Abstract
Pax3/7 paired homeodomain transcription factors are important markers of muscle stem cells. Pax3 is required upstream of myod for lateral dermomyotomal cells in the amniote somite to form particular muscle cells. Later Pax3/7-dependent cells generate satellite cells and most body muscle. Here we analyse early myogenesis from, and regulation of, a population of Pax3-expressing dermomyotome-like cells in the zebrafish. Zebrafish pax3 is widely expressed in the lateral somite and, along with pax7, becomes restricted anteriorly and then to the external cells on the lateral somite surface. Midline-derived Hedgehog signals appear to act directly on lateral somite cells to repress Pax3/7. Both Hedgehog and Fgf8, signals that induce muscle formation within the somite, suppress Pax3/7 and promote expression of myogenic regulatory factors (MRFs) myf5 and myod in specific muscle precursor cell populations. Loss of MRF function leads to loss of myogenesis by specific populations of muscle fibres, with parallel up-regulation of Pax3/7. Myod is required for lateral fast muscle differentiation from pax3-expressing cells. In contrast, either Myf5 or Myod is sufficient to promote slow muscle formation from adaxial cells. Thus, myogenic signals act to drive somite cells to a myogenic fate through up-regulation of distinct combinations of MRFs. Our data show that the relationship between Pax3/7 genes and myogenesis is evolutionarily ancient, but that changes in the MRF targets for particular signals contribute to myogenic differences between species.
Keywords: Pax3, Pax7, muscle, zebrafish, hedgehog, somite, fgf8, dermomyotome, myf5, myod, col1a2, dlx2a, sox10
Introduction
Pax genes encode transcription factors that are broadly expressed in developing animals and often specify cell identity (reviewed in Chi and Epstein, 2002). In amniotes, the closely-related Pax3 and Pax7 genes are expressed in various dorsal tissues and Pax3 is required for patterning of the dorsal central nervous system, neural crest and skeletal muscle (Epstein et al., 1991; Franz et al., 1993; Goulding et al., 1991). In Pax3 mutant mice, migratory muscle precursors fail to exit the dermomyotome, a superficial epithelium of the somite, causing a lack of limb and certain head muscles (Borycki et al., 1999; Epstein et al., 1991). However, Pax3 mutants show only mild somitic muscle defects, despite widespread Pax3 expression in young somites (Franz et al., 1993; Goulding et al., 1994; Tajbakhsh et al., 1997; Williams and Ordahl, 1994). Elimination of Pax3 and its close homologue Pax7 has little effect on early somite myogenesis but ablates later muscle (Relaix et al., 2005). Congruently, over-expression of Pax3 can drive myogenesis (Maroto et al., 1997). Lineage tracing shows that Pax3/7-expressing cells in the dermomyotome are capable of generating muscle and dermis (Ben-Yair and Kalcheim, 2005; Gros et al., 2005; Relaix et al., 2005). So Pax3/7 is necessary for most somite-derived myogenesis. In addition to their early functions, both Pax3 and Pax7 appear to mark late myogenic cells (Kassar-Duchossoy et al., 2005). Pax7 is required for maintenance and function of many satellite stem cells (Relaix et al., 2004; Relaix et al., 2005; Seale et al., 2000). Thus, the Pax3/7-expressing amniote dermomyotome gives rise to most, if not all, body skeletal muscle and to the dermis of the back.
The evolutionary origin of the dermomyotome is obscure. Although actinopterygian fish have a dermis, a distinct myogenic dermomyotome has not been demonstrated. The lateral surface of the zebrafish somite is covered with a thin layer of external cells of unknown embryonic affiliation (Waterman, 1969). As in amniotes, zebrafish pax3 marks immature somites and subsequently becomes restricted to cells on the outer surface of the somite, raising the possibility that fish also have a dermomyotome capable of generating muscle (Devoto et al., 2006; Groves et al., 2005; Seo et al., 1998). However, the nature of the pax3/7-expressing cells remains equivocal because other potentially pax3/7-expressing cells, namely neural crest cells, traverse the superficial somite during their migration on the so-called lateral pathway (reviewed in Kelsh and Raible, 2002). Here we show that expression of both pax3 and pax7 is intrinsic to the zebrafish somite and marks the proliferative external cells that lie superficial to the myotome.
Myogenesis from amniote dermomyotome depends on myogenic regulatory factors (MRFs) of the MyoD family, several of which appear to drive formation of specific populations of early muscle fibres (Kassar-Duchossoy et al., 2004; Rudnicki et al., 1993). These populations rapidly become indistinguishable in the myotome. In the zebrafish, several early populations of muscle fibres generated within a few hours of somite formation have been precisely identified (reviewed in Stickney et al., 2000). Two populations of slow fibres derive from the medial adaxial cells of the presomitic mesoderm. The superficial slow fibres (SSFs) then migrate laterally to lie on the outer myotome surface, whereas the slow muscle pioneer fibres remain medial at the horizontal myoseptum (Devoto et al., 1996). Formation of both cell types depends on Hedgehog (Hh) signals from the ventral midline, which also promote adaxial MRF expression (Blagden et al., 1997; Coutelle et al., 2001; Du et al., 1997). At least two cell populations generate fast fibres from more lateral presomitic mesoderm cells. The lateral fast myoblasts (LFMs) appear to form dependent upon Fgf8-driven myod expression in the posterior somite. Other fast fibres in the somite do not require Fgf8 (Groves et al., 2005). In addition, a fifth muscle fibre population, the Hedgehog-independent slow fibres are formed at the epaxial and hypaxial extremes of the somite and therefore could originate from a dermomyotome-like tissue (Barresi et al., 2001). In amniotes, the signals that control pax3/7 expression and myogenesis are intimately linked. We set out to investigate the relationship between pax3/7, MRFs and formation of these early fibres in zebrafish.
Pax3 expression is dependent on signals that impinge on the dermomyotome from neighbouring tissues (Dietrich et al., 1999; Dietrich et al., 1998; Goulding et al., 1994; Pourquie et al., 1995). Midline-derived Hh signals repress Pax3, and ectopic Hh in the chick somite increases MyoD at the expense of Pax3 mRNA (Amthor et al., 1999; Johnson et al., 1994). Similarly, Pax7 is negatively regulated by notochord and Hh signalling (Incardona et al., 1998; Otto et al., 2006). Conversely, Pax3 expression levels are elevated and Myf5 mRNA and epaxial myogenesis diminished in mice with defective Hh signalling (Chiang et al., 1996). Importantly, loss of Pax3 combined with mutation at the Myf5/Mrf4 locus leads to complete failure of body myogenesis (Tajbakhsh et al., 1997).
Here we show that zebrafish early slow and fast myogenesis depends on Myf5 and Myod. Hh or Fgf8, signals that induce muscle by promoting MRF expression, suppress pax3/7 expression. In the absence of these MRFs, the number of pax3/7-expressing dermomyotome-like cells on the lateral somite surface increases. Our data reveal conservation of evolutionarily ancient mechanisms regulating somitic myogenesis between teleosts fish and mammals.
MATERIALS AND METHODS
Zebrafish lines and maintenance
Mutant lines aceti282a (Reifers et al., 1998), smub641 (Varga et al., 2001), syubx392 (Schauerte et al., 1998), ubotp39 (Baxendale et al., 2004) and cls m618 (Malicki et al., 1996) and transgenic line Tg(acta1::GFP) (Higashijima et al., 1997) were maintained on King’s wild type background. Staging and husbandry were as described previously (Westerfield, 1995).
In situ mRNA hybridisation and immunohistochemistry
In situ mRNA hybridisation was as described previously (Coutelle et al., 2001; Groves et al., 2005). Probes were mylz2 (Xu et al., 1999), pax3, pax7 (Seo et al., 1998), sox10 (Dutton et al., 2001), fgf8 (Groves et al., 2005), dlx2a (Kelsh and Eisen, 2000), col1a2 (Le Guellec et al., 2004), myod (Weinberg et al., 1996). Embryos for immunohistochemistry were fixed in 4% PFA for 30 min or Carnoy’s (Barresi et al., 2000) and cryosectioned and stained as described (Blagden et al., 1997; Groves et al., 2005). Primary antibodies were MF20 (Sigma), A4.1025 (Blagden et al., 1997), F59, S58 (Devoto et al., 1996), H3P (Upstate Bioscience), EB165 (Blagden et al., 1997), monoclonal Pax7 (anti-chicken, DSHB) or Pax3/7 (DP312, Davis et al., 2005). Rabbit polyclonal anti-human Myf5 detects Myod in the zebrafish, as shown in Fig. 6A, and is hereafter referred to as anti-Myod (Santa Cruz, C-20, sc-302). HRP-conjugated (Vector) or Alexa-conjugated subclass-specific (Molecular Probes) secondary antibodies were used with Citifluor mountant (Agar). Confocal images were collected on a Zeiss LSM510. For electron microscopy, embryos were fixed in 2.5% glutaraldehyde for 4 hours, postfixed in osmium tetroxide for 90 min at 4°C, embedded, sectioned at 0.1 μM, stained with uranyl acetate and lead citrate and viewed on a Hitachi H7600.
