Abstract
IL-12 is a prominent Th1 differentiator and leukocyte activator. Ample studies showed suppression of IL-12 production by numerous stress factors, including prostaglandins, catecholamines, glucocorticoids, and opioids, but did so in vitro and in the context of artificial leukocyte activation, not simulating the in vivo setting. In a recent study we reported in vivo suppression of plasma IL-12 levels by behavioral stress and surgery. The current study aims to elucidate neuroendocrine mechanisms underlying this phenomenon in naïve F344 rats. To this end, both adrenalectomy and administration of specific antagonists were used, targeting the aforementioned stress factors. The results indicated that corticosterone and prostaglandins are prominent mediators of the IL-12-suppressing effects of stress and surgery, apparently through directly suppressing leukocyte IL-12 production. Following surgery, endogenous prostaglandins exerted their effects mainly through elevating corticosterone levels. Importantly, stress-induced release of epinephrine or opioids had no impact on plasma IL-12 levels, while pharmacological administration of epinephrine reduced plasma IL-12 levels by elevating corticosterone levels. Last, a whole blood in vitro study indicated that prostaglandins and corticosterone, but not epinephrine, suppressed IL-12 production in non-stimulated leukocytes, and only corticosterone did so in the context of CpG-C-induced IL-12 production. Overall, the findings reiterate the notion that results from in vitro or pharmacological in vivo studies cannot indicate the effects of endogenously released stress hormones under stress/surgery conditions. Herein, corticosterone and prostaglandins, but not catecholamines or opioids, were key mediators of the suppressive effect of stress and surgery on in vivo plasma IL-12 levels in otherwise naïve animals.
Keywords: interleukin-12, stress, surgery, glucocorticoid, catecholamine, epinephrine, prostaglandin, opioid, in vivo
Introduction
Interleukin-12 (IL-12) is a prominent activator of cell-mediated immunity and promoter of Th1 responses (Yoo et al., 2002; Trinchieri, 2003). Specifically, IL-12 is considered the most significant cytokine in stimulating natural killer (NK) cells and CD4+ T cells to release interferon-γ (IFN-γ), and a potent promoter of immunocyte proliferation, specifically of cytotoxic T lymphocytes (CTL), B and NK cells (Metzger et al., 1997). Additionally, IL-12 exerts anti-malignant effects through the induction of anti-angiogenic chemokines (e.g., IFN-γ-induced protein 10 (IP-10) and Mig) (Kanegane et al., 1998).
Numerous stress and surgery-related factors have been suggested by in vitro studies to suppress or modulate IL-12 production, including the prevalent catecholamines (CAs), glucocorticoids, opioids, and prostanoids, although their endogenous in vivo role is yet unclear. First, several β2-adrenergic agonists inhibited in vitro production of IL-12 and Th1 differentiation in human leukocytes (Panina-Bordignon et al., 1997). Second, glucocorticoids were implicated in regulating IL-12 production, as in vitro exposure of pre-activated human monocytes (Blotta et al., 1997) and murine macrophages (DeKruyff et al., 1998) to dexamethasone (a synthetic glucocorticoid agonist) resulted in reduced production of IL-12, decreased capacity of T cells to produce IFN-γ, and down-regulated IL-12 receptor levels in T and NK cells (1996; Elenkov et al., 2000). Third, prostaglandins (PGs), which are abundantly secreted in the context of tissue damage (e.g., surgery & burns), were shown to reduce in vitro IL-12 production by activated human monocytes (van der Pouw Kraan et al., 1995; Iwasaki et al., 2003). Last, opioids were suggested to suppress IL-12 production and promote Th2 differentiation (Sacerdote, 2003), but opposite effects were also reported.
In vivo assessment of baseline levels of Th1 cytokines in naïve animals is often impractical, due to their low undetectable plasma levels. Therefore, the few studies that have assessed the impact of stress hormones on plasma IL-12 levels did so employing animals exposed to biological-response-modifiers, rather than naïve animals. In a recent study we showed that unlike most other Th1 cytokines, baseline IL-12 levels are detectable in naïve Fischer 344 (F344) rats, and that several behavioral stress paradigms and surgery profoundly suppressed plasma IL-12 levels (Shaashua et al., 2012). Additionally, in vivo administration of pharmacological doses of corticosterone (CORT), epinephrine, and PGE2, induced similar effects, suggesting their potential involvement in mediating the effects of stress (Shaashua et al., 2012). However, thus far, no study has implicated an endogenous release of specific stress hormones in mediating the effects of stress exposure on plasma IL-12 levels. Additionally, as PGE2, CORT, opioids and epinephrine are known to mutually affect each other levels and interact in their impact on other systems (Ehrhart-Bornstein et al., 1998; Mohn et al., 2005), it is crucial to elucidate each factor’s exclusive and direct impact on IL-12 levels, as well as the interdependent impact of different stress factors in the in vivo milieu.
In the clinical context, patients are subjected to psychological and physiological stressors, and oncological patients often undergo surgery, all of which result in endogenous secretion of stress factors that may adversely impact IL-12 levels and consequently affect immune competence, immediate health outcomes, and long-term oncological outcomes. Thus, elucidating specific mediating mechanisms could endorse optimal interventions of clinical applicability. Therefore, the aims of the current study were to (i) elucidate specific endogenous endocrine and paracrine factors underlying in vivo suppression of plasma IL-12 levels by behavioral and physiological stressors; (ii) identify mechanisms, direct and indirect, through which the identified factors exert their impact on the final outcome of plasma IL-12 levels, and (iii) test pharmacological prophylactic measures with potential clinical applicability.
Methods
Animals and counterbalancing
Three month old male F344 rats (Harlan Laboratories, Jerusalem, Israel), were housed 3-4 per cage in our vivarium with ad-lib access to food and water on a 12:12 light:dark cycle at 22 ±1°C. Animals were handled daily during the last week prior to experimentation to reduce potential procedural stress. Body weight, and the order of drug administration and blood withdrawal were counterbalanced in each study. Housing conditions were monitored by the Institutional Animal Care and Use Committee of Tel-Aviv University, which also approved all studies described herein.
The “wet-cage exposure” stress paradigm
Animals were placed in cages filled with 2cm of room-temperature water, with ad-lib access to food and water, for 4–10 hrs (varying between experiments). Our previous studies indicated that during this stress paradigm CORT levels increased by approximately 6-fold in males, an effect that completely dissipated within 2 hrs of stress cessation (Shaashua et al., 2012).
Experimental laparotomy
The procedure has been described in detail elsewhere (Page et al., 1994). Briefly, rats were anesthetized with 2.5% isoflurane and a 4cm midline abdominal incision was performed. The small intestine was externalized, rubbed with a gauze pad soaked with phosphate-buffered-saline (PBS), and left hydrated for 40 minutes. Lastly, the intestine was internalized and the abdomen was sutured.
Adrenalectomy
Rats were anesthetized with 2.5% isoflurane, and a 4cm midline abdominal incision was performed. Both adrenal glands were removed, and the abdomen was sutured. To facilitate the animals’ recovery, CORT (3 mg/kg) was administered subcutaneously (SC) following the surgery. Subsequently, adrenalectomized (ADX) animals received saline (0.9% NaCl) supplemented with CORT (15 mg/1 lt) as a replacement for their drinking water. This paradigm was reported to result in low levels of CORT in the serum of adrenalectomized rats, which mimic the lower levels of the circadian rhythmus in normal rats (Zagron and Weinstock, 2006). To prevent potential exposure to CORT during the experiment, saline alone was given as a replacement from 4 hrs before the initiation of the experiment until its end. Control animals underwent a sham operation, in which a 4cm midline abdominal incision was performed and sutured, without the removal of the adrenal glands. ADX animals were used for experimentation 4 weeks following this procedure.
Blood withdrawal and plasma collection
Blood was withdrawn by cardiac puncture or from the tail end. To this end, animals were euthanized/anesthetized with isoflurane, and 1 or 1/2 ml of blood was withdrawn from the heart/tail end, respectively, within less than 3 min of approaching the animals, using EDTA-containing syringes (1.8 mg/1ml blood). To obtain blood samples from the tail end, animals were placed on a heating pad (which enhance blood circulation), and a 2mm cut was performed at the end of the tail. Blood was then centrifuged for 20 min at 930g, 4°C, for plasma separation, which was collected and stored at −20°C until assayed for IL-12 and/or CORT levels.
