Abstract
Objective:
We examined the complex relationship between depression, anxiety, and seizure control and quality of life (QOL) outcomes after epilepsy surgery.
Methods:
Seven epilepsy centers enrolled 373 patients and completed a comprehensive diagnostic workup and psychiatric and follow-up QOL evaluation. Subjects were evaluated before surgery and then at 3, 6, 12, 24, 48, and 60 months after surgery. Standardized assessments included the Quality of Life in Epilepsy Inventory–89, Beck Depression Inventory (BDI), and Beck Anxiety Inventory (BAI). A mixed-model repeated-measures analysis was used to analyze associations of depression, anxiety, seizure outcome, and seizure history with overall QOL score and QOL subscores (cognitive distress, physical health, mental health, epilepsy-targeted) prospectively.
Results:
The groups with excellent and good seizure control showed a significant positive effect on the overall QOL compared to the groups with fair and poor seizure control. The BDI and BAI scores were both highly and negatively associated with overall QOL; increases in BDI and BAI scores were associated with decreased overall QOL score.
Conclusions:
Depression and anxiety are strongly and independently associated with worse QOL after epilepsy surgery. Interestingly, even partial seizure control, controlling for depression and anxiety levels, improved QOL. Management of mood and anxiety is a critical component to postsurgical care.
Quality of life (QOL) in people with epilepsy is lowered by antiepileptic drug (AED) side effects, cognitive impairment, underlying neurologic disease, perceived stigma, comorbid psychiatric illness, and poor seizure control.1 While QOL improves with seizure freedom,2–6 reductions in seizure frequency have a small effect on QOL.7–9 In patients with epilepsy, mood and anxiety are often the strongest predictors of QOL.10–13 However, cross-sectional studies demonstrating that higher depression and anxiety scores strongly correlate with higher QOL cannot assess relationships among mood, anxiety, QOL, and seizure control over time.
In a prospective multicenter study of resective epilepsy surgery, we found clinically significant improvements in QOL in patients who were seizure-free 5 years after surgery6; however, such gains were not observed in individuals with incomplete seizure control. Subsequently, we found that when incomplete seizure control is stratified, there are differences in the course of depression between individuals with “good” seizure outcomes (incomplete control over 5 years but 2 or more years of seizure freedom) and “fair” outcomes (only 1 year of seizure freedom).14 Those with good seizure control showed less depression than those with fair seizure control 5 years after surgery.14,15 It remains unclear how these mood improvements affect overall QOL, particularly with incomplete seizure control. In the current study, we examined the association between depression, anxiety, and seizure outcomes and QOL over time. We hypothesized that better seizure control and improvement in depressive and anxiety symptoms would be independently associated with gains in all QOL domains.
METHODS
The design, measures, and subject recruitment of the Epilepsy Multicenter Study were detailed elsewhere.16 Seven tertiary epilepsy centers enrolled 396 patients and completed a comprehensive diagnostic workup that included an extensive medical history and physical examination; neuropsychological, neuroimaging, and neurophysiologic testing; and a psychiatric as well as QOL evaluation. Subjects were evaluated at baseline, before surgery, and then 3, 6, 12, 24, 48, and 60 months after surgery.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards for each respective center approved all procedures in the study.
Subjects.
Subjects were enrolled as part of a 7-center prospective observational study of resective epilepsy surgery outcomes, the details of which have been published previously.16 Subjects, older than 12 years, were recruited between June 1996 and January 2001, at the time of their initial referral, for possible resective epilepsy surgery. They were included if they had no prior epilepsy surgical treatment, had continuing complex partial seizures with or without secondarily generalized seizures despite treatment with 2 or more AEDs, and with more than 20 seizures in the 2 years before recruitment. Evaluation was standardized across the 7 centers.16 For this analysis, only adult subjects (17 years or older) at the time of the surgery (N = 379) were included.
Measures.
