Abstract
Adenosine is a wide-spread endogenous neuromodulator. In the central nervous system it activates A1 and A2A receptors (A1Rs and A2ARs) which have differential distributions, different affinities to adenosine, are coupled to different G-proteins, and have opposite effects on synaptic transmission. Although effects of adenosine are studied in detail in several brain areas, such as hippocampus and striatum, the heterogeneity of the effects of A1R and A 2A R activation and their differential distribution preclude generalization over brain areas and cell types. Here we study adenosine's effects on excitatory synaptic transmission to layer 2/3 pyramidal neurons in slices of the rat visual cortex. We measured effects of bath application of adenosine receptor ligands on evoked EPSPs, miniature EPSPs (mEPSPs), and membrane properties. Adenosine reduced the amplitude of evoked EPSPs and EPSCs, and reduced frequency of mEPSPs in a concentration dependent and reversible manner. Concurrent with EPSP/C amplitude reduction was an increase in the paired-pulse ratio. These effects were blocked by application of the selective A1R antagonist DPCPX, suggesting that activation of presynaptic A1Rs suppresses excitatory transmission by reducing release probability. Adenosine (20 μM) hyperpolarized the cell membrane from 65.3±1.5 to -67.7±1.8 mV, and reduced input resistance from 396.5±44.4 to 314.0±36.3 MOhm (~20%). These effects were also abolished by DPCPX, suggesting postsynaptic A1Rs. Application of the selective A2AR antagonist SCH-58261 on the background of high adenosine concentrations revealed an additional decrease in EPSP amplitude. Moreover, application of the A2AR agonist CGS-21680 led to an A1R-dependent increase in mEPSP frequency. Dependence of the A2AR effects on the A1R availability suggests interaction between these receptors, whereby A2ARs exert their facilitatory effect on synaptic transmission by inhibiting the A1R mediated suppression.
Our results demonstrate functional pre and postsynaptic A1Rs and presynaptic A2ARs in layer 2/3 of the visual cortex, and suggest interaction between presynaptic A2ARs and A1Rs.
Keywords: Neocortex, Synaptic transmission, Modulation, Adenosine, A1 and A2A Receptors, Presynaptic
Adenosine (Ado) is an endogenous neuromodulator which is widespread in the central nervous system. It is a metabolite of ATP, hence its ubiquitous presence, yet this purine nucleoside has an importance that surpasses cellular energy maintenance. It serves in a multitude of functions in the nervous system, which range from sleep homeostasis and regulation of cortical slow oscillations (Bjorness and Greene, 2009; Halassa et al., 2009) to neuroprotective response to potentially traumatizing events such as hypoxia, ischemia and excitotoxicity (Mendonça et al., 2000; Dunwiddie and Masino, 2001; Cunha, 2005; Gomes et al., 2011). The effects of adenosine receptor activation have also been exploited in the treatment of epilepsy (Dale and Frenguelli, 2009; Masino et al., 2011).
Adenosine and ATP are released by neurons and astrocytes in an activity-dependent manner (Pascual et al., 2005; Wall and Dale, 2008; Halassa et al., 2009; Lovatt et al., 2012). In the extracellular space, the formation of adenosine from nucleotides is completed with the breakdown of AMP by Ecto-5’-nucleotidase, which is also the rate limiting step in these processes (Dunwiddie et al., 1997). Four types of G-protein coupled adenosine receptors have been identified in the brain (A1, A2A, A2B, and A3), the A1Rs and A2ARs being the most abundant and well-studied. A1Rs and A2ARs have distinct patterns of expression in different brain regions, different affinities to adenosine, are coupled to different G-proteins and have opposing effects on synaptic transmission (Fredholm et al., 2001a,b; Dunwiddie and Masino, 2001). A1Rs are expressed throughout the brain; high concentrations have been found in the cerebral cortex, subcortical structures, cerebellum, and the spinal cord (Dixon et al., 1996, Fredholm et al. 2001a). Activation of A1Rs has a generally suppressive effect on synaptic transmission and cell excitability. In the hippocampus A1R activation reduces release probability (Dunwiddie and Haas, 1985; Scanziani et al., 1992) by inhibiting calcium influx in the presynaptic terminal through N and P/Q type calcium channels (Gundlfinger et al., 2007; Wu and Saggau, 1994, 1997). Decrease of release probability was also reported at excitatory and inhibitory connections to layer 5 pyramids (Murakoshi et al., 2001; Kruglikov and Rudy, 2008) and inhibitory connections in layer 1 of the neocortex (Kirmse et al., 2008). Further suppression of neuronal excitability by A1Rs is mediated via activation of G-protein coupled inwardly-rectifying potassium channels which leads to hyperpolarization of the cell membrane and decrease of input resistance (Trussel and Jackson, 1985, 1987; Thompson et al., 1992; Takigawa and Alzheimer, 1999, 2002). A2ARs are expressed most densely within the striatum, but have been also found in other brain structures including cortex (Dixon et al., 1996; Fredholm et al. 2001a; Dunwiddie and Masino 2001). A2ARs have lower affinity to adenosine than A1Rs (Fredholm et al., 2001b), and their activation has a facilitatory effect on synaptic transmission (Marchi et al., 2002; Cunha, 2005). In synaptosomes from cortical tissue, facilitation of glutamate release by A2ARs has been reported (Marchi et al., 2002), as well as antagonistic interaction between A2ARs and A1Rs (Lopes et al., 1999). Some in vitro work has corroborated these actions of A2ARs in the hippocampus (Li and Henry, 1998; Lopes et al., 2002), but a demonstration of A2ARs effects on neocortical synaptic transmission is lacking.
