Abstract
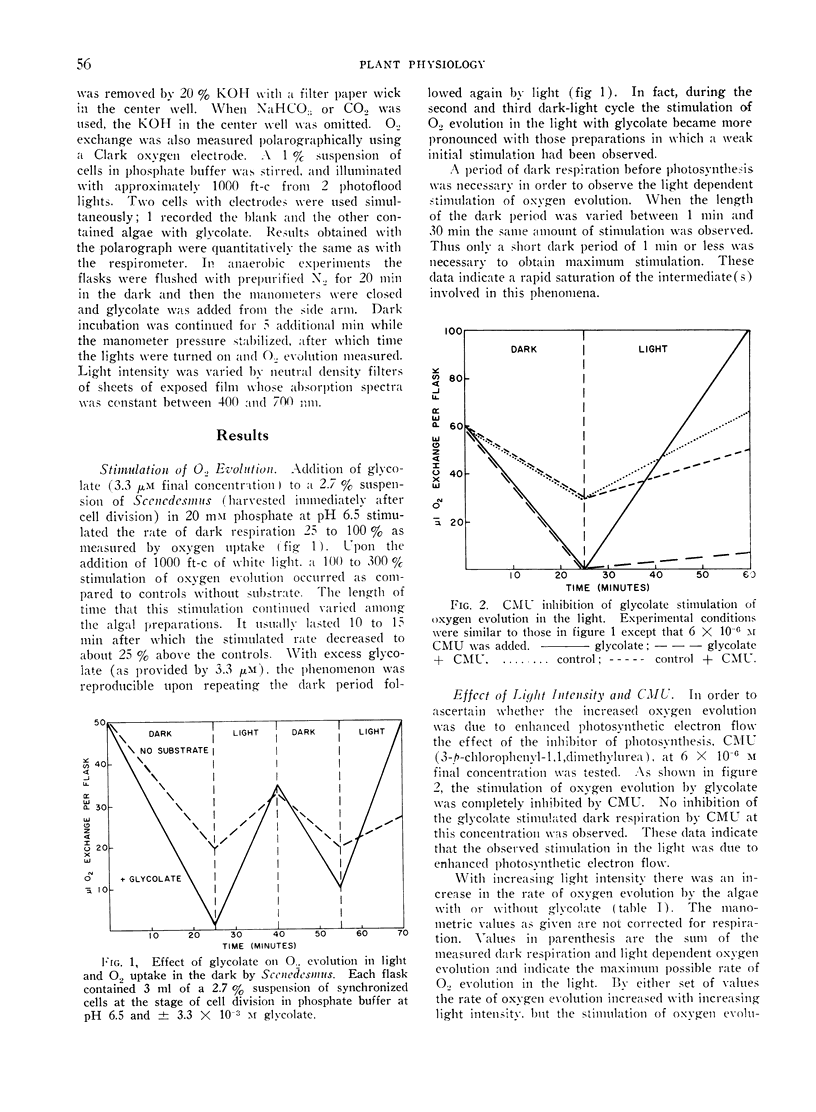
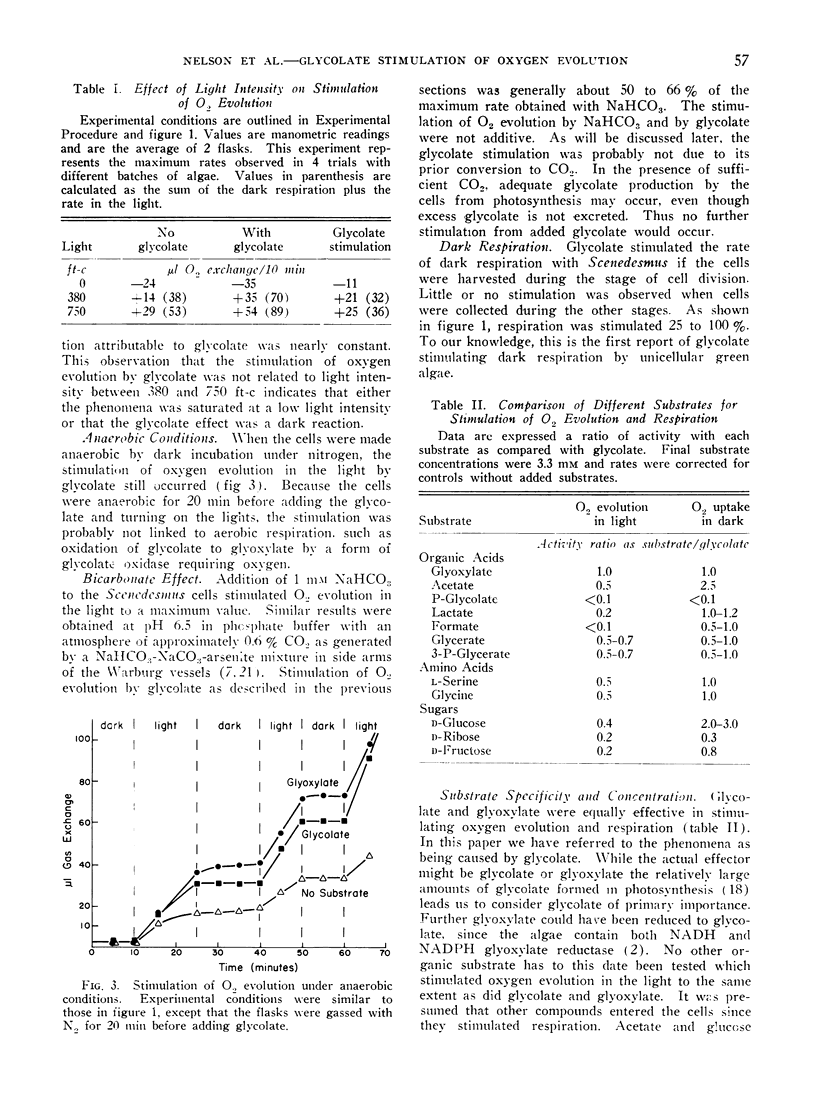
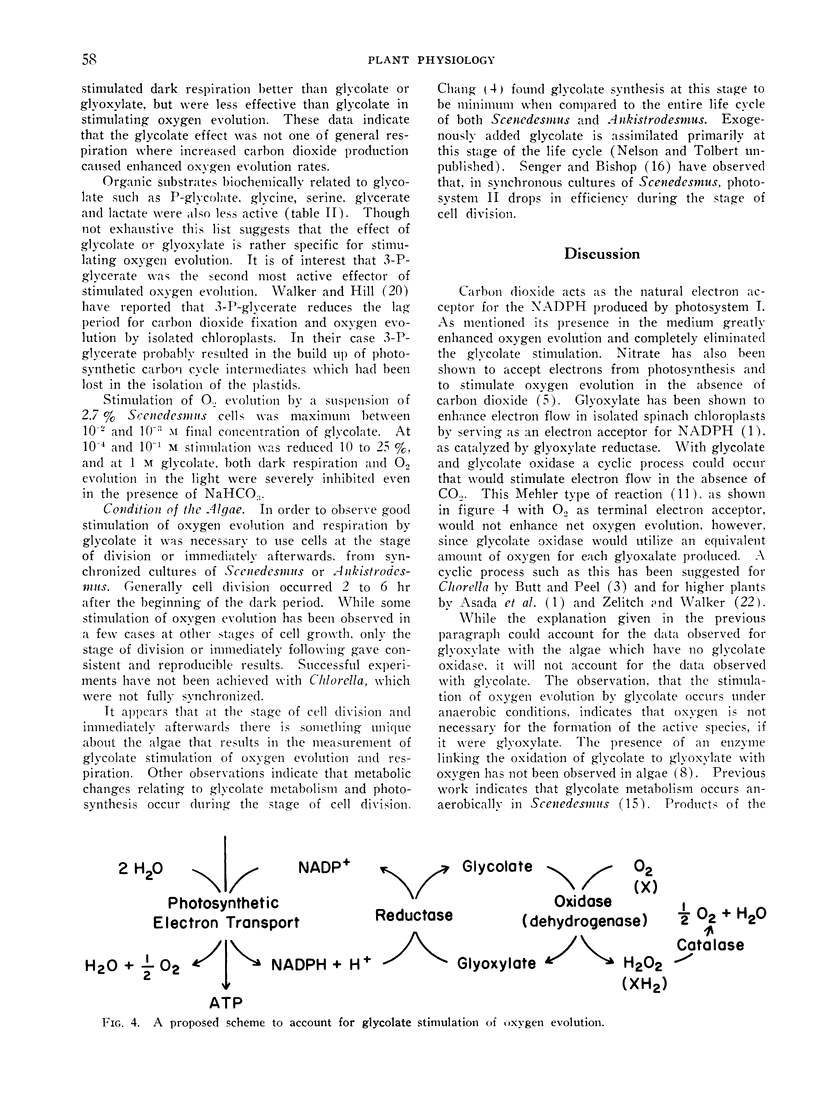
Glycolate and glyoxylate stimulated 100% to 300% the rate of oxygen evolution by Scenedesmus in the light in the absence of added carbon dioxide. This stimulation occurred either aerobically or anaerobically, and was sensitive to CMU. Aerobic dark respiration was stimulated 25% to 100% by glycolate. This phenomenon was best demonstrated with synchronized Scenedesmus at the stage of cell division. For glycolate stimulation of oxygen evolution, a dark preincubation of 1 minute or less was necessary. In comparative test with other compounds of metabolism and photosynthesis, the stimulation of oxygen evolution was greatest by glycolate and glyoxylate. In a proposed scheme glyoxylate serves as a terminal hydrogen acceptor from NADPH produced