Abstract
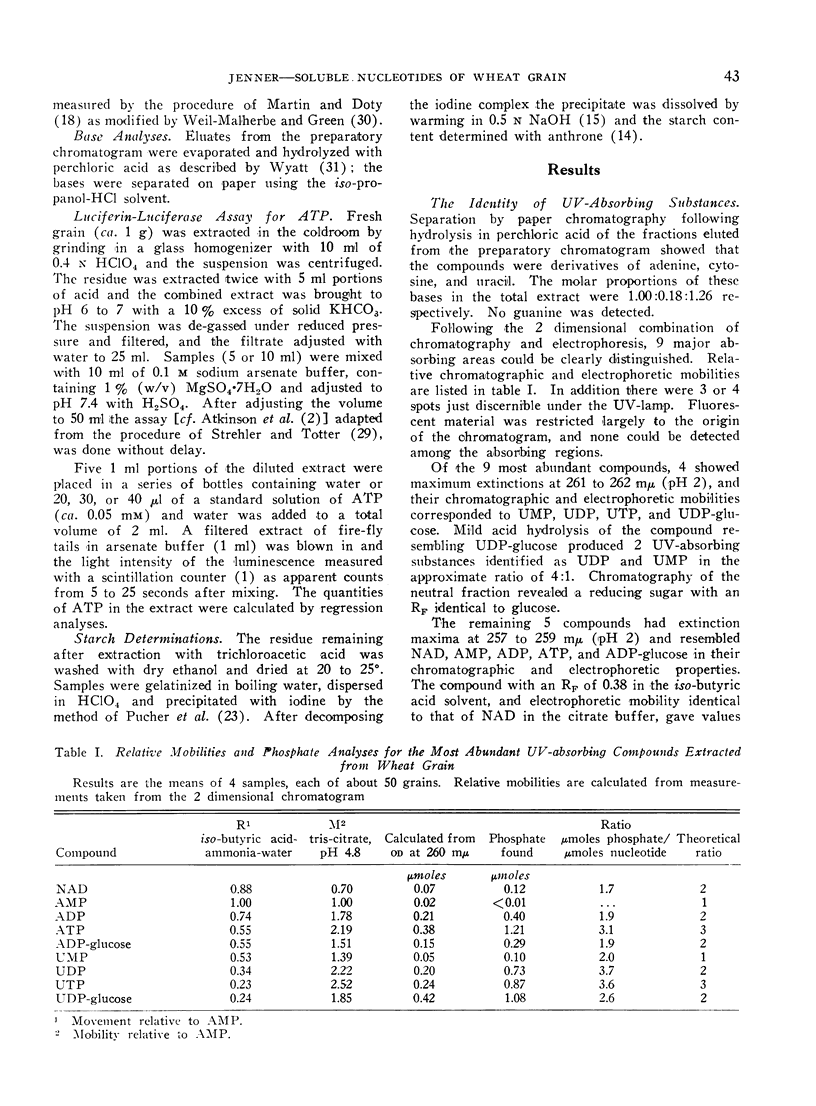
A method is described for the extraction and measurement of soluble nucleotides from wheat grain. Nucleotides were separated (80-90% recovery) by paper chromatography followed by electrophoresis. The nucleotides extracted were ADP-glucose, ATP, ADP, AMP, and NAD; UDP-glucose, UTP, UDP, and UMP with smaller quantities of cytidine nucleotides.