The opposite actions of A1Rs and A2ARs allow bidirectional regulation of synaptic transmission by adenosine, the net effect depending on the local adenosine concentration and the presence of A1 and/or A2A receptors. The distribution of A1Rs and A2ARs is area specific in the brain (Dixon et al., 1996; Fredholm et al., 2001a) and layer-specific in the cortex (Fastbom et al., 1987; Svenningsson et al., 1997; Chaudhuri et al., 1998). Moreover, both A1Rs and A2ARs can form heteromers with other receptors and influence their ligand affinity, subsequently affecting various intracellular cascades (Ciruela et al., 2011; Orrú et al., 2011). Therefore, effects of adenosine on synaptic transmission and membrane properties of neurons of a specific type cannot be established through generalization.
Here we set to characterize adenosine's effects on excitatory synaptic transmission to layer 2/3 pyramidal neurons of the rat visual cortex, and determine which receptors mediate these effects. Layer 2/3 pyramids play a central role in cortical operations, serving as one of the intracortical stages in feedforward processing of sensory inputs from the thalamus, but also providing connections between cortical areas which mediate long-range interactions and multisensory integration in the cortex. We show that suppression of excitatory synaptic transmission to these neurons is mediated by presynaptic A1Rs. In contrast to results reported for other cortical layers, we found that A1R activation also leads to a robust decrease of input resistance and hyperpolarization of layer 2/3 pyramids. Furthermore, in our preparation ambient adenosine acting on A1Rs exerts an inhibitory tone on basal transmission. We also show that activation of A2ARs facilitates transmission in an A1R-dependent manner, providing what is to our knowledge the first demonstration of A2AR mediated actions on basal transmission in the neocortex.
1. EXPERIMENTAL PROCEDURES
1.1. Slice Preparation
All experimental procedures used in this study are in compliance with the US National Institutes of Health regulations and were approved by the Institutional Animal Care and Use Committee of the University of Connecticut. Details of slice preparation and recording were similar to those used in previous studies (Volgushev et al., 2000; Lee et al., 2012). Wistar rats (15-32 days old, Charles-River or Harlan) were anaesthetized with isoflurane, decapitated, and the brain was quickly removed and placed into an ice-cold oxygenated artificial cerebrospinal fluid solution (ACSF), containing, in mM: 125 NaCl, 25 NaHCO3, 25 glucose, 3 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, bubbled with 95% O2/5% CO2 , pH 7.4. Coronal slices (350 μm thickness) containing the visual cortex were prepared from the right hemisphere. Slices were allowed to recover for at least one hour at room temperature. For recording, individual slices were transferred to a recording chamber mounted on an Olympus BX-50WI microscope equipped with infrared differential interference contrast (IR-DIC) optics. In the recording chamber slices were submerged in oxygenated ACSF at 28°-32°C.
1.2. Intracellular recording and synaptic stimulation
Layer 2/3 pyramidal cells from visual cortex were selected for recording in the whole cell configuration. Identification of pyramidal neurons using DIC microscopy was reliable as demonstrated in our previous work with biocytin labelling and morphological reconstruction of recorded neurons (Volgushev et al., 2000). Intracellular pipette solution contained, in mM: 130 K-Gluconate, 20 KCl, 10 HEPES, 10 Na-Phosphocreatine, 4 Mg-ATP, 0.3 Na2-GTP, (pH 7.4 with KOH). In experiments for Fig 3, Cs-based intracellular solution was used (in mM): 130 Cs-CH2-S03, 20 KCl, 10 HEPES, 10 Na-Phosphocreatine, 4 Mg-ATP, 0.3 Na2-GTP, 5mM EGTA, (pH 7.4 with KOH). In voltage-clamp experiments the cells were held at a holding potential of-70 mV or -80 mV throughout the recording.
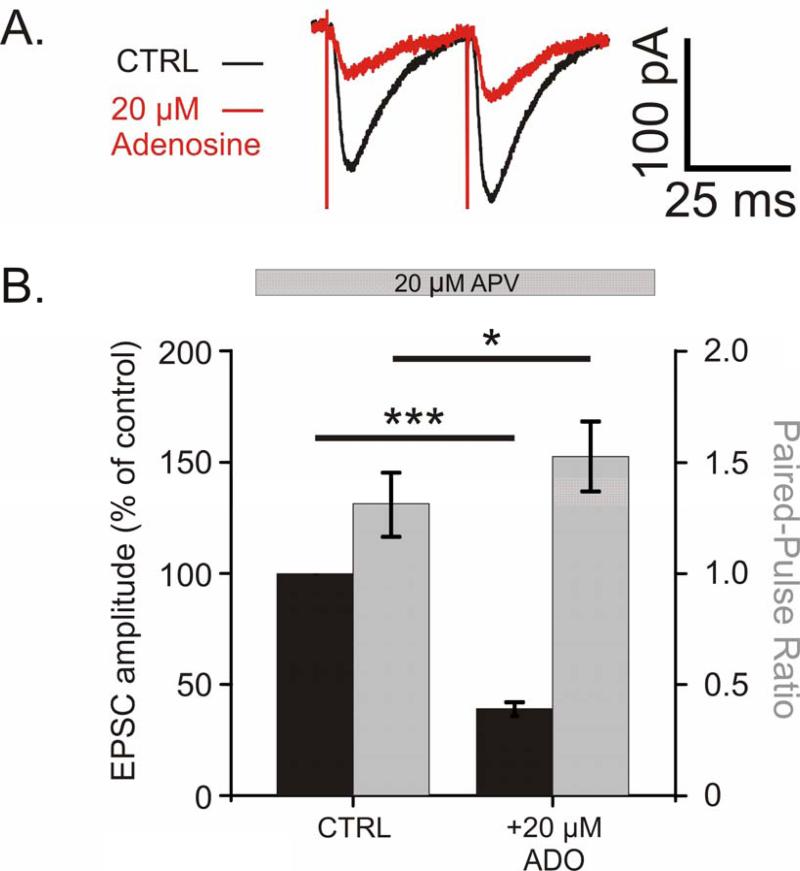
Fig 3. Adenosine reduces the amplitude of EPSCs and increases the paired-pulse ratio.
