Abstract
Mitochondria were prepared from the spadices of skunk cabbage (Symplocarpus foetidus) whose respiratory rate with succinate and malate showed 15% to 30% sensitivity to cyanide inhibition, and which showed respiratory control by added ADP. The observed respiratory control ratios ranged from 1.1 to 1.4. The change in pH of the mitochondrial suspension was recorded simultaneously with oxygen uptake: alkalinization of the medium, expected for phosphorylation of ADP, coincided with the period of acceleration in oxygen uptake caused by addition of an ADP aliquot. The ADP/O ratios obtained were 1.3 for succinate and 1.9 for malate. In the presence of 0.3 mm cyanide, the ADP/O ratio for succinate was zero, while that for malate was 0.7. These results are consistent with the existence of an alternate oxidase which interacts with the flavoprotein and pyridine nucleotide components of the respiratory chain and which, in the presence of cyanide, allows the first phosphorylation site to function with an efficiency of about 70%. In the absence of respiratory inhibitors, the efficiency of each phosphorylation site is also about 70%. This result implies that diversion of reducing equivalents through the alternate oxidase, thereby bypassing the 2 phosphorylation sites associated with the cytochrome components of these mitochondria, occurs to a negligible extent during the oxidative phosphorylation of ADP or State 3.
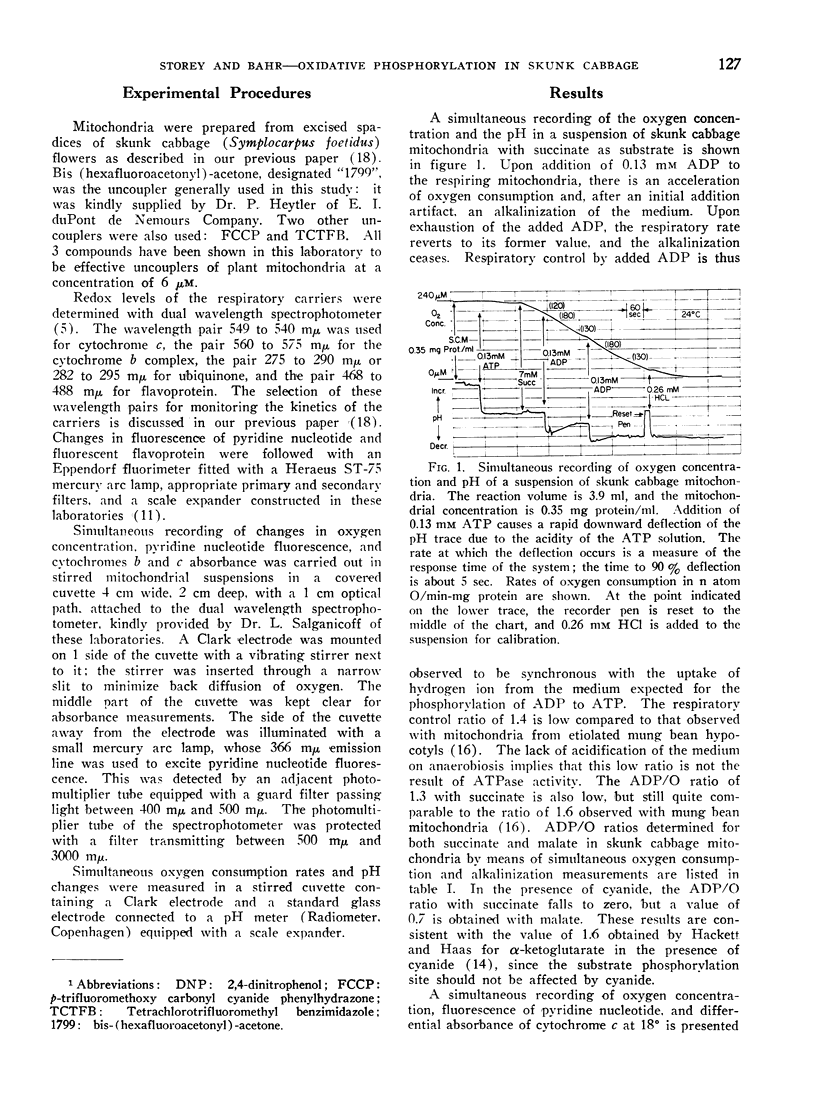
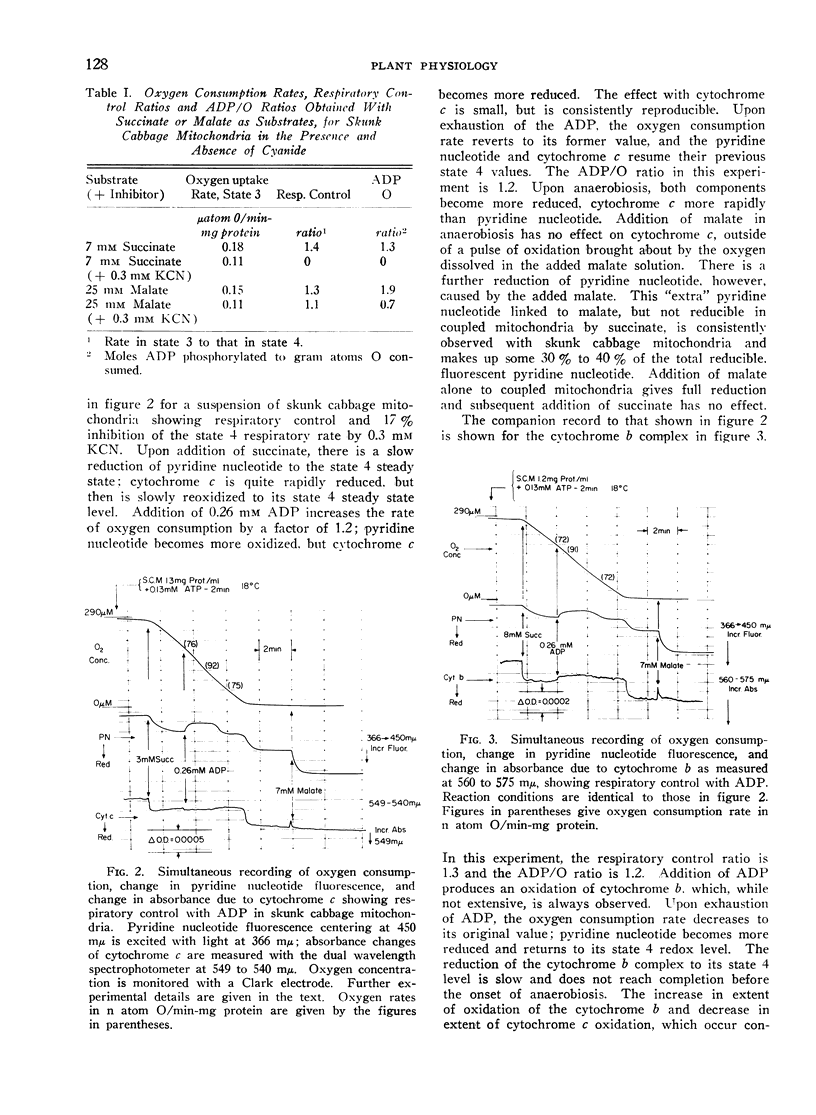
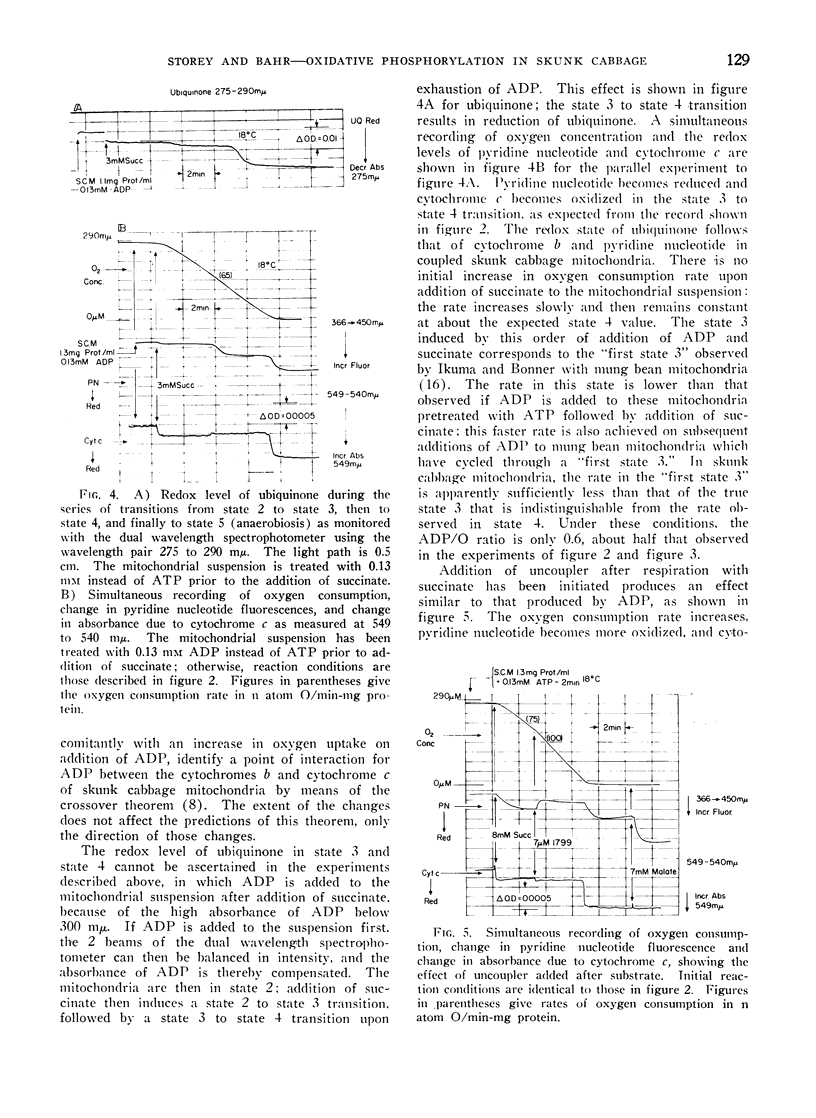
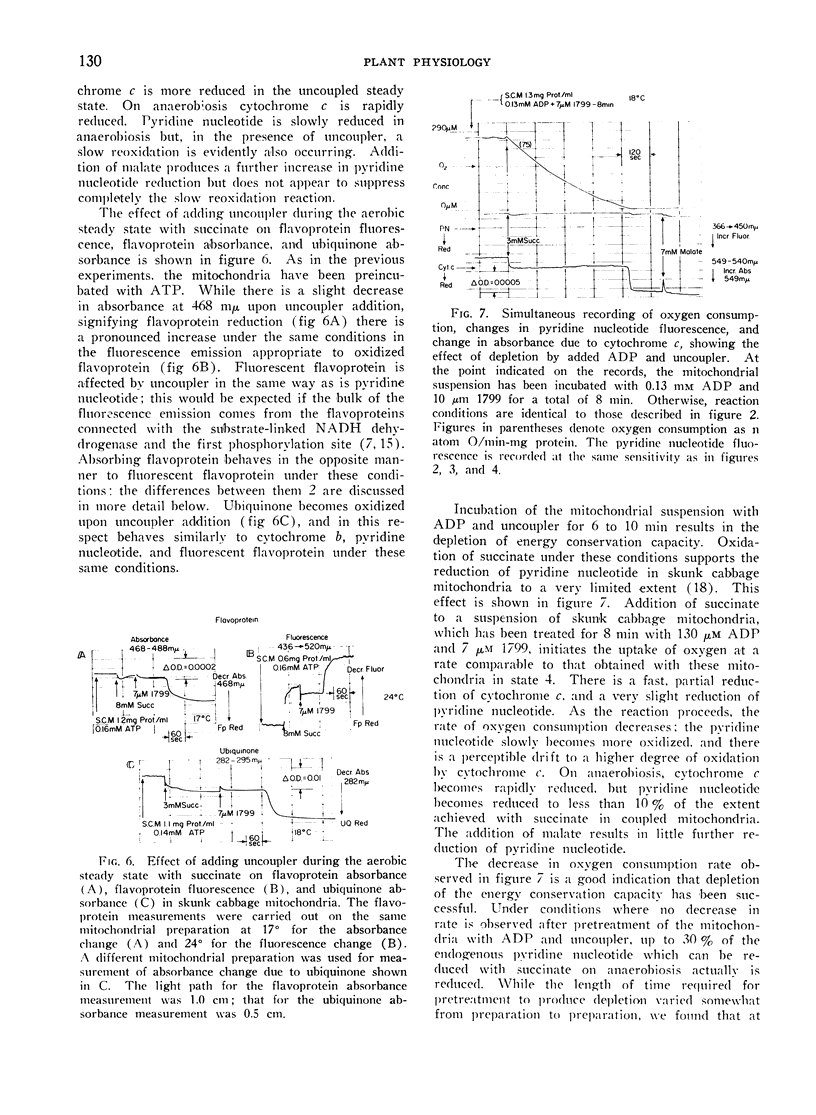
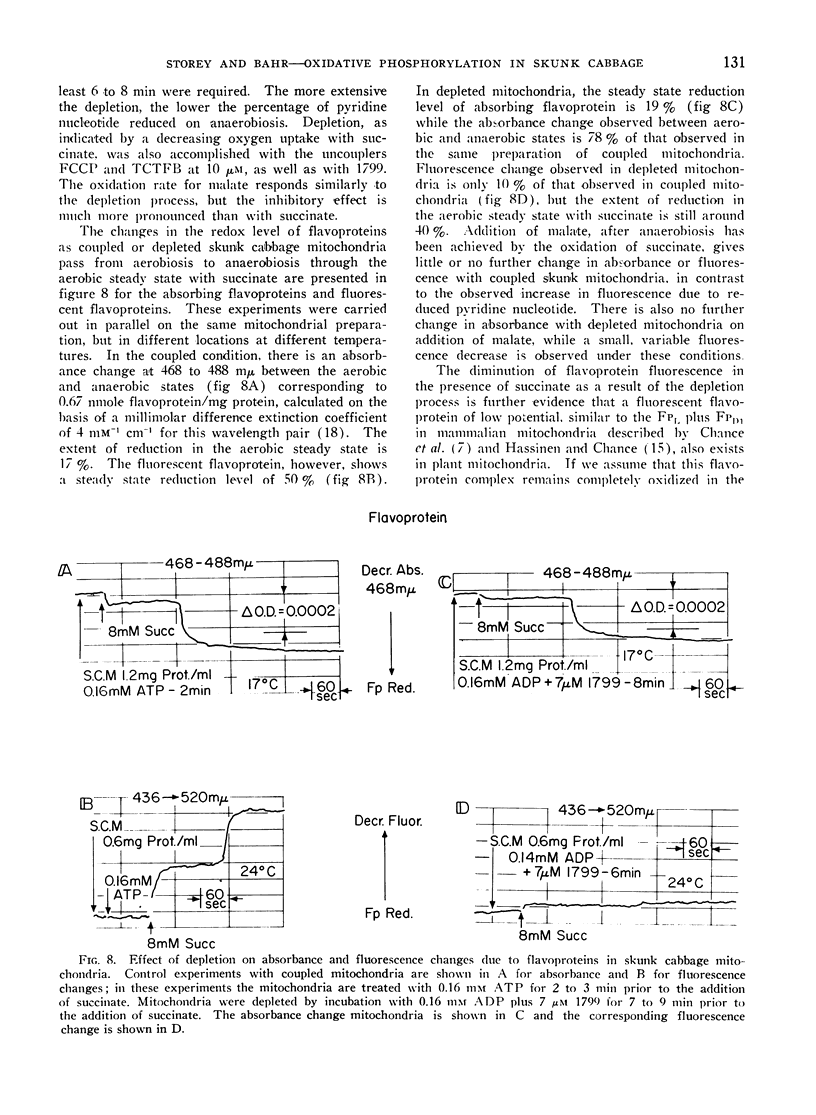
Addition of ADP or uncoupler to skunk cabbage mitochondria respiring in the controlled state or State 4, results in reduction of cytochrome c and the oxidation of the cytochromes b, ubiquinone and pyridine nucleotide. A site of interaction of