An extensive preoperative interview and medical record review provided demographic, historical, and seizure-related information on all patients. We included adult subjects who had seizure control and QOL outcome data after epilepsy surgery. Standardized postoperative assessments included a measure of QOL, the Quality of Life in Epilepsy Inventory–89 (QOLIE-89), Beck Depression Inventory (BDI), and Beck Anxiety Inventory (BAI). The QOLIE-89 is a self-report, comprehensive, epilepsy-targeted health-related QOL instrument that includes a generic core, the 36-Item Short Form Health Survey. Reliability, validity, and responsiveness to change have been reported for the QOLIE-89.17 The QOLIE-89 yields an overall score, 17 multi-item scales, and 4 dimension scores (cognitive distress, physical health, mental health, epilepsy-targeted) derived from a previously published factor analysis of the 17 multi-item scales and reported in an article describing the initial development of the QOLIE-89. Our analysis used the overall and 4 dimension scores, converting factor scores to T scores (mean, 50; SD, 10) for ease of interpretation. Greater T-score values indicate better QOL. The BDI and BAI are reliable and validated general measures of depression and anxiety18 that are scaled from 0 to 63 with higher scores reflecting more severe symptoms. The QOLIE-89, BAI, and BDI were administered at baseline, within 3 months of surgery, and at intervals approximately 1, 2, 3, 4, and 5 years after surgery.
Seizure control was operationalized using 2 different models. The first seizure control definition, “5-year control,” is a fixed covariate that captures relative seizure control between subjects across the 5 years of follow-up. For the 5-year control variable, seizure outcome was classified into 1 of 4 categories: “excellent” for subjects seizure-free (and no auras) for all 5 years, “good” for 2 consecutive years but not all 5, “fair” if subjects were seizure-free for 1 year but never 2 consecutive years, and “poor” if subjects never had a 1-year period of seizure freedom. The second seizure control definition, “seizure breakthrough,” is a time-varying indicator that captures when a subject is no longer seizure-free within the 5-year period. Starting at baseline, this variable was coded as 0 and would remain 0 throughout the follow-up or switch to 1 at an assessment (and all subsequent assessments) when a seizure was reported. For instance, if a subject had a seizure at 9 months (which they reported at the 12-month assessment), they would have a time-varying vector for the seizure breakthrough variable of 0 0 1 1 1 1 1 corresponding to months 0, 3, 12, 24, 36, 48, 60, respectively.
Statistical analysis.