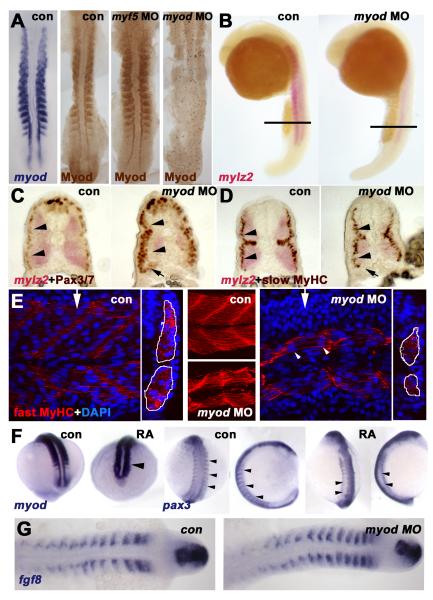
Figure 6. Myod is required for normal fast muscle differentiation and suppression of Pax3/7.
In situ mRNA hybridisation for myod, mylz2 myosin light chain, fgf8 or pax3 or immunodetection of Myod, Pax3/7, slow or fast MyHC viewed in dorsal flatmount (A,G 15s), lateral wholemount (B, 26s dorsal to right), transverse section of wholemount (C,D, 26s dorsal to top), confocal stack (E, middle) or section in lateral view (E, 24hpf medial somite anterior to left, dorsal at top, with transverse projection at white arrows shown to right) and wholemount (F, mid somitogenesis, anterior to top). A. Myod-specific immunoreaction co-localises with myod mRNA. Injection of myod morpholino oligonucleotide (MO) ablates Myod protein, whereas myf5 MO enhances Myod immunoreaction. B-D. Myod MO reduces mylz2 mRNA in fast muscle (B-D), without affecting slow muscle differentiation (D). Cryosections at the level indicated in B reveal reduced lateral migration of slow fibres (D), correlating with increased lateral Pax3/7 expressing cells (C). Pax3/7 expression in neural tube/crest appears unaffected. Note that all fast fibres appear medial to slow fibres, that most Pax3/7+ cells lie lateral to slow fibres (arrowheads C,D), but that Pax3/7+ cells accumulate ventrally next to the residual fast muscle (arrows C,D). E. Similarly, myod MO reduces the quantity of muscle labelled for fast MyHC (outlined in white in transverse projections). Note that some fibres still appear multinucleate (arrowheads). F. Exposure of embryos to 10−7 M retinoic acid up-regulates myod mRNA in lateral presomitic mesoderm and somite (arrowhead, left pair, 15s dorsal view of tailbud), RA reduces pax3 mRNA in nascent somites of 13s embryos (arrowheads, shown at left in dorsal view and to right in lateral view). Note the up-regulation of pax3 signal in neural tissue. G. Myod MO has no effect on fgf8 mRNA accumulation.
Western Blots
Dechorionated, deyolked 24 hpf embryos were homogenized manually on ice for 10 min in standard lysis buffer, centrifuged and the equivalent of 10 embryos run on each lane of a 10% acrylamide denaturing gel. After electroblotting onto nitrocellulose (Amersham), strips were blocked in 5% milk powder for 1 hour, washed, and incubated with A4.1025 (1:20), anti-Pax7 (1:20) or DP312 (1:200) for 1 hr at room temperature. After washing, primary antibody was detected with horseradish peroxidase-conjugated goat anti-mouse IgG F(ab)2 and ECL kit (Amersham).
Embryo Manipulations
Embryos were injected at 1-2 cell stage with 2.5 mg/ml stock morpholino to fgf8 (gagtctcatgtttatagcctcagta, 7-10 ng), myod (atatccgacaactccatcttttttg, 2-4 ng), myf5 (gatctgggatgtggagaatacgtcc, 2-4 ng) and rhodamine dextran as described (Westerfield, 1995). Cyclopamine (100 μM or ethanol/solanidine controls) was added to dechorionated embryos placed in 1% agarose-coated dishes from 50% epiboly unless otherwise stated. Embryos were incubated in chorions in 10−7 M all-trans RA (Sigma) from 75% epiboly to 13 or 15s in the dark, before fixation (Hamade et al 2006). Cell quantitations were compared by one way ANOVA followed by the indicated t-tests (two tailed, unbalanced, unequal variance).
RESULTS
Pax3/7 gene expression marks the superficial somite surface
Several reports have shown that pax3/7 genes are expressed in developing zebrafish somites (Devoto et al., 2006; Groves et al., 2005; Seo et al., 1998). We examined the relative timing and location of pax3 and pax7 expression during somitogenesis with respect to the terminal differentiation of muscle. Pax3 expression initiates in the anterior presomitic mesoderm shortly after tailbud stage, and is broadly expressed in both anterior and posterior regions of newly-formed somites until the end of somitogenesis (Fig. 1A). As each somite matures, pax3 transcripts rapidly become down-regulated in the posterior somite, where myod is expressed (Fig. 1A; Groves et al., 2005). Pax3 mRNA is not detected in adaxial cells in the posterior presomitic mesoderm (Fig.1A, right panels). Pax7 expression commences in each somite several hours later than pax3, first appearing between the 7 and 12 somite stages, when it is observed in the anterolateral region of the oldest somites in similar cells to those expressing pax3 (Fig. 1B,D). Thus, pax3/7 mRNA appears to be excluded from regions of the somite that strongly express myogenic regulatory factors (MRFs).
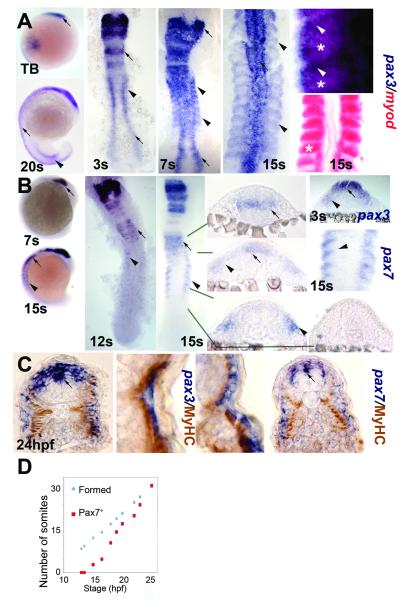
Figure 1. Pax3 and pax7 expression becomes restricted to the surface of zebrafish somites.
In situ mRNA hybridisation for pax3 (A,C left, blue), pax7 (B, C right) and myod (A, red) or immunodetection of slow muscle (C, brown). Wholemounts (A,B) are lateral views, dorsal to left. Flatmounts (A,B) are dorsal view, anterior to top. Transverse cryosections of wholemount-stained embryos (B,C) have dorsal to top. A,B. Pax3 and pax7 mRNA in neural tissue (arrows) and somites (arrowheads). Wholemount embryos show how pax3 is broadly expressed in new somites, whereas pax7 increases after pax3 mRNA becomes restricted to the anterolateral somite. In high magnification views of somites, note the distinct location of myod in posterior somite (asterisks) and pax3 in anterior (arrowheads). C. Pax3 and pax7 expression is superficial to slow myosin. Low magnification caudal somite; high magnification rostral somite. D. Plot of number of somites with pax7 mRNA signal in embryos of each developmental stage. Expression is always in a single series of rostral somites. Note the lag in pax7 expression compared to somite formation.
Fast muscle differentiation and lateral migration of slow fibres begins in rostral somites at mid-somitogenesis and rapidly progresses caudally in an apparent wave. By about 20s, the oldest somites consist mainly of muscle fibres extending from anterior to posterior somite border (Henry and Amacher, 2004). During this time, a change in pax3/7 expression also passes along the somites from rostral to caudal, clearing pax3 and pax7 mRNA from most of the anterior somite and restricting it to the lateral somite surface, superficial to slow MyHC (Fig. 1B,C). Expression of pax3/7 in superficial somite cells is confirmed by the presence of Pax3/7 proteins, recognised with a family-specific antibody, in the nuclei of cells of this layer (Fig. 2A-C). Whereas Pax7 protein is clearly present in this layer, the absence of a Pax3-specific antibody means we can not be sure whether Pax3 protein is present in superficial cells (Fig. 2B,C). Pax3/7 protein is also detected in neurectodermal tissue, and is particularly abundant in neural crest cells, a few of which commence migration over the lateral somite surface by 24 hpf (Fig. 2B, see below). There is strikingly intense Pax3/7 antibody labelling of small numbers of neural crest cell nuclei in the dorsal region at this stage, whereas more numerous weakly labelled nuclei are uniformly distributed over the somite surface, suggesting a separate somite cell layer (Fig. 2B). Pax3/7 gene expression thus undergoes dynamic changes correlating in space and time with muscle fibre patterning and migration in successively formed somites.