Assessment of IL-12 p70+p40 levels using ELISA
As IL-12 has a long biological t1/2 time (2–5 hrs in rats) (Stern et al., 1996), the blood withdrawal for IL-12 assessment was conducted at least 4 hrs following stress initiation, in all experiments. The anti rat IL-12 p70+p40 ELISA kit (BioSource International, Camarillo, CA) was used to assess IL-12 levels from plasma and supernatants, based on the manufacturer’s instructions. The detection range of this kit is 15 to 1000 pg/ml. The intra assay coefficient of variability was less than 5%, as reported by the manufacturer.
Assessment of plasma corticosterone levels
Plasma CORT levels were measured by radioimmunoassay (RIA) (ImmuChem double antibody corticosterone 125I RIA kit, MP Biomedicals, Orangeburg, NY), per manufacturer’s instructions. The intra assay coefficient of variability was less than 5%, as reported by the manufacturer.
Drugs and their administration
Drug sources - all drugs and substances (i.e., propranolol, mannide monooleate, mineral oil, nadolol, doxazosin, naltrexone, mifepristone, PGE2, epinephrine, CpG-C, and corticosterone) were obtained from Sigma-Aldrich, Rehovot, Israel, except for etodolac, which was kindly donated by Taro, Israel.
Slow-release emulsion (SRE) – the emulsion is based on a mixture of PBS, mineral oil and mannide monooleate (a non-ionic surface active emulsifier), in a 4:3:1 ratio, respectively. Drugs were dissolved in the PBS or the oil fraction of the emulsion, the emulsifying agent (mannide monooleate) was then added, and a rigorous vortexing was conducted to create the emulsion. Rats were always injected SC with 0.5 ml of the emulsion. Unpublished data from our laboratory have shown that drugs delivered in this emulsion, were released slowly and their effects were evident for at least 12 hs. For instance, propranolol dissolved in the slow-release emulsion (SRE) was effective for at least 12 hrs in blocking the effects of metaproterenol on heart rate in mice, while the same dose of propranolol dissolved in PBS was effective for less than 6 hs.
Propranolol – a non-selective β-adrenergic blocker that crosses the blood brain barrier. The drug was dissolved in the slow-release emulsion (see above), and administered SC (1.5 mg/kg). See above for the duration of propranolol effect in a SRE.
Nadolol – a hydrophilic non-selective β-adrenergic blocker that does not crosses the BBB. The drug was dissolved in PBS, and administered SC (0.4 mg/kg). The t1/2 of nadolol in humans is 14 hrs (Schafer-Korting et al., 1984).
Doxazosin – an α1 adrenergic-receptor blocker. The drug was dissolved in Dimethyl sulfoxide (DMSO), and, given its short t1/2 time in rats (1.2 hs) (Kaye et al., 1986), was administered SC twice, immediately before stress initiation (3 mg/kg) and 2.5 hrs later (2 mg/kg).
Etodolac – a selective cyclooxygenase-2 (COX-2) inhibitor (Jones, 1999). The drug was dissolved in corn oil and administered SC (12.5 mg/kg). The t1/2 of etodolac in rats is 18 hrs (Shi et al., 2004).
Mifepristone (RU-38486) – a glucocorticoid (and progesterone) competitive receptor antagonist. A fine powder of the drug was dissolved in corn oil, and administered SC (25 mg/kg). The t1/2 of mifepristone in rat serum is relatively short (1–2 hrs following oral administration), compared to 30 hrs in humans. However, in rats, mifepristone is collected in adipose tissues in high concentrations and therefore may redistribute continuously from the adipose tissues, resulting in long-lasting low serum levels (Heikinheimo et al., 1994). In our laboratory, we found that 25 mg/kg of mifepristone was effective in ameliorating 75% of the impact of 3 mg/kg corticosterone on lung-tumor-retention (LTR) at 3 and 8 hrs following its administration, and reducing 30% of such effects at 18 hrs (Ben-Eliyahu, 2010).
Naltrexone – a non-selective opioid receptor antagonist. The drug was dissolved in PBS, and administered SC (10 mg/kg). The t1/2 of naltrexone in rats is 4.6 hrs (Yoburn et al., 1986).
Epinephrine – in Exp. 3a & 3b the drug was dissolved in the slow-release emulsion (see above) and administered SC (1 mg/kg). For the in vitro study (Exp. 9) epinephrine was dissolved in complete media, and the concentrations used were chosen to simulate physiological levels. As the level in control animals was reported to be equal to 5.5×10−10M (DeTurck and Vogel, 1980), and as the concentration in stressed animals was reported to be at least 10-fold higher (Gray and Young, 1956), the concentrations in our in vitro study ranged from the typically employed high concentrations (5.5×10−6M) to the above physiological concentrations (5.5×10−10M).
Corticosterone – the active glucocorticoid in rats. A fine powder of the drug was dissolved in corn oil, and administered SC (3 or 9 mg/kg) or employed in vitro in Exp. 9. The concentrations chosen for the in vitro study intended to simulate physiological levels, which were reported in control animals to be approximately 3×10−7M (Haim et al., 2003) and in stressed animals up to 3×10−6M (Neeman et al., 2012a). Thus, the concentrations in our in vitro study ranged from these physiological concentrations to lower concentrations (3×10−8M/3×10−9M), which aim to reflect the lower level of active CORT molecules that are not bound to corticosteroid-binding globulins (CBG).
Prostaglandin E2 (PGE2) - the drug was dissolved in ethanol and diluted 1:10 in PBS. The concentrations used in the in vitro study were chosen to reproduce physiological levels in control and in inflammatory conditions. As the level in naïve animals was reported to be equal to 2×10−10M (Yakar et al., 2003), and as the concentration in inflammatory sites was reported to be 2×10−8M (Goodwin and Webb, 1980), the concentrations in our in vitro study ranged from the typically employed high concentrations (2×10−6M) to these physiological-relevant concentrations (2×10−10M).
CpG-C oligodeoxynucleotides (ODNs) – CpG-C is an oligodeoxynucleotide (ODN) that stimulate intracellular toll-like-receptor-9 (TLR-9), leading to the activation of B, NK, and antigen-presenting cells. CpG-C induces the release of various Th1 cytokines, potently elevates IL-12 and IFN-α levels, and becoming a widespread immuno-adjuvant in patients given its relatively mild adverse effects (Weiner, 2000). CpG-C ODN (ODN 2395:5′-TCGTCGTTTTCGGCGCGCG CCG-3′) with a phosphorothioate backbone was used. CpG-C was dissolved in PBS and employed in a final concentration of 5 μg/ml in the in vitro experiment. CpG-C endotoxin levels (as measured by the limulus amebocyte lysate assay) were undetectable.
Complete Media - RPMI-1640 media supplemented with 10% heat-inactivated fetal calf serum, 50 μg/ml of gentamicin, 2 mM of L-glutamine, 0.1 mM of nonessential amino-acids, and 1 mM of sodium pyruvate.
In vitro CpG-C-induced production of IL-12
Half ml of whole blood was washed once with PBS (4-fold dilution, 10min at 456g, followed by supernatant removal to restore the original volume), and twice with complete media (see above) to discard endogenous IL-12. A 500 μl washed blood aliquot was then added to a well containing 500 μl of complete media with CpG-C, reaching a final concentration of 5 μg CpG-C/ml. Samples were incubated at 100% humidity, 5% CO2, and 37°C for 24 hrs. The supernatants were then harvested and stored at −20°C until assayed for IL-12 levels.
In vitro spontaneous release of IL-12
To assess the spontaneous release of IL-12 without a specific activation, a similar procedure to the induced production approach described above was conducted, without adding CpG-C.
Statistical Analyses
A one- two- or three-way factorial analysis of variance (ANOVA), with a predetermined significance level of 0.05 was conducted. Provided significant group differences were found, Fisher’s Protected Least Significant Differences (Fisher’s PLSD) contrasts were performed to compare pair-wise comparisons, based on a priori hypotheses, and Tukey’s Honestly Significant Differences (Tukey’s HSD) were performed to compare unplanned pair-wise comparisons.