A. Example traces of evoked EPSCs recorded with Cs-based pipette solution in control (black) and with 20 μM adenosine in the bath (red; average of 20 traces for each condition).
B. Average reduction in EPSC amplitude as percent of baseline (black bars, n = 10, p<0.001), and average increase in PPR (grey bars, p<0.05) after application of 20 μM adenosine.
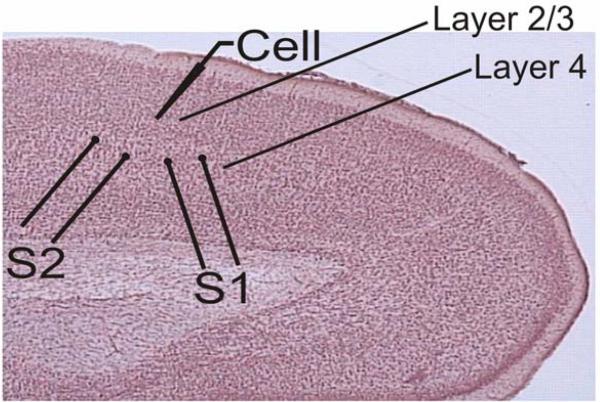
Two pairs of stimulating electrodes (S1 and S2) were placed in Layer 4, below the Layer 2/3 recording site (Fig. 1). Stimulation current intensities were adjusted to evoke monosynaptic excitatory postsynaptic potentials (EPSPs) in the recorded neuron. We used a paired-pulse stimulation protocol with a 50 ms inter-pulse interval. Paired stimuli were applied to S1 and S2 in alternating sequence once per 7.5 seconds, so that each input was stimulated with paired pulses each 15 seconds. To test for the possible contribution of inhibition, evoked PSPs were recorded at depolarized potentials between −50 and −40 mV. Only those PSPs that were still depolarizing at this membrane potential were considered excitatory and included in the analysis.
Fig 1. Scheme of the location of stimulation and recording electrodes in neocortical slices.
Recordings were made from layer 2/3 pyramidal neurons while the two stimulation electrodes (S1 and S2) were placed in layer 4 below the recording site.
All drugs were bath applied. For experiments measuring miniature EPSPs (mEPSPs), tetrodotoxin (TTX; 0.1-0.5 μM; Tocris, Bristol UK) was added to the extracellular solution at least 25 minutes before recordings were started. TTX was dissolved in water to a 0.5 mM stock before being added to the bath. In some mEPSP experiments (where mentioned), picrotoxin (100 μM; Sigma, St. Louis MO, USA) was used to block inhibitory transmission. Picrotoxin was dissolved in the ACSF directly. APV (Tocris, Bristol UK) was dissolved in water to a 50 mM stock before being added. Adenosine (Sigma, St. Louis MO, USA) was dissolved in ACSF to a 1 mM stock before being applied to the bath. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX; Sigma, St. Louis MO, USA) was dissolved in a 60% ethanol solution to a 0.5 mM stock or >99.9% DMSO to a 1 mM stock. Final ethanol concentration in the bath ranged from 1.8×10−4 to 1.8×10−2% for 1.5-150 nM DPCPX. SCH-58261 (Tocris, Bristol UK) was dissolved in 100% ethanol to a 2.5mM stock or in >99.9% DMSO to a 1 mM stock. CGS-21680 (Tocris, Bristol UK) was dissolved in >99.9% DMSO to a 1 mM stock. Final bath concentration of ethanol in SCH-58261 experiments was 12×10−4%. Final concentration of DMSO in bath was <0.05%. Experiments to test for any biological actions of vehicle revealed no differences between the use of ethanol and DMSO (data not shown).
1.3. Data analysis
Data analysis was made using custom-written programs in MatLab (© The MathWorks, Natick MA, USA). All inputs included in the analysis fulfilled the criteria of (1) stability of EPSP amplitudes during the control period, (2) stability of the membrane potential throughout the recording, and (3) stability of the onset latency and kinetics of the rising slope of the EPSP. EPSP amplitudes were measured as the difference between the mean membrane potential during two time windows. The first time window was placed before the EPSP onset and the second time window was placed on the rising slope of the EPSP, just before the peak. Amplitude of the second EPSP in paired-pulse stimulation paradigm was measured using windows of the same duration, but shifted by the length of the inter-pulse interval (50 ms).
Miniature EPSPs were detected as following. Two windows (a window 1, ‘baseline’, 2.5 ms in width and a window 2, ‘amplitude’ 1.5 ms in width) were spaced 2.5 ms and swept the recorded trace. Within each window the mean membrane potential was measured. When the difference between the mean membrane potential in the ‘amplitude’ and the ‘baseline’ window (window 2 – window 1) was at least 0.2 mV but no more than 2mV, an event was detected. After this automatic detection, all detected events were visually checked for their shape, and erroneously detected traces were excluded from analysis. We measured frequency of mEPSCs, amplitude of each event and compiled their amplitude distributions. From the amplitude distributions, we calculated the the skew of the distribution and determined median.
Significance tests were accomplished utilizing Student's t-tests or one-way ANOVAs with Post-hoc comparisons (Dunnett's and Tukey's HSD). Error bars represent the standard error of the mean (± SEM).
