Abstract
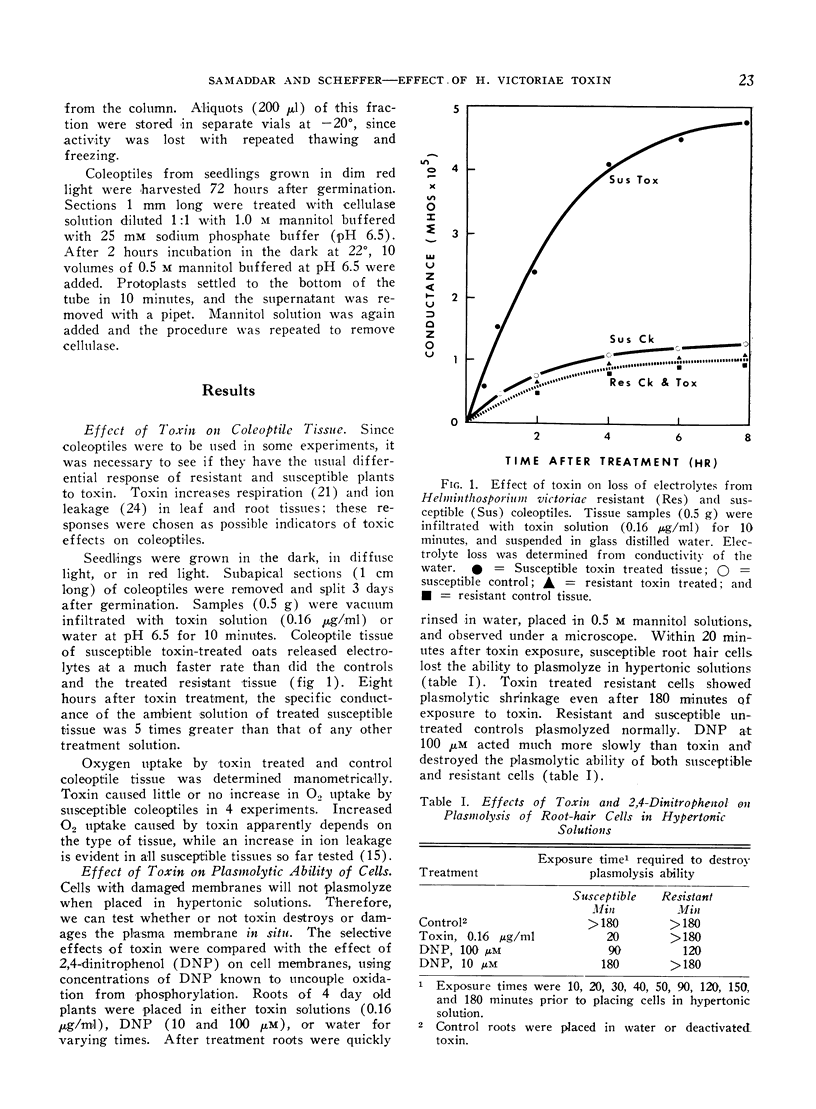
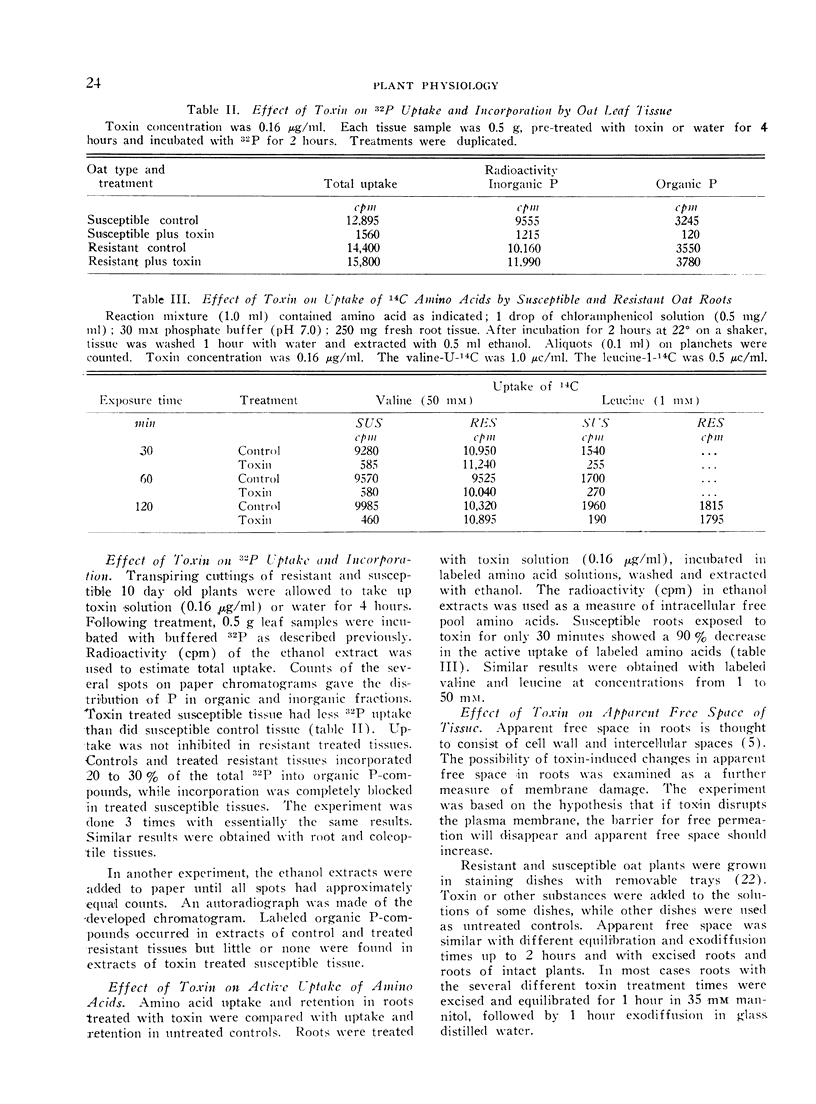
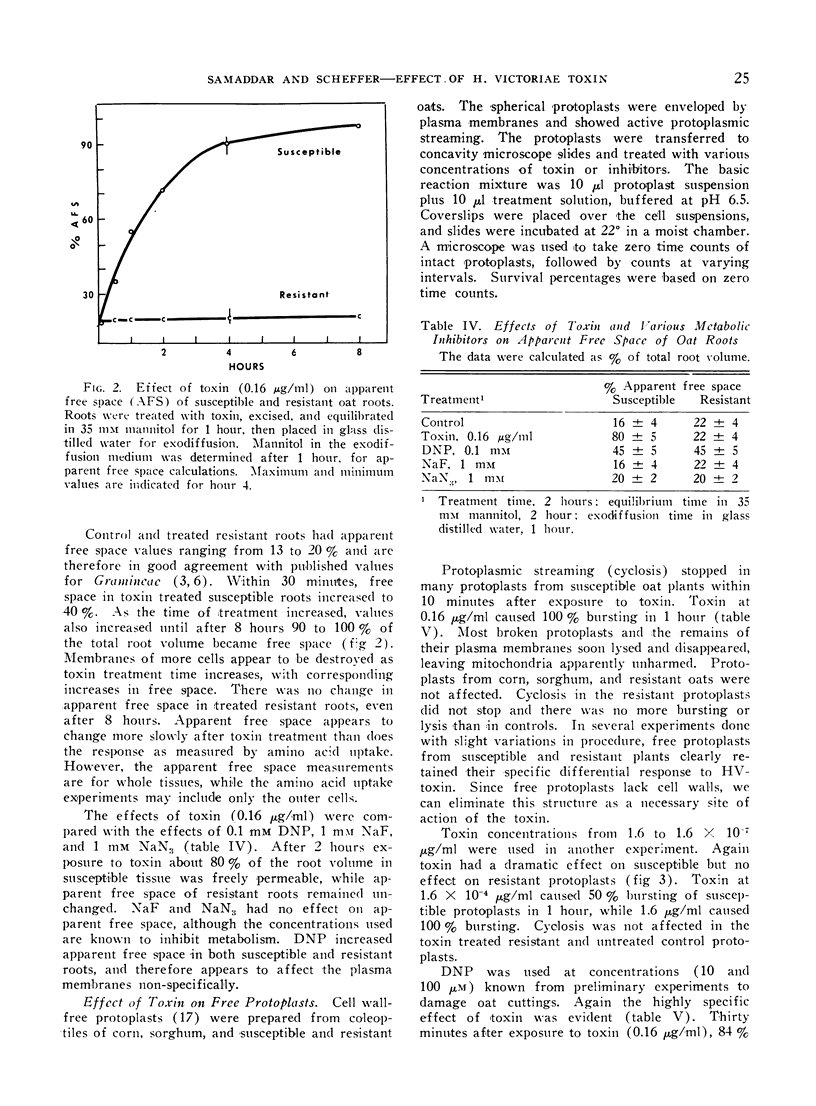
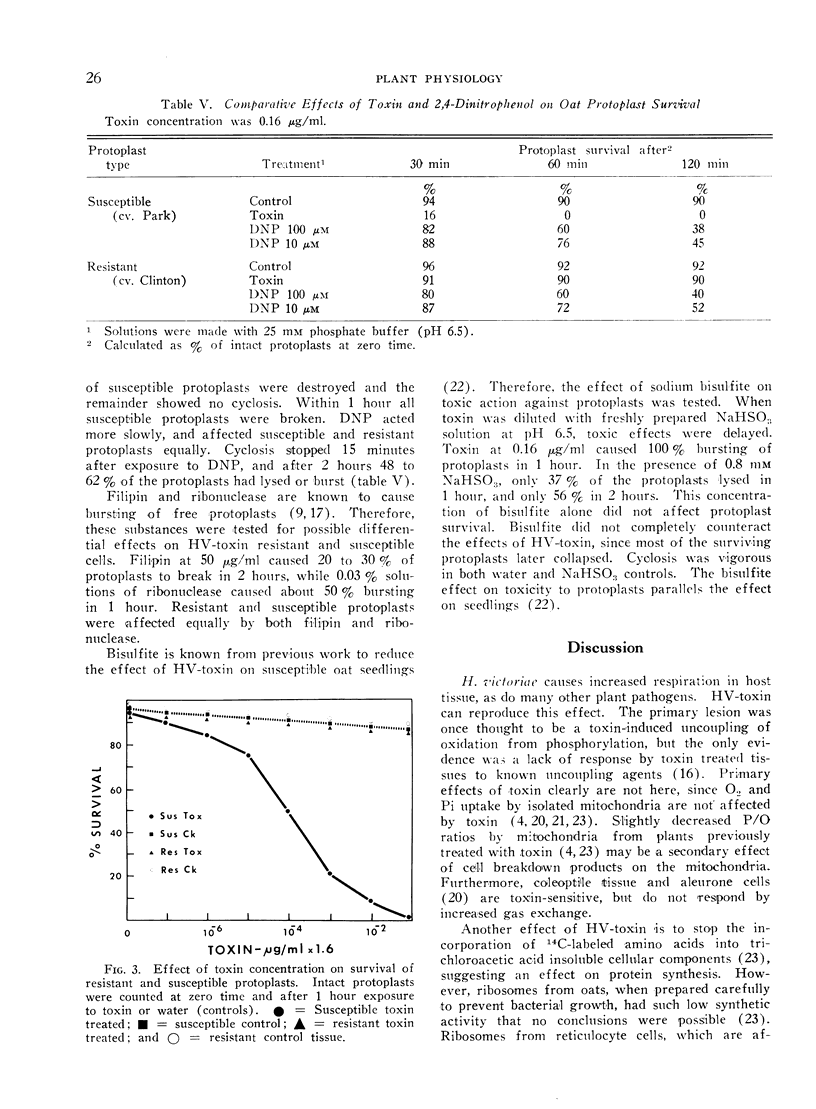
Helminthosporium victoriae toxin, which affects only hosts of the toxin-producing fungus, causes loss of electrolytes from roots, leaves, and coleoptiles of treated plants. Root hair cells lost the ability to plasmolyze after 20 minutes exposure to toxin in solution; comparable resistant cells retained plasmolytic ability during 3 hours exposure. Toxin stopped uptake of exogenous amino acids and Pi by susceptible but not by resistant tissue. Incorporation of 32P into organic-P and 14C-amino acids into protein was blocked in susceptible but not in resistant tissue. Apparent free space increased in susceptible but not in resistant roots. The increase was evident within 30 minutes, and reached 80% free space after 2 hours exposure to toxin. When cell wall-free