Results
Experiment 1: Adrenal hormones mediate the effects of wet-cage exposure
Design and procedure
40 male rats underwent either adrenalectomy or sham operation and maintained thereafter as described in the Methods section. Following the recovery period, the two groups were further subdivided to undergo 4 hrs of wet-cage exposure (a behavioral stress paradigm) or to serve as home cage controls. Blood was withdrawn immediately after stress cessation for IL-12 and CORT level assessments.
Results
IL-12 levels
A 2×2 (adrenalectomy by stress exposure) ANOVA revealed a significant increase in IL-12 levels in the ADX animals compared to sham-operated animals [F(1,36)=40.043, p<0.05]. In addition, a significant interaction was found between adrenalectomy and stress exposure [F(1,36)=4.747, p<0.05]. Specifically, in the sham-operated animals, wet-cage exposure reduced IL-12 levels (Fisher’s post hoc PLSD, p<0.05), while having no impact in the ADX animals (Fig. 1). n=9–12 per group.
Figure 1. Adrenal hormones mediate the effects of wet-cage exposure.
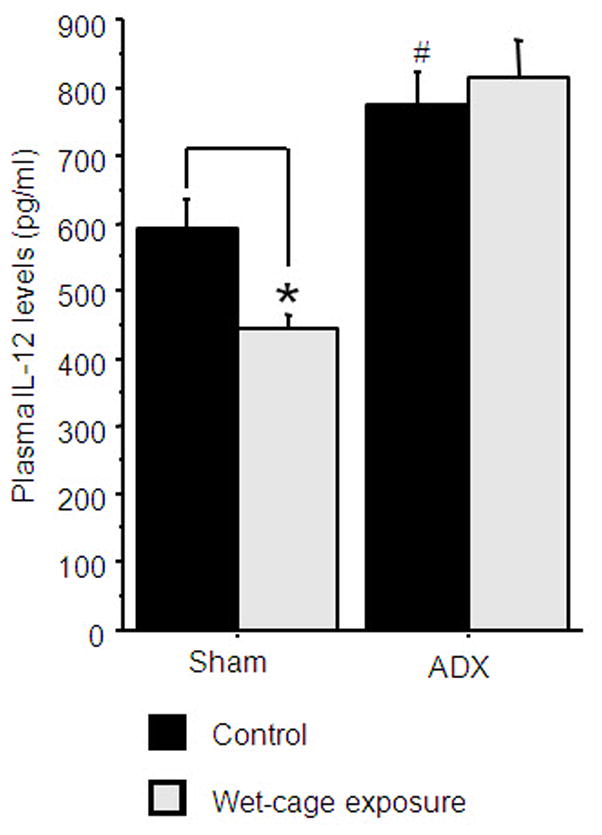
Four hrs of wet-cage exposure reduced IL-12 levels in sham operated animals but not in adrenalectomized (ADX) animals, immediately at the end of the stress paradigm. * indicates a significant reduction compared to the matched control group. # indicates a significant elevation compared to the matched sham group. Data presented as mean + SEM.
CORT levels
A 2×2 ANOVA revealed a significant interaction [F(1,36)=181.338, p<0.05]. Specifically, in the sham-operated animals, wet-cage exposure caused a 5-fold increase in CORT levels (increasing from 214 ±130 to 1060 ±137; mean ±SD), while causing no increase in ADX animals (73 ±84 to 10 ±12).
Experiments 2a & 2b: CORT, but not epinephrine, mediates the effects of wet-cage exposure on IL-12 levels
Design and procedure
An identical design was used in the following two experiments (2a and 2b). In each experiment, male rats (n(2a)=64, n(2b)=62) were administered with 25 mg/kg of mifepristone, 0.4 mg/kg nadolol, both drugs, or vehicle (corn oil), 1 hr prior to stress initiation. Each of these four groups was further subdivided to undergo wet-cage exposure or to serve as home-cage control. In Exp. 2a animals were exposed to 5 hrs of the wet-cage paradigm, and IL-12 levels were assessed 8 hrs following stress initiation. In Exp. 2b animals were exposed to 10 hrs of the wet-cage paradigm and IL-12 levels were assessed at 12 hrs following stress initiation. n=7–9 per group.
Results
In both experiments, a 4×2 (drug treatment by stress exposure) ANOVA showed decreased IL-12 levels following wet-cage exposure [Exp. (2a) F(1,56)=26.692, p<0.05] [Exp. (2b) F(1,54)=45.005, p<0.05] and no interaction. Fisher’s post hoc PLSD revealed an attenuated reduction in IL-12 levels by mifepristone, but not by nadolol (p<0.05) (Fig. 2).
Figure 2. CORT, but not epinephrine, mediates the effects of wet-cage exposure on IL-12.
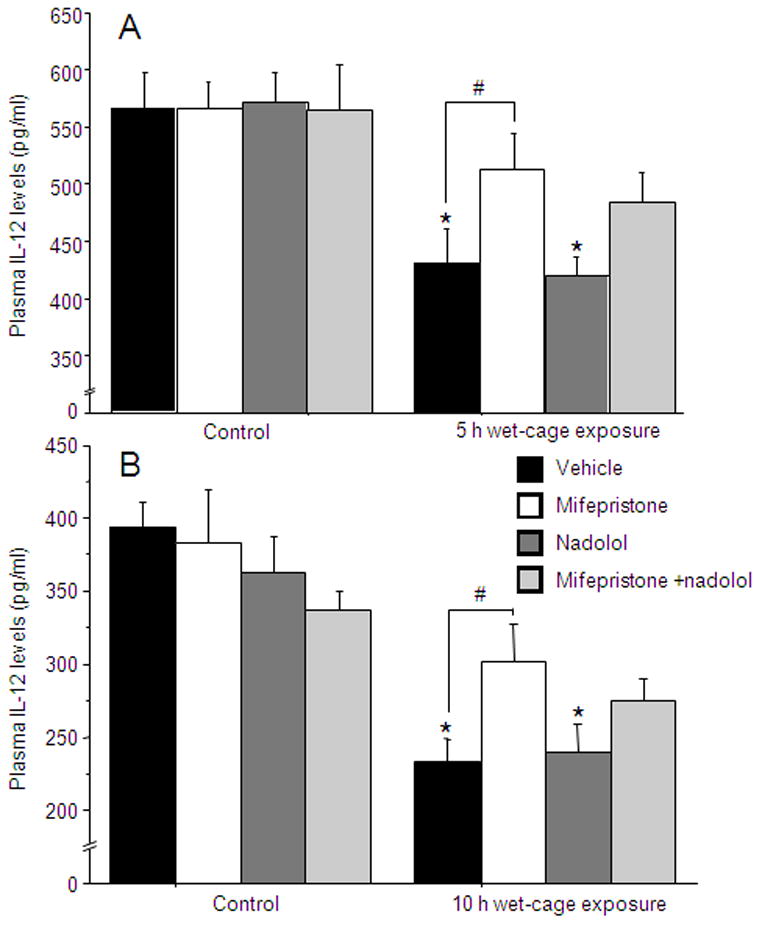
(A) Five hrs of wet-cage exposure reduced IL-12 levels at 8 hrs following stress initiation. This effect was attenuated by mifepristone but not by nadolol. (B) Ten hrs of wet-cage exposure reduced IL-12 levels at 12h following stress initiation. This effect was attenuated by mifepristone but not by nadolol. * indicates a significant reduction compared to the matched control group. # indicates a significant difference from the matched vehicle group. Data presented as mean + SEM.
Experiment 3a & 3b: The reduction of IL-12 levels by pharmacological epinephrine administration is mediated through the release of adrenal CORT
Two studies were conducted to address the effects of epinephrine administration, the first employing specific antagonists to stress hormones, and the second comparing sham-operated to ADX animals.
3a – Design and procedure
59 male rats were administered with 25 mg/kg mifepristone, 0.4 mg/kg nadolol, both drugs or vehicle (corn oil). One hour later, half of the animals in each group were injected with 1 mg/kg of epinephrine dissolved in the SRE, while the other half was injected with vehicle (SRE). 12 hrs following epinephrine injection, blood was withdrawn to assess plasma IL-12 levels. n=6–9 per group.
