Summary
Quorum sensing (QS) is a process of bacterial cell-cell communication that relies on the production, detection, and population-wide response to extracellular signal molecules called autoinducers. The QS system commonly found in vibrios and photobacteria consists of the CqsA synthase/CqsS receptor pair. Vibrio cholerae CqsA/S synthesizes and detects (S)-3-hydroxytridecan-4-one (C10-CAI-1), whereas Vibrio harveyi produces and detects a distinct but similar molecule, (Z)-3-aminoundec-2-en-4-one (Ea-C8-CAI-1). To understand the signaling properties of the larger family of CqsA-CqsS pairs, here, we characterize the Photobacterium angustum CqsA/S system. Many photobacterial cqsA genes harbor a conserved frameshift mutation that abolishes CAI-1 production. By contrast, their cqsS genes are intact. Correcting the P. angustum cqsA reading frame restores production of a mixture of CAI-1 moieties, including C8-CAI-1, C10-CAI-1, Ea-C8-CAI-1 and Ea-C10-CAI-1. This signal production profile matches the P. angustum CqsS receptor ligand-detection capability. The receptor exhibits a preference for molecules with 10-carbon tails, and the CqsS Ser168 residue governs this preference. P. angustum can overcome the cqsA frameshift to produce CAI-1 under particular limiting growth conditions presumably through a ribosome slippage mechanism. Thus, we propose that P. angustum uses CAI-1 signaling for adaptation to stressful environments.
Keywords: quorum sensing, sensor kinase, signal transduction, gene-regulation
Introduction
Bacteria coordinate group behaviors by producing, detecting, and collectively responding to extracellular signaling molecules called autoinducers. This process is called quorum sensing (QS). Bacteria living in heterogeneous populations presumably encounter mixtures of autoinducers produced by themselves and their unrelated neighbors. Therefore, perceiving and integrating the information contained in autoinducer blends could enable bacteria to facilitate intraspecies, intragenus, and interspecies communication, ultimately controlling niche-specific behaviors (Fuqua and Greenberg, 2002; Novick and Geisinger, 2008; Ng and Bassler, 2009; Rutherford and Bassler, 2012).
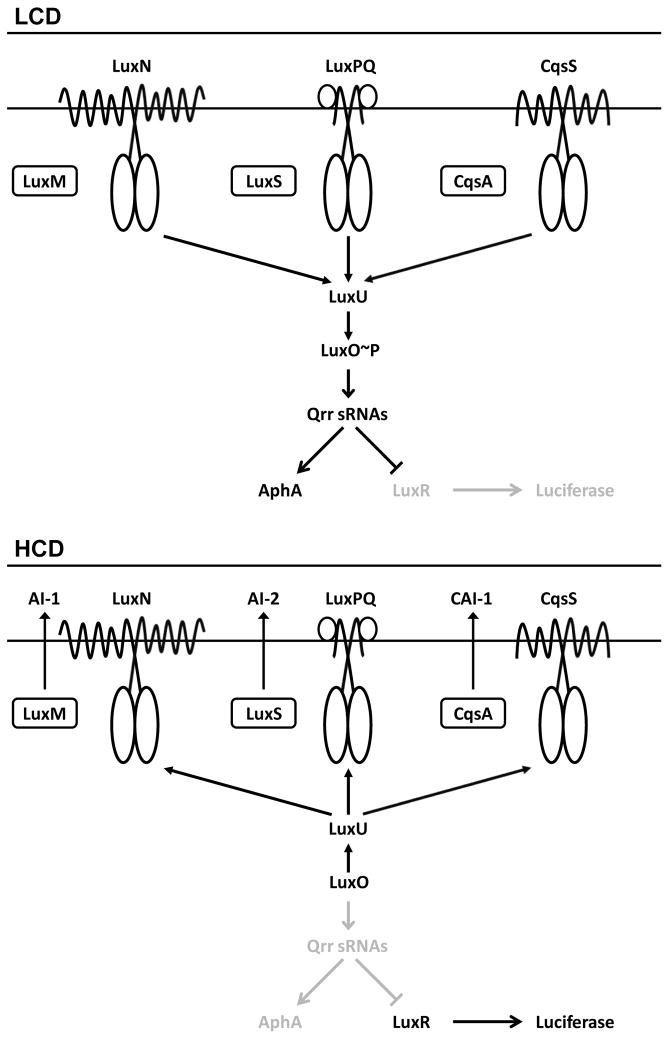
The Vibrio harveyi QS circuit is studied as a model to define the mechanisms that allow bacteria to interpret the information encoded in mixtures of autoinducers. V. harveyi has three QS systems and each is composed of an autoinducer synthase and a two-component receptor (Henke and Bassler, 2004) (Fig. 1). The LuxM synthase produces Autoinducer-1 (AI-1) (Cao and Meighen, 1989; Bassler et al., 1993), an acyl homoserine lactone (AHL) used for intraspecies communication. LuxS synthesizes Autoinducer-2 (AI-2) (Surette et al., 1999; Schauder et al., 2001; Chen et al., 2002), a furanosyl borate diester involved in interspecies communication. CqsA produces (Z)-3-aminoundec-2-en-4-one (Ea-C8-CAI-1) that is used for intragenus communication (Ng et al., 2011). AI-1, AI-2, and Ea-C8-CAI-1 bind to the LuxN, LuxPQ, and CqsS receptors, respectively (Henke and Bassler, 2004).
Fig. 1. The V. harveyi quorum-sensing system.
Top, low cell density (LCD). Bottom, high cell density (HCD). See text for details of the signal relay mechanism. Important for the present work is that at HCD, LuxR activates expression of the luciferase operon.
When V. harveyi is at low cell density (LCD), there is little autoinducer present and thus, the receptors are unliganded. Under this condition, the receptors’ autokinase activities dominate (Fig. 1, top). Phosphorelay to LuxU occurs (Freeman and Bassler, 1999a), followed by phospho-transfer to the response regulator, LuxO (Freeman and Bassler, 1999b). LuxO~P activates transcription of genes encoding five regulatory small RNAs (Qrr sRNAs) (Lenz et al., 2004), which post-transcriptionally activate expression of the LCD transcription factor AphA (Rutherford et al., 2011) and repress production of LuxR (Pompeani et al., 2008), the master high cell density (HCD) transcription factor. Thus, at LCD (i.e., AphA present, LuxR absent), AphA controls the regulon of genes underpinning individual behaviors.
At HCD (Fig. 1, bottom), autoinducers accumulate and bind to their cognate receptors. Binding inhibits the receptors’ autokinase activities (Freeman et al., 2000). This step reverses the phospho-flow and consequently drains phosphate from LuxO. Dephosphorylated LuxO cannot activate transcription of the qrr genes, which reduces production of AphA and allows LuxR to be translated. LuxR regulates hundreds of genes required for group behaviors (van Kessel et al., 2013). For example, the luciferase operon is activated by LuxR in V. harveyi. Therefore, cells are dark at LCD and bright at HCD. Luciferase, due to its large dynamic range and ease of measurement, has been used as the canonical QS readout in V. harveyi.
V. harveyi CqsA (CqsAVh) exclusively produces Ea-C8-CAI-1 and CqsSVh appears to detect only that molecule (Ng et al., 2011). By contrast, V. cholerae CqsA (CqsAVc) produces C10-CAI-1, and Ea-C8-CAI-1 is produced as a minor product (Higgins et al., 2007; Kelly et al., 2009; Ng et al., 2011). CqsSVc detects CAI-1 molecules harboring both 10-carbon and 8-carbon tails, with C10-CAI-1 being the preferred ligand. To understand how bacteria decode the information contained in structurally similar molecules, here, we continue our investigation of the signaling properties of CqsA-CqsS pairs.