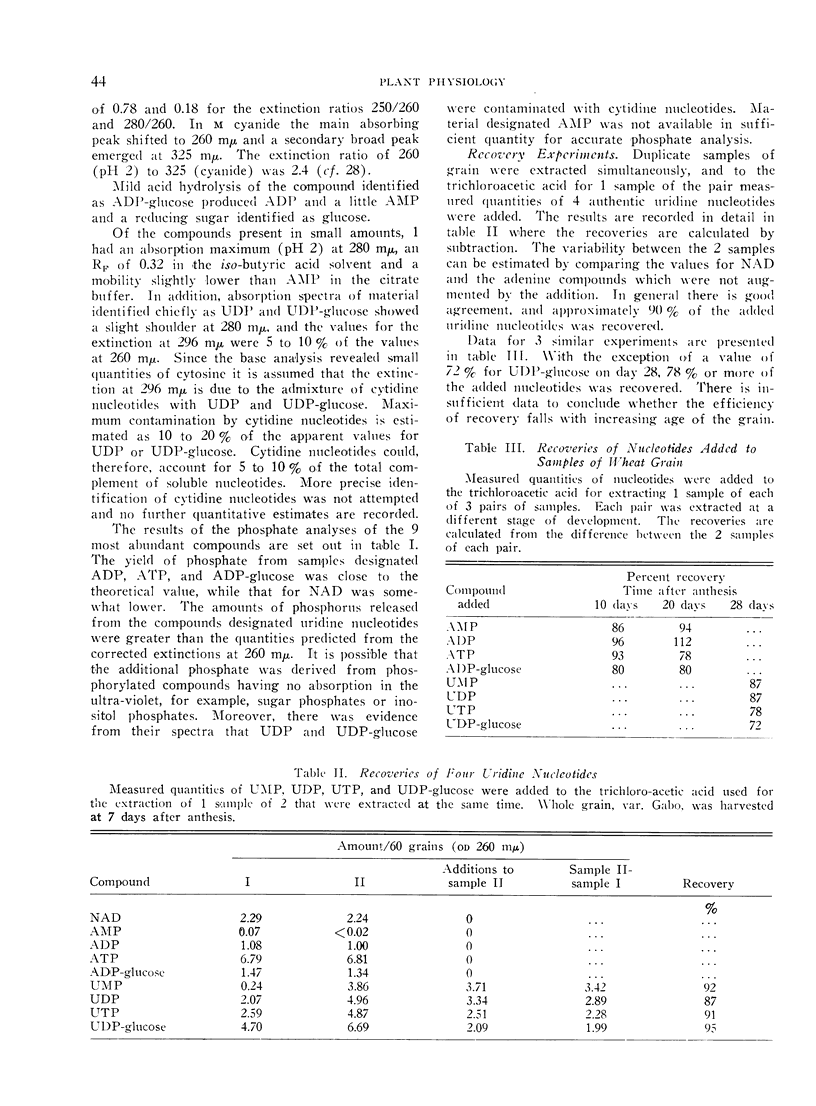
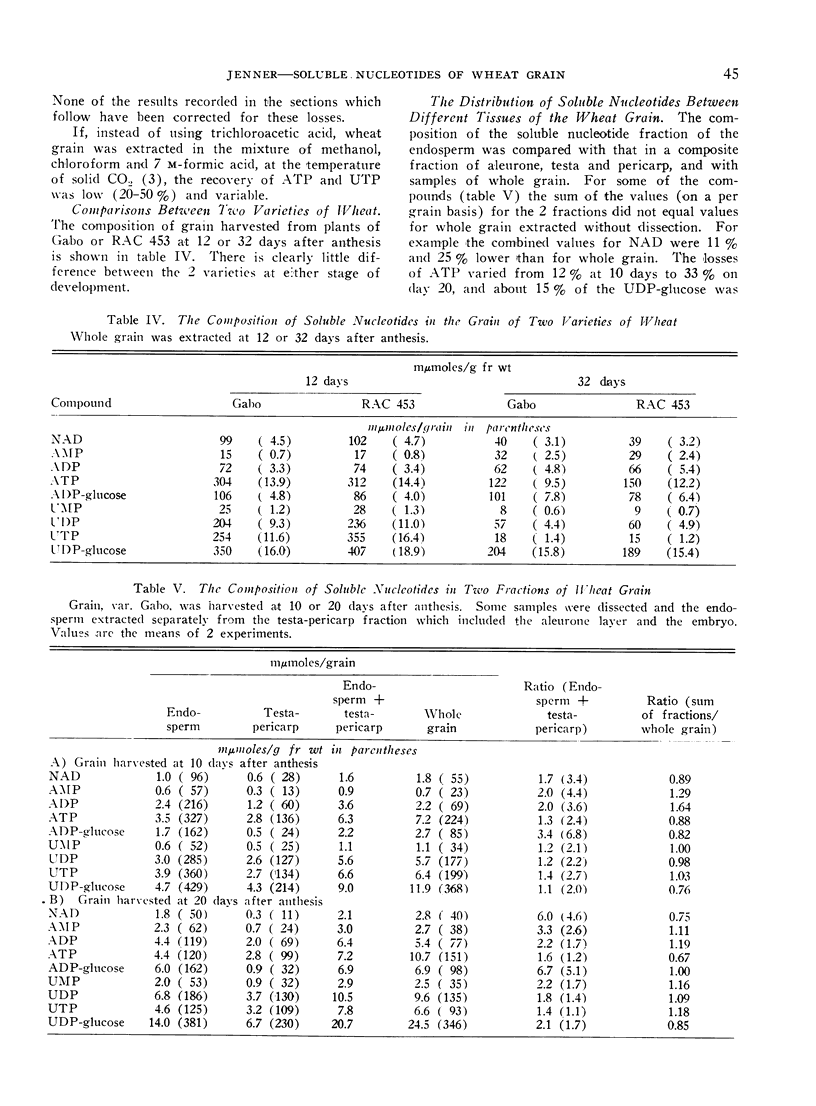
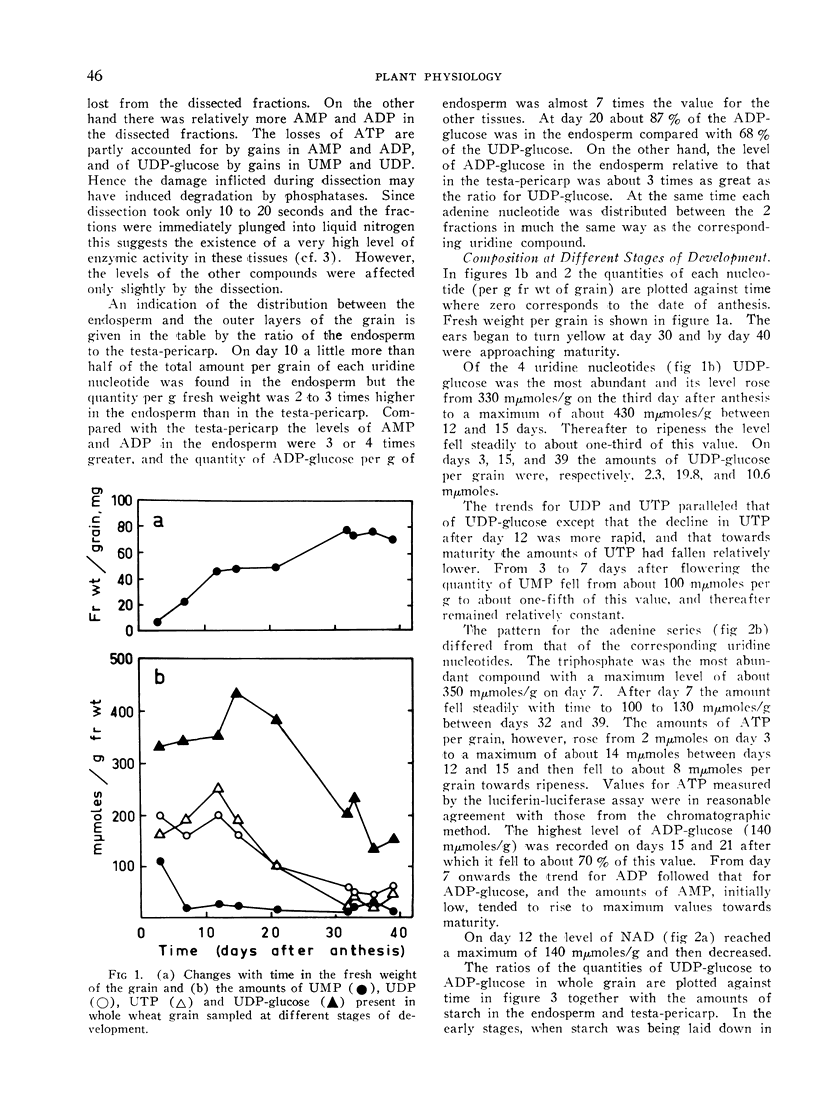
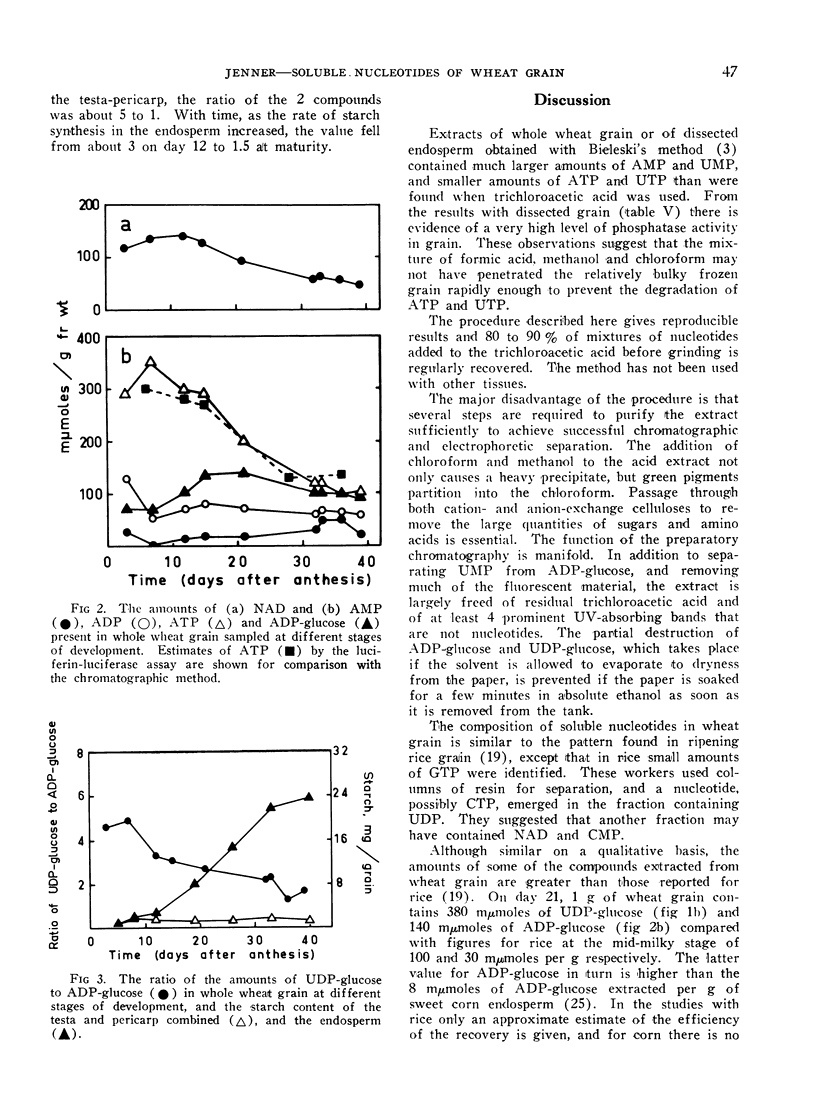
In grain sampled at 20 days after anthesis, 70% of the UDP-glucose was present in the endosperm and the remainder in the testa and pericarp; 90% of the ADP-glucose was found in the endosperm. Of the four uridine nucleotides UDP-glucose was the most plentiful and the level rose from about 330 mμmoles per g fresh weight on the third day after flowering to 430 mμmoles/g on day 12 and then fell steadily to about 140 mμmoles/g just before complete ripening. Levels of 250 mμmoles and 200 mμmoles per g fresh weight were recorded for UTP and UDP on day 12. Thereafter the content of UTP fell relatively more rapidly than either UDP or UDP-glucose.
ATP was the most abundant adenine nucleotide and from 7 days after anthesis to day 40 the quantity per g fresh weight fell from about 350 mμmoles to 100 mμmoles. The level of ADP-glucose rose to a maximum of 140 mμmoles/g between days 15 and 21 and then fell slightly towards maturity while ADP varied between 50 and 80 mμmoles/g. On day 20, coinciding with the maximum rate of starch synthesis in the endosperm, the concentration of ADP-glucose in this tissue was about 0.3 mm, and that of UDP-glucose 0.7 mm.