2. RESULTS
2.1. Adenosine reduces the amplitude of evoked EPSPs and increases the paired pulse ratio
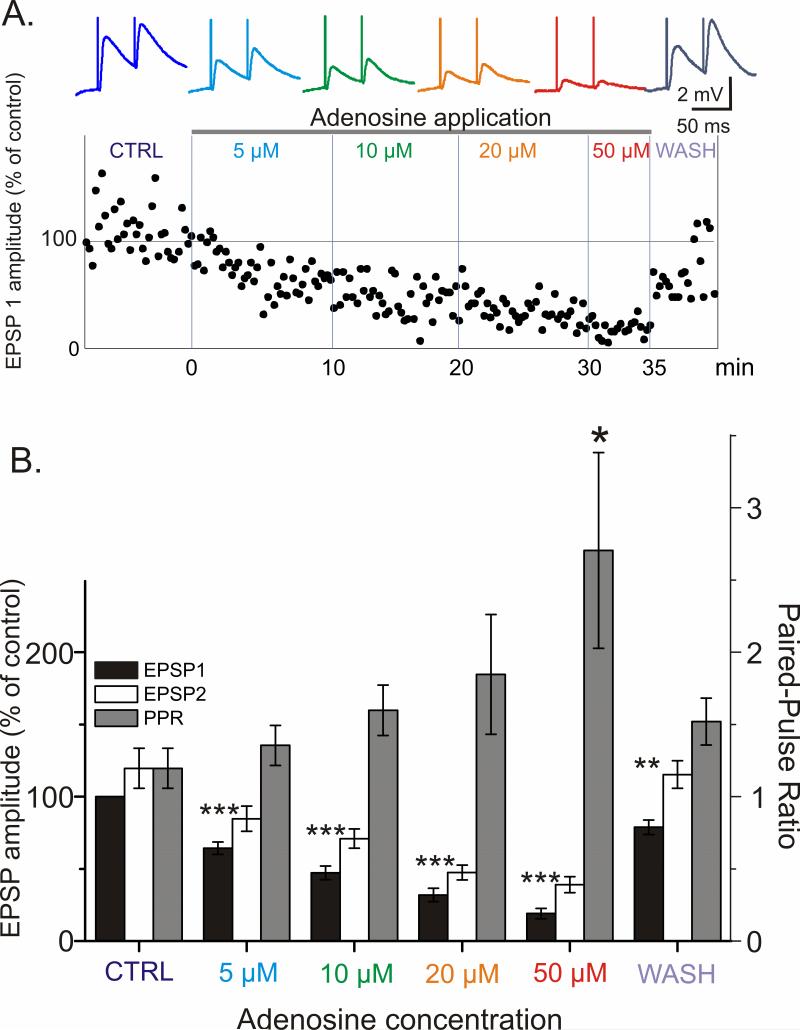
To study the effects of adenosine on synaptic transmission to layer 2/3 pyramidal neurons we recorded EPSPs evoked by paired-pulse electric stimuli in control and during bath application of adenosine at different concentrations. To determine an effective concentration, we used bath application of 5 μM to 50 μM adenosine (Fig 2). Already with the lowest tested concentration of 5 μM, adenosine induced a clear decrease of the EPSP amplitude to 64.3 ± 4.3% of baseline (p <0.001). Application of increasing concentrations of adenosine led to a progressive reduction of the EPSP amplitude (Fig 2A, B). 20 μM of adenosine had a robust effect, reducing the EPSP amplitude to 32 ± 4.6% of baseline (p < 0.001). This concentration was selected for further experiments.
Fig 2. Adenosine reduces evoked EPSP amplitude and increases paired-pulse ratio (PPR) in a reversible and concentration dependent manner.
A. (Above) Traces of averaged EPSPs evoked in a layer 2/3 neuron from visual cortex by paired stimuli (50 ms interpulse interval) in control and through increasing concentrations of adenosine. (Below) The time course of amplitude changes of the responses to the first pulse in a pair (EPSP1, % of control). Data for same cell.
B. Changes of the amplitude of EPSP1, EPSP2 and paired-pulse ratio (PPR) induced by increasing concentration of adenosine. EPSP amplitudes were normalized by the amplitude of the EPSP1 in control for each cell, and then averaged for N=5 neurons (10 inputs). Adenosine reduces EPSP amplitude and increases PPR in a concentration dependent and reversible manner.
Significance denoted as * p<0.05; **p<0.01; *** p<0.001. Significance for EPSP2 mirrored EPSP1 for all concentrations of adenosine tested, yet “***” is omitted for clarity.
The reduction in EPSP amplitude by adenosine was accompanied by an increase in the paired-pulse ratio (PPR; Figure 2B). The PPR is an index of release that is inversely related to the release probability at a synapse (Stevens, 1993; Voronin, 1993). The increase in the PPR is suggestive of a decrease in release probability.
To confirm the presynaptic locus of adenosine's effects, we made voltage clamp recordings with Cs-based pipette solution to block potassium channels and remove the possible contribution of postsynaptic potassium currents from adenosine's effect (Fig 3). Evoked excitatory postsynaptic currents (EPSCs) were recorded in the presence of 20 μM APV to block NMDA receptors (Fig 3). In 10 inputs, 20 μM adenosine reduced the amplitude of EPSCs to 39.1 ± 3.1% of baseline (a change from an average of -76.6 ± 10.9 pA to 30.1 ± 10.3 pA; n = 10, p < 0.001), and increased the PPR from 1.31 ± 0.14 to 1.53 ± 0.16 (Fig 3B; p < 0.05). These data with postsynaptic potassium channels blocked and AMPA EPSCs pharmacologically isolated confirm adenosine acts presynaptically to suppress excitatory transmission.