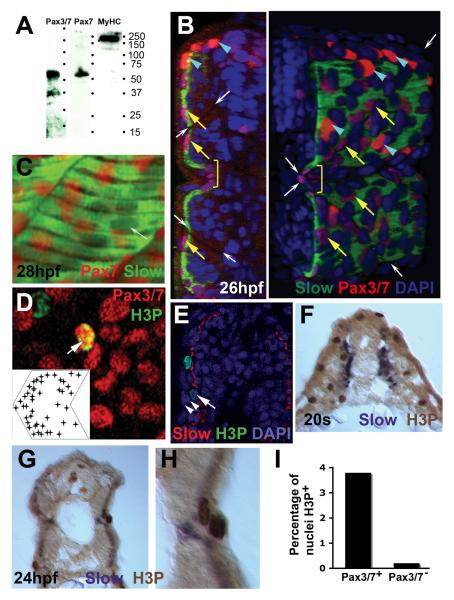
Figure 2. Pax3/7 protein accumulates in nuclei on the somite surface.
Immunodetection of slow muscle (B,C, green; E, red; F-H, purple), Pax7 (C, red), Pax3/7 (B,D, red) or H3P (D,E, green; F-H, brown). Wholemount 24 hpf confocal images (B-E) in transverse reconstruction (B,E left), lateral view (C,D, anterior to left) or rendered 3D reconstruction (E right) or transverse cryosection at 20 som (F) or 24 hpf (G,H) are shown with dorsal to top. A. Western blot of 24 hpf embryo proteins reveals a similar ~50 kD band with both Pax7 and Pax3/7 antibodies. MyHC shows the specificity of the reagents. B. Confocal images reveal two types of Pax3/7 expressing nuclei (red/purple). Left panel, transverse section of a yolk extension level somite showing superficial location of Pax expressing cells. Right panel, rendered ¾ view showing infrequent intensely-labelled red nuclei located dorsal to the neural tube and lateral to the dorsal somite (blue arrowheads). More abundant weakly-labelled purple nuclei are evenly spread over the lateral somite surface (yellow arrows), but tend to congregate near somite borders (indicated by white arrows) and at the horizontal myoseptum (yellow bracket). Note weak Pax3/7 immunoreactivity in nuclei of dorsal neural tube, floor plate and between somite and neural tube. The latter may be medially-migrating neural crest. C. A short confocal stack showing unlabelled slow muscle fibre nuclei (white arrow) and Pax7 immunoreactivity (red). D. Pax3/7 (red) and phosphohistone H3 (green) dual stained nuclei (arrows). Inset plots distribution of Pax3/7 and H3P dual labelled nuclei on a schematised somite from analysis of somites 12 and 13 in 20 wild type fish. Nuclei with only H3P are generally in the epidermis. E. The non-epidermal nature of an H3P-labelled nucleus (arrow) superficial to the slow fibre layer is demonstrated by the two layers of overlying epidermal cell nuclei (arrowheads). F-I. At 20s stage, abundant H3P is present in the lateral somite, which consists chiefly of undifferentiated fast muscle precursors (F). After fast fibre formation and slow fibre migration, H3P is generally restricted to the somite surface lateral to slow myosin (G). Note the presence of two dividing cells in adjacent cell layers, confirming that somitic external cells divide (H). Quantification of the fraction of DAPI-labelled nuclei in 80 somites 12-16 of 24 hpf embryos that express H3P in Pax3/7+ or Pax3/7− cells. Total H3P+ nuclei counted were 114 and 38, respectively. Somites in this region and stage contain 271 nuclei ± 19 sem, n=4, estimated from confocal stacks (I).
Pax3/7 expressing cells are proliferative
The superficial layer of pax3/7 expressing cells underlies the ectoderm and overlies the slow muscle fibres as shown by the location of pax3 and pax7 transcripts and protein immediately adjacent to slow myosin heavy chain (MyHC)-expressing cell layer (Figs 1C,2B; Devoto et al., 2006; Groves et al., 2005). A proportion of cells containing Pax3/7 express the mitotic marker, phosphohistone H3 (H3P: Fig. 2D-I). Moreover, mitotic cells are present within the somite superficial to slow muscle both early, prior to slow fibre migration to the lateral myotome surface, and late when only the external cell layer remains undifferentiated. H3P-expressing cells are rarely observed within the myotome itself at these stages, but are spread uniformly across the dorsoventral extent of the lateral somite surface in nuclei weakly positive for Pax3/7 (Fig. 2D-I). Quantification shows that almost 4% of Pax3/7 expressing somite cells contain H3P, whereas only 0.2% of somite nuclei lacking Pax3/7 react for H3P. As H3P marks nuclei in M-phase, a period that occupies a small fraction of total cell cycle time, many, perhaps most, Pax3/7 expressing somite cells are in the cell cycle. Thus, there is a correlation between Pax3/7 expression and cell replication.
Pax gene expression also marks dorsal neural tissue
Pax3 and pax7 are each expressed in the dorsal neural tube at various rostrocaudal locations, including spinal cord (Figs 1A-C,2B). Neural expression of pax3 mRNA and protein commences prior to somite condensation at each rostrocaudal level. However, pax7 expression is delayed relative to pax3, such that dorsal neural expression begins anterior to the newer somites. At 15s, no neural keel expression of pax7 is observed posterior to the hindbrain, where pax7 appears to mark rhombomeres in a complex and dynamic striped pattern (Fig. 1B). Subsequently, pax7 mRNA and protein begins to be expressed in a domain of dorsal neural tissue in the spinal cord but, in contrast to pax3, this expression is not as high as in the emerging neural crest cells. Expression of the pax genes persists until at least 24 hpf in dorsal neural tube, including at least some precursors of neural crest cells, which migrate out of the dorsal spinal cord and follow medial and lateral migratory paths around the nascent somites (Raible et al., 1992).
Superficial somitic Pax3/7 precedes neural crest cell migration on medial and lateral pathways
To address the possibility that some pax gene expression associated with somites is accounted for by neural crest cells, we first examined independent markers of neural crest cells. Ectomesenchymal neural crest marked by dlx2a is absent from trunk and tail levels except in the tail tip during caudal fin formation until after 27 hpf (Fig. 3A). Non-ectomesenchymal neural crest is found all along the body axis and requires expression of Sox10 transcription factor for its migration out of the neural tube (Dutton et al., 2001). Sox10 is expressed in the dorsal neural tube at trunk levels from 5s, and is then observed to spread ventrally in cells adjacent to over the somites on both their medial aspect, between somite and neural tube/notochord complex, and their lateral aspect next to the surface ectoderm (Fig. 3A). However, comparison with pax gene expression reveals that expression of both pax3 and pax7 covers the surface of somites at rostrocaudal levels where sox10 expression has yet to begin to spread ventrally (Fig. 3A). Congruently, electron microscopy reveals two morphologies of cells between the most superficial muscle and the epidermis, thin external cells closely apposed to the SSFs and rounded cells with less obvious adhesion to the somite. The rounded cells are more prominent in rostral somites at 24 hpf and are frequently apposed to the lateral surface of external cells (Fig. 3B and data not shown). Thus, neural crest does not appear to account for superficial somitic expression of pax3/7 genes: somite cells themselves express pax genes.
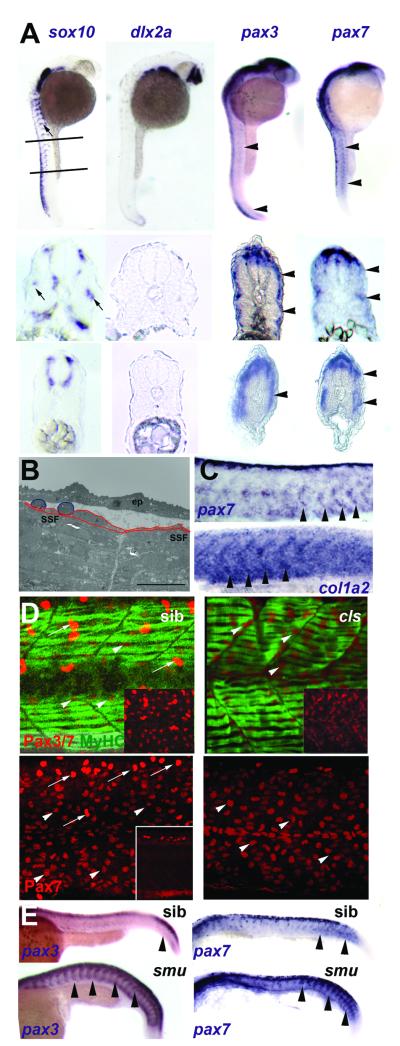
Figure 3. Neural crest does not account for superficial somite pax3/7 expression.