In addition to vibrios, the closely-related photobacteria have cqsA and cqsS genes. Indeed, these genes are present in four of the five sequenced photobacterial genomes, (the exception is Photobacterium damselae). Legionellacea, Burkholderiacea, and Chlorobiaceae, also contain CqsA-CqsS homologs (Tiaden et al., 2010). Only the vibrio CqsA enzyme catalysis mechanism has been investigated (Higgins et al., 2007; Kelly et al., 2009; Ng et al., 2011). In vibrios, the sixth trans-membrane (TM) helices of CqsS receptors play crucial roles in ligand recognition (Ng et al., 2010). Strikingly, however, the amino acid sequences of the sixth TM helicies in photobacterial CqsS receptors differ significantly from those of vibrio CqsS receptors (Fig. S1). Additionally, a crucial Cys170/Phe175 residue is present in vibrio CqsS receptors, and it specifies the ligand chain length (Ng et al., 2011). Most photobacterial CqsS receptors (the exception is Photobacterium profundum CqsS) contain a Ser in place of the Cys/Phe (Fig. S1). These differences suggest distinct CAI-1 detection possibilities for photobacteria.
Here we focus on Photobacterium angustum, sometimes referred to as Vibrio angustum, to examine CqsA signal production and CqsS signal detection. P. angustum is a copiotrophic bacterium that has been reported to use QS to regulate the carbon starvation response (Srinivasan et al., 1998 and hereto). Consistent with this notion, cell-free extracts prepared from carbon-starved P. angustum upregulated carbon-starvation-induced proteins and re-activated growth. The P. angustum genome contains a hypothetical luxMN operon, but no AHL autoinducer has been detected. AI-2 signaling has been suggested to be involved in P. angustum stress adaptation because culture fluids from stressed cells induced bioluminescence in a V. harveyi AI-2 detector strain (McDougald et al., 2003). However, no obvious LuxPQ receptor exists. By contrast, both cqsA and cqsS are present in the P. angustum genome. To our knowledge, they have not yet been studied. The P. angustum CqsA-CqsS system could possess QS signaling capacity, and thus, could provide the link to the observed starvation responses. To investigate these possibilities, here, we examine ligand detection by CqsS, identify the P. angustum CAI-1 molecule, and evaluate its production by CqsA. No genetic tools exist that enable examination of Cqs function in photobacterium. Thus, we define the Cqs QS signaling properties using a heterogeneous vibrio system.
Results
Wild-type P. angustum produces no CAI-1
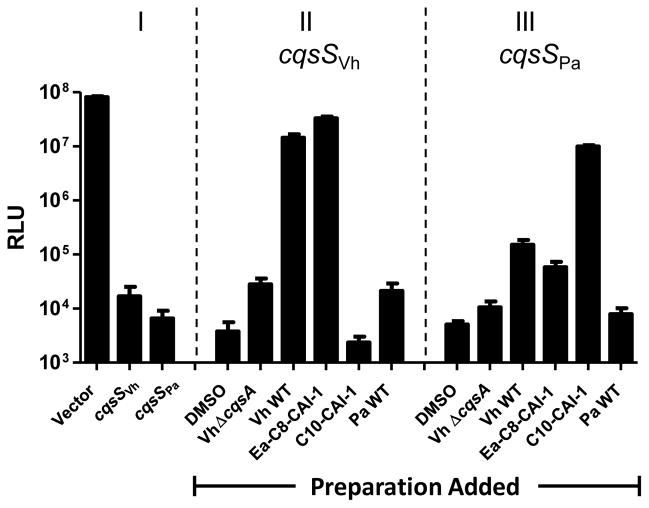
It is not possible to know the structures of small molecule autoinducers from the sequences of the genes encoding their synthases. Typically, autoinducers have been identified via extraction from cell-free culture fluids followed by structure determination by NMR, mass spectrometry, or crystallography. To begin our analysis of P. angustum CAI-1 signal production, we constructed a V. harveyi reporter strain to monitor CAI-1 activity. The reporter strain lacks all three endogenous QS receptors (ΔluxN ΔluxPQ ΔcqsSVh), so it is incapable of autoinducer detection. It also lacks cqsA (ΔcqsAVh) so it produces no CAI-1. This reporter strain produces light constitutively because the lack of QS receptors eliminates phosphorylation of LuxO, so LuxR is constitutively produced, and it activates expression of the luciferase operon (See Fig. 1 bottom, Fig. 2 Panel I). Consistent with this circuitry, addition of wild-type V. harveyi or P. angustum cell-free culture fluids does not alter light production in this strain. Introduction of cqsSVh on a vector renders the strain dark because CqsSVh functions as a kinase in the absence of autoinducer, leading to repression of the luciferase operon (Fig. 2 Panel I). Addition of nothing, DMSO or cell-free culture fluid from a V. harveyi ΔcqsA mutant (Vh ΔcqsA) does not induce light production in the reporter strain carrying cqsSVh (Fig.2 compare Panel I to Panel II). However, when the reporter strain carrying cqsSVh is provided cell-free culture fluid prepared from wild-type V. harveyi (Vh WT), which contains Ea-C8-CAI-1, the reporter produces light. This result shows that the V. harveyi CqsS receptor is capable of switching from kinase to phosphatase mode when bound by Ea-C8-CAI-1. The reporter strain carrying cqsSVh also produced light when synthetic Ea-C8-CAI-1 but not C10-CAI-1 is added which is consistent with the known CqsSVh detection specificity (Fig. 2 Panel II). When we introduced cqsSPa instead of cqsSVh, the reporter strain became dark showing that CqsSPa functions as a kinase in the absence of ligand (Fig. 2 Panel I). Addition of DMSO or culture fluid from the ΔcqsA V. harveyi strain did not induce light production (Fig. 2 compare Panel I to Panel III). Addition of WT V. harveyi culture fluid or synthetic Ea-C8-CAI-I induced modest increases in light production (~10 to 100-fold), whereas addition of synthetic C10-CAI-1 induced maximal light production (~1000-fold) (Fig. 2 Panel III). The results show that the kinase activity of CqsSPa is inhibited in the presence of ligand, and also that CqsSPa detects the V. harveyi and V. cholerae CAI-1 autoinducers. We return to the basis for the different levels of detection below. Cell-free culture fluid collected from wild-type P. angustum (Pa WT) failed to induce light production in the reporter strain carrying cqsSVh, cqsSPa or cqsSVc (Fig. 2, Pa WT Panel II and III, and data not shown). These data indicate that, under our conditions, wild-type P. angustum is either incapable of producing CAI-1 or the P. angustum autoinducer cannot be detected by any of the CqsS receptors examined.
Fig. 2. P. angustum does not produce CAI-1 activity.
A V. harveyi bioluminescent reporter strain (ΔluxN ΔluxPQ ΔcqsASVh) expressing a vector, cqsSVh, or cqsSPa was used to detect CAI-1 activity. Panel I: The reporter strain carrying a vector or the vector with cloned cqsSVh or cqsSPa when no molecules or culture fluids are added. Panel II: The reporter strain carrying cqsSVh. Panel III: The reporter strain carrying cqsSPa. In panels II and III, DMSO was used as the control, cell-free culture fluids from WT V. harveyi, a V. harveyi ΔcqsA mutant, or WT P. angustum were provided at 10% v/v and Ea-C8-CAI-1 or C10-CAI-1 were supplied at 5 μM. RLU, Relative Light Units (normalized to OD). Error bars represent standard deviations for three replicates.
CqsSPa preferentially detects CAI-1 molecules with C10 tails
To examine CqsSPa ligand detection specificity, we measured dose-dependent responses of CqsSVh, CqsSVc, and CqsSPa to a set of CAI-1-type molecules (C8-CAI-1, Ea-C8-CAI-1, C10-CAI-1 and Ea-C10-CAI-1) using the above reporter strain expressing either CqsSVh, CqsSPa, or CqsSVc (Figs 3 and S2). Consistent with our understanding of CqsS signaling, the CqsSVh receptor is stringent in ligand detection and only responds to the cognate V. harveyi autoinducer, Ea-C8-CAI-1 (EC50 = 100 nM). The CqsSVc reporter responds maximally to Ea-C10-CAI-1 (EC50 = 150 nM), and efficiently detects C10-CAI-1 and Ea-C8-CAI-1 (EC50 of 400 nM and 1 μM, respectively), showing that it has relaxed specificity. The CqsSPa receptor exhibits intermediate ligand stringency. Similar to CqsSVc, it responds robustly to Ea-C10-CAI-1 and C10-CAI-1 (EC50 = 4 nM and 12 nM, respectively), but it shows a modest response to Ea-C8-CAI-1 (Figs 3 and S2). Sub-maximal light is produced in response to molecules with C8 tails, and so EC50 values could not be reliably determined. These data indicate that CqsSPa strongly prefers CAI-1 molecules carrying C10 tails over those carrying C8 tails.
