2013; 127:2336-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993; 43:132-137 [DOI] [PubMed] [Google Scholar]
- Grubb BP. Postural tachycardia syndrome. Circulation. 2008; 117:2814-2817 [DOI] [PubMed] [Google Scholar]
- Stewart JM. Postural tachycardia syndrome and reflex syncope: similarities and differences. J Pediatr. 2009; 154:481-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999; 317:75-77 [DOI] [PubMed] [Google Scholar]
- Raj SR. The postural tachycardia syndrome (pots): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006; 6:84-99 [PMC free article] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011; 21:69-72 [DOI] [PubMed] [Google Scholar]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin‐aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005; 111:1574-1582 [DOI] [PubMed] [Google Scholar]
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007; 69:790-798 [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO, III, Sharabi Y, Esler MD, Eisenhofer G. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002; 106:2358-2365 [DOI] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011; 58:167-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kem DC, Reim S, Khan M, Vanderlinde‐Wood M, Zillner C, Collier D, Liles C, Hill MA, Cunningham MW, Aston CE, Yu X. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012; 59:402-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Stavrakis S, Hill MA, Huang S, Reim S, Li H, Khan M, Hamlett S, Cunningham MW, Kem DC. Autoantibody activation of beta‐adrenergic and muscarinic receptors contributes to an “autoimmune” orthostatic hypotension. J Am Soc Hypertens. 2012; 6:40-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G, Nissen E, Morwinski R, Muller J. Autoantibodies against the beta‐ and muscarinic receptors in cardiomyopathy. Herz. 2000; 25:261-266 [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Boivin V, Stork S, Angermann CE, Ertl G, Lohse MJ, Jahns R. A novel fluorescence method for the rapid detection of functional beta1‐adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol. 2007; 50:423-431 [DOI] [PubMed] [Google Scholar]
- Herda LR, Felix SB, Boege F. Drug‐like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br J Pharmacol. 2012; 166:847-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwazue VC, Raj SR. Confounders of vasovagal syncope: postural tachycardia syndrome. Cardiol Clin. 2013; 31:101-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin ST, Black BK, Biaggioni I, Paranjape SY, Orozco C, Black PW, Dupont WD, Robertson D, Raj SR. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. 2012; 9:1484-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plash WB, Diedrich A, Biaggioni I, Garland EM, Paranjape SY, Black BK, Dupont WD, Raj SR. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond). 2013; 124:109-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MA, Zou H, Davis MJ, Potocnik SJ, Price S. Transient increases in diameter and [Ca(2+)](i) are not obligatory for myogenic constriction. Am J Physiol Heart Circ Physiol. 2000; 278:H345-H352 [DOI] [PubMed] [Google Scholar]
- Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013; 61:793-801 [DOI] [PubMed] [Google Scholar]
- Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med. 1988; 111:326-335 [PubMed] [Google Scholar]
- Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002; 105:2274-2281 [DOI] [PubMed] [Google Scholar]
- Jordan J, Shannon JR, Diedrich A, Black BK, Robertson D. Increased sympathetic activation in idiopathic orthostatic intolerance: role of systemic adrenoreceptor sensitivity. Hypertension. 2002; 39:173-178 [DOI] [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine‐transporter deficiency. N Engl J Med. 2000; 342:541-549 [DOI] [PubMed] [Google Scholar]
- Wang XL, Ling TY, Charlesworth MC, Figueroa JJ, Low P, Shen WK, Lee HC. Autoimmunoreactive iggs against cardiac lipid raft‐associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res. 2013; 162:34-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody‐mediated cell signaling in autoimmune myocarditis. J Immunol. 2006; 177:8234-8240 [DOI] [PubMed] [Google Scholar]
- Chiale PA, Garro HA, Schmidberg J, Sanchez RA, Acunzo RS, Lago M, Levy G, Levin M. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006; 3:1182-1186 [DOI] [PubMed] [Google Scholar]