Analyses were performed in SAS 9.3, and 3D surface plots were created in SAS (SAS Institute, Cary, NC) using SAS Macro Program: surface.sas.19 Mixed-model repeated-measures analyses (implemented with SAS PROC MIXED) were used to evaluate the relations of depression, anxiety, and either 5-year seizure control or seizure breakthrough on trajectories of change in several measures of QOL (overall QOL score and the 4 dimension subscores: cognitive distress, physical health, mental health, epilepsy-targeted) over time.20 Depression (BDI score) and anxiety (BAI score) were both included as time-varying covariates allowing their values to change upon annual reassessment. Models also included fixed effects for sex, race, number of years with epilepsy before surgery, education, laterality of seizure focus, resection location, number of AEDs taken at each follow-up period postsurgery, and time. Interactions of primary independent variables (i.e., seizure-control variables, depression, and anxiety) with time were included to determine whether they modified the slope of change in QOL outcomes. Random effects were included for intercepts and slopes to allow for the dependence of observations obtained from the same individuals. Regression estimates from these models reflect the difference in the QOL outcome for a 1-unit difference in the independent variable.
RESULTS
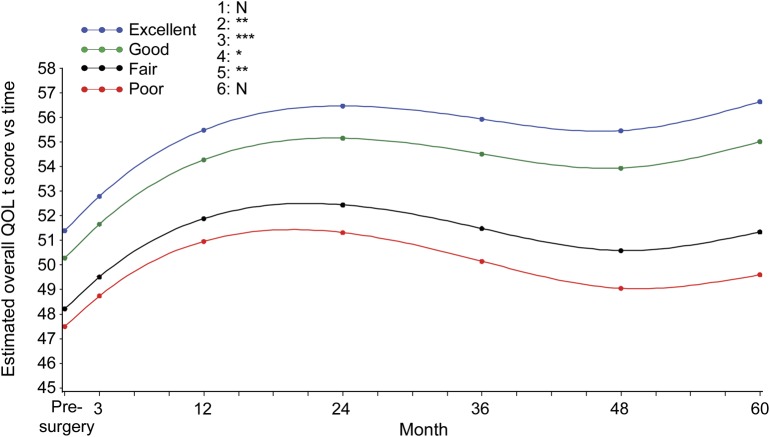
Detailed demographics, seizure history, and seizure location are shown in table 1. Of the 379 adults who were included in the analysis, 373 subjects completed a baseline QOL assessment, 359 subjects had baseline QOL assessment and at least one QOL assessment during follow-up, and 255 of those who completed a baseline QOL assessment had 5-year follow-up (table e-1 on the Neurology® Web site at Neurology.org). We examined the difference in QOL scores between subjects who withdrew from the study and those who completed each follow-up interval, and we found no significant difference (no p value was <0.13). Of the 373 subjects who completed at least one QOL assessment, 200 subjects were female, 60 were not white, 150 subjects had high school–level education or less, 325 subjects (87.1%) had temporal lobe resection, 182 subjects had left-sided resections, and 108 subjects were seizure-free 5 years after their surgery. Overall QOL and each of the QOL subscores improved over time (p < 0.001; see figures 1 and e-1 and tables 2 and e-2) as evidenced by the positive regression estimates for time. Both quadratic and cubic polynomials of time were significant, suggesting (as illustrated in figures 1 and e-1) that QOL changes over time plots on a complex curve as opposed to a straight line. At baseline, the groups with excellent and good seizure control had higher overall QOL compared with the groups with fair and poor seizure control (p < 0.0001). There was a significant interaction between seizure control and time (p = 0.02) whereby the rate of increase in overall QOL was greater in the “excellent” and “good” groups. For example, the group with “excellent” seizure control demonstrated a 0.05-unit per month greater increase in QOL score compared with the group with “poor” seizure control. Over a period of 5 years this translates