A. In situ mRNA hybridisation at 27 hpf for sox10, dlx2a, pax3 and pax7. Upper panels show lateral views, dorsal to left anterior to top, lines indicate locations of cryosections in lower panels. Note the punctate distribution of sox10 expression (arrows) on lateral pathway only in anterior sections compared with uniform distribution of pax3/7 mRNA at all axial levels (arrowheads). B. Electron micrograph of longitudinal section of 24 hpf somite revealing rounded cells (blue) overlying flattened external cells (red) superficial to myofibril-containing slow fibres (SSF). ep, epidermis. Scale bar = 10 μm. C. In situ hybridisation of 27 hpf embryos reveal the increase of col1α2 as pax7 declines (arrowheads). D. Lateral view, dorsal to top anterior to left of confocal stacks of superficial region of wholemount immunodetection of Pax3/7 (red, upper panels) or Pax7 (red, lower panels) and myosin (MF20, green) of 24 hpf sibling (left) and colourless (right) embryos. Note the strong Pax-expressing cells (putative neural crest cells, arrows) are missing in cls, whereas the abundant weak Pax-expressing cells are still present (arrowheads). Insets show Pax channel alone at superficial (upper insets) or deep (lower inset) level within the somite. E. Lateral views of wholemount in situ mRNA hybridisation for pax3 and pax7 in smoothened mutants and control siblings at 24 hpf. Note the persistence of abundant mRNA in the somitic region as somites mature in the absence of slow muscle (arrowheads).
Shortly after somite expression of pax7 commences, expression of collagen1α2, a marker of dermal and other connective tissue, increases in a chevron pattern reminiscent of somitic cells (Fig. 3C). Analysis of Pax7+ nuclei also suggests a distinction between neural crest cells expressing pax genes and the superficial somite cells. A wave of large irregularly-shaped nuclei with strong Pax7 signal could be observed sweeping over the somite in successively more anterior somites. Beneath these cells are a layer of more numerous, but smaller nuclei with weaker Pax7 protein labelling (Figs 2B,C;3D). These data strongly suggest that neural crest cells migrate directly over the pax-expressing superficial somite cells.
Neural crest cells do not account for pax3/7 expression on the somite surface
To prove that pax expression in somitic regions is not neural crest derived, we examined colourless (cls) mutant fish, which have a null mutation in the sox10 gene that is essential for non-ectomesenchymal fates, the sole derivatives of trunk neural crest (Dutton et al., 2001). Cls somites have no change in early trunk pax3 or pax7 expression or weak Pax3/7+ cells, but lack the large strongly Pax3/7+ nuclei at later stages (Fig. 3D and data not shown). These data confirm that most somitic pax3 and pax7 expression is not in neural crest cells, yet appears to be in cells superficial to the differentiated muscle of the zebrafish somite.
Slow muscle fibres are not required for pax3/7 expression
To address the possibility that lateral pax3/7 expression is in SSFs, we examined co-localisation of Pax3/7 with slow markers. SSFs migrate to the surface of the myotome at about the time that pax3 expression becomes restricted to the superficial somite surface (Devoto et al., 1996; Groves et al., 2005). SSFs have a single large oval nucleus that is usually located in the centre of the fibre (Roy et al., 2001). As the weak Pax-expressing nuclei are small, rounded and often lie near the ends of fibres at the somite borders, they are unlikely to represent SSF nuclei (Fig. 2C). In addition, there are twice as many weak Pax3/7 nuclei as SSF nuclei (around 40 compared to 20 per somite, Devoto et al., 1996; Figs 2B and 4B). To eliminate the possibility that some Pax3/7 expression might be in SSFs, we examined pax expression in smu (smoothened) mutants that entirely lack slow muscle due to the failure of Hedgehog signalling (Barresi et al., 2000; Chen et al., 2001; Varga et al., 2001). Mutants have apparently up-regulated pax3 and pax7 mRNA and Pax3/7 protein at the somite surface at stages after fast muscle differentiation (Figs 3E,4B). Thus, superficial pax expression does not require slow muscle.
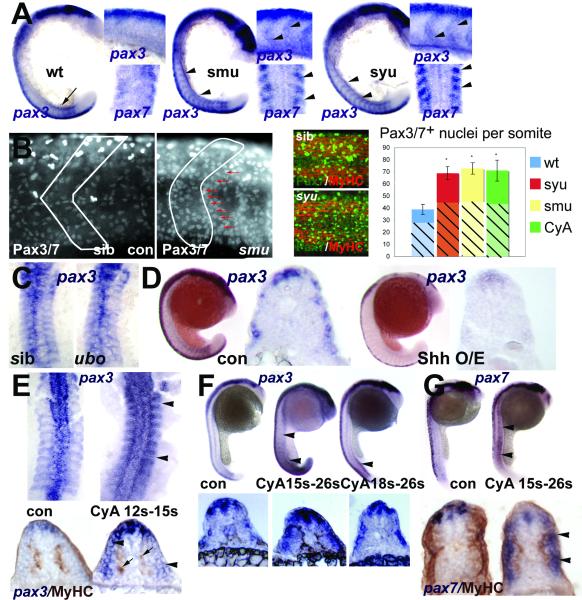
Figure 4. Hedgehog signalling positively regulates somitic pax3/7 gene expression.
A. Flatmounts of wholemount in situ mRNA hybridisation for pax3 (at 18s main panel, dorsal to left; upper inset, dorsal to top) and pax7 (at 15s lower inset dorsal view of somites 3-6) in wild type, smu and syu mutant embryos. Note the transient expression of high level pax3 in nascent somites in wild type (arrow), and its persistence in anterior somites of mutants, particularly at the somite borders (arrowheads). B. Confocal immunodetection of Pax3/7 protein increase in somites of 24 hpf smu (left panels, somite border outlined) and syu (central panels) embryos. Note the clustering of Pax-expressing cells near the anterior somite border (red arrows). Graph shows quantification of number of nuclei labelled with Pax3/7 (plain strong colour) or Pax7 (hatched pastel shade) in each condition from somites 12-16. Note that the Pax7 antibody gives less signal and more background, which probably explains the lower counts. Counts of three somites in 4-7 animals, error bars show standard deviation. Asterisks: P < 0.001. C. Pax3 expression is indistinguishable in dorsal flatmounts of 15s u-boot and sibling embryos. D. Sonic hedgehog mRNA injection into the early embryo decreases pax3 mRNA at 18s. Left panels wholemount, dorsal to left. Right panels, wholemount cryosection at yolk extension level. E-G. Transient exposure to cyclopamine at the indicated stages leads to localised up-regulation of somitic pax3 (E,F) and pax7 (G). Upper panels wholemounts, dorsal to left. Lower panels, wholemount cryosection, dorsal to top. E. Dorsal flatmount showing that treatment from 12s to 15s up-regulates pax3 in all somites (arrowheads) without affecting slow muscle formation (arrows, lower panels). F. Treatment from 15s up-regulates pax3 along most of rostrocaudal axis at 26s (arrowheads), whereas treatment from 18s has little effect in anterior somites, shown in transverse section below. G. Cyclopamine from 15s up-regulates pax7 throughout the axis at 26s, but only in somitic tissue superficial to the slow muscle (arrowheads).
Hedgehog signalling suppresses pax3/7 expression in the lateral somite
In smu embryos, or those treated with cyclopamine, a Smoothened inhibitor, expression of pax3 and pax7 is not only increased at 24hpf, it is clearly up-regulated from early stages at both mRNA and protein levels (Fig. 4A,B). This up-regulation reflects an increase in the number of expressing nuclei (Fig. 4B), raising the possibility that adaxial cells failing to become slow fibres might contribute to an enhanced pax domain by remaining in a precursor state. However, neither adaxial cells nor differentiating slow fibres express pax7 (Fig. 1B, right panels). To address this possibility more fully, we examined syu mutants that lack Sonic hedgehog and slow muscle in the tail yet have relatively normal numbers of SSFs in anterior somites (Coutelle et al., 2001; Lewis et al., 1999b). In these embryos, pax3 and pax7 are up-regulated as strongly as in smu throughout the axis, despite the presence of near-normal numbers of SSFs in anterior somites (Fig. 4A,B). Conversely, ubo mutants that experience Hh signalling but have defective slow muscle due to the lack of the downstream transcription factor Prdm1 (Blimp1) do not show a pax3 change (Fig. 4C) (Baxendale et al., 2004). Thus, the up-regulation of pax expression appears independent of slow fibres.
Over-expression of Shh by mRNA injection converts much of the somite to slow muscle (Blagden et al., 1997; Du et al., 1997). Shh over-expression also suppresses pax3 and pax7 in the somite, confirming that these genes are not expressed in slow muscle (Fig. 4D and data not shown).
Hh signalling is required until the time of fast muscle differentiation to suppress pax expression
We employed the drug cyclopamine, that blocks Hh signalling by binding to Smoothened (Chen et al., 2002), to determine when Hh signalling is required for pax down-regulation. Application of cyclopamine at any stage prior to 18s leads to more pax3 and pax7 mRNA and Pax3/7 protein (Fig. 4B,E-G). Early application phenocopies smu, as expected (Fig. 4B). Transient application after slow fibre formation still up-regulates pax3/7, without affecting slow fibre differentiation (Fig. 4E,G). Somitic up-regulation occurs throughout the rostrocaudal axis after application of drug at 15s (Fig. 4F,G). Strikingly, however, application at 18s up-regulates pax genes in posterior but not anterior somites, again without affecting SSF formation (Fig. 4F,G). Application of cyclopamine after 20s has no effect on either pax gene (data not shown). Thus, there appears to be a critical event occurring in each somite that sweeps from rostral to caudal around the 18s stage, after which the somitic pax expression becomes Hh signalling insensitive. This timing coincides with the initiation of fast muscle terminal differentiation (Blagden et al., 1997).