Fig. 3. P. angustum CqsS preferentially detects CAI-1 moieties with C10 tails.
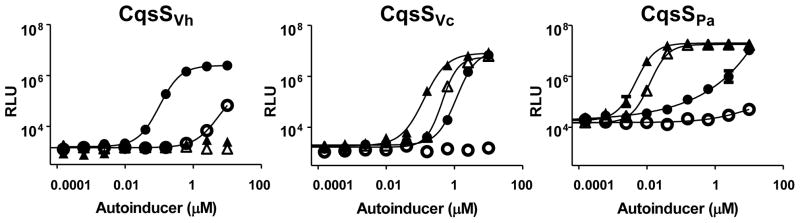
Bioluminescence from a V. harveyi reporter strain expressing cqsSVh, cqsSVc, or cqsSPa was measured in response to Ea-C8-CAI-1 (closed circles); C8-CAI-1 (open circles); Ea-C10-CAI-1 (closed triangles); C10-CAI-1 (open triangles).
Ser168 defines the CqsSPa ligand detection preference
Previous analyses of CqsSVh and CqsSVc show that Phe175 restricts ligand detection in CqsSVh to CAI-1 moieties carrying C8 tails, whereas the smaller Cys170 residue in the analogous position in CqsSVc relaxes stringency, enabling detection of CAI-1-type molecules carrying C8 and C10 tails (Fig. S1). We wondered if Ser168 at the corresponding position in CqsSPa plays the crucial role in determining chain-length preference. To examine this possibility, we exchanged the residues in the different CqsS receptors and measured their responses to CAI-1 molecules (Fig. S2). Consistent with previous results, when Phe175 was changed to Cys or Ser (CqsSVhF175C and CqsSVhF175S), the CqsSVh receptor gained the ability to detect CAI-1 moieties carrying 10-carbons. CqsSVcC170S detected CAI-1 molecules with both C8 and C10 tails, whereas CqsSVcC170F had greatly reduced detection of CAI-1 molecules with C10 tails.
We compared the above results to those for CqsSPa carrying analogous substitutions. CqsSPaS168C retained the ability to detect Ea-C10-CAI-1 and C10-CAI-1, but did not gain any ability to detect CAI-1 molecules with C8 tails (Fig. S2). Replacement with other small amino acids such as Gly, Ala, Thr resulted in the same phenotype. The CqsSPaS168F and CqsSPaS168V variants with Ser168 replaced with bulky hydrophobic residues showed no response to any CAI-1-type molecule in our collection (Fig. S2). These experiments demonstrate that Ser168 is important for CAI-1 detection by CqsSPa, but this residue alone does not specify the chain-length preference. We reason that in CqsSPa, ligand specificity is determined by Ser168 and residues that do not exist in CqsSVh or CqsSVc. To investigate this region further, we replaced the stretch of seven amino acids surrounding Ser168 (TGIASHY) in the CqsSPa receptor with the corresponding residues from the CqsSVh receptor (FGNLFYF) or the CqsSVc receptor (FGNLCFF). Neither of these chimeric mutant receptors was functional.
A two-nucleotide deletion causes a frameshift mutation in the P. angustum cqsA gene
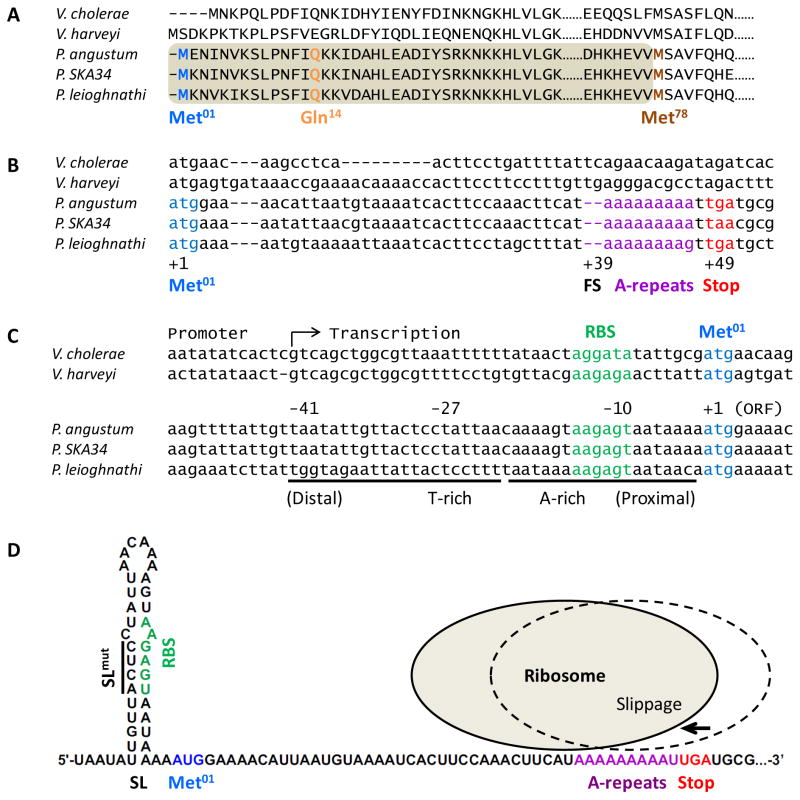
The above analyses show that CqsSPa is capable of detecting specific CAI-1-type molecules, however, curiously, CAI-1 activity is absent in P. angustum culture fluids. We wondered if the P. angustum cqsA gene (cqsAPa) was defective. Genomic analysis revealed extensive homology between cqsAPa and vibrio cqsA genes. However, the annotated cqsAPa ORF encodes a protein of 313 amino acids, significantly shorter than vibrio CqsA proteins that contain approximately 390 amino acids (see Met78 in Fig. 4A). This CqsAPa protein sequence, beginning with Met78, aligns well with an internal portion of vibrio CqsA proteins. We name this shorter ORF cqsAMet78. The DNA sequence homology between cqsAPa and vibrio cqsA genes extends well upstream of the annotated start site of cqsAMet78 (Fig. 4B). We can identify a putative translation start site for cqsAPa in this extended upstream region and this start codon corresponds closely to those of vibrio cqsA genes. We assign this putative upstream CqsAPa start site as Met01 (Fig. 4A and B). Additionally, a predicted RBS is located upstream of Met01 (Fig. 4C; RBS). We hypothesize that the Met01 start site, if used, could enable translation of a functional CqsA protein of 390 amino acids; the same length as other CqsA proteins. We name the ORF starting at Met01 cqsAMet01. However, in this putative gene, two consecutive nucleotides are missing at +39 relative to Met01, which corresponds to a TC in cqsAVc and a TG in cqsAVh gene (Fig. 4B; FS). Restoration of a TC would introduce a conserved Gln at the 14th residue, whereas a TG would introduce a Glu (Fig. 4A). To simplify our nomenclature, we assume the deleted dinucleotide to be TC in cqsAPa, and we name the frameshift site Gln14. The naturally occurring deletion causes a frameshift at Gln14 and introduces a stop codon at position +49 relative to Met01 (Fig. 4B; Stop). Thus, even if Met01 is used, this frameshift would make the CqsAPa protein only 16 amino acids long, ending with Lys14-Lys15-Asn16. Introduction of TC at the frameshift would restore the reading frame to generate an ORF encoding a protein of 390 amino acids. We call this corrected ORF cqsAMet01+TC. We engineered cqsAPa constructs carrying the various features described above (Fig. 5A), and measured the resulting CAI-1 activity using the V. harveyi reporter strain harboring CqsSPa (Fig. 5B).