into a greater increase of 3.0 units. This relation was apparent in the seizure (p < 0.001) and physical (p = 0.008) QOL subscores. The seizure breakthrough model (table e-2) demonstrated similar relations of seizure control with QOL. The transition from seizure-free to the presence of a seizure was associated with a decrease in overall QOL (p < 0.0001) and all subscores (p < 0.001) with the exception of cognitive (p = 0.09). For seizure breakthrough, with the exception of the mental health subscore, interactions of the time-varying indicator with time were not significant, indicating that the effect of the transition was persistent for the remainder of follow-up. The positive estimate of this interaction for mental health suggested that the immediate effect of having a seizure attenuated over time.
Table 1.
Demographic characteristics and seizure surgical experience of subjects (N = 373)

Figure 1. QOL stratified by seizure control over time.
Least squares mean plot based on the modeling results shown in table 2 (5-year seizure-control variable) demonstrates the relationship between quality of life (QOL) and seizure control over time. The y-axis provides overall QOL T scores and color codes the seizure-control status (excellent, good, fair, and poor). The x-axis shows time in months, with the 0 point designated as the presurgical evaluation. Six pairwise comparisons of overall effect of different seizure-control groups over QOL T score: 1 = excellent vs good; 2 = excellent vs fair; 3 = excellent vs poor; 4 = good vs fair; 5 = good vs poor; 6 = fair vs poor. The p value is represented as N = not significant; *p < 0.05; **p < 0.01; *** p < 0.001.
Table 2.
Multivariable repeated-measures regression estimates of the effects of 5-year seizure control, depression, and anxiety on overall QOL and QOL subscores
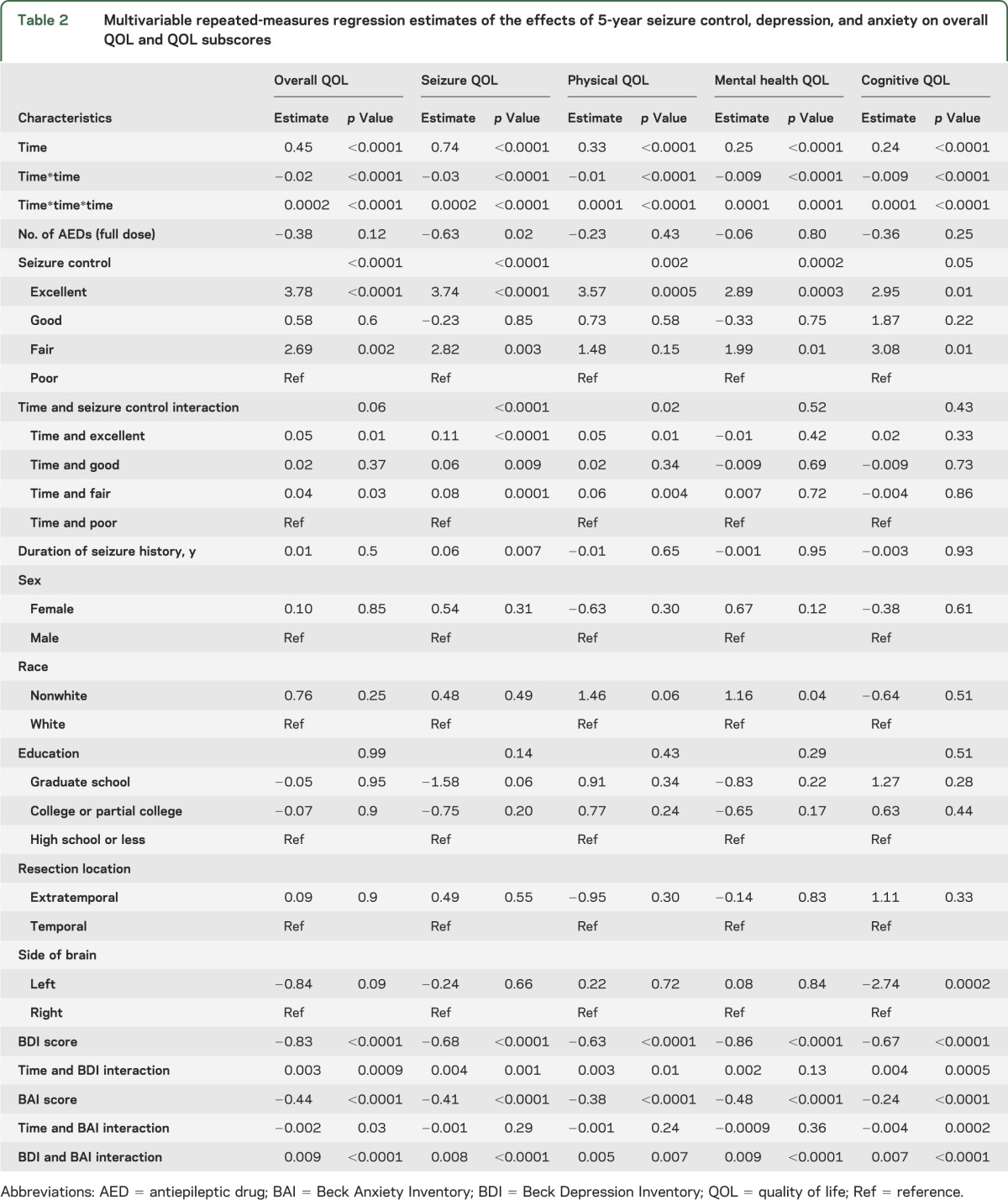
As illustrated in figures 2 and 3 (and tables e-2 and e-3), BDI, BAI, and overall QOL varied across time. Higher BDI and BAI scores were associated with lower QOL scores (p < 0.0001) when either 5-year seizure control or seizure breakthrough was included in the model. The effect of BDI on overall QOL diminished with time (p = 0.001) as indicated by its positive interaction with time, whereas the inverse effect of BAI on overall QOL increased with time (p = 0.04). Although the BDI was associated with lower QOL subscores, the diminished effect of BDI over time was only consistently demonstrated on the seizure and cognitive subscores. Similarly, BAI was associated with lower QOL subscores, but the change over time seen on the overall QOL was only significantly demonstrated in the cognitive subscore. Relations of BDI and BAI were not significantly modified by either 5-year seizure control (p = 0.12, p = 0.79, respectively) or seizure breakthrough (p = 0.50, p = 0.07, respectively).
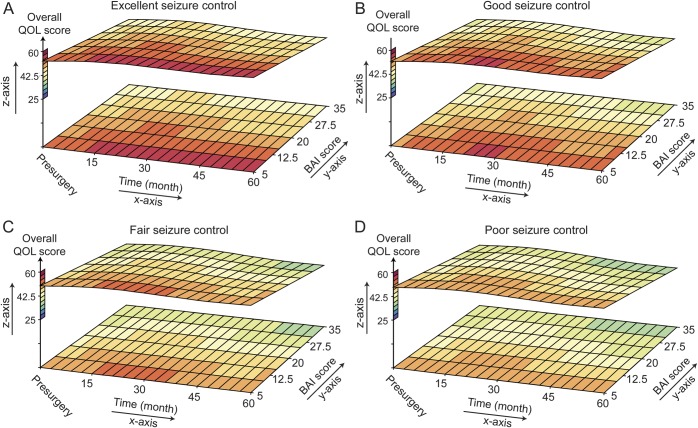
Figure 2. Estimated least squares mean of overall QOL and BDI score over time.