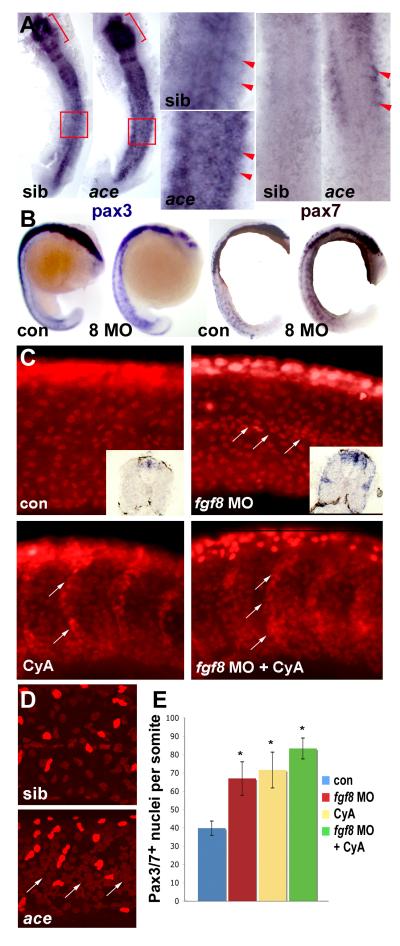
Fgf8 signalling suppresses pax3/7 expression
We previously reported that treatment of embryos with the Fgf receptor antagonist SU5402 leads to pax3 up-regulation (Groves et al., 2005), similar to that caused by reduced Hh signalling. This up-regulation appears to be due to lack of Fgf8 signalling, because Fgf8 morpholino-injected or ace (fgf8) mutant embryos have raised pax3 mRNA, particularly in the anterior somite where expression is normally most abundant at 15 som (Fig. 5A,B). Moreover, pax7 mRNA and Pax3/7 protein are also elevated in situations where Fgf8 signalling is disrupted (Fig. 5A-D). There is a twofold increase in the number of somite cells with Pax3/7 in their nuclei at 24 hpf, and increased mRNA persists at the lateral somite surface at 33 hpf (Fig. 5E). The extra Pax3/7-expressing cells are located preferentially around the horizontal myoseptum, in contrast to the somite border location of the extra cells when Hh signalling is reduced, perhaps because the extra external cells fill available space (Figs 4,5).
Figure 5. Fgf8 signalling negatively regulates pax3/7 expression.
In situ mRNA hybridisation for pax3, pax7 or immunodetection of Pax3/7 in ace mutants or embryos injected with fgf8 MO or vehicle, followed by treatment with cyclopamine or vehicle and analyzed at 15s (A, dorsal flatmount anterior to top), 18s (B, lateral wholemount dorsal to left), 24 hpf (C) or 26 hpf (D, lateral confocal stacks of midbody somites, dorsal to top, anterior to left). A. Both pax3 and pax7 mRNA are up-regulated in ace mutants, which were identified by their midhindbrain pattern defects (brackets). Note pax3/7 increase in the anterior of somites (arrowheads, boxes are enlarged), relative to neural tube. B. Pax3 and pax7 up-regulation in all somites after fgf8 MO injection. C. Pax3/7-containing nuclei are increased in number by fgf8 MO, cyclopamine or both. Fgf8 MO particularly increases staining at the horizontal myoseptum, in contrast to cyclopamine which increases cells on the somite borders (arrows). Note the lack of change in intensity of immunofluorescence and the lack of significant neural crest migration in both control and treated posterior somites. Insets show pax3 mRNA up-regulation persisting to 33 hpf. D. Ace mutant embryos (identified by cerebellar morphology) have more weak Pax3/7 immunoreactive nuclei than siblings. Note clustering around the horizontal myoseptum (arrows) and more extensive neural crest migration in these older embryos compared to C. E. Quantification of Pax3/7-expressing cells per somite after treatments indicated. Data represent mean ± standard deviation of counts from four somites in at least 4-7 embryos. Asterisks indicate significant difference from control, P < 0.001.
Myod drives lateral fast myogenesis and suppresses pax3/7
We have postulated that Fgf8 signalling blocks pax3 expression and promotes fast myogenesis by inducing myod expression in LFMs (Groves et al., 2005). To begin to test this hypothesis, we used a morpholino (MO) against myod to knock down Myod protein. Myod protein is accumulated in the nuclei of all cells that express mRNA at 15 som (Fig. 6A). Myod MO ablates all detectable protein, whereas a myf5 MO significantly increases Myod accumulation (Fig. 6A). Myod MO reduces fast fibre formation in a manner similar to that caused by Fgf8 blockade (Fig. 6B-E). Myod MO also enhances Pax3/7 expression, mimicking Fgf8 blockade (Fig. 6C). However, myod MO has no effect on fgf8 expression itself (Fig. 6G). As loss of Myod enhances Pax3/7 expression, we asked whether the converse is also true: does up-regulation of Myod suppress pax3? Transient retinoic acid (RA) treatment causes over-expression of Fgf8 and myod up-regulation in the LFMs (Hamade et al., 2006). We find that RA suppresses pax3 expression in the somite, paralleling the up-regulation of myod mRNA (Fig. 6F). Taken together, these data indicate that the effect of Fgf8 on Pax3/7 is most simply explained by Fgf8 action on myod expression.
Myod, Fgf8 and Hh suppress Pax3/7 in the anterior somite
As lack of Fgf8 prevents myoD expression in lateral fast myoblasts (LFMs) at the posterolateral border of nascent somites, we asked whether increased pax expression is in LFMs. Myod MO causes pax3 and pax7 mRNA up-regulation, but primarily in the anterior somite (Fig. 7A-D). Similarly, after loss of Fgf8 signalling pax3 and pax7 expression increases most markedly in the anterior somite at early stages, and widespread on the somite surface later (Fig. 5A-D). As myod mRNA is not detected in the anterolateral somite (Weinberg et al., 1996), the data raise the possibility of an indirect effect of loss of Myod on pax3/7-expressing cells.
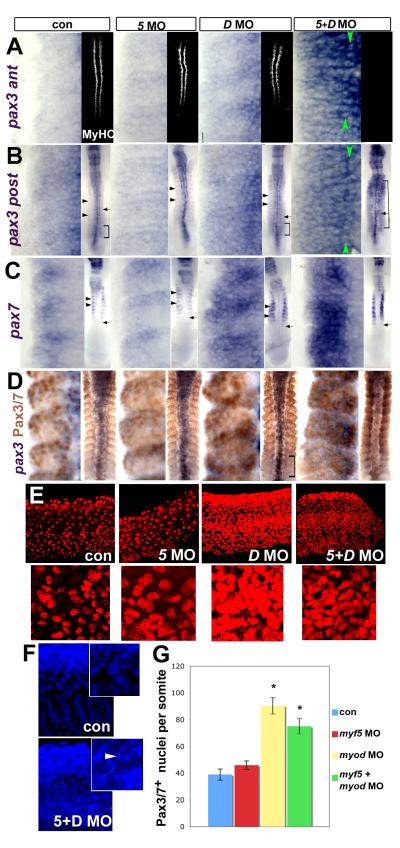
Figure 7. MRFs act to suppress Pax3/7 expression during somite development.
Immunodetection of MyHC or Pax3/7 or in situ mRNA hybridisation for pax3 or pax7 in embryos injected with MRF MOs viewed in dorsal flatmount (A-D 15s anterior to top), lateral confocal short stack of yolk extension somites at 24hpf (E, magnified in insets) and confocal stack in lateral view (F, 24hpf, DAPI). A insets. Injection of both myf5 and myod MOs ablates muscle, whereas each MO alone has no effect on MyHC. A-C. Myod MO or double MO up-regulate pax3 both in anterior (som 8-10, A) and posterior (som 11-13, B) somites and pax7 (C) mRNA. Green arrowheads indicate adaxial cell rows. Insets show the normal timing of pax7 accumulation after pax3 (arrows). Brackets show somites in which pax3 is widely expressed. Black arrowheads indicate posterior region of more mature somites in which pax3/7 mRNA is low relative to the anterior region. Myf5 MO is like controls. D. Dual immunodetection of pax3 mRNA and Pax3/7 protein. E. Pax3/7 protein is up-regulated in many lateral somite cells by either myod MO alone or double MO, but is little affected by myf5 MO. Insets show a single region at higher magnification. F. DAPI reveals numerous somite nuclei with condensed chromatin (arrowhead), suggesting apoptosis in double MO embryos. Note that the apparent reduction in tissue mass may lead to an underestimate of the extent of the Pax3/7 increase. G. Counts at 24hpf represent mean ± standard deviation of four somites in 4-6 embryos. Asterisks indicate significant difference from control, P < 0.001.