2.2. A1R blockade prevents adenosine's effects on evoked EPSPs
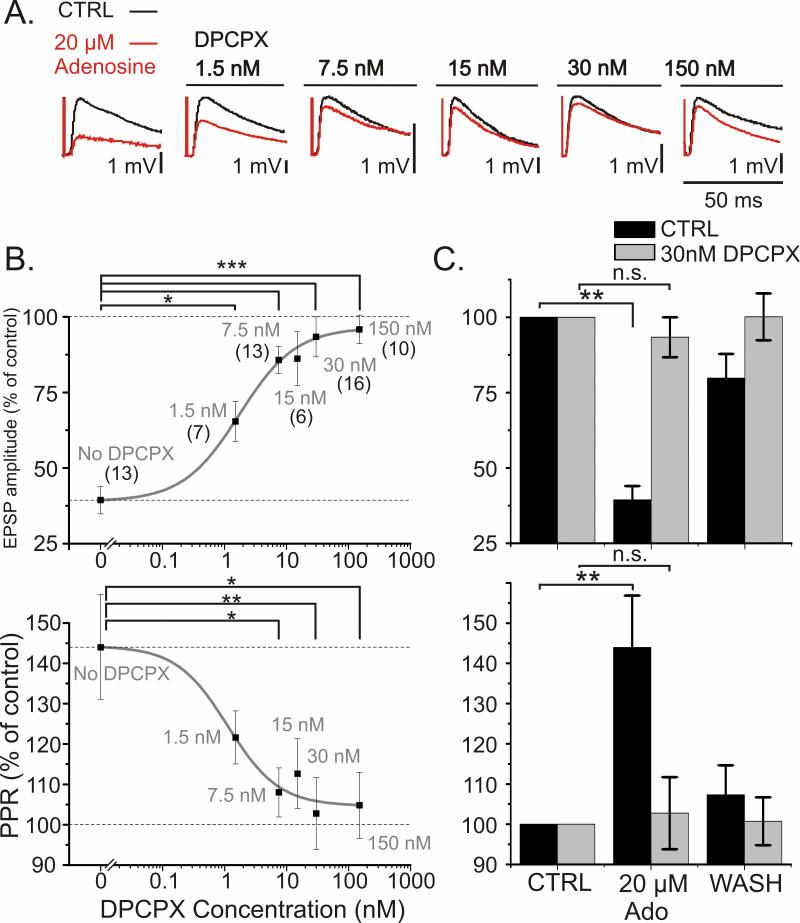
Which receptors mediate adenosine's effects on synaptic transmission? In situ hybridization, reverse transcription-polymerase chain reaction, binding studies, and other biochemical methods have demonstrated high level of expression of A1Rs in the neocortex (Dixon et al., 1996; Fredholm et al., 2001a). These receptors are classically responsible for the inhibitory actions of adenosine. Therefore we first studied how A1R blockade affects the inhibitory actions of adenosine on synaptic transmission. We applied 20 μM adenosine on the background of varying concentrations of the selective A1R antagonist DPCPX. DPCPX blocked the adenosine-induced reduction in EPSP amplitude in a concentration dependent manner (Fig 4). The effect of DPCPX was clear even at the lowest concentration tested (1.5 nM), and in the presence of 30 nM DPCPX, application of 20 μM adenosine failed to induce significant reduction of EPSP amplitude. Figure 4B displays summary data on the EPSP amplitude and PPR changes during application of 20 μM adenosine as a percent of baseline. In control conditions (no DPCPX), 20 μM adenosine reduced the EPSP amplitude to 39.4 ± 4.6% of baseline (Fig 4B, C; p<0.01, n= 13; significant reduction from baseline denoted in C). On the background of 1.5 nM DPCPX adenosine reduced the EPSP amplitude to 65.4 ± 6.7% of baseline. This reduction was significantly smaller than in the control group (Fig 4B, p < 0.05). On the background of 30nM DPCPX, adenosine reduced the EPSP amplitude only slightly, to 93.4 ± 6.6% of baseline, which was significantly less than the reduction without DPCPX (Fig 4B; p < 0.001). In fact, 30 nM DPCPX was sufficient to completely block the effect of adenosine on EPSP amplitude. Application of 20 μM adenosine in the presence of 30 nM DPCPX did not lead to a significant reduction in EPSP amplitude (Fig 4C, top).
Fig 4. A1 receptor antagonist DPCPX blocks adenosine's effects on synaptic transmission.
A. Example traces of evoked EPSPs before (black) and after application of 20 μM adenosine (red) on the background of DPCPX. Data for different concentrations of DPCPX are from different cells.
B. Concentration dependence of the blockade of adenosine's effects on synaptic transmission by DPCPX. Changes of EPSP1 amplitude (top) and PPR (bottom) induced by bath application of 20 μM adenosine in control (no DPCPX) and on the background of different concentrations of DPCPX. Number of synaptic inputs studied with each DPCPX concentration is indicated in parentheses in the top plot. Solid curves show sigmoid fit to the data points. Significance was calculated for the difference between percent reduction of EPSP amplitude by 20 μM adenosine in control group vs. the reduction in the presence of DPCPX.
C. Effects of 20 μM adenosine on synaptic transmission are completely blocked by 30 nM DPCPX. Data from B, but shown with washout of adenosine. Changes of EPSP1 amplitude (top) and PPR (bottom) during application and washout of 20 μM adenosine, in control (black bars) and in the presence of 30 nM DPCPX (grey bars). Note that Y-axes in B, C do not start from zero.