Surface plots demonstrate the relationship between quality of life (QOL) and depression scores (Beck Depression Inventory [BDI]) over time. The z-axis provides QOL T scores and color codes lowest scores blue to highest scores red. The x-axis shows changes in QOL and BDI over time in months, with the 0 point designated as the presurgical evaluation. The y-axis shows the BDI scores, with high scores indicating more severe depression. Figure parts A, B, C, and D represent subjects with excellent, good, fair, and poor seizure control, respectively. The scatter plots clearly show more dark red, reflecting better QOL, in subjects with better seizure control. Furthermore, with the exception of fair seizure control, the QOL improves (becomes more red) with time. Finally, there is improved QOL with lower BDI scores across each level of seizure control.
Figure 3. Estimated least squares mean of overall QOL and BAI score over time.
Surface plots demonstrate the relationship between quality of life (QOL) and anxiety scores (Beck Anxiety Inventory [BAI]) over time. The z-axis provides QOL T scores and color codes lowest scores blue to highest scores red. The x-axis shows changes in QOL and BAI over time in months, with the 0 point designated as the presurgical evaluation. The y-axis shows the BAI scores, with high scores indicating more severe anxiety. Figure parts A, B, C, and D represent subjects with excellent, good, fair, and poor seizure control, respectively. The scatter plots clearly show more dark red, reflecting better QOL, in subjects with better seizure control. Furthermore, with the exception of fair seizure control, the QOL improves (becomes more red) with time. Furthermore, there is improved QOL with lower BAI scores across each level of seizure control.
DISCUSSION
Our findings prospectively demonstrate that improved but incomplete seizure control is associated with a significant improvement in QOL. Prior studies showed that QOL improved only with complete seizure freedom3,5,6; however, we found that good control with only rare breakthrough seizures also improves QOL. Most studies showing a poor correlation between seizure frequency and QOL were cross-sectional, before treatment intervention, and their results were affected by biased recall and subjects with more severe disease. Our finding that QOL improved in the group with incomplete seizure control over time is likely attributable to the increased sensitivity of repeated-measures linear mixed-effect analysis, in which subjects serve as their own controls. While the ultimate clinical goal remains seizure freedom, subjects with a rare breakthrough seizure can show significant QOL improvements from epilepsy surgery. Our findings support a recent International League Against Epilepsy consensus recommendation suggesting that a single breakthrough seizure within 1 year of an intervention or within 3 times the length of the prior longest seizure-free interval should not constitute treatment failure.21
Our large sample size, prospective design, and linear mixed-effect statistical analysis allowed us to untangle the complex interactions among anxiety, depression, seizure control, and QOL longitudinally, up to 5 years after epilepsy surgery. We demonstrated that improvement in anxiety and depression was independently associated with QOL scores across levels of seizure control over time. Additionally, we showed that when depression and anxiety symptoms are analyzed separately, there is an interaction between seizure control and depression severity on QOL, as well as an interaction between seizure control and anxiety severity on QOL. These findings demonstrate that the effect of seizure control on QOL varies as a function of depression and anxiety severity. In other words, QOL gains with better seizure control are diminished when depression and anxiety symptoms are elevated.
Both depression and anxiety symptoms improve after epilepsy surgery.15,22–24 Because depression and anxiety are highly comorbid and share many symptoms, improvement in both sets of scores may result from an interaction effect or colinearity. Anxiety and depression scores were highly correlated in our analyses, but when the interaction was controlled for, both anxiety and depression independently were associated with QOL scores.
Anxiety scores had a slightly stronger independent effect on QOL than depression scores over time; however, it is unclear whether this effect is clinically significant. The partial or complete amygdala resection in more than 90% of patients in this study may contribute to this finding. The role of amygdala in emotional processing and, specifically, the psychopathology of anxiety disorders is well established. Dysfunctional, epileptogenic amygdala may be associated with heightened anxiety symptoms, and surgical resection of the injured amygdala may account for the improvement in anxiety symptoms. Only one other study attempted to systematically explore differences in psychiatric outcome between temporal lobe and extratemporal lobe resection.25 In contrast to our study, the authors did not find any association between psychopathology and seizure control 1 and 3 months postoperatively. However, among the 43 subjects with temporal lobe epilepsy, 23% had anxiety before surgery, and among the 17 subjects with extratemporal lobe epilepsy, 18% had anxiety before surgery; thus, this study was poorly powered to demonstrate the interactions among seizure control, temporal lobe epilepsy vs extratemporal lobe epilepsy, and anxiety. They did not report data related to amygdala resections.