Absence of Hh signalling causes a similar quantitative increase in Pax3/7 cells to that caused by loss of Fgf8 function (Fig. 5C,E). But loss of Hh signalling does not lead to loss of myod expression in the LFMs. Nevertheless, myod mRNA levels are reduced in older somites of Hh signalling mutant fish (Lewis et al., 1999b; van Eeden et al., 1996). As with Fgf8 loss, the extra pax3/7 expression in the absence of Hh signals is predominantly in the anterior somite. Moreover, blockade of both pathways simultaneously fails to give a fully additive effect (Fig. 5C,E). These findings suggest that both signals impinge on a similar cell population, probably in the anterior somite.
Myf5 or myod is required for slow fibre formation and suppression of Pax3/7
In contrast to LFMs, which contain myf5 mRNA only transiently during development, adaxial slow fibre precursors express myf5 until the time of their terminal differentiation (Coutelle et al., 2001; Groves et al., 2005). Neither myod MO nor myf5 MO alone has any obvious effect on formation of slow fibres (Fig. 7A). Injection of both MOs, however, completely ablates slow muscle formation, in addition to the reduction of fast muscle caused by myod MO alone (Figs 6B-D, 7A). Thus, Myf5 and Myod are the MRFs primarily responsible for early myogenesis in the zebrafish.
Expression of pax3 and pax7 mRNA and Pax3/7 protein is enhanced by either double MO or myod MO alone (Fig. 7A-E,G). Myf5 MO alone has little effect (Fig. 7A-E,G). Double MO embryos are significantly thinner, paralleling the reduction in muscle mass, and have more apoptotic nuclei, as judged by condensed and fragmented chromatin revealed by DAPI stain (Fig. 7F). Strikingly, pax3 up-regulation differs between myod MO and double MO embryos. Double MO leads to persistent pax3 up-regulation in all somitic compartments, whereas myod MO only transiently increases pax3 mRNA in the posterior LFM region in nascent somites. In more anterior somites, pax3 mRNA remains up-regulated in anterolateral cells, but declines in posterior cells (Fig. 7A,B). Analysis of pax7 expression confirms this regional difference: myod MO drives pax7 up-regulation mainly in anterior cells in somites in which it is normally expressed whereas double MO expands expression into the posterior somite region (Fig. 7C). In controls, Pax3/7 proteins accumulate a few somites anterior to the early pax3 mRNA and are restricted to anterolateral cells. In myod MO injected embryos Pax3/7 is enhanced in the anterior somite, mainly in epithelial cells. Double MO gives widespread Pax3/7 expression in block-like somites (Fig. 7D). After either myod or double MO, more than twice the number of Pax3/7+ cells persist at 24hpf, after fast muscle differentiation in controls (Fig. 7E,G).
Discussion
Dermomyotome in fish
We have shown that pax3 and pax7 are expressed in the superficial layer of external cells on the zebrafish somite, first described by Waterman (Waterman, 1969), and which we suggest are homologous to amniote dermomyotome. Here we show that these cells are distinct from neural crest and SSFs, are proliferative and share expression of a collagen gene with later dermal tissue (Figs 1, 2; Le Guellec et al., 2004). In various fish, cells in this location express MRFs, molecules that drive myogenesis in amniotes and zebrafish (Devoto et al., 2006) Although we have not shown by lineage tracing that these cells contribute to later muscle or dermis formation, based on location and marker expression we hereafter refer to these pax3/7-expressing external cells as dermomyotome-like. Such cells have not been reported in Amphioxus, even though AmphiPax3/7 is expressed in somites (Holland et al., 1999). It seems that dermomyotome-like tissue arose early in evolution of vertebrates prior to the divergence of sarcopterygian and actinopterygian fish.
Formation of these dermomyotomal cells does not depend on two nearby cell populations: the early pax3/7-expressing cells are distinct from both SSFs and neural crest cells. In the absence of either of these cell populations pax3/7-expressing external cells remain. External cells can also be distinguished from both SSFs and migrating crest by the morphology, size and location of their nuclei, which are small, and contain lower levels of Pax3/7 proteins. The thin dermomyotomal cells are located across the entire somite surface and frequently abut one another. However, they are not apparent in all electron micrographs and so may not form a continuous epithelial sheet (data not shown). Their nuclei are often aligned dorsoventrally in rows near the ends of the muscle fibres. In contrast, the ~20 SSFs have a uniform distribution with large oval nuclei located near the centre of the fibres (Roy et al., 2001), and lack Pax3/7 proteins. Neural crest cells expressing high levels of Pax3/7 protein are less common, have irregular-shaped nuclei and migrate gradually from the dorsal region (Raible et al., 1992). The accumulation of Pax3/7+ cells near the edges of the dermomyotome-like layer may be related to regional differences in myogenesis, for example at dermomyotomal lips or elsewhere (Barresi et al., 2001; Devoto et al., 2006). Thus, the Pax3/7+ cells that persist in the lateral somite are dermomyotome-like.
Dermomyotome-like cells are abundant at the horizontal myoseptum. Muscle pioneer slow fibres reside in this location but constitute a distinct population expressing Engrailed and Prox1 (Roy et al., 2001), but not Pax3/7 (Minchin and Hughes, unpublished observation). Although a concentration of pax3/7-expressing cells at the border between epaxial and hypaxial somite has not been reported in amniotes, pax3 and pax7 are not uniformly expressed within the dermomyotome layer. Murine Pax7 is more abundant in the medial region of the somite and Pax3 at the somitic extremes (Relaix et al., 2004; Sporle, 2001). In amniotes, cells contributing to the myotome arise from the medial dermomyotome and are Pax3/7-dependent (Ben-Yair and Kalcheim, 2005; Gros et al., 2005; Relaix et al., 2005). Fish also show significant evidence of new fibre formation near the horizontal myoseptum in a layer of cells between the SSFs and the deep fast muscle region, that may arise by inward migration of Pax3/7+ cells and generate the ‘pink’ muscle (Rowlerson et al., 1995). Thus, distinct dermomyotomal regions may behave differently in later myogenesis.
Hh reciprocally regulates fast muscle and dermomyotome
The temporal correlation between the appearance of superficial pax3/7-expression and the wave of slow fibre migration from their original adaxial position to the somite surface raised the possibility that either pax3/7 is expressed within the slow fibres themselves or that slow cell migration is required for superficial pax3/7 expression. Our data show this is not the case. Indeed, pax3 expression seems unaffected in ubo mutants lacking slow fibres. But our results are consistent with the view that fast fibre differentiation drives pax gene suppression. The reduced differentiation of fast muscle correlates with an increase in pax3- and pax7-expression. Moreover, blockade of fast muscle differentiation by MRF MO injection enhances pax3/7 expression. In mice, lack of muscle does not obviously increase Pax3 mRNA (Kablar et al., 1999). Indeed, the murine evidence is the opposite, that Pax3 is required for MRF expression (Tajbakhsh et al., 1997; Maroto et al., 1997; Bajard et al., 2006). Whether Pax3 promotes MRF expression in fish is unclear, but up-regulation of pax3 when Fgf8 and/or Myod function are reduced argues that fish somitic myogenesis differs from mouse. The reciprocal relationship between muscle formation and dermomyotome character in fish supports our contention that Pax3/7+ cells are myogenic.
In amniotes, Hh signals influence pax3 expression and myogenesis in some lateral myogenic populations (Chiang et al., 1996; Duprez et al., 1998; Johnson et al., 1994; Li et al., 2004). Similarly, we find that Hh suppresses fish dermomyotome character and enhances fast myogenesis in the anterior somite independent of effects on slow fibres, in agreement with the recent report of Feng et al. (2006). Five lines of our evidence lead to this conclusion. First, pax3 is up-regulated in syu mutants or after late cyclopamine treatment, both of which leave slow fibres intact. Conversely, ubo mutants lacking slow muscle do not have altered pax3/7 expression. Second, patched genes, which are often up-regulated by Hh, are highly expressed during fast myogenesis in a region of wild type somites distinct from slow fibres (Lewis et al., 1999a). Third, reduction in Hh signalling up-regulates pax3/7 and depletes myod mRNA in older somites, but has no effect on expression in LFMs on the posterior border of nascent somites (Coutelle et al., 2001; Lewis et al., 1999b; van Eeden et al., 1996). Fourth, although fast muscle, probably LFMs, are reported to commence differentiation on schedule in Hh signalling mutants (Lewis et al., 1999b), at later stages such mutants have less or delayed fast muscle development, accompanied by increased cell death (Chen et al., 2001; Du and Dienhart, 2001; Henry and Amacher, 2004; Hirsinger et al., 2004). Loss of slow muscle is not accompanied by increased apoptosis of adaxial slow precursors (Coutelle et al., 2001; Hirsinger et al., 2004). Yet there is extensive cell death after MRF blockade, suggesting that failure to undergo correct fast myogenesis explains the increased apoptosis when Hh signalling fails. Fifth, the quantity of residual myod expression is significantly less in embryos with reduced Hh and Fgf signalling than after Fgf blockade alone (Groves et al., 2005). The most parsimonious explanation of these observations is that Hh drives differentiation of a population of fast muscle precursor cells that are distinct from the LFMs and slow fibres, and thereby reduces pax3/7-expression. Feng et al. (2006) show that Hh acts cell autonomously within the somite to prevent cells becoming dermomyotome, a view supported by our data.