The attenuation of the adenosine-induced reduction in EPSP amplitude in the presence of DPCPX was accompanied by an attenuation of the adenosine-induced increase in PPR. Figure 4B (bottom) shows a summary of the PPR changes induced by application of 20 μM adenosine on the background of different concentrations of DPCPX. In control conditions (no DPCPX), application of 20 μM adenosine increased the PPR to 144 ± 12.9% of baseline (Fig 4B, C; p < 0.01, significant increase denoted in C). In the presence of 1.5 nM DPCPX, the increase was smaller (122 ± 6.6%), and became significantly different from the no-DPCPX group at 7.5 nM (108 ± 6.1% of baseline; p < 0.05). With 30 nM DPCPX, adenosine did not elicit any significant increase in the PPR, just as it did not depress the EPSP amplitude (Fig 4B,C).
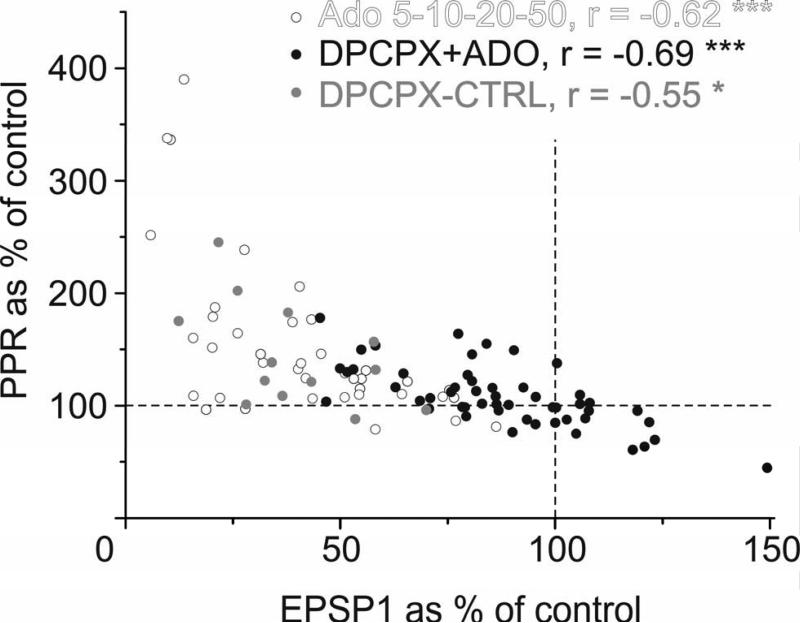
In experiments with adenosine applied alone or in the presence of DPCPX, the changes in EPSP amplitude were inversely proportional to the PPR changes (Fig 5). A strong negative correlation between EPSP amplitude changes and PPR changes was found in DPCPX experiments (r = −0.693, n = 52, p < 0.001), as well as in experiments in which adenosine was applied alone at different concentrations (r = −0.620, n = 39, p < 0.001 for concentration dependence experiments and r = −0.546, n = 13, p < 0.05 for the control group for DPCPX experiments). The correlation remained strong when all groups were pooled together (r = −0.644, n = 104, p < 0.001) indicating that the relationship between EPSP/PPR change is maintained when manipulating the activation or blockade of A1Rs by various ligands. These results suggest that presynaptic A1Rs are mediating adenosine's effects on EPSP amplitude by modifying release probability.
Fig 5. Adenosine induced changes in EPSP amplitude are inversely related to changes in PPR.
Changes in PPR plotted against changes in the EPSP1 amplitude. The changes in PPR and EPSP1 amplitude were correlated in all three groups of experiments: Experiments with the application of adenosine at different concentrations (5-50 μM, open circles, r = -0.62, n = 39, p < 0.001); experiments utilizing application of 20 μM adenosine on the background of different concentrations of DPCPX (1.5 - 150 nM, closed circles; r = -0.69, n = 52, p < 0.001); and the control group for these experiments, which utilized 20 μM adenosine but no DPCPX (grey circles, r = −0.55, n = 13, p < 0.05). The changes in EPSP amplitude and PPR are inversely related during all manipulations of A1R activation.