Results
A 4×2 (drug treatment by epinephrine administration) ANOVA revealed a reduction in IL-12 levels following epinephrine injection [F(1,51)=38. 234, p<0.001], and a main effect for drug treatment [F(3,51)=6.158, p<0.01]. Fisher’s post hoc PLSD showed that mifepristone and nadolol, when administered separately or simultaneously significantly attenuated the epinephrine-induced reduction of IL-12 levels (p<0.05) (Fig 3). As the drugs used also seem to impact baseline levels of IL-12 (although not significantly), the following experiment was conducted employing ADX animals that cannot mount adrenal stress hormones under any experimental condition.
Figure 3. The reduction of IL-12 levels by epinephrine administration is partially mediated through the release of CORT.
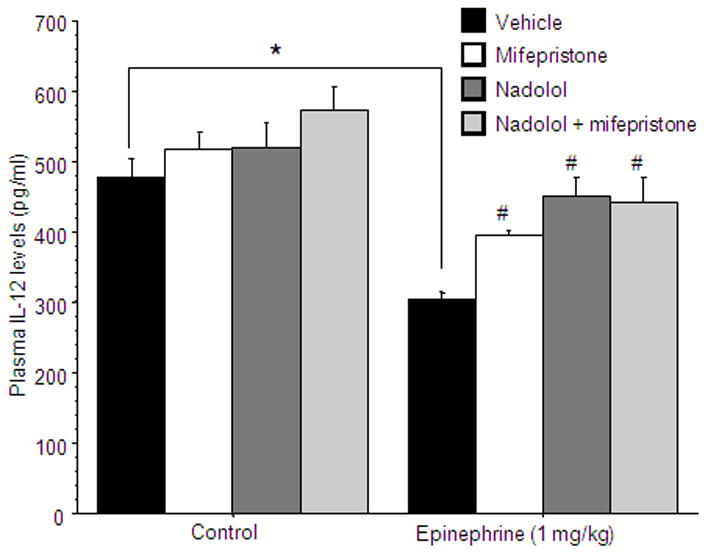
IL-12 levels were reduced by epinephrine administration (indicated by *). Mifepristone significantly attenuated the epinephrine-induced reduction of IL-12 levels, and nadolol was even more effective. The joint administration did not alter IL-12 levels compared to each drug alone. (# indicates a significant elevation compared to vehicle). Data presented as mean + SEM.
3b – Design and procedure
60 male rats underwent either adrenalectomy or sham operation. Three weeks later, ADX and sham-operated animals were subdivided to receive one of four injections: 3 mg/kg CORT, 1 mg/kg epinephrine (in SRE), CORT & epinephrine, or vehicle (SRE). Blood was withdrawn 12 hrs following the administration of these stress factors for IL-12 level assessment. n=6–8 per group.
Results
A 2×4 (adrenalectomy by stress factor administration) ANOVA revealed a significant interaction [F(3,52)=6.653, p<0.05]. Specifically, in the sham-operated animals, IL-12 levels were reduced when injected with CORT, epinephrine, or both drugs (Fisher’s post hoc PLSD, p<0.05). However, in the ADX animals, only CORT, when given alone or combined with epinephrine, reduced IL-12 levels, while epinephrine alone did not have any effect on IL-12 levels (Fig 4).
Figure 4. Adrenalectomy prevents the effect of epinephrine administration on IL-12 levels.
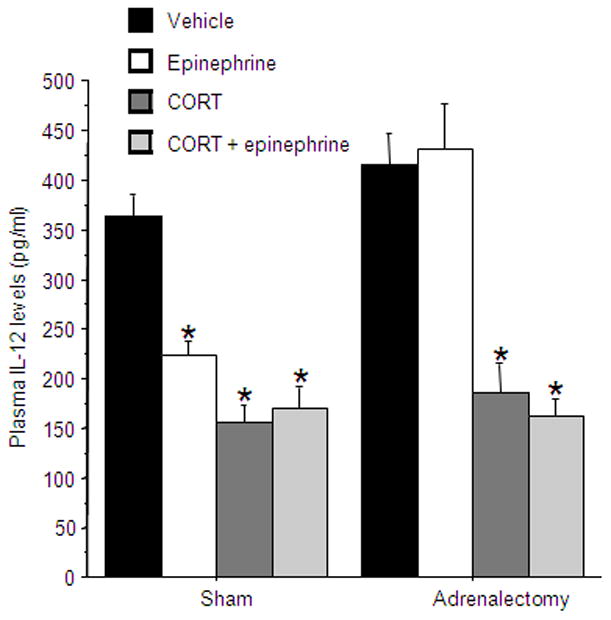
CORT, when given alone or combined with epinephrine, reduced IL-12 levels in both sham-operated and ADX rats, while epinephrine reduced IL-12 levels only in the sham-operated group. * indicates a significant difference compared to the matched vehicle group. Data presented as mean + SEM.
Experiments 4a–4d: Catecholamines and opioids do not mediate the effects of stress and surgery on IL-12 levels
In Exp. 2, nadolol was shown to be ineffective in blocking the IL-12 reduction by wet-cage exposure. In the following experiments, different adrenergic and opioid antagonists were used in conjunction with the wet-cage paradigm, and nadolol was also employed in the context of surgery (laparotomy).
Design and procedure
An identical 2×2 design was used in all four experiments, in which animals (n(4a)=19, n(4b)=34, n(4c)=27, n(4d)=28) were administered with an antagonist or vehicle 1 hour prior to stress initiation (6 hrs of wet-cage exposure in Exp. 4a–c and surgery in Exp. 4d). Each of these groups was subdivided to undergo the relevant stress paradigm or served as home-cage control. IL-12 levels were assessed 12 hrs following stress initiation. The antagonists used were the β-adrenergic antagonist propranolol (1.5 mg/kg; Exp. 4a), the α1-adrenergic antagonist doxazosin (5 mg/kg; Exp. 4b), the opioid antagonist naltrexone (10 mg/kg; Exp. 4c), and the β-adrenergic antagonist nadolol (0.4 mg/kg; Exp. 4d). n=4–9 per group.
Results
In all four experiments a 2×2 (drug treatment by stress exposure) ANOVA was used and revealed a significant reduction in IL-12 levels following the stress paradigms [Exp. (a) F(1,15)=10.27, p<0.05] [Exp. (b) F(1,30)=35.487, p<0.05] [Exp. (c) F(1,23)=322.787, p<0.05] [Exp. (d) F(1,24)=11.744, p<0.05]. However, none of the antagonists attenuated this effect, as no significant interaction was evident. Additionally, excluding doxazosin, no main effects of drug was evident. Doxazosin caused a significant reduction in IL-12 levels, independently of the effects of stress (data are presented in Table I).
Table I.
Various antagonists that did not affect IL-12 levels following stress
| Exp. # | Stress type | Control groups | Stress groups | ||
|---|---|---|---|---|---|
| 4a | Wet-cage | vehicle 414 ± 28 |
propranolol 368 ± 21 |
vehicle* 307 ± 20 |
propranolol 290 ± 32 |
| 4b | Wet-cage | vehicle 355 ± 24 |
doxazosin 313 ± 18 |
vehicle* 257 ± 13 |
doxazosin 194 ± 17 |
| 4c | Wet-cage | vehicle 441 ± 17 |
naltrexone 409 ± 13 |
vehicle* 199 ± 12 |
naltrexone 205 ± 9 |
| 4d | Surgery | vehicle 487 ± 28 |
nadolol 474 ± 29 |
vehicle* 350 ± 52 |
nadolol 335 ± 47 |
Plasma IL-12 levels were assessed at 12 hrs following stress initiation and are presented as mean ± SEM.
indicates a significant reduction by exposure to stress paradigm
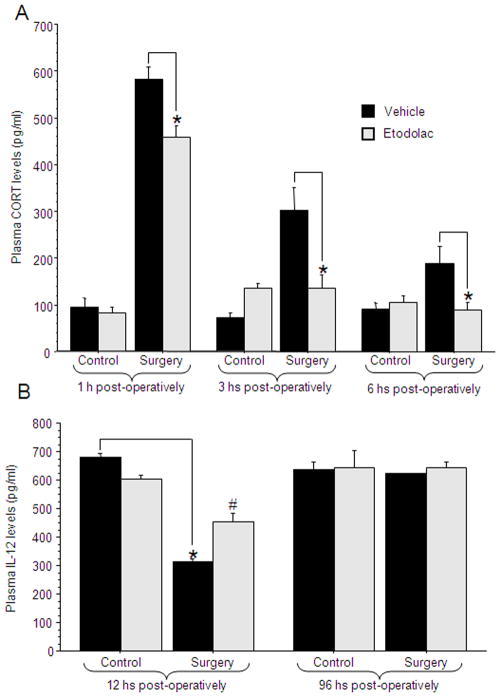
Experiment 5: CORT and PGs mediate the effects of surgery on IL-12 levels
Design and procedure
50 male rats were administered with 25 mg/kg of mifepristone, 12.5 mg/kg of etodolac, both drugs, or vehicle (corn oil), and 3 hrs later were further subdivided to undergo experimental laparotomy or to serve as home-cage controls. Blood for IL-12 level assessments was withdrawn from the tail end of each rat at 12 hrs post-operatively, and by cardiac puncture at 24 hrs postoperatively. n=5–7 per group.