The relationship of these results to the mechanism of transfer of hexose units from sucrose to starch is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki S., Sotos J. F., Rearick P. D. Rapid determination of picomole quantities of ATP with a liquid scintillation counter. Anal Biochem. 1966 Feb;14(2):261–264. doi: 10.1016/0003-2697(66)90135-7. [DOI] [PubMed] [Google Scholar]
- Atkinson M. R., Eckermann G., Grant M., Robertson R. N. Salt accumulation and adenosine triphosphate in carrot xylem tissue. Proc Natl Acad Sci U S A. 1966 Mar;55(3):560–564. doi: 10.1073/pnas.55.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIELESKI R. L. THE PROBLEM OF HALTING ENZYME ACTION WHEN EXTRACTING PLANT TISSUES. Anal Biochem. 1964 Dec;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- FRYDMAN R. B. STARCH SYNTHETASE OF POTATOES AND WAXY MAIZE. Arch Biochem Biophys. 1963 Aug;102:242–248. doi: 10.1016/0003-9861(63)90177-2. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Cardini C. E. Studies on the biosynthesis of starch. II. Some properties of the adenosine diphosphate glucose:starch glucosyltransferase bound to the starch granule. J Biol Chem. 1967 Jan 25;242(2):312–317. [PubMed] [Google Scholar]
- Frydman R. B., De Souza B. C., Cardini C. E. Distribution of adenosine diphosphate D-glucose: alpha-1,4-glucan alpha-4-glucosyltransferase in higher plants. Biochim Biophys Acta. 1966 Mar 7;113(3):620–623. doi: 10.1016/s0926-6593(66)80023-1. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Biosynthesis of starch in spinach chloroplasts. Biochemistry. 1965 Jul;4(7):1354–1361. doi: 10.1021/bi00883a020. [DOI] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., MORTON R. K. The chemical composition of microsomes and mitochondria from silver beet. Biochem J. 1956 Oct;64(2):221–235. doi: 10.1042/bj0640221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURATA T., MINAMIKAWA T., AKAZAWA T., SUGIYAMA T. ISOLATION OF ADENOSINE DIPHOSPHATE GLUCOSE FROM RIPENING RICE GRAINS AND ITS ENZYMIC SYNTHESIS. Arch Biochem Biophys. 1964 Jul 20;106:371–378. doi: 10.1016/0003-9861(64)90202-4. [DOI] [PubMed] [Google Scholar]
- MURATA T., SUGIYAMA T., AKAZAWA T. ENZYMIC MECHANISM OF STARCH SYNTHESIS IN RIPENING RICE GRAINS. II. ADENOSINE DIPHOSPHATE GLUCOSE PATHWAY. Arch Biochem Biophys. 1964 Jul;107:92–101. doi: 10.1016/0003-9861(64)90274-7. [DOI] [PubMed] [Google Scholar]
- Murata T., Sugiyama T., Minamikawa T., Akazawa T. Enzymic mechanism of starch synthesis in ripening rice grains. 3. Mechanism of the sucrose-starch conversion. Arch Biochem Biophys. 1966 Jan;113(1):34–44. doi: 10.1016/0003-9861(66)90153-6. [DOI] [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECONDO E., DANKERT M., LELOIR L. F. Isolation of adenosine diphosphate D-glucose from corn grains. Biochem Biophys Res Commun. 1963 Jul 26;12:204–207. doi: 10.1016/0006-291x(63)90190-6. [DOI] [PubMed] [Google Scholar]
- RECONDO E., GONCALVES I. R., DANKERT M. SODIUM CARBONATE-SODIUM BICARBONATE BUFFER FOR THE ELECTROPHORETIC SEPARATION OF NUCLEOTIDES AND PHOSPHORIC ESTERS. J Chromatogr. 1964 Nov;16:415–416. doi: 10.1016/s0021-9673(01)82509-7. [DOI] [PubMed] [Google Scholar]
- RECONDO E., LELOIR L. F. Adenosine diphosphate glucose and starch synthesis. Biochem Biophys Res Commun. 1961 Nov 1;6:85–88. doi: 10.1016/0006-291x(61)90389-8. [DOI] [PubMed] [Google Scholar]
- RONGINE DE FEKETE M. A., LELOIR L. F., CARDINI C. E. Mechanism of starch biosynthesis. Nature. 1960 Sep 10;187:918–919. doi: 10.1038/187918a0. [DOI] [PubMed] [Google Scholar]
- RONGINEDEFEKETE M. A., CARDINI C. E. MECHANISM OF GLUCOSE TRANSFER FROM SUCROSE INTO THE STARCH GRANULE OF SWEET CORN. Arch Biochem Biophys. 1964 Jan;104:173–184. doi: 10.1016/s0003-9861(64)80052-7. [DOI] [PubMed] [Google Scholar]
- SIEGEL J. M., MONTGOMERY G. A., BOCK R. M. Ultraviolet absorption spectra of DPN and analogs of DPN. Arch Biochem Biophys. 1959 Jun;82(2):288–299. doi: 10.1016/0003-9861(59)90124-9. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., TOTTER J. R. Firefly luminescence in the study of energy transfer mechanisms. I. Substrate and enzyme determination. Arch Biochem Biophys. 1952 Sep;40(1):28–41. doi: 10.1016/0003-9861(52)90070-2. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]