Fig. 4. The P. angustum cqsA gene contains a conserved frameshift mutation and a stem-loop in the 5′UTR occludes ribosome binding.
A) Alignment of the CqsAPa sequence with vibrio CqsA proteins. Dashes represent gaps in the alignment. Dots denote amino acids that are not shown. The annotated cqsAPa ORF begins at Met78 (brown), and would encode a protein shorter than vibrio CqsA proteins and it would lack the amino acids in the shaded area. A protein beginning at the Met01 site (blue) would have a frameshift at Gln14 (orange). B) Alignment of vibrio and photobacterial cqsA genes. The cqsAPa ORF starting at +1 (blue, Met01) contains a two-nucleotide frameshift at +39 (FS), resulting in a stop codon at +49 (red, Stop) that immediately follows an adenosine-repeat (purple, A-repeats). C) Alignment of 5′UTRs of vibrio and photobacterial cqsA genes. A-rich and T-rich regions with dyad symmetry (underline) exist in cqsAPa, which could form a stem loop in the mRNA that blocks the conserved ribosome binding site (RBS, green). D) Predicted cqsAPa mRNA folding. Colors are as in panel C and D. The putative stem-loop (SL) is shown. Sites of mutations engineered to disrupt the stem-loop are shown by the line (SLmut). The cartoon depicts how ribosome retraction by two nucleotides could restore cqsA expression.
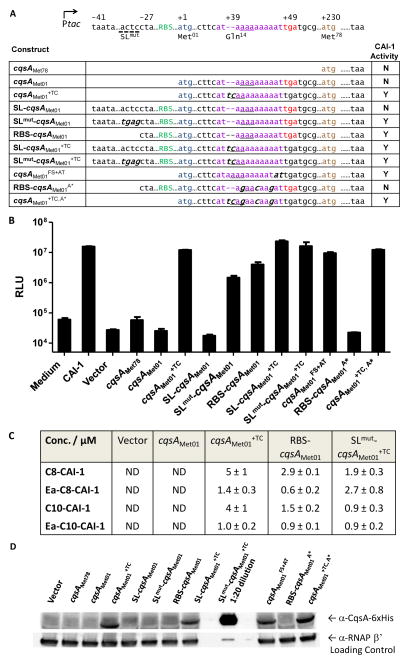
Fig. 5. CAI-1 activity is restored when the frameshift mutation is corrected and the 5′UTR stem-loop is disrupted.
A) P. angustum cqsA constructs expressed in E. coli. Color designations are the same as in Fig. 4. Mutated nucleotides are noted in italics. Underlined trinucelotides represent the first codon that would be specified following the frameshift. CAI-1 activity shown in panel B is summarized as Y (yes) or N (no). B) E. coli harboring the P. angustum cqsA constructs in panel A were assayed for CAI-1 activity using the bioluminescent reporter strain carrying cqsSPa. Cell-free culture fluids were added at 1% v/v. Error bars represent standard deviations for three replicates. C) CAI-1–type molecules detected by GC-MS in culture fluids prepared from E. coli strains expressing the designated cqsAPa constructs. ND, none detected. D) Western blot analysis of 6x-His tagged CqsA protein detected in lysates made from E. coli carrying the designated cqsAPa constructs. The SLmut-cqsAMet01+TC sample was diluted 20-fold relative to the other samples on the gel because of the high CqsA protein production.
First, we overexpressed cqsAMet78 and cqsAMet01 in E. coli. No CAI-1 activity was produced as judged by the lack of induction of light production by the CqsSPa detector strain following treatment with cell-free culture fluids from these strains (Fig. 5B). Analogous overexpression of the cqsAVh or cqsAVc ORF in E. coli resulted in CAI-1 production (Kelly et al., 2009; Ng et al., 2011). cqsAMet01+TC with the frameshift repaired, when overexpressed in E. coli, produced CAI-1 that could induce light production in the reporter strain (Fig. 5A and B). Restoring the reading frame and simultaneously replacing the annotated Met78 with a Thr codon (denoted cqsAMet01+TC, M78T) maintained CAI-1 production, indicating that Met78 is not employed as the start site (Fig. S3). However, CAI-1 activity depended on translation from Met01, because introduction of a nonsense mutation at the Ser08 codon between Met01 and Met78 (cqsAMet01+TC, S08*) abolished CAI-1 activity (Fig. S3). Together, these results show that the naturally occurring frameshift inactivates cqsAPa, and restoring both the length and the reading frame of the cqsA gene is necessary for CAI-1 production.
Intriguingly, a stretch of nine consecutive adenosine nucleotides (A-repeats) is located immediately downstream of the Gln14 (+39) frameshift in cqsAPa (Fig. 4B). Insertion or deletion of adenosines could occur when errors happen during replication or transcription. If so, the cqsAPa reading frame could be restored. Indeed, insertion of two additional adenosine nucleotides into cqsAMet01 to replace the deleted TC (denoted cqsAMet01+AA), which introduces a Lys at the Gln14 site, resulted in CAI-1 production (Fig. S3). However, deletion of one adenosine nucleotide in the A-repeats (denoted cqsAMet01ΔA), while restoring the reading frame, makes a CqsA protein that lacks an amino acid at the conserved Gln14 position. This alteration did not result in CAI-1 activity (Fig. S3). Thus, having a residue at the Gln14 site is necessary for CqsA function. Together, our results support the idea that Met01 is the correct start site, and a correct reading frame is essential for CqsA enzymatic activity. We infer that wild-type P. angustum and other photobacteria are cqsA null mutants, due to the acquisition of dinucleotide deletions. We discuss the biological implications of this finding below.
P. angustum CqsA synthesizes a mixture of CAI-1-type molecules
The above results show that correcting the reading frame of the P. angustum cqsAPa gene is sufficient to produce a functional CqsA protein. Thus, the cqsAPa+TC construct provides us a means to identify which CAI-1 moieties the P. angustum CqsA enzyme produces. Culture fluid was prepared from the E. coli strain overexpressing cqsAMet01+TC. Preparations were extracted with dichloromethane and analyzed by triple quadrupole gas-chromatography mass spectrometry (GC-MS). We identified molecules with masses corresponding to known CAI-1 molecules (Fig. 5C). Quantification with a C9-CAI-1 standard showed that C8-CAI-1 and C10-CAI-1 are the predominant CqsAPa products and were present at 5 μM and 4 μM, respectively. Ea-C8-CAI-1 and Ea-C10-CAI-1 were also present, each at around 1 μM. The composition of CAI-1-type molecules in the preparations strongly tracked with the ability of the preparations to induce bioluminescence in the V. harveyi reporters carrying CqsS receptor variants that preferentially detect particular CAI-1 family molecules (Fig. 5B and S4). No CAI-1 molecules were detected by GC-MS or via activation of the bioluminescent reporter strain in preparations made from E. coli carrying the empty vector or the cqsAMet01 construct harboring the 2-nucleotide deletion (Figs 5B, C, and S4). Together, these results demonstrate that the repaired cqsAPa gene enables production of CAI-1-like moieties. Although unlikely, we cannot exclude the possibility that other CAI-1-like molecules are also produced that do not match our synthetic GC-MS standards or induce our set of reporter strains.
Regulation of P. angustum CAI-1 production
Given that CqsSPa functions, it is curious then that a dinucleotide frameshift exists in the P. angustum cqsA gene that eliminates CAI-1 production. The dinucleotide deletion and the A-repeats exist in the cqsA locus of two other P. angustum isolates that we have sequenced (S14 and B70) and genomic database analyses show that this exact frameshift coupled with the A-repeats is also present in Photobacterium sp. SKA34 and Photobacterium leiognathi, but absent in Photobacterium profundum (Fig. 4B). Several polymorphisms exist in the sequences of the photobacterial cqsA genes, but no detrimental mutations other than the dinucleotide deletion appear to be present. The other components of the QS circuit, luxU, luxO, qrr1, luxR, and aphA are present in all sequenced P. angustum strains and appear to be intact. These findings suggest that there could be some selective advantage to maintain a DNA sequence that can be capable of producing full-length CqsA.