Results
A 4×2 (drug treatment by surgery) ANOVA revealed a significant interaction between the effects of surgery and drug treatment at both 12 hrs [F(3,42)=11.342, p<0.05] and 24 hrs [F(3,42)=2.884, p<0.05] postoperatively. Specifically, PLSD indicated that both etodolac and mifepristone significantly attenuated the surgery-induced reduction in IL-12 levels. The combined administration was more effective than each drug alone at 12 hrs, and completely abolished the effect of surgery at 24 hrs (p<0.05) (Fig. 5).
Figure 5. CORT and PGs mediate the effects of surgery on IL-12 levels.
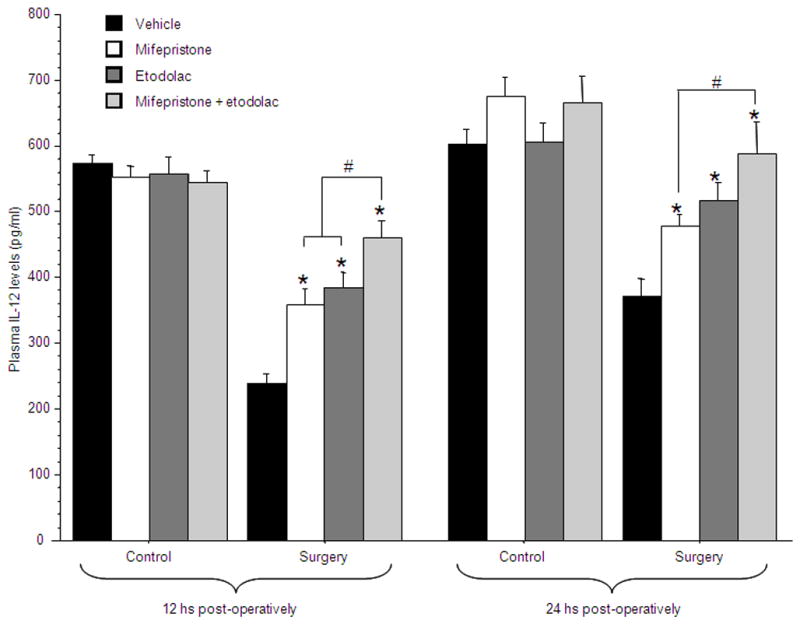
Surgery significantly reduced IL-12 levels at 12 and 24 hrs post-operatively. Either mifepristone or etodolac were effective in attenuating this reduction (indicated by *), and their combined administration was significantly more effective than each drug alone (indicated by #), restoring IL-12 to baseline levels. Data presented as mean + SEM.
Experiment 6: IL-12 reduction by endogenous PGs release is partially mediated through increased CORT levels
The main focus of this experiment was to assess whether PG synthesis inhibition (with etodolac) can reduce surgery-induced elevated CORT levels. The time points chosen for CORT assessment are those that enable the detection of surgery-induced elevated CORT levels. To verify the previously evident effect of surgery on IL-12 levels, blood was randomly withdrawn from selected animals also at 12 or 96 hrs post-operatively.
Design and procedure
72 male rats were administered with either 12.5 mg/kg of etodolac or vehicle (corn oil). One hour later, animals were further subdivided to undergo experimental laparotomy or to serve as home-cage controls. The surgery was performed at 1, 3 or 6 hrs before blood was collected for CORT level assessment from the tail end. To overcome potential impacts of circadian rhythm on CORT levels, blood was collected from all animals at the same time point of the day (approximately 4 hrs after light onset). For IL-12 level assessments, blood was withdrawn at 12 or 96 hrs post-operatively by cardiac puncture.
Results
CORT levels
A 2×2×3 (surgery by drug treatment by time point) ANOVA revealed a significant interaction between surgery and drug treatment [F(1,60)=15.627, p<0.05]. Specifically, at all time points surgery elevated CORT levels (PLSD p<0.05 in all three comparisons) and etodolac decreased this effect (PLSD p<0.05 in all three comparisons) without affecting non-operated animals. It is noteworthy that etodolac completely abolished the effects of surgery on CORT levels at the 3 and 6 hrs time points, but not at the 1 hour time point (Fig. 6a). n=4–10 per group.
Figure 6. IL-12 reduction by PGs is partially mediated by increased CORT levels.
(A) Etodolac attenuated the elevation of CORT levels following surgery (indicated by *) at all time points measured (1/3/6 hrs post-operatively). This study was conducted once; n=4–10 per group. (B) At 12 hrs post-operatively surgery reduced IL-12 levels (indicated by *) and etodolac attenuated this effect (indicated by #). At 96 hrs post-operatively no differences were found in IL-12 levels. Data presented as mean + SEM.
IL-12 levels
A 2×2×2 (surgery by drug treatment by time point) ANOVA revealed a significant two-way interaction between surgery and drug treatment [F(1,25)=6.61, p<0.05]. 12 hrs post-operatively, surgery reduced IL-12 levels, and etodolac attenuated its effect. At 96 hrs post-operatively no effects of surgery on IL-12 were evident (Fig. 6b). n=2–7 per group.
Experiment 7: surgery may affect IL-12 levels also through a CORT-independent pathway
Design and procedure
48 male rats underwent either adrenalectomy or sham operation and maintained thereafter as detailed in the Methods section. Following the recovery period, animals were further subdivided to either undergo experimental laparotomy or to serve as home-cage controls. 12 hrs following the initiation of surgery, blood was withdrawn for IL-12 level assessment. n=11–13 per group.
Results
A 2×2 (adrenalectomy by surgical procedure) ANOVA revealed a significant main effect for surgery (laparotomy) [F(1,44)=19.06, p<0.05], and a marginally significant interaction [F(1,44)=3.881, p=0.055]. Specifically, laparotomy significantly reduced IL-12 levels in sham-operated group (Fisher’s post hoc PLSD, p<0.05), apparently less so in the ADX group [F(1,44)=3.881, p=0.055], but some effects of laparotomy were still evident in ADX animals (Fisher’s post hoc PLSD, p=0.09) (Fig 7).
Figure 7. surgery may also affect IL-12 levels through a CORT-independent pathway.
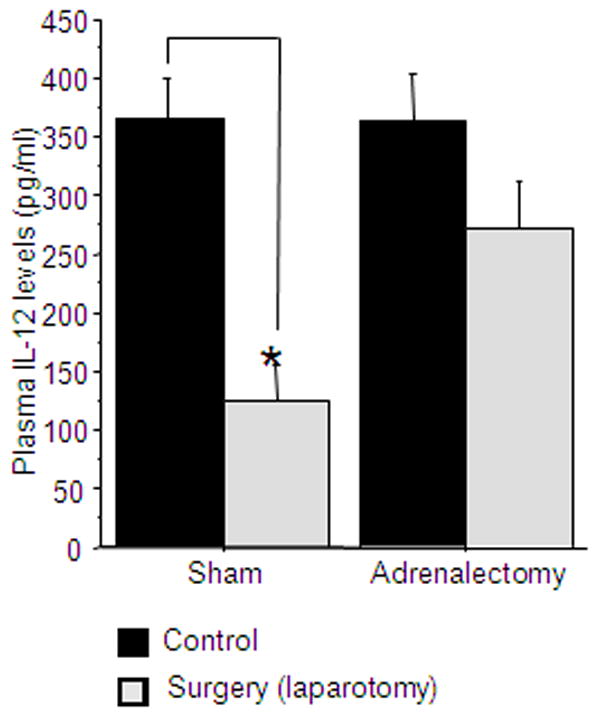
Surgery significantly reduced IL-12 levels in both sham operated and ADX animals. * indicates a significant reduction compared to the matched control group. Data presented as mean + SEM.