ADP with the respiratory chain between cytochromes b and cytochrome c is thereby identified by means of the crossover theorem. Flavoprotein measured by fluorescence is also oxidized upon addition of ADP or uncoupler, but flavoprotein measured by optical absorbance changes becomes more reduced under these conditions. Depletion of the mitochondria by pretreatment with ADP and uncoupler prevents reduction of most of the fluorescent flavoprotein by succinate. These results indicate that skunk cabbage mitochondria contain both high and low potential flavo-proteins characterized by different fluorescence/absorbance ratios similar to those demonstrated to be part of the respiratory chain in mitochondria from animal tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. D., Jr, Plesnicar M. Electron transport carriers in plant mitochondria. Nature. 1967 May 6;214(5088):616–617. doi: 10.1038/214616a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Ernster L., Garland P. B., Lee C. P., Light P. A., Ohnishi T., Ragan C. I., Wong D. Flavoproteins of the mitochondrial respiratory chain. Proc Natl Acad Sci U S A. 1967 May;57(5):1498–1505. doi: 10.1073/pnas.57.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W., MAITRA P. K. A fluorimetric method for the quantitative microanalysis of adenine and pyridine nucleotides. Anal Biochem. 1962 May;3:369–382. doi: 10.1016/0003-2697(62)90065-9. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W. Oxidative Phosphorylation and Functional Cytochromes in Skunk Cabbage Mitochondria. Plant Physiol. 1958 Jan;33(1):27–32. doi: 10.1104/pp.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Chance B. Azide inhibition of mitochondrial electron transport. I. The aerobic steady state of succinate oxidation. Biochim Biophys Acta. 1967 May 9;131(3):421–430. doi: 10.1016/0005-2728(67)90002-3. [DOI] [PubMed] [Google Scholar]