2.3. Adenosine reduces the frequency of miniature EPSPs
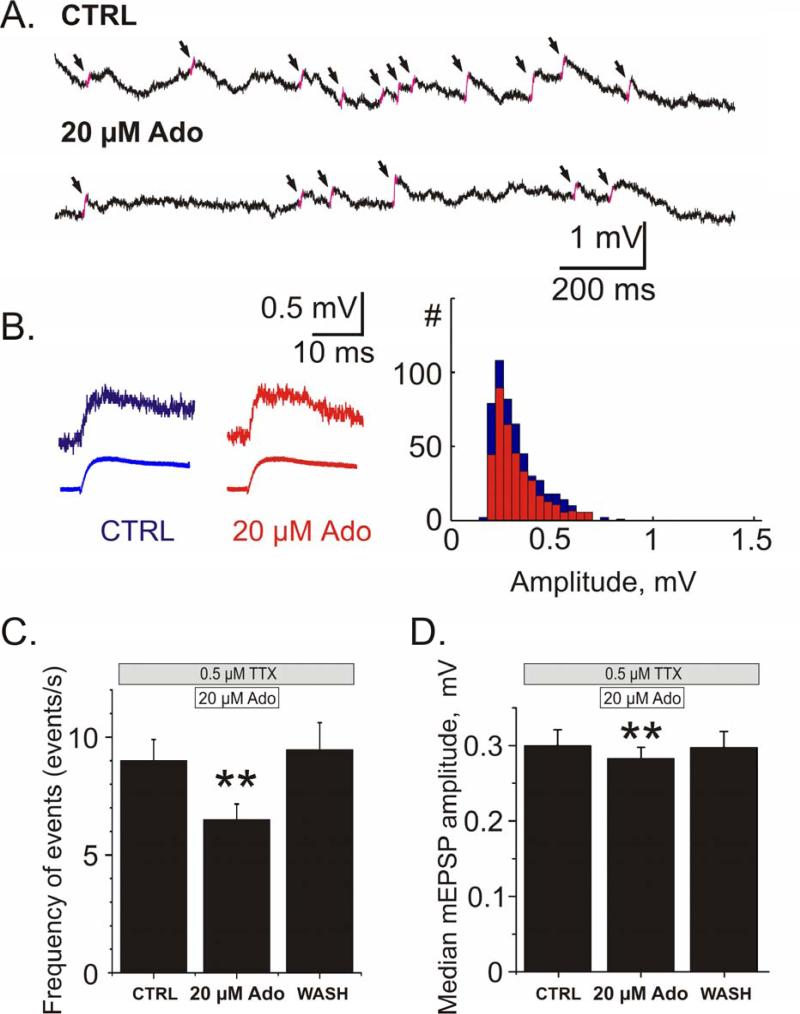
The involvement of presynaptic mechanisms in adenosine's effects on synaptic transmission is corroborated by results of recordings of miniature EPSPs in the presence of 0.5 μM TTX in the bath. Figure 6A illustrates membrane potential traces and detected mEPSPs. Application of adenosine (20 μM) led to a decrease of the frequency of mEPSPs (Fig 6A, C). On average, mEPSP frequency decreased from 9.0 ±0.9 events/sec in control to 6.5 ±0.7 events/sec (when normalized, 80.4 ±4.5% of baseline frequency) during adenosine application (Fig 6C; n = 20, p < 0.01). After washout of adenosine, mEPSP frequency recovered to 9.5 ± 1.2 events/sec (104.3 ± 9.8% of baseline frequency).
Fig 6. Adenosine reduces miniature EPSP (mEPSP) frequency and decreases the median amplitude of events.
A. Example traces of spontaneous activity from one cell in control and in the presence of 20 μM adenosine. The slopes of detected mEPSPs are highlighted in pink and marked with arrows.
B. Examples of individual (top) and averaged (bottom; N=509 events for CTRL; N=355 events for Ado) miniature EPSPs and their amplitude distributions from 2.5 min recordings in control and during application of 20 μM adenosine.
C, D. Changes of the frequency of mEPSPs (C) and their median amplitude (D) during application of 20 μM adenosine and washout. Both the reduction in frequency of events and median amplitude of events recovered after washout of adenosine. Averaged data for N=18 cells.
Adenosine application led to reduction of the frequency of mEPSPs of all amplitudes; the amplitude distributions were scaled down, but their skew did not change (2.9 ±0.3 vs 3.1 ±0.4; n.s.). There was however a very small yet significant reduction in the median amplitude of events, from 0.29 ±0.02 mV to 0.28 ±0.02 mV, (95.0 ±1.2% of baseline; Fig 6D n = 20, p < 0.01). This reduction of the median mEPSP amplitude may be attributable to a ~20% decrease of the input resistance of neurons during adenosine application (see 2.4 below).
Adenosine-induced decrease of the frequency of mEPSPs was also found to be mediated by A1 receptors. On the background of 30 nM DPCPX, application of 20 μM adenosine failed to induce any changes of the frequency of mEPSPs (9.8 ±1.5 vs. 9.6 ±1.6 events/sec, n= 15, data not shown).
To uncover any tonic activation of A1Rs, DPCPX was applied to the slice without the application of adenosine. The application of 30 nM DPCPX on the background of 0.1μM TTX and 100 μM picrotoxin led to a significant increase of excitatory mEPSP frequency from 6.8 ± 1.0 events/sec to 7.1 ± 1.0 events/sec (n = 11, p < 0.05). An increase in mEPSP frequency suggests that the blockade of A1Rs by DPCPX relieves a tonic inhibition of release by adenosine. The present results confirm physiologically relevant tonic levels of adenosine in the neocortex in vitro.
Taken together, the results of experiments with DPCPX demonstrate that the inhibitory effects of adenosine on synaptic transmission to pyramidal neurons in layer 2/3 are mediated via activation of presynaptic A1 receptors which have a suppressive effect on release probability.