Experiment 8: The effects of CORT are not mediated or modulated by PGs or CAs
Design and procedure
39 male rats were administered with 12.5 mg/kg etodolac and 1.5 mg/kg propranolol (in SRE), or with vehicle (SRE). One hour later, each group was subdivided and administrated with either 9 mg/kg CORT or vehicle. n=9–10 per group.
Results
A 2×2 (drug treatment by CORT administration) ANOVA showed significantly reduced plasma IL-12 levels following CORT administration [F(1,35)=14.151, p<0.05], and no effect of drug treatment (Fig. 8). This suggests that CORT does not act through increasing PGs and/or CAs levels when reducing IL-12 levels.
Figure 8. The effects of CORT are not mediated by PGs or CAs.
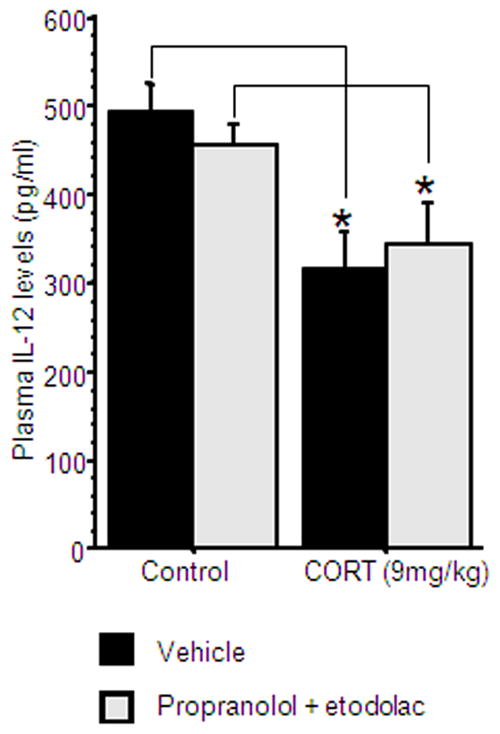
Injection of CORT significantly reduced IL-12 levels, and was not attenuated by combined administration of propranolol and etodolac. * indicates a significant reduction compared to the matched control group. Data presented as mean + SEM.
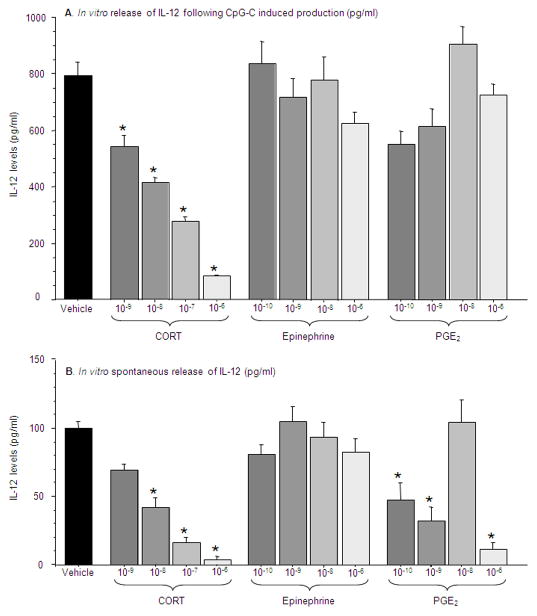
Experiment 9: In vitro assessment of IL-12 levels following exposure to CORT, epinephrine, and PGE2
In order to suggest a direct effect of specific hormones on leukocyte production of IL-12, the following in vitro experiment was conducted employing whole blood assay. As most in vitro studies reported in the literature have used BRMs to induce the release of IL-12, and as our in vivo studies were conducted in animals not subjected to BRMs, we herein used the two conditions in vitro. n=4 per condition for each replica (conducted twice).
Design and procedure
blood was withdrawn from 11 rats, pooled, and washed (see Methods). Aliquots of 500 μl washed blood were mixed with 500 μl of complete media, and incubated with/without CpG-C, and with one stress factor/vehicle in a specific concentration. The stress factors used were CORT, epinephrine, and PGE2. For each factor, four different concentrations were used (see Methods). The study was conducted in quadruplicates and was repeated twice. Plates were incubated for 24 hs, and supernatants were collected for IL-12 level assessment.
Results
IL-12 levels following CpG-C induced production
A one-way ANOVA showed significant differences in supernatant IL-12 levels following incubation with different stress factors at different concentrations [F(12,44)=15.01, p<0.0001]. Specifically, as indicated by Tukey’s HSD, the only stress factor that reduced IL-12 production by leukocytes activated by CpG-C was CORT, at all concentrations tested (3×10−6M – 3×10−9M), while neither PGE2 nor epinephrine had such an effect (Fig. 9a).
Figure 9. In vitro assessment of IL-12 levels following exposure to CORT, epinephrine, and PGE2.
(A) In the CpG-C induced production condition only CORT at all concentrations measured reduced IL-12 production (B) In the spontaneous release condition, CORT and PGE2 reduced IL-12 levels at most concentrations measured. * indicates a significant reduction compared to vehicle. Data presented as mean + SEM.
Spontaneous release of IL-12
A one-way ANOVA showed significant differences of plasma IL-12 levels following incubation with different stress factors at different concentrations [F(12,47)=20.25, p<0.0001]. Specifically, as indicated by Tukey’s HSD, in leukocytes not stimulated by CpG-C, both PGE2 (at the dose of 2×10−6M, 2×10−9M and 2×10−10M) and CORT (at all concentrations tested - 3×10−6M – 3×10−9M) reduced IL-12 levels, while epinephrine had no effect (Fig. 9b).
Discussion
Ample in vitro and few pharmacological in vivo studies reported suppression of IL-12 production by numerous stress hormones, including PGs, CAs, glucocorticoids, and opioids (van der Pouw Kraan et al., 1995; Blotta et al., 1997; Panina-Bordignon et al., 1997; Sacerdote, 2003). However, most of these studies assessed IL-12 levels following exposure to BRMs in vitro or in vivo, rather than in naïve animals. A recent study of our group was the first to show stress- and surgery-induced suppression of plasma IL-12 levels in otherwise naïve rats, suggesting the involvement of endogenously released stress hormones in this suppression (Shaashua et al., 2012). The current study aimed at elucidating specific underlying neuroendocrine mechanisms of stress- and surgery-induced suppression of plasma IL-12 levels. As elaborated below, and in contrast to most in vitro and pharmacological in vivo studies (Panina-Bordignon et al., 1997; Hasko et al., 1998; Sacerdote, 2003), our results indicated that CAs and opioids do not mediate the effects of behavioral stress or surgery. On the other hand, CORT and PGs were shown to be crucial mediators of these in vivo effects. Thus, this study also reinforces the notion that results from in vitro or pharmacological in vivo studies cannot reliably predict the effects of endogenously released stress hormones under stressful conditions, as was previously shown (Greenfeld et al., 2007; Meron et al., 2013).
In vivo, endogenous hormonal stress responses are mutually interactive, and the impact of a particular stress hormone may actually be mediated through regulating other stress responses. Therefore, in the current study we also attempted to elucidate both the independent effects of each hormone, as well as its interdependent effects through interactions with other hormones. Specifically, interactions between CORT and epinephrine are known to occur at several physiological levels (Ehrhart-Bornstein et al., 1998). For example, the assumed classical adrenal morphological segregation has been disproved by findings that located epinephrine-secreting cells (chromaffin cells) scattered in the adrenal cortex, and glucocorticoid-releasing cells in the adrenal medulla. This proximity enables proven paracrine relations between these cells, such as epinephrine-induced CORT release (Bornstein and Ehrhart-Bornstein, 1992; Bornstein et al., 1994). In addition, an IL-1α-induced secretion of CORT, which is a surgery-related process, was shown to be catecholamine-dependent at the adrenal gland level (Gwosdow et al., 1992; O’Connell et al., 1994). Concurrent with such interactions, in our previous study, a systemic pharmacological administration of epinephrine markedly elevated endogenous CORT levels (Shaashua et al., 2012). An additional interaction was suggested between epinephrine and PGs by in vitro studies reporting epinephrine-induced release of PGs from epithelial cells (Liedtke, 1986). Last, interactions between PGs and CORT were also reported. For instance, in-vitro, adrenocorticotropic-hormone-induced adrenal CORT release was shown to be mediated by PGE2 (Mohn et al., 2005), and in the current study, a PG synthesis inhibitor attenuated surgery-induced CORT secretion. Therefore, in the current study we employed several in vivo approaches to elucidate the exclusive and interdependent effects of endogenous PGs, CORT, epinephrine and opioids on plasma IL-12 levels, using ADX/sham-operated rats and administration of specific antagonists targeting these factors.