To examine regulation and possible reactivation of cqsAPa, we first focused on the 5′ UTR. The 5′ UTRs of photobacterial cqsA genes are highly similar, and in all cases, the conserved ribosome binding site is centered at the -10 position relative to Met01 (Fig. 4C, D). The sequences surrounding the Shine-Dalgarno motif are AT-rich and possess dyad symmetry, potentially forming a stem loop that occludes the ribosome (Fig. 4D; denoted SL). The analogous 5′ UTR sequences of vibrio cqsA genes are not similar to the P. angustum 5′UTR sequence, and they do not appear capable of forming extended stem loops. We wondered if the putative structure in the cqsAPa 5′UTR influences regulation. To test this idea, we engineered a P. angustum cqsA construct harboring the native 41-nucleotide 5′ UTR region that is capable of forming a stem-loop (denoted SL-cqsAMet01 in Fig. 5A). Overexpression of SL-cqsAMet01 did not result in CAI-1 activity (Fig. 5B). Mutating this region to eliminate stem-loop formation (denoted SLmut-cqsAMet01) partially restored CAI-1 production, as indicated by light production from the V. harveyi CqsSPa reporter (Fig. 5A and B). Rather than a structural motif, the palindromic sequence in the cqsAPa 5′UTR could encode a repressor DNA binding site, and if so, the mutation in SLmut-cqsAMet01 could have eliminated repressor binding and derepressed cqsAPa expression. We do not favor this possibility because there are less than two-fold differences in transcript levels between the cqsAMet01, SL-cqsAMet01, and SLmut-cqsAMet01 constructs according to qRT-PCR (Fig. S5). We therefore conclude that the differences in CAI-1 activity produced by the various constructs are primarily a consequence of post-transcriptional regulation.
The above results suggest that eliminating the stem-loop reveals the native cqsA RBS and partially overcomes the frameshift mutation. Possibly, the lack of a stem loop enables some ribosome recruitment and transit through the gene enabling modest CqsA production even when the frameshift mutation is present. To examine this possibility, we engineered a cqsAPa construct harboring the region of the 5′ UTR that preserves the native RBS, but prevents stem-loop formation because the distal, complementary sequence is not included in the construct (denoted RBS-cqsAMet01 in Fig. 5A). Overexpression of this construct in E. coli indeed resulted in CAI-1 activity consistent with the idea that the presence of the native RBS is sufficient to override the frameshift mutation (Fig. 5B). We discuss possible mechanisms by which this could occur below. Mutations in the RBS abolished CAI-1 activity (Fig. S3; RBSmut-cqsAMet01) and CAI-1 production also depended on translation from Met01, because no activity was produced when the Met01→Thr mutation was present (denoted RBS-cqsAMet01M01T, Fig. S3). By contrast, alteration of Met78 to Thr (RBS-cqsAMet01M78T, Fig. S3) maintained CAI-1 activity. GC-MS analysis demonstrated that the mixture of CAI-1 molecules present in the cell-free culture fluids prepared from the E. coli strain overexpressing RBS-cqsAMet01 was similar to that produced by the cqsAMet01+TC strain (Fig. 5C). The concentrations of CAI-1 moieties were, however, only half as high as those present in culture fluids prepared from the cqsAMet01+TC strain harboring the restored reading frame. Introduction of the stem-loop upstream of the repaired cqsAMet01+TC did not reduce CAI-1 production (SL-cqsAMet01+TC; Fig. 5A and B). We reason that, in this construct, the RBS that is supplied by the Ptac promoter is sufficient for cqsAPa expression when the frameshift is restored. Repairing the frameshift in the context of disruption of the stem-loop (denoted SLmut-cqsAMet01+TC) also resulted in full bioluminescence induction and CAI-1 production (Fig. 5A, B and C). All four CAI-1-type molecules were produced (Fig. 5C). We note, however, that overexpression of this construct impaired E. coli growth by three fold, suggesting that E. coli lacked the capacity to produce as much CAI-1 as the construct could support. Thus, if we adjust for cell growth, we expect the CqsA production per cell in E. coli carrying the SLmut-cqsAMet01+TC construct is higher than that in the E. coli strain carrying the SL-cqsAMet01+TC construct. We provide proof for this assertion in the next section. Collectively, our results show that disruption of the native stem-loop combined with restoration of the cqsAPa ORF fully de-repressed cqsA expression, and furthermore, the stem-loop functions in post-transcriptional control.
To verify the above results, we measured CqsA protein in the above E. coli strains using Western blotting with an antibody targeting a 6x-His tag fused to the C-terminus of CqsA. Our strategy ensured that only fully translated CqsA protein is detected (Fig. 5D). No CqsA protein was present in lysates of E. coli strains harboring the empty vector, cqsAMet78, or the cqsAMet01 construct lacking the native 5′ UTR but carrying the frameshift, or the SL-cqsAMet01 construct containing the stem-loop and the frameshift mutation. Low levels of CqsA protein were produced by E. coli expressing the SLmut-cqsAMet01 construct with the stem-loop disrupted and by E. coli harboring the RBS-cqsAMet01 construct with the truncated 5′UTR (Figs. 5D and S6A). When the reading frame was restored, however, (see cqsAMet01+TC) the construct lacking the native 5′UTR produced about ten-fold higher CqsA protein. Introducing the 5′ stem-loop upstream of cqsA+TC (see SL-cqsAMet01+TC) did not significantly alter CqsA protein production, which matches the bioluminescence assay results and suggests the RBS supplied by the vector is sufficient. However, 500-fold more CqsA protein was produced when the stem-loop was disrupted and the frameshift was simultaneously repaired (SLmut-cqsAMet01+TC) compared to the construct with the restored frameshift but carrying the stem-loop (SL-cqsAMet01+TC) (Figs. 5D and S6B). These results show that disrupting the 5′UTR stem-loop structure or restoring the frameshift allows CqsA protein production, but combining these two features results in maximal CqsA protein production. Indeed E. coli carrying the SLmut-cqsAMet01+TC construct displayed a severe growth defect due to the high level production of CqsA. This growth defect accounts for why less CAI-1 activity is present in cell-free culture fluids than would be predicted (Fig. 5C) given that the Western shows that this strain produces the highest amount of CqsA protein per-cell (Fig. 5D). We note that the different assays we use to track cqsA expression and CAI-1 activity have different sensitivities and different dynamic ranges. The assays in Fig. 5 measure stages along a signal transduction cascade (protein levels → CAI-1 production → response to CAI-1). The outputs of each step are not linearly related so one cannot make a one to one comparison of the different graphs. In every case, the cqsA expression and the CAI-1 activity from each construct track with one another. Our results indicate that both the stem-loop that inhibits ribosome binding and the frameshift need to be overcome to achieve maximal cqsA expression. We assume the stem-loop can be overcome by a sRNA or other RNA regulatory process. Regarding the frameshift, one possibility is that ribosome slippage, in which the ribosome slips back and forth to alter the register, could occur, enabling reuse or bypass of nucleotides. Repeats often facilitate ribosome slippage, and in the context of cqsAPa, a conserved stretch of A-repeats immediately follows the two-nucleotide deletion (Fig. 4B and D). To test whether a slippage mechanism is plausible, we introduced an AT dinucleotide upstream of the stop codon at the +49 site in the cqsAMet01 construct to mimic the outcome of two-nucleotide ribosome slippage to the left, with translation resuming with the correct reading-frame after Asn16. This construct (denoted cqsAMet01FS+AT) encodes a protein that differs from the native CqsAPa (encoded by cqsAMet01+TC) by two amino acids (IKKNID instead of IQKKID) (Fig. 5A). Importantly, this construct does not possess the putative stem-loop in the cqsAPa 5′ UTR. CAI-1 activity was detected in cell-free culture fluid prepared from the E. coli carrying this construct (Fig. 5B), and CqsA protein was present at levels comparable to that found in E. coli lysates carrying cqsAMet01+TC with the restored reading frame (Fig. 5D). We assume that the CAI-1 produced from the RBS-cqsAMet01 construct occurred through a similar ribosome slippage mechanism, which would depend on continuous adenosines in the A-repeat region. Indeed, when we engineered synonymous mutations in the wobble nucleotides of the codons in the A-repeat region to prevent ribosome slippage, CAI-1 activity and CqsA protein production were abolished (RBS-cqsAMet01A*, Fig. 5A, B and D). By contrast, this mutation did not affect CAI-1 production or CqsA levels when the frameshift was repaired (cqsAMet01+TC, A*) (Fig. 5A, B and D). Our data suggest that a ribosome slippage model is a plausible mechanism for overriding the frameshift mutation present in the P. angustum cqsA gene.