by photosynthesis, and it thereby stimulates oxygen evolution when carbon dioxide is not available. Transformation of glycolate to glyoxylate in these cells would have to occur in the absence of oxygen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis E. A. Nitrate Reduction by Chlorella. Plant Physiol. 1953 Jul;28(3):539–544. doi: 10.1104/pp.28.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E. Carbon Dioxide & the Hill Reaction. Plant Physiol. 1963 May;38(3):298–304. doi: 10.1104/pp.38.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate pathway in algae. Plant Physiol. 1967 Mar;42(3):371–379. doi: 10.1104/pp.42.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER E. On the role of manganese in the oxygen-evolving system of photosynthesis. Arch Biochem Biophys. 1955 Dec;59(2):527–529. doi: 10.1016/0003-9861(55)90519-1. [DOI] [PubMed] [Google Scholar]
- Senger H., Bishop N. I. Quantum yield of photosynthesis in synchronous Scenedesmus cultures. Nature. 1967 Apr 8;214(5084):140–142. doi: 10.1038/214140a0. [DOI] [PubMed] [Google Scholar]
- TANNER H. A., BROWN T. E., EYSTER C., TREHARNE R. W. A manganese dependent photosynthetic process. Biochem Biophys Res Commun. 1960 Aug;3:205–210. doi: 10.1016/0006-291x(60)90224-2. [DOI] [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]
- Walker D. A., Hill R. The relation of oxygen evolution to carbon assimilation with isolated chloroplasts. Biochim Biophys Acta. 1967 Mar 8;131(2):330–338. doi: 10.1016/0005-2728(67)90146-6. [DOI] [PubMed] [Google Scholar]
- Zelitch I., Walker D. A. The Role of Glycolic Acid Metabolism in Opening of Leaf Stomata. Plant Physiol. 1964 Sep;39(5):856–862. doi: 10.1104/pp.39.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]