QOL is a multidimensional construct, and the measure of QOL we used is comprehensive, encompassing mental health as well as other domains. Depression and anxiety are aspects of mental health, and thus measures of depression and anxiety are expected to be associated with the mental health domain of the QOLIE-89. Nonetheless, our analyses demonstrated that anxiety and depression were strongly and independently associated with epilepsy-targeted, physical health, and cognitive components of QOL.
Left-sided resections were associated with less overall QOL gains than right-sided resections; however, this effect was driven by the cognitive dimension, because none of the other QOL dimensions were related to side of surgery. This finding is consistent with prior research that showed greater cognitive morbidity after left-sided surgery (recently reviewed, reference 26), which can worsen QOL, particularly (from our cohort) in the context of persistent seizures.27 While the adverse side effects of AEDs negatively affect QOL28 and decreasing AED-related adverse side effects improves QOL,29 we were only able to accurately obtain number of AEDs used at each of the follow-up intervals. Number of AEDs may serve as a surrogate to capture the effect of AED side effects on QOL. Interestingly, number of AEDs had an independent negative effect on seizure-related QOL, not mental, cognitive, or physical components of QOL as would be expected (table 2). However, the negative of number of AEDs was not significant enough to affect overall QOL. We suspect that the number of AEDs may more reflect how “refractory” the seizures were, hence worse seizure-related QOL scores, rather than adverse effect of drugs.
In the context of excellent or good seizure control, our results show that health-related QOL can return to a level equivalent with the normal population; however, this potential is lessened by persistent anxiety or depression symptoms. In a prior study, complete and incomplete seizure control in individuals without depression was associated with a higher QOL than individuals with depression without a history of epilepsy.2 This highlights the importance of affective symptoms on subjective appraisal of QOL. In fact, affective symptoms show up to 2 times larger contribution to QOL in epilepsy than seizure control.30
While our study demonstrates the complex association and effect of depression, anxiety, and seizure control on QOL, there are a few limitations in our methods. BDI and BAI are self-reported scales that quantify psychiatric symptoms but do not provide clinical diagnoses. However, we validated, in this population, the BDI and BAI using the World Health Organization Composite International Diagnostic Interview.14 We cannot determine whether depression or anxiety relates to poor QOL through another confounder or mediator such as loss of sleep, medication noncompliance, or drug/alcohol use. Furthermore, because QOL is subjective and therefore sensitive to patients' expectations, perception of their illness, and coping skills, depression and anxiety can bias appraisal of their treatment response in a negative way.
Our study suggests that people who have medically resistant epilepsy and comorbid depression or anxiety generally may have improved QOL with successful epilepsy surgery. Comorbid depression and anxiety should not delay referral for epilepsy surgery.
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- BAI
Beck Anxiety Inventory
- BDI
Beck Depression Inventory
- QOL
quality of life
- QOLIE-89
Quality of Life in Epilepsy Inventory–89
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Hamid contributed to the design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript. Dr. Blackmon and Dr. Atlas contributed to the analysis and interpretation of the data, drafting and revising the manuscript. Dr. Cong contributed to the analysis and interpretation of the data, design and creation of figures and tables, drafting and revising the manuscript. Dr. Berg, Dr. Vickrey, Dr. Sperling, Dr. Shinnar, Dr. Langfitt, and Dr. Walczak contributed to the design and conceptualization of the study, analysis and interpretation of the data, and revising the manuscript. Dr. Dziura contributed to the design of the study, analysis and interpretation of the data. Dr. Bazil contributed to the interpretation of the data, and revising the manuscript. Dr. Devinsky contributed to the analysis and interpretation of the data, drafting and revising the manuscript.
STUDY FUNDING
Supported by the NIH (National Institute of Neurological Disorders and Stroke, RO1-NS32375, to A.T.B, M.R.S., C.W.B., J.T.L, and T.S.W.). Funding did not have a role in the design, analysis, interpretation, or writing of this study.