P. angustum produces CAI-1 activity under stress conditions
Collectively, our results show that the cqsAPa gene harbors the potential to be functional even in the face of a putative mRNA stem loop that occludes the RBS and a conserved dinucleotide frameshift that inserts a stop codon at position +49. However, Fig. 2 shows that stationary phase cultures of P. angustum do not contain any CAI-1 activity, suggesting environmental factors that are not mimicked under laboratory conditions, could be involved in enabling cqsAPa expression presumably through melting of the 5′ stem loop and/or ribosome slippage. Previous studies suggest that QS controls the P. angustum response to carbon starvation and stress adaption (Srinivasan et al., 1998). During carbon and energy starvation, a relA-dependent surge in ppGpp synthesis occurs in P. angustum (Flärdh et al., 1994). Ribosome slippage in E. coli is induced by amino acid limitation and is regulated by RelA (Masucci et al., 2002). To test if carbon starvation influences ribosome slippage in P. angustum, we grew P. angustum in Biolog plates containing IF-0a medium supplemented with different carbon sources. CAI-1 activity was measured using the V. harveyi bioluminescent reporter supplied with the P. angustum Biolog culture suspension. Thirty out of the ninety-five different carbon sources tested supported more than two bacterial doublings compared to a no carbon control (Fig. S7A). As expected, many sugars were efficiently used by P. angustum for growth with D-galactose supporting the highest growth, to an OD of 1.3 (Fig. S7A). Several amino acids, central carbon metabolites, nucleosides and dipeptides, were also sufficient as sole carbon sources (Fig S7A).
CAI-1 activity could be detected in cultures grown on some of the above carbon sources (Fig S7B). Intriguingly, the sugars (Glc, Gal, Fru, etc.) and amino acids (Ser, Ala) that enabled the highest growth resulted in the lowest CAI-1 production. This result could explain our lack of detection of CAI-1 in P. angustum grown in rich medium. Likewise, Asn, Glu and Gln, while stimulating high CAI-1 production, supported only a few cell doublings. The trend appears to be that slowly growing P. angustum cells produce high CAI-1 activity. This result is consistent with the notion that sub-optimal growth conditions, or specifically carbon starvation, could stimulate ribosome slippage and enable CqsA production. There were a few exceptions to this pattern. Thymidine and Ala-Gly supported significant bacterial growth and CAI-1 production.
Discussion
The CqsA-CqsS QS system coupled to downstream signal relay components provides photobacteria and vibrios an apparatus capable of detecting and responding to extracellular signal molecules to orchestrate collective behaviors. Vibrios use the CqsA/S QS system to regulate bioluminescence, virulence, and EPS production (Ng and Bassler, 2009; Rutherford and Bassler, 2012). While we do not understand what processes photobacteria control with CqsA-CqsS, several previous studies have related the carbon starvation response of P. angustum to QS regulation. Our analyses identify CqsA-CqsS as the candidate QS system to consider in this context and that CAI-1 signal production is coupled to metabolic stress. The lack of genetic techniques for manipulating photobacterium hinders our ability to define its QS outputs. Nonetheless, we could characterize CqsAPa and CqsSPa function via expression and analysis in heterologous systems.
CqsSPa detects CAI-1-type molecules, indicating that the overall architecture of the CqsSPa ligand binding pocket is similar to that of vibrio CqsS receptors, despite striking sequence differences in the key ligand-detection transmembrane domain (TM6). Vibrios discriminate between CAI-1 tail lengths by controlling the ligand binding pocket size. A Phe175 in CqsSVh restricts detection to CAI-1 molecules with C8 tails. The smaller Cys170 at the corresponding position in CqsSVc enables detection of CAI-1 molecules with C8 and C10 tails. By contrast, CqsSPa strongly favors CAI-1 molecules with C10 tails over those with C8 tails. Our analyses suggest that CqsSPa ligand discrimination is accomplished by a different mechanism than that used by vibrio receptors. While the Ser168 residue in CqsSPa is essential for detection of C10-CAI-1, it does not account for the tail-length preference. Possibly, ligands with short, C8, tails do not make sufficient contacts with the residues lining the CqsSPa ligand binding pocket, resulting in reduced binding affinity. Alternatively, C8-CAI-1 could bind CqsSPa with high affinity, but be incapable of inducing the requisite conformational change to initiate signal transduction. Chemical-genetic analyses (Ng et al., 2010) could distinguish between these possibilities.
CqsSPa and CqsSVc both detect Ea-C10-CAI-1 and C10-CAI-1, suggesting these molecules could be involved in communication between photobacteria and vibrios. It is intriguing that P. angustum does not produce CAI-1 under normal laboratory conditions. We attribute this inability to a frameshift mutation in the cqsAPa ORF and an inhibitory regulatory stem-loop in the 5′UTR that prevents ribosome access. These same features are conserved in several photobacterial species. Retaining the capability to detect exogenous antoinducers without the intrinsic ability to produce the corresponding autoinducer could be an “eavesdropping” strategy that P. angustum uses to garner information about its neighbors. For example, P. angustum could “tune in” to vibrios producing CAI-1 and use that information to execute appropriate behaviors in heterogeneous bacterial communities.
If photobacteria exclusively relies on eavesdropping for cell-cell communication, it could simply lose the cqsA gene thereby ensuring that CAI-1 production never occurs. This is not what we observe from genome sequence analyses. Harboring a full-length cqsAPa gene preserves the potential to produce CAI-1. An alternative explanation could be that P. angustum actively regulates CAI-1 production. The CqsA enzyme employs S-adenosylmethionine (SAM) and fatty acids for CAI-1 synthesis (Kelly et al., 2009; Wei et al., 2011). Possibly, it is advantageous to divert these substrates to other uses under many of the environmental circumstances encountered by photobacteria. Perhaps, relatively extreme repression mechanisms have evolved that ensure CAI-1 production remains off, albeit with the possibility of reactivation. We favor a hypothesis along these lines because our results show that CAI-1 production is restored when the frameshift is repaired and the stem-loop is disrupted. We speculate that the conserved A-repeats located near Gln14 in CqsAPa presumably supply the necessary track to overcome the frameshift. A change could occur during transcription or translation. During transcription, insertion of two adenosine nucleotides into the A-repeat region would produce an mRNA transcript with the correct reading frame, permanently restoring CAI-1 activity. In this scenario, CAI-1 production could still be subject to negative regulation via the 5′ stem-loop. During translation, the correct reading frame could be restored by ribosome slippage and/or usage of overlapping codons. Ribosome frameshifting is enhanced in an E. coli relA mutant during amino acid starvation (Masucci et al., 2002). Under nutrient limiting conditions, RelA and SpoT increase synthesis of (p)ppGpp, which in turn, activates the stringent response (Boutte and Crosson, 2013). In V. cholerae, three synthases, RelA, SpoT, and RelV, modulate (p)ppGpp metabolism depending on nutrient availability (Das et al., 2009). RelAVc activity increases during amino acid starvation, whereas SpoTVc and RelVVc synthesize (p)ppGpp during carbon and fatty acid starvation. Homologs of the three synthases exist in P. angustum and presumably regulate (p)ppGpp dynamics depending on growth conditions (Flärdh et al., 1994). It is known that glucose upshift of carbon-starved P. angustum S14 causes amino acid starvation and induction of the stringent response (Flärdh and Kjelleberg, 1994). Possibly, carbon starvation conditions influence P. angustum (p)ppGpp levels, promote ribosome slippage in cqsAPa, and restore CAI-1 production. If so, this mechanism could link the stress response to QS, as suggested previously. An alternative mechanism could occur under conditions of aminoacyl-tRNA shortage, which stimulates ribosome frameshifting at corresponding codons. This mechanism enables alternative and overlapping reading frames to be used (Yelverton et al., 1994; Barak et al., 1996). One could imagine that when P. angustum is challenged with amino acid limitation, ribosome slippage occurs at a site near the conserved frameshift. In cqsAPa, the frameshift is located at the Gln14 codon and causes a stop codon following the codon specifying Asn16. Asn and Gln are the amino acids that act as sentinels to reflect carbon and nitrogen availability. Nutrient starvation could cause accumulation of uncharged tRNA-Asn and promote ribosome slippage at this location.