MRFs Myf5 and Myod are required for early myogenesis in zebrafish
Knockdown of Myf5 has no obvious effect on zebrafish myogenesis, a result in striking contrast to the failure of epaxial myogenesis in myf5 null mice (Braun et al., 1992; Kassar-Duchossoy et al., 2004). In contrast to a previous report (Chen and Tsai, 2002), no brain or epiboly defects were observed at the MO doses used. Although it is possible that the fish null phenotype is more severe, our data argue for a shift in the roles of MRFs during evolution. The myf5 MO clearly works as it triggers Myod protein up-regulation, although myod mRNA level appears unaltered (Osborn, Hinits and Hughes, unpublished observation). Moreover, in combination with myod MO, myf5 MO prevents differentiation of the adaxial slow muscle in zebrafish. The need to ablate both Myf5 and Myod to stop early myogenesis is congruent with the high level mRNA from both genes in fish adaxial cells (Coutelle et al., 2001; Weinberg et al., 1996). In contrast, mice do not express myoD in early myoblasts (Braun et al., 1994). Avian myoD genes are expressed much earlier after somite formation than murine myod, at least in some somites (Borycki et al., 1997). Moreover, early myod expression in both fish and bird is Hedgehog-dependent (Borycki et al., 1998; Coutelle et al., 2001; Lewis et al., 1999b; van Eeden et al., 1996). As adaxial myogenesis seems to be a vertebrate synapomorphy (Devoto et al., 2006; Grimaldi et al., 2004), these cells were either lost during murine evolution or else became delayed in their differentiation, perhaps through loss of myod expression.
Myod drives fast muscle differentiation
We previously reported a correlation between reduction in Fgf8-dependent myod expression in posterolateral somite cells, the LFMs, and loss of some fast muscle in older embryos (Groves et al., 2005). The findings reported here, that myod MO phenocopies Fgf8 loss of function in the somite, prove that loss of Myod causes the somitic fast muscle deficit. Lack of myod in mice reduces myogenesis in the lateral somite and in limb muscle, which is derived from hypaxial dermomyotome (Kablar et al., 1997; Rudnicki et al., 1993). Thus, there is a striking similarity in the potent role of MyoD in the lateral somite across the vertebrate phylum.
How does induction of muscle suppress Pax3/7?
Knockdown of Myf5 and Myod causes loss of early myogenesis in the zebrafish. Terminal differentiation of both the adaxial slow and lateral fast muscle fails. Double knockdown also triggers a widespread up-regulation of pax3 and pax7 expression in the somite, followed by significant apoptosis. In amniotes, prevention of muscle differentiation also increases apoptosis in the somite (Kablar et al., 2003). Pax3 up-regulation in the LFMs, in which myf5 and myod are normally expressed after pax3, probably reflects persistence of a precursor state. Similarly, although adaxial cells express little if any, pax3 or pax7 mRNA, they accumulate transcript if Myf5 and Myod cannot function, perhaps because they revert to a default state. Pax up-regulation in these cells may be a cell autonomous effect.
In contrast, the anterolateral region of unmanipulated nascent somites normally does not detectably express myf5 or myod, but instead expresses pax3 at moderate levels (Coutelle et al., 2001; Weinberg et al., 1996; this work). How does loss of Myod function cause a partial loss in fast muscle and up-regulation of pax3/7 in cells that express little if any myod? One hypothesis is that anterior somite cells do in fact express myod at low levels or late. In this cell autonomous model, the fast muscle defect caused by Fgf8 blockade would not arise from failure of LFM differentiation (which might be maintained by Myf5). Instead, lack of Fgf signalling might prevent myod expression in anterior somite cells and lead to lack of fast muscle and pax3/7 up-regulation. Such a role for Fgf8, which is itself expressed in the anterior somite, is reminiscent of the ‘community effect’ described in Xenopus (Fisher et al., 2002; Standley et al., 2001). Interestingly, the evidence discussed above that Hh directly induces fast muscle formation in anterior somite cells, raises the possibility that the similar pax3/7 increase when either Hh or Fgf8 or Myod function is abolished reflects loss of myogenesis by the same anterior somite cells. Although some fast muscle forms in Hh mutants, it is morphologically defective and may contain fewer cells (Chen et al., 2001; Du and Dienhart, 2001; Henry and Amacher, 2004, Feng et al., 2006).
An alternative non-cell autonomous hypothesis is that suppression of pax3 mRNA in anterior somite cells occurs in the absence of MRF function in those cells, through lack of a signal from MRF-expressing cells, possibly LFMs. The elegance of this hypothesis is that the observed loss of Myod in LFMs in embryos lacking Fgf signalling would account for the up-regulation of pax3/7 expression elsewhere. Such a model would suggest that signalling from the early myotome to the dermomyotome occurs in fish. The models can be distinguished by comparing the fate of LFMs and anterior fast muscle precursors in wild type and manipulated embryos. Whatever the case, our data show that myogenesis suppresses Pax3/7 expression in fish somites.
Acknowledgement
Thanks to Rezaur Abdullah who participated in early experiments, to Stephen Devoto and Xavier Cousin for communicating unpublished results and to Nipam Patel for DP312. SMH is a member of MRC External Scientific Staff with Programme Grant support. CLH and JENM had MRC PhD studentships.
Footnotes
Note added in proof While this article was under review Lin et al (doi:10.1016/j.ydbio.2006.08.042) published that a myf5 morpholino up-regulates myod mRNA, a finding consistent with our anti-Myod antibody results generated with a distinct myf5 morpholino, but which we did not observe at 15 s. Lin et al. report effects of MRF MOs on head myogenesis, some of which we have also noted.
References
- Amthor H, Christ B, Patel K. A molecular mechanism enabling continuous embryonic muscle growth - a balance between proliferation and differentiation. Development. 1999;126:1041–53. doi: 10.1242/dev.126.5.1041. [DOI] [PubMed] [Google Scholar]
- Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;20:2450–64. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi MJ, D’Angelo JA, Hernandez LP, Devoto SH. Distinct mechanisms regulate slow-muscle development. Curr Biol. 2001;11:1432–8. doi: 10.1016/s0960-9822(01)00428-6. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–2199. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Baxendale S, Davison C, Muxworthy C, Wolff C, Ingham PW, Roy S. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat Genet. 2004;36:88–93. doi: 10.1038/ng1280. [DOI] [PubMed] [Google Scholar]
- Ben-Yair R, Kalcheim C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development. 2005;132:689–701. doi: 10.1242/dev.01617. [DOI] [PubMed] [Google Scholar]
- Blagden CS, Currie PD, Ingham PW, Hughes SM. Notochord induction of zebrafish slow muscle mediated by Sonic Hedgehog. Genes Dev. 1997;11:2163–2175. doi: 10.1101/gad.11.17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycki A-G, Strunk KE, Savary R, Emerson CP., Jr. Distinct signal/response mechanisms regulate pax1 and QmyoD activation in sclerotomal and myotomal lineages of quail somites. Dev Biol. 1997;185:185–200. doi: 10.1006/dbio.1997.8555. [DOI] [PubMed] [Google Scholar]
- Borycki A-G, Li J, Jin F, Emerson CP, Epstein JA. Pax3 functions in cell survival and in pax7 regulation. Development. 1999;126:1665–74. doi: 10.1242/dev.126.8.1665. [DOI] [PubMed] [Google Scholar]
- Borycki A-G, Mendham L, Emerson CP., Jr. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 1998;125:777–790. doi: 10.1242/dev.125.4.777. [DOI] [PubMed] [Google Scholar]
- Braun T, Bober E, Rudnicki MA, Jaenisch R, Arnold HH. MyoD expression marks the onset of skeletal myogenesis in Myf-5 mutant mice. Development. 1994;120:3083–92. doi: 10.1242/dev.120.11.3083. [DOI] [PubMed] [Google Scholar]
- Braun T, Rudnicki MA, Arnold HH, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–82. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Burgess S, Hopkins N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–96. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- Chen YH, Tsai HJ. Treatment with Myf5-morpholino results in somite patterning and brain formation defects in zebrafish. Differentiation. 2002;70:447–56. doi: 10.1046/j.1432-0436.2002.700807.x. [DOI] [PubMed] [Google Scholar]
- Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002;18:41–7. doi: 10.1016/s0168-9525(01)02594-x. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Coutelle O, Blagden CS, Hampson R, Halai C, Rigby PW, Hughes SM. Hedgehog signalling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev Biol. 2001;236:136–50. doi: 10.1006/dbio.2001.0193. [DOI] [PubMed] [Google Scholar]