DISCLOSURE
H. Hamid serves as the managing editor of the Journal of Muslim Mental Health, and receives funding from the National EpiFellows Foundation and Epilepsy Foundation. K. Blackmon, X. Cong, J. Dziura, and L. Atlas report no disclosures. B. Vickrey serves on the editorial board of Neurorehabilitation & Neural Repair and is a section editor for Stroke. She serves on the advisory boards of Sports Concussion Institute, National Institute of Neurological Disorders and Stroke (NINDS)-funded ROSE phase III clinical trial (UCSF) data safety monitoring board, the National Institute on Aging–funded study, Pharmacological Management of Delirium (Indiana University), data safety and monitoring board. She has consulted for and received travel funding from CHDI, National Parkinson Foundation, and EMD Serono. She receives funding from the National Institute on Aging/NIH, 1RC4AG038804, principal investigator; VA Health Services Research and Development Service, NRI 08-370-1, co-principal investigator, 2009–present; NINDS/NIH, R37 NS031146, co-investigator/subaward PI, 2007–present; American Heart Association–Pharmaceutical Roundtable 0875133N, Outcomes Research Center, principal investigator, 2008–present; and NINDS/NIH, U54NS081764, principal investigator. A. Berg receives support from NINDS grant R37-NS31146. She has received travel funding and honoraria from Eisai, the British Pediatric Neurological Association, and the Epilepsy Research Center (Melbourne); travel funding from UCB, the American Epilepsy Society, and the International League Against Epilepsy; awards from the American Epilepsy Society and British Pediatric Neurological Association; and consulting fees from Dow Agro Science. She serves on the editorial boards of Epileptic Disorders and Epilepsy & Behavior. She is past chair of the ILAE's Commission on Classification and Terminology, current chair of the ILAE's Task Force on Classification-Diagnostic Manual, member of the ILAE's Pediatric Commission's Task Force on Autism, Member of the AES's Commission on Nonepileptic Seizures, member ad hoc Task Force of the ILAE Commission on Therapeutic Strategies, member of the AES Suicidality Task Force, and Steward for the NINDS Benchmarks in Epilepsy Research. C. Bazil is the principal investigator of a drug trial supported by Pfizer and has received honoraria for speaking and consulting from Pfizer. J. Langfitt receives support from the NIH (NINDS U01 NS 045686 and R01 NS035929) and has received honoraria from Lundbeck A/S. T. Walczak reports no disclosures. M. Sperling has consulted for Accorda Therapeutics and electroCore, and has research support from Eisai, Marinus, Vertex Pharmaceuticals, H. Lundbeck A/S, Medtronic, Inc., NeuroPace, Sunovion, and UCB Pharma. S. Shinnar has served on scientific advisory boards for Questcor and Upsher-Smith; serves on the editorial board of Pediatric Neurology; receives royalties from publication of Febrile Seizures (Academic Press, 2002); serves as a consultant for Impax, Questcor, and Upsher-Smith; serves on a data safety monitoring board for Pfizer and UCB Pharma and receives research support from the CURE Foundation, NIH (NINDS R01 NS43209 [principal investigator], NINDS R01 NS 045911 [coinvestigator], NINDS 1U10NS077308 [site co-principal investigator], and NINDS P20 NS080181 [coinvestigator]). O. Devinsky serves on a speakers bureau for UCB; has served as a consultant for Medivation, Inc.; serves as an associate editor of Epilepsy & Behavior; and receives research support from the NIH/NINDS (R01 NS053998 [phenome core leader]). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jacoby A, Snape D, Baker GA. Determinants of quality of life in people with epilepsy. Neurol Clin 2009;27:843–863 [DOI] [PubMed] [Google Scholar]
- 2.Vickrey BG, Hays RD, Rausch R, Sutherling WW, Engel J, Jr, Brook RH. Quality of life of epilepsy surgery patients as compared with outpatients with hypertension, diabetes, heart disease, and/or depressive symptoms. Epilepsia 1994;35:597–607 [DOI] [PubMed] [Google Scholar]
- 3.Markand ON, Salanova V, Whelihan E, Emsley CL. Health-related quality of life outcome in medically refractory epilepsy treated with anterior temporal lobectomy. Epilepsia 2000;41:749–759 [DOI] [PubMed] [Google Scholar]
- 4.Birbeck GL, Hays RD, Cui X, Vickrey BG. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia 2002;43:535–538 [DOI] [PubMed] [Google Scholar]