P. angustum could use these CAI-1 restoration mechanisms to react to carbon- and nitrogen-limited situations to induce QS to initiate its stress adaptation response. Additionally, production of the CAI-1 signal could mimic vibrio QS, and P. angustum could uses this tactic to “trick” neighboring vibrios into running their QS programs under inappropriate conditions. This idea is supported by our finding that P. angustum produces but does not detect C8-CAI-1 and Ea-C8-CAI-1, two molecules that are detected by V. harveyi. Such a strategy could, for example, trigger vibrios to produce extracellular enzymes that are beneficial public goods that photobacteria can exploit, enabling it to thrive at the cost of its competitors.
Beyond the conserved frameshift, a putative stem-loop at the 5′UTR of cqsAPa provides another layer of post-transcriptional control over CAI-1 production. This stem-loop could function in vivo to inhibit cqsAPa translation with release under appropriate conditions. We envision several mechanisms by which the stem-loop could be regulated. One possibility is that a small regulatory RNA base pairs with and destabilizes the self-inhibitory stem-loop structure. In a variety of bacteria, regulatory RNAs (DsrA, GlmZ, RNAIII, RprA, RyhB, and Qrr1-5) pair with and disrupt inhibitory stem-loops in the 5′ regions of target mRNAs to promote translation by an ‘anti-antisense mechanism’ (Majdalani et al., 1998; Prévost et al., 2007; Hammer and Bassler, 2007; Fröhlich and Vogel, 2009). Bacterial genomes typically possess 100–200 sRNAs, any one of which in P. angustum could be the cqsAPa activator. One obvious candidate to fulfill this role is the P. angustum qrr1 sRNA because there exist predicted base-pairing regions between Qrr1 and the cqsAPa stem-loop. If qrr1 activates cqsA expression, CAI-1 production would occur at LCD but not at HCD, perhaps increasing sensitivity to CAI-1 at the onset of QS. We overexpressed qrr1Pa in E. coli carrying cqsAPa but no activation occurred as measured by bioluminescence. We know our qrr1Pa construct was functional because it repressed V. harveyi luxR translation (Fig. S8). Alternatively, another small RNA for example, one analogous to Caulobacter crescentus CrfA, that is specifically induced upon carbon starvation could be involved (Landt et al., 2010). A riboswitch mechanism could also be used to regulate the cqsAPa stem-loop. In these cases, small molecules bind to mRNA riboswitch modules to alter mRNA structure, or changes in physical-chemical conditions, such as temperature, occur to stabilize alternative mRNA conformations. These changes lead to alterations in gene activity (Henkin, 2008). Known riboswitch-binding molecules include metabolites such as nucleotides, SAM, amino acids, sugars, and metal ions as well as larger polymers such as uncharged tRNAs (Serganov and Nudler, 2013). We predict a riboswitch regulatory mechanism would promote or repress CAI-1 production under particular metabolic regimes. A third possibility is that dedicated RNA binding regulatory proteins interact with and modulate cqsAPa stem-loop folding, and such proteins could themselves be subject to environmental regulation. Our preliminary results suggest that CAI-1 production is elevated in P. angustum cultures grown on non-ideal carbon sources. Screening more diverse stimuli such as pH, temperature, osmolarity, or antibiotic stress could reveal specific conditions in which CAI-1 is robustly produced. Such experiments could define the environmental cues governing CAI-1 production thereby allowing us to study the P. angustum QS response.
Material and Methods
Bacterial strains and culture conditions
P. angustum S14 and P. angustum B70 were generous gifts from Edward F. DeLong. The cqsAS sequences from these two strains are similar, and no difference in CAI-1 activity or in CqsS response could be detected. P. angustum S14 genomic DNA was used to clone cqsAPa and cqsSPa, except for in the experiment in Fig. S2, in which P. angustum B70 genomic DNA was used. cqsSVh and cqsSVc were cloned from the V. harveyi BB120 (Vh WT, Bassler et al., 1997) and V. cholerae El Tor C6706 (Thelin and Taylor, 1996). E. coli S17-1 (λ-pir) and XL-10 Gold were used as recipients in all cloning procedures. V. harveyi strain JMH603 (Vh ΔcqsA) is a ΔcqsA::Cmr mutant that does not produce CAI-1. The V. harveyi reporter strain WN1397 (ΔcqsAS::Cmr ΔluxPQ ΔluxN) carrying pLAFR2-cqsS was used in bioluminescence assays, and cqsSVh, cqsSPa and cqsSVc were expressed under the native cqsSVh promoter. Genotypes of strains and plasmids are provided in the Supporting information. Unless specified, E. coli was grown in LB medium at 37°C with shaking, and V. harveyi and P. angustum were grown in LM medium at 30°C with shaking. Antibiotic concentrations are: kanamycin, 100 mg L−1; chloramphenicol and tetracycline, 10 mg L 1 unless otherwise specified.
DNA manipulation, site-directed mutagenesis, and mutant construction
Standard procedures (Sambrook et al., 1989) were used for DNA manipulation. Oligonucleotide sequences employed in PCR, site-directed mutagenesis, and sequencing reactions will be provided upon request. cqsS constructs were each fused to the cqsSVh promoter and cloned into pLAFR2 using XbaI and BamHI. Point mutations were engineered into cqsS by overlap extension PCR (Ho et al., 1989). Plasmids carrying wild-type and mutant cqsS genes were introduced into V. harveyi WN1397 via conjugation, and the mutant cqsS alleles were maintained as exogenotes. cqsAPa variants (cqsAMet78, cqsAMet01, SL-cqsAMet01, RBS-cqsAMet01) were cloned into pEVS143 using AvrII and BamHI. Expression was controlled by an IPTG inducible Ptac promoter. All mutations engineered upstream of cqsAPa were made with the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent) according to the manufacturer’s instructions.
Bioluminescence assay
Cell-free culture fluids from V. harveyi and P. angustum were prepared from overnight cultures unless otherwise specified. Overnight cultures of E. coli strains carrying cqsAPa constructs were diluted 1:1000 in LB medium containing 50 mg l−1 kanamycin and grown at 30°C with shaking. cqsA expression was induced with 0.2 mM IPTG for ~8 hrs. Cell-free culture fluids were subsequently collected at OD600 = 1.5. For CAI-1 activity assessment in bioluminescence assays, overnight cultures of reporter strains carrying pLAFR2-cqsS were grown in LM medium with 5 mg l−1 tetracycline and diluted 50-fold with sterile medium. Cell-free culture fluids were added from 1–20% (v/v) to the diluted reporter strains. Bioluminescence and OD600 were measured using an Envison Multilabel Reader following 4.5 h incubation at 30°C with shaking. Synthetically prepared CAI-1 analogues were dissolved in DMSO.
Western blot analyses
E. coli strains carrying cqsAPa constructs were grown as described for the bioluminescence assay. 1 ml of cells were collected and resuspended in 100 μl of Bugbuster (Novagen) with 50 ug ml−1 lysozyme (Sigma) and 10 U ml−1 Benzonase Nuclease (Novagen), and combined with 4x SDS-PAGE protein loading buffer. Samples were electrophoresed on 4–20% Mini-Protein Gels (Bio-Rad), and subsequently transferred to nitrocellulose membranes. Membranes were blotted with anti-RNA polymerase beta subunit (NeoClone) and with monoclonal anti-polyhistidine antibody produced in mice (Sigma). Following exposure, films were scanned and analyzed using ImageJ software (NIH).