Our findings clearly demonstrate that endogenous CORT release plays a crucial and independent role in mediating the effects of stress and surgery. Both adrenalectomy and the glucocorticoid-receptor blocker, mifepristone, significantly attenuated the IL-12 suppressing effects of stress and surgery in all experimental conditions tested. Additionally, in the context of CORT administration [3 mg/kg, which induces high physiological levels (Haim et al., 2003)], neither the simultaneous blockade of CAs and PGs, nor adrenalectomy, attenuated its effect. These findings suggest that the in vivo effects of CORT are not mediated through PGs, CAs or other adrenal factors (e.g., opioids). Evidence suggesting a direct effect of CORT on leukocyte secretion of IL-12 are the current findings that in vitro exposure to CORT in concentrations of 3×10−6M to 3×10−9M, equal to or profoundly lower than its physiological systemic total levels (3×10−6M to 3×10−7M), markedly reduced IL-12 levels in leukocytes that spontaneously released IL-12 or that were activated by CpG-C. It has been shown that CORT suppress IL-12 transcription through a Nuclear Factor-KappaB (NFκB) pathway. Upon binding of the glucocorticoid and its cytosolic receptor complex to the NFκB transcription factor, the translocation of NFκB to the nucleus is inhibited and the IL-12p40 unit is not produced (Murphy et al., 1995; Barnes, 1996).
PGs mediated the IL-12 suppressive effect of surgery, but this effect was at least partly dependent on PGs-induced increase in CORT levels, while the adrenal-independent effects of surgery are inconclusively PGs-mediated. Specifically, the administration of the COX-2 inhibitor, etodolac, markedly attenuated the IL-12-suppressing effect of surgery in all experiments tested, indicating a mediating role for PGs. However, etodolac also markedly reduced the release of CORT following surgery, both in the current study and in previous studies (Glasner et al., 2010), implying that its beneficial effects are at least partially mediated through attenuating post-operative CORT levels. Nevertheless, converging lines of evidence suggest an additional effects of PGs, which are adrenal and CORT independent: (i) the combined blockade of CORT and PG synthesis in operated animals was significantly more effective than each of these interventions alone; (ii) a marginally significant reduction of IL-12 levels was caused by surgery in ADX animals, where PGs cannot induce an adrenal CORT release (or other adrenal hormones); and (iii) the in vitro study indicated that PGE2 (at concentrations as low as 2×10−10M which are physiologically relevant) can directly suppress IL-12 production in leukocytes not stimulated with BRMs (but not in CpG-stimulated leukocytes).
The current study also indicates that endogenous CAs release (specifically epinephrine and norepinephrine) has no role in mediating the effects of stress or surgery on IL-12 levels. Specifically, the α- and/or β-adrenergic blockers did not counteract the IL-12-reducing effects of behavioral stress and of surgery. Neither propranolol nor nadolol that differ in their ability to cross the BBB had any ameliorating effects. It is worthy to note that the stress paradigms used in this study profoundly activate the SNS and were shown to cause immune perturbations that were blocked by propranolol or nadolol (Ben-Eliyahu et al., 2000; Ben-Eliyahu, 2010). In addition, and contrary to other studies, we did not observe any in vitro effect of epinephrine on IL-12 production, using concentrations that ranged from 5.5×10−6M to 5.5×10−10M, employing a whole blood assay, with or without leukocyte stimulation. The inconsistencies with other studies may be related to the use of epinephrine in our study, compared to synthetic β-agonists in other studies, and to the use of different immune stimulators in other studies (LPS and IFN-γ) in contrast to the TLR-9 agonist, CpG-C, or to non-stimulated leukocytes used herein. Interestingly, following a pharmacological administration of epinephrine, a marked reduction in plasma IL-12 levels was observed. However, this reduction was completely mediated through elevating CORT levels as indicated by the following lines of evidence: (i) CORT levels markedly increased following a pharmacological epinephrine administration (Shaashua et al., 2012); (ii) mifepristone (a glucocorticoid receptor blocker) significantly attenuated the IL-12-reducing effects of epinephrine administration, and (iii) the effects of the pharmacological epinephrine administration on IL-12 levels were completely abolished by adrenalectomy. Thus, a disparity between the effects of an in vivo pharmacological approach and endogenously elevated CA levels is evident in this study.
Opioids also do not seem to mediate the IL-12-reducing effects of stress, as naltrexone administration did not attenuate these effects. This result is incongruent to findings from in vitro and ex-vivo studies showing a decrease in IL-12 levels and a promotion of Th2 differentiation by opioids (Sacerdote, 2003). It is also worthy to note that other in vitro studies showed opposite effects of opioids (Messmer et al., 2006).
One can suggest that the dosages of the antagonists used herein were not sufficient to achieve prophylactic effects. However, the same dosage of nadolol was highly effective in Exp. 3 of this study, following the pharmacological administration of epinephrine, and both nadolol and propranolol were effective in the same dosages in many of our previous in vivo studies (Benish et al., 2008; Glasner et al., 2010). The 10 mg/kg dose of naltrexone used herein is commonly used in our laboratory, and was shown to be effective in other in vivo studies (Shavit et al., 1987).
The biological and clinical consequences of suppressed IL-12 levels may be numerous, especially given that IL-12 is a prominent activator of NK cells and other aspects of cell-mediated immunity. For example, in the clinical setting of excising a primary tumor, stress factors and PGs were implicated in promoting cancer metastasis through suppressing anti-metastatic immunity (including NK cytotoxicity) and through other mechanisms (Sloan et al., 2010; Neeman and Ben-Eliyahu, 2013). Potentially mediated through surgery-induced suppression of IL-12 levels, elevated post-operative PG levels were associated with reduced NK cytotoxicity in patients (Baxevanis et al., 1993). Additionally, a decreased NK-dependent resistance to experimental metastasis was caused by PGE2 administration in rats (Yakar et al., 2003). CORT was also implicated in promoting deleterious effects of stress and surgery on metastatic progression (Shakhar and Ben-Eliyahu, 2003), and the current findings suggest a mediating mechanism through reduced IL-12 levels and suppressed cell-mediated immunity.
Overall, our findings indicate that in the context of natural or clinical stressors, a profound suppression of IL-12 is induced through elevated CORT and PGs levels, but not through other neuroendocrine or paracrine responses. In the clinical setting of surgery, the complete blockade of CORT has clear contraindications, and thus partial blockade through various interventions, including the PGs system may be more feasible (Seltzer et al., 1988), as we have recently reviewed (Neeman et al., 2012b) and are currently employing in cancer patients.
Acknowledgments
Role of the funding source
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This work was supported by NIH/NCI grant # CA125456 (SBE), and by the Israel-USA binational Science Foundation # 2005331 (SBE & GGP).
Footnotes
Contributors
Conceived and designed the experiments: Lee Shaashua, Shamgar Ben-Eliyahu.
Performed the experiments: Lee Shaashua, Ella Rosenne, Elad Neeman, Liat Sorski, Luba Sominsky, Pini Matzner.
Contributed reagents/materials: Gayle G. Page.
Analyzed the data: Lee Shaashua & Shamgar Ben-Eliyahu.
Wrote the paper: Lee Shaashua & Shamgar Ben-Eliyahu.
All authors have read and approved the final manuscript.