protoplasts were exposed to 0.16 μg toxin/ml, protoplasmic streaming stopped and all plasma membranes of susceptible protoplasts broke within 1 hour. Resistant protoplasts were not affected significantly. Data support the hypothesis of a primary lesion of toxin in the plasma membrane. Effects on synthesis could result from lack of transport of exogenous solutes to sites of synthesis. It is possible that all other observed effects of toxin are secondary to membrane damage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein L., Nieman R. H. Apparent Free Space of Plant Roots. Plant Physiol. 1960 Sep;35(5):589–598. doi: 10.1104/pp.35.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Effect of polyene antibiotics on protoplasts of Neurospora crassa. J Bacteriol. 1962 Feb;83:351–358. doi: 10.1128/jb.83.2.351-358.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman O. E., Spiegelman S. ENZYME FORMATION IN PROTOPLASTS OF BACILLUS MEGATERIUM. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):698–704. doi: 10.1073/pnas.41.10.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of Organic Acids in Corn Roots II. The Cytoplasmic Pool of Malic Acid. Plant Physiol. 1966 Apr;41(4):713–717. doi: 10.1104/pp.41.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke H. H., Warmke H. E., Hanchey P. Effects of the pathotoxin victorin on ultrastructure of root and leaf tissue of Avena species. Phytopathology. 1966 Oct;56(10):1178–1183. [PubMed] [Google Scholar]
- Ruesink A. W., Thimann K. V. Protoplasts from the Avena coleoptile. Proc Natl Acad Sci U S A. 1965 Jul;54(1):56–64. doi: 10.1073/pnas.54.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. Permeability Characteristics and Amino Acid Incorporation during Senescence (Ripening) of Banana Tissue. Plant Physiol. 1966 Apr;41(4):701–708. doi: 10.1104/pp.41.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]