- 5.Lowe AJ, David E, Kilpatrick CJ, et al. Epilepsy surgery for pathologically proven hippocampal sclerosis provides long-term seizure control and improved quality of life. Epilepsia 2004;45:237–242 [DOI] [PubMed] [Google Scholar]
- 6.Spencer SS, Berg AT, Vickrey BG, et al. Health-related quality of life over time since resective epilepsy surgery. Ann Neurol 2007;62:327–334 [DOI] [PubMed] [Google Scholar]
- 7.McLachlan RS, Rose KJ, Derry PA, Bonnar C, Blume WT, Girvin JP. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol 1997;41:482–489 [DOI] [PubMed] [Google Scholar]
- 8.Kellett MW, Smith DF, Baker GA, Chadwick DW. Quality of life after epilepsy surgery. J Neurol Neurosurg Psychiatry 1997;63:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malmgren K, Sullivan M, Ekstedt G, Kullberg G, Kumlien E. Health-related quality of life after epilepsy surgery: a Swedish multicenter study. Epilepsia 1997;38:830–838 [DOI] [PubMed] [Google Scholar]
- 10.Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology 2004;62:258–261 [DOI] [PubMed] [Google Scholar]
- 11.Tracy JI, Dechant V, Sperling MR, Cho R, Glosser D. The association of mood with quality of life ratings in epilepsy. Neurology 2007;68:1101–1107 [DOI] [PubMed] [Google Scholar]
- 12.Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia 2004;45:544–550 [DOI] [PubMed] [Google Scholar]
- 13.Luoni C, Bisulli F, Canevini MP, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia 2011;52:2181–2191 [DOI] [PubMed] [Google Scholar]
- 14.Hamid H, Liu H, Cong X, et al. Long-term association between seizure outcome and depression after resective epilepsy surgery. Neurology 2011;77:1972–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology 2005;65:1744–1749 [DOI] [PubMed] [Google Scholar]
- 16.Berg AT, Vickrey BG, Langfitt JT, et al. The multicenter study of epilepsy surgery: recruitment and selection for surgery. Epilepsia 2003;44:1425–1433 [DOI] [PubMed] [Google Scholar]
- 17.Devinsky O, Vickrey BG, Cramer J, et al. Development of the quality of life in epilepsy inventory. Epilepsia 1995;36:1089–1104 [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100 [Google Scholar]
- 19.Friendly MW, Wolfinger R. Draw color 3D surface plot with contours in X-Y plane [SAS Macro Programs: surface]; 2012 [Google Scholar]
- 20.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis: Probability and Statistics. Hoboken, NJ: Wiley-Interscience; 2004:187–236 [Google Scholar]
- 21.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077 [DOI] [PubMed] [Google Scholar]
- 22.Cankurtaran ES, Ulug B, Saygi S, Tiryaki A, Akalan N. Psychiatric morbidity, quality of life, and disability in mesial temporal lobe epilepsy patients before and after anterior temporal lobectomy. Epilepsy Behav 2005;7:116–122 [DOI] [PubMed] [Google Scholar]
- 23.Hermann BP, Wyler AR. Depression, locus of control, and the effects of epilepsy surgery. Epilepsia 1989;30:332–338 [DOI] [PubMed] [Google Scholar]
- 24.Reuber M, Andersen B, Elger CE, Helmstaedter C. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure 2004;13:129–135 [DOI] [PubMed] [Google Scholar]
- 25.Wrench J, Wilson SJ, Bladin PF. Mood disturbance before and after seizure surgery: a comparison of temporal and extratemporal resections. Epilepsia 2004;45:534–543 [DOI] [PubMed] [Google Scholar]
- 26.Sherman EM, Wiebe S, Fay-McClymont TB, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia 2011;52:857–869 [DOI] [PubMed] [Google Scholar]
- 27.Langfitt JT, Westerveld M, Hamberger MJ, et al. Worsening of quality of life after epilepsy surgery: effect of seizures and memory decline. Neurology 2007;68:1988–1994 [DOI] [PubMed] [Google Scholar]
- 28.Taylor RS, Sander JW, Taylor RJ, Baker GA. Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review. Epilepsia 2011;52:2168–2180 [DOI] [PubMed] [Google Scholar]
- 29.Uijl SG, Uiterwaal CS, Aldenkamp AP, et al. Adjustment of treatment increases quality of life in patients with epilepsy: a randomized controlled pragmatic trial. Eur J Neurol 2009;16:1173–1177 [DOI] [PubMed] [Google Scholar]
- 30.Park SP, Song HS, Hwang YH, Lee HW, Suh CK, Kwon SH. Differential effects of seizure control and affective symptoms on quality of life in people with epilepsy. Epilepsy Behav 2010;18:455–459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.