- Davis GK, D’Alessio JA, Patel NH. Pax3/7 genes reveal conservation and divergence in the arthropod segmentation hierarchy. Dev Biol. 2005;285:169–84. doi: 10.1016/j.ydbio.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Melançon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Stoiber W, Hammond CL, Steinbacher P, Haslett JR, Barresi MJ, Patterson SE, Adiarte EG, Hughes SM. Generality of vertebrate developmental patterns: evidence for a dermomyotome in fish. Evol Dev. 2006;8:101–10. doi: 10.1111/j.1525-142X.2006.05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Gruss P, Lumsden A. The role of the notochord for epaxial myotome formation in the mouse. Cell Mol Biol (Noisy-le-grand) 1999;45:601–16. [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A. Specification of the hypaxial musculature. Development. 1998;125:2235–49. doi: 10.1242/dev.125.12.2235. [DOI] [PubMed] [Google Scholar]
- Du SJ, Devoto SH, Westerfield M, Moon RT. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF–β gene families. J. Cell Biol. 1997;139:145–156. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Dienhart M. Gli2 mediation of hedgehog signals in slow muscle induction in zebrafish. Differentiation. 2001;67:84–91. doi: 10.1046/j.1432-0436.2001.067003084.x. [DOI] [PubMed] [Google Scholar]
- Duprez D, Fournier-Thibault C, Le Douarin N. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development. 1998;125:495–505. doi: 10.1242/dev.125.3.495. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–25. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–74. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]; Fisher ME, Isaacs HV, Pownall ME. eFGF is required for activation of XmyoD expression in the myogenic cell lineage of Xenopus laevis. Development. 2002;129:1307–15. doi: 10.1242/dev.129.6.1307. [DOI] [PubMed] [Google Scholar]
- Feng X, Adiarte EG, Devoto SH. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev. Biol. 2006;300 doi: 10.1016/j.ydbio.2006.08.056. doi:10.1016/j.ydbio.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Fisher ME, Isaacs HV, Pownall ME. eFGF is required for activation of XmyoD expression in the myogenic cell lineage of Xenopus laevis. Development. 2002;129:1307–15. doi: 10.1242/dev.129.6.1307. [DOI] [PubMed] [Google Scholar]
- Franz T, Kothary R, Surani MA, Halata Z, Grim M. The Splotch mutation interferes with muscle development in the limbs. Anat Embryol (Berl) 1993;187:153–60. doi: 10.1007/BF00171747. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermamyotome and its role in muscle development. Development. 1994;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J.l. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi A, Tettamanti G, Martin BL, Gaffield W, Pownall ME, Hughes SM. Hedgehog regulation of superficial slow muscle fibres in Xenopus and the evolution of tetrapod trunk myogenesis. Development. 2004;131:3249–3262. doi: 10.1242/dev.01194. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–8. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Groves JA, Hammond CL, Hughes SM. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development. 2005;132:4211–22. doi: 10.1242/dev.01958. [DOI] [PubMed] [Google Scholar]
- Hamade A, Deries M, Begemann G, Bally-Cuif L, Genet C, Sabatier F, Bonnieu A, Cousin X. Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev Biol. 2006;289:127–40. doi: 10.1016/j.ydbio.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Henry CA, Amacher SL. Zebrafish slow muscle cell migration induces a wave of fast muscle morphogenesis. Dev Cell. 2004;7:917–23. doi: 10.1016/j.devcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol. 1997;192:289–99. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- Hirsinger E, Stellabotte F, Devoto SH, Westerfield M. Hedgehog signaling is required for commitment but not initial induction of slow muscle precursors. Dev Biol. 2004;275:143–57. doi: 10.1016/j.ydbio.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Schubert M, Kozmik Z, Holland ND. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol Dev. 1999;1:153–65. doi: 10.1046/j.1525-142x.1999.99019.x. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–62. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kablar B, Krastel K, Tajbakhsh S, Rudnicki MA. Myf5 and MyoD activation define independent myogenic compartments during embryonic development. Dev Biol. 2003;258:307–18. doi: 10.1016/s0012-1606(03)00139-8. [DOI] [PubMed] [Google Scholar]
- Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development. 1997;124:4729–4738. doi: 10.1242/dev.124.23.4729. [DOI] [PubMed] [Google Scholar]
- Kablar B, Krastel K, Ying C, Tapscott SJ, Goldhamer DJ, Rudnicki MA. Myogenic determination occurs independently in somites and limb buds. Dev Biol. 1999;206:219–31. doi: 10.1006/dbio.1998.9126. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Gayraud-Morel B, Gomes D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature. 2004;431:466–71. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–31. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RN, Eisen JS. The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives. Development. 2000;127:515–25. doi: 10.1242/dev.127.3.515. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Raible DW. Specification of zebrafish neural crest. Results Probl Cell Differ. 2002;40:216–36. doi: 10.1007/978-3-540-46041-1_11. [DOI] [PubMed] [Google Scholar]
- Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio) Int J Dev Biol. 2004;48:217–31. doi: 10.1387/ijdb.15272388. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Concordet JP, Ingham PW. Characterisation of a second patched gene in the zebrafish Danio rerio and the differential response of patched genes to Hedgehog signalling. Dev. Biol. 1999a;208:14–29. doi: 10.1006/dbio.1998.9169. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Currie PD, Roy S, Schauerte H, Haffter P, Ingham PW. Control of muscle cell-type specification in the zebrafish embryo by Hedgehog signalling. Dev Biol. 1999b;216:469–80. doi: 10.1006/dbio.1999.9519. [DOI] [PubMed] [Google Scholar]
- Li X, Blagden CS, Bildsoe H, Bonnin M-A, Duprez D, Hughes SM. Hedgehog can drive terminal differentiation of amniote slow skeletal muscle. BMC Dev Biol. 2004;4:9. doi: 10.1186/1471-213X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J, Schier AF, Solnica-Krezel L, Stemple DL, Neuhauss SCF, Stainier DYR, Abdelilah S, Rangini Z, Zwartkruis F, Driever W. Mutations affecting development of the zebrafish ear. Development. 1996;123:275–283. doi: 10.1242/dev.123.1.275. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–48. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Breant C, Le Douarin NM. Control of somite patterning by signals from the lateral plate. Proc. Natl Acad. Sci. USA. 1995;92:3219–3223. doi: 10.1073/pnas.92.8.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–53. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Mascarello F, Radaelli G, Veggetti A. Differentiation and growth of muscle in the fish Sparus aurata: II. Hyperplastic and hypertrophic growth of lateral muscle from hatching to adult. J. Muscle Res. Cell Motility. 1995;16:223–236. doi: 10.1007/BF00121131. [DOI] [PubMed] [Google Scholar]
- Roy S, Wolff C, Ingham PW. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001;15:1563–76. doi: 10.1101/gad.195801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–9. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strahle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–86. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Seo H-C, Saetre BO, Havik B, Ellingsen S, Fjose A. The zebrafish Pax3 and Pax7 homologues are highly conserved, encode multiple isoforms and show dynamic segment-like expression in the developing brain. Mech. Dev. 1998;70:49–63. doi: 10.1016/s0925-4773(97)00175-5. [DOI] [PubMed] [Google Scholar]
- Sporle R. Epaxial-adaxial-hypaxial regionalisation of the vertebrate somite: evidence for a somitic organiser and a mirror-image duplication. Dev Genes Evol. 2001;211:198–217. doi: 10.1007/s004270100139. [DOI] [PubMed] [Google Scholar]; Standley HJ, Zorn AM, Gurdon JB. eFGF and its mode of action in the community effect during Xenopus myogenesis. Development. 2001;128:1347–57. doi: 10.1242/dev.128.8.1347. [DOI] [PubMed] [Google Scholar]
- Standley HJ, Zorn AM, Gurdon JB. eFGF and its mode of action in the community effect during Xenopus myogenesis. Development. 2001;128:1347–57. doi: 10.1242/dev.128.8.1347. [DOI] [PubMed] [Google Scholar]
- Stickney HL, Barresi MJ, Devoto SH. Somite development in zebrafish. Dev Dyn. 2000;219:287–303. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1065>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–38. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–64. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Waterman RE. Development of the lateral musculature in the teleost, Brachydanio rerio: a fine structural study. Amer. J. Anat. 1969;125:457–494. doi: 10.1002/aja.1001250406. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–80. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book - a guide for the laboratory use of zebrafish (Danio rerio) University of Oregon Press; 1995. [Google Scholar]
- Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994;120:785–796. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- Xu Y, He J, Tian HL, Chan CH, Liao J, Yan T, Lam TJ, Gong Z. Fast skeletal muscle-specific expression of a zebrafish myosin light chain 2 gene and characterization of its promoter by direct injection into skeletal muscle. DNA Cell Biol. 1999;18:85–95. doi: 10.1089/104454999315655. [DOI] [PubMed] [Google Scholar]