Conflict of Interest Statement: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes PJ. Mechanisms of action of glucocorticoids in asthma. Am J Respir Crit Care Med. 1996;154:S21–26. doi: 10.1164/ajrccm/154.2_Pt_2.S21. discussion S26–27. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Missitzis I, Papamichail M. Elevated prostaglandin E 2production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993;72:491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Rosenne E, Sorski L, Levi B. Second Thoughts on The Role of Glucocorticoids in The In Vino Suppression of NK Activity Following Stress. 17th Annual Meeting of The PsychoNeuroImmunology Research Society; Dublin, Ireland. 2010. p. S23. Abstract # 292. [DOI] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- Bornstein SR, Ehrhart-Bornstein M. Ultrastructural evidence for a paracrine regulation of the rat adrenal cortex mediated by the local release of catecholamines from chromaffin cells. Endocrinology. 1992;131:3126–3128. doi: 10.1210/endo.131.6.1446648. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Gonzalez-Hernandez JA, Ehrhart-Bornstein M, Adler G, Scherbaum WA. Intimate contact of chromaffin and cortical cells within the human adrenal gland forms the cellular basis for important intraadrenal interactions. J Clin Endocrinol Metab. 1994;78:225–232. doi: 10.1210/jcem.78.1.7507122. [DOI] [PubMed] [Google Scholar]
- DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- DeTurck KH, Vogel WH. Factors influencing plasma catecholamine levels in rats during immobilization. Pharmacol Biochem Behav. 1980;13:129–131. doi: 10.1016/0091-3057(80)90132-x. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP, Wilder RL. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann N Y Acad Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108:374–381. [PubMed] [Google Scholar]
- Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Webb DR. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980;15:106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Gray I, Young JG. Biochemical response to trauma. III. Epinephrine and norepinephrine levels in plasma of rats subjected to tumbling trauma. Am J Physiol. 1956;186:67–70. doi: 10.1152/ajplegacy.1956.186.1.67. [DOI] [PubMed] [Google Scholar]
- Greenfeld K, Avraham R, Benish M, Goldfarb Y, Rosenne E, Shapira Y, Rudich T, Ben-Eliyahu S. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21:503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Gwosdow AR, O’Connell NA, Spencer JA, Kumar MS, Agarwal RK, Bode HH, Abou-Samra AB. Interleukin-1-induced corticosterone release occurs by an adrenergic mechanism from rat adrenal gland. Am J Physiol. 1992;263:E461–466. doi: 10.1152/ajpendo.1992.263.3.E461. [DOI] [PubMed] [Google Scholar]
- Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26:1013–1022. doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Salzman AL, Vizi ES. Stimulation of beta-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J Neuroimmunol. 1998;88:57–61. doi: 10.1016/s0165-5728(98)00073-3. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Pesonen U, Huupponen R, Koulu M, Lahteenmaki P. Hepatic metabolism and distribution of mifepristone and its metabolites in rats. Hum Reprod. 1994;9(Suppl 1):40–46. doi: 10.1093/humrep/9.suppl_1.40. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Noguchi K, Endo H, Kondo H, Ishikawa I. Prostaglandin E 2downregulates interleukin-12 production through EP4 receptors in human monocytes stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans and interferon-gamma. Oral Microbiol Immunol. 2003;18:150–155. doi: 10.1034/j.1399-302x.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jones RA. Etodolac: an overview of a selective COX-2 inhibitor. Inflammopharmacology. 1999;7:269–275. doi: 10.1007/s10787-999-0010-3. [DOI] [PubMed] [Google Scholar]
- Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, Farber JM, Liao F, Liu L, Tosato G. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukoc Biol. 1998;64:384–392. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- Kaye B, Cussans NJ, Faulkner JK, Stopher DA, Reid JL. The metabolism and kinetics of doxazosin in man, mouse, rat and dog. Br J Clin Pharmacol. 1986;21(Suppl 1):19S–25S. doi: 10.1111/j.1365-2125.1986.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke CM. Interaction of epinephrine with isolated rabbit tracheal epithelial cells. Am J Physiol. 1986;251:C209–215. doi: 10.1152/ajpcell.1986.251.2.C209. [DOI] [PubMed] [Google Scholar]
- Meron G, Tishler Y, Shaashua L, Rosenne E, Levi B, Melamed R, Gotlieb N, Matzner P, Sorski L, Ben-Eliyahu S. PGE(2) suppresses NK activity in vivo directly and through adrenal hormones: Effects that cannot be reflected by ex vivo assessment of NK cytotoxicity. Brain Behav Immun. 2013;28:128–138. doi: 10.1016/j.bbi.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer D, Hatsukari I, Hitosugi N, Schmidt-Wolf IG, Singhal PC. Morphine reciprocally regulates IL-10 and IL-12 production by monocyte-derived human dendritic cells and enhances T cell activation. Mol Med. 2006;12:284–290. doi: 10.2119/2006-00043.Messmer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DW, McNutt RM, Collins JT, Buchanan JM, Van Cleave VH, Dunnick WA. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-gamma-dependent and -independent mechanisms. Eur J Immunol. 1997;27:1958–1965. doi: 10.1002/eji.1830270820. [DOI] [PubMed] [Google Scholar]
- Mohn CE, Fernandez-Solari J, De Laurentiis A, Prestifilippo JP, de la Cal C, Funk R, Bornstein SR, McCann SM, Rettori V. The rapid release of corticosterone from the adrenal induced by ACTH is mediated by nitric oxide acting by prostaglandin E2. Proc Natl Acad Sci U S A. 2005;102:6213–6218. doi: 10.1073/pnas.0502136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(Suppl):S32–40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Shaashua L, Benish M, Page GG, Zmora O, Ben-Eliyahu S. Stress and skin leukocyte trafficking as a dual-stage process. Brain Behav Immun. 2012a;26:267–276. doi: 10.1016/j.bbi.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012b;18:4895–4902. doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell NA, Kumar A, Chatzipanteli K, Mohan A, Agarwal RK, Head C, Bornstein SR, Abou-Samra AB, Gwosdow AR. Interleukin-1 regulates corticosterone secretion from the rat adrenal gland through a catecholamine-dependent and prostaglandin E2-independent mechanism. Endocrinology. 1994;135:4.467–60. doi: 10.1210/endo.135.1.8013385. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994;8:241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P. Effects of in vitro and in vivo opioids on the production of IL-12 and IL-10 by murine macrophages. Ann N Y Acad Sci. 2003;992:129–140. doi: 10.1111/j.1749-6632.2003.tb03144.x. [DOI] [PubMed] [Google Scholar]
- Schafer-Korting M, Bach N, Knauf H, Mutschler E. Pharmacokinetics of nadolol in healthy subjects. Eur J Clin Pharmacol. 1984;26:125–127. doi: 10.1007/BF00546720. [DOI] [PubMed] [Google Scholar]
- Seltzer JL, Goldberg ME, Larijani GE, Ritter DE, Starsnic MA, Stahl GL, Lefer AM. Prostacyclin mediation of vasodilation following mesenteric traction. Anesthesiology. 1988;68:514–518. doi: 10.1097/00000542-198804000-00007. [DOI] [PubMed] [Google Scholar]
- Shaashua L, Sominsky L, Levi B, Sorski L, Reznick M, Page GG, Ben-Eliyahu S. In vivo suppression of plasma IL-12 levels by acute and chronic stress paradigms: potential mediating mechanisms and sex differences. Brain Behav Immun. 2012;26:996–1005. doi: 10.1016/j.bbi.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Martin FC, Yirmiya R, Ben-Eliyahu S, Terman GW, Weiner H, Gale RP, Liebeskind JC. Effects of a single administration of morphine or footshock stress on natural killer cell cytotoxicity. Brain Behav Immun. 1987;1:318–328. doi: 10.1016/0889-1591(87)90034-1. [DOI] [PubMed] [Google Scholar]
- Shi JM, Lai SG, Xu CJ, Duan GL, Li D. Pharmacokinetic difference between S-(+)- and R-(−)-etodolac in rats. Acta Pharmacol Sin. 2004;999–996(8):25. [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AS, Magram J, Presky DH. Interleukin-12 an integral cytokine in the immune response. Life Sci. 1996;58:639–654. doi: 10.1016/s0024-3205(96)80003-8. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GJ. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J Leukoc Biol. 2000;68:455–463. [PubMed] [Google Scholar]
- Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10:469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Cohen AH, Inturrisi CE. Pharmacokinetics and pharmacodynamics of subcutaneous naltrexone pellets in the rat. J Pharmacol Exp Ther. 1986;237:126–130. [PubMed] [Google Scholar]
- Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]