Measurement of the concentration of CAI-1-type molecules in culture fluids
E. coli carrying cqsAPa constructs grown in LB medium with kanamycin at 37°C overnight with shaking. Cultures were diluted 1000-fold in fresh LB medium and incubated at 30°C with shaking. At OD600 = 0.25, the cultures were induced with 0.2 mM IPTG and allowed to grow for an additional 6 h to an OD600 = ~2.0. Cells were removed by centrifugation. Synthetic C9-CAI-1 was added to 9 ml of collected cell-free fluids at 1 μM. The mixtures were extracted into 500 μl of dichloromethane. The organic layer was separated. This extract was diluted 1:1 with dichloromethane, and samples were subjected to GC-MS analysis. Calibration curves and correction factors were obtained for each molecule by preparing samples containing known concentrations of the CAI-1 type molecule together with an internal standard (C9-CA1-1) in LB medium (Ng et al., 2011). Correction factors (normalized to C9-CAI-1) were as follows: C8-CAI-1 (1.8 ± 0.7), Ea-C8-CAI-1 (0.7 ± 0.2), C10-CAI-1(1.0 ± 0.4), Ea-C10-CAI-1 (1.4 ± 0.4).
P. angustum growth and CAI-1 activity on Biolog PM Plates
P. angustum S14 was grown in LM medium with shaking overnight. Cells were collected by centrifugation at 10,000 x g. The cells were washed and resuspended in IF-0a (Biolog) containing 0.3 M NaCl and 1x Biolog redox dye mix F to reach a final OD600 of 0.06-0.07. The cell suspension was used to inoculate PM1 Microplates (100 μl per well for each carbon source). Absorbance at OD510 was measured after 36 hrs incubated at 30°C with shaking. CAI-1 activity was measured by bioluminescence assay as described using the WN1397 reporter strain carry CqsSPa supplied with 50% v/v of the P. angustum cell suspension.
Supplementary Material
Acknowledgments
We thank members of the Bassler laboratory for insightful discussions and suggestions, and especially Julie S. Valastyan for critical reading of this work. We also thank the Groves lab for use of the GC-MS. This work was supported by the Howard Hughes Medical Institute, National Institutes of Health (NIH) Grant 5R01GM065859 and National Science Foundation (NSF) Grant MCB-0343821 to B.L.B. and an HHMI International Student Research fellowship to X.K.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional supporting information may be found in the online version of this article.
References
- Barak Z, Lindsley D, Gallant J. On the mechanism of leftward frameshifting at several hungry codons. J Mol Biol. 1996;256:676–684. doi: 10.1006/jmbi.1996.0117. [DOI] [PubMed] [Google Scholar]
- Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutte CC, Crosson S. Bacterial lifestyle shapes stringent response activation. Trends Microbiol. 2013;21:174–180. doi: 10.1016/j.tim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JG, Meighen EA. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- Das B, Pal RR, Bag S, Bhadra RK. Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol Microbiol. 2009;72:380–398. doi: 10.1111/j.1365-2958.2009.06653.x. [DOI] [PubMed] [Google Scholar]
- Flärdh K, Axberg T, Albertson NH, Kjelleberg S. Stringent control during carbon starvation of marine Vibrio sp. strain S14: molecular cloning, nucleotide sequence, and deletion of the relA gene. J Bacteriol. 1994;176:5949–5957. doi: 10.1128/jb.176.19.5949-5957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flärdh K, Kjelleberg S. Glucose upshift of carbon-starved marine Vibrio sp. strain S14 causes amino acid starvation and induction of the stringent response. J Bacteriol. 1994;176:5897–5903. doi: 10.1128/jb.176.19.5897-5903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JA, Bassler BL. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol. 1999a;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999b;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- Fröhlich KS, Vogel J. Activation of gene expression by small RNA. Curr Opin Microbiol. 2009;12:674–682. doi: 10.1016/j.mib.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc Natl Acad Sci USA. 2007;104:11145–11149. doi: 10.1073/pnas.0703860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin TM. Riboswitch RNAs: using RNA to sense cellular metabolism. Genes Dev. 2008;22:3383–3390. doi: 10.1101/gad.1747308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–886. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kelly RC, Bolitho ME, Higgins DA, Lu W, Ng WL, Jeffrey PD, et al. The Vibrio cholerae quorum-sensing autoinducer CAI-1: analysis of the biosynthetic enzyme CqsA. Nat Chem Biol. 2009;5:891–895. doi: 10.1038/nchembio.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel JC, Rutherford ST, Shao Y, Utria AF, Bassler BL. Individual and combined roles of the master regulators AphA and LuxR in control of the Vibrio harveyi quorum-sensing regulon. J Bacteriol. 2013;195:436–443. doi: 10.1128/JB.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt SG, Lesley JA, Britos L, Shapiro L. CrfA, a small noncoding RNA regulator of adaptation to carbon starvation in Caulobacter crescentus. J Bacteriol. 2010;192:4763–4775. doi: 10.1128/JB.00343-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JP, Gallant J, Lindsley D, Atkinson J. Influence of the relA gene on ribosome frameshifting. Mol Genet Genomics. 2002;268:81–86. doi: 10.1007/s00438-002-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougald D, Srinivasan S, Rice SA, Kjelleberg S. Signal-mediated cross-talk regulates stress adaptation in Vibrio species. Microbiology. 2003;149:1923–1933. doi: 10.1099/mic.0.26321-0. [DOI] [PubMed] [Google Scholar]
- Ng WL, Perez LJ, Wei Y, Kraml C, Semmelhack MF, Bassler BL. Signal production and detection specificity in Vibrio CqsA/CqsS quorum-sensing systems. Mol Microbiol. 2011;79:1407–1417. doi: 10.1111/j.1365-2958.2011.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL, Wei Y, Perez LJ, Cong J, Long T, Koch M, et al. Probing bacterial transmembrane histidine kinase receptor-ligand interactions with natural and synthetic molecules. Proc Natl Acad Sci USA. 2010;107:5575–5580. doi: 10.1073/pnas.1001392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- Pompeani AJ, Irgon JJ, Berger MF, Bulyk ML, Wingreen NS, Bassler BL. The Vibrio harveyi master quorum-sensing regulator, LuxR, a TetR-type protein is both an activator and a repressor: DNA recognition and binding specificity at target promoters. Mol Microbiol. 2008;70:76–88. doi: 10.1111/j.1365-2958.2008.06389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost K, Salvail H, Desnoyers G, Jacques JF, Phaneuf E, Massé E. The small RNA RyhB activates the translation of shiA mRNA encoding a permease of shikimate, a compound involved in siderophore synthesis. Mol Microbiol. 2007;64:1260–1273. doi: 10.1111/j.1365-2958.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, van Kessel JC, Shao Y, Bassler BL. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 2011;25:397–408. doi: 10.1101/gad.2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. doi: 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Ostling J, Charlton T, de Nys R, Takayama K, Kjelleberg S. Extracellular signal molecule(s) involved in the carbon starvation response of marine Vibrio sp. strain S14. J Bacteriol. 1998;180:201–209. doi: 10.1128/jb.180.2.201-209.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiaden A, Spirig T, Hilbi H. Bacterial gene regulation by alpha-hydroxyketone signaling. Trends Microbiol. 2010;18:288–297. doi: 10.1016/j.tim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Wei Y, Perez LJ, Ng WL, Semmelhack MF, Bassler BL. Mechanism of Vibrio cholerae autoinducer-1 biosynthesis. ACS Chem Biol. 2011;6:356–365. doi: 10.1021/cb1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelverton E, Lindsley D, Yamauchi P, Gallant JA. The function of a ribosomal frameshifting signal from human immunodeficiency virus-1 in Escherichia coli. Mol Microbiol. 1994;11:303–313. doi: 10.1111/j.1365-2958.1994.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.