2.4. Adenosine reversibly hyperpolarizes the membrane and decreases the input resistance of layer 2/3 pyramidal neurons
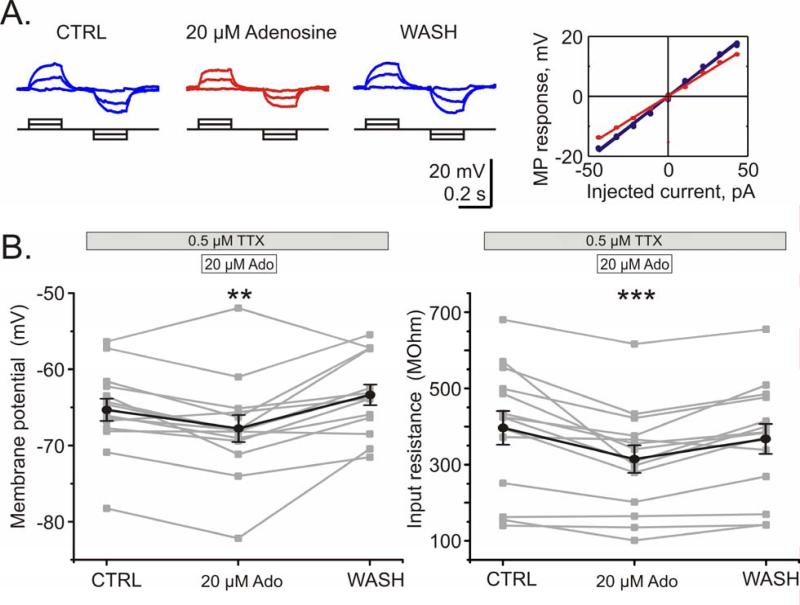
In addition to the presynaptically mediated effects on synaptic transmission, adenosine application led to a decrease of the input resistance and hyperpolarization of the cell membrane in layer 2/3 pyramidal neurons. Input resistance was calculated from membrane potential responses to small steps of positive and negative current applied through the recording electrode (Fig 7A). During application of 20 μM adenosine the input resistance of the neurons decreased by 19.5% ± 4.0% from 396.5 ± 44.4 MOhm to 314.0 ± 36.3 MOhm (Fig 7B, right; n = 14 p < 0.001). The decrease of the input resistance could have been the reason for the minor decrease of the median amplitude of mEPSPs during adenosine application. The resting membrane potential was hyperpolarized by 2.3 ± 0.8 mV during adenosine application from - 65.3 ± 1.5 mV to -67.7 ± 1.8 mV (Fig 7B, left; n = 14 p < 0.01). In experiments with A1 receptor blocker the input resistance and resting membrane potential changed in the opposite direction: application of 30 nM DPCPX in the absence of exogenous adenosine increased the input resistance by 10.5 ± 5.4% (n = 15, p < 0.05) and depolarized the resting potential by 2.8 ± 1.2 mV (n = 15, p < 0.05, data not shown).
Fig 7. Adenosine decreases input resistance and causes hyperpolarization in pyramidal neurons from layer 2/3.
A. Membrane potential response of a pyramidal neuron from layer 2/3 to current steps in control, during application of 20 μM adenosine, and after washout. Decreased slope of the voltage-current relationship during adenosine application (red) indicates a decrease of the input resistance.
B. Changes of the membrane potential (left) and input resistance (right) during adenosine application and washout. Individual data for N=14 neurons (grey) and their average (black). 20 μM adenosine hyperpolarizes cells and decreases their input resistance. Washout of adenosine demonstrates the reversibility of these effects.
2.5. Effects of high concentration of adenosine on synaptic transmission
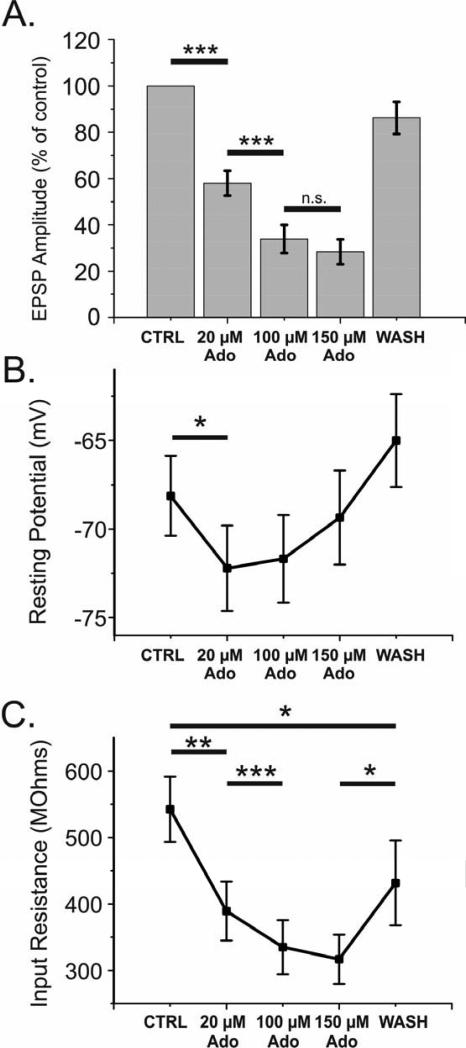
In order to discover a maximal effect of adenosine, we conducted a series of experiments which included high concentrations of adenosine (100 and 150 μM). On 18 inputs from 14 cells, we bath applied 20, 100 and 150 μM adenosine and recorded evoked activity and membrane properties (Fig 8). Increasing adenosine concentration from 20 μM to 100 μM led to a significant decrease of EPSP amplitude from 58.0 ± 5.3% to 33.9 ± 6.1 % of the control (Fig 8A, p<0.001). In 2 out of 18 cases, responses were completely abolished during application of 100 μM adenosine, but recovered upon wash out. Further increase of adenosine concentration to 150 μM did not result in a further significant decrease of response (28.3 ± 5.4% of the baseline), and did not increase the proportion of inputs in which responses were completely abolished.
Fig 8. High concentrations of adenosine reveal a saturating effect on reduction of EPSP amplitude, hyperpolarization of membrane, and decrease in input resistance.
A. Average reduction in evoked EPSP amplitude as percent of baseline during a bath application of 20, 100, and 150 μM adenosine reveal an upper limit on suppression of the EPSP. 11% (2 of 18) inputs were totally silenced by 100 μM adenosine, yet returned upon washout.
B, C. Average changes in membrane potential (B) and input resistance (C) before, during, and after adenosine application. Concentrations of adenosine needed to achieve a maximal effect on hyperpolarization were lower in comparison to concentrations needed to reach maximal effects on reduction of EPSP amplitude and input resistance.
The input resistance of the membrane followed similar concentration dependence as the EPSP (Fig 8C): significantly decreasing from control to 20 μM adenosine, and again from 20 μM to 100 μM adenosine. There was no significant change between 100-150 μM adenosine however, suggesting a saturating or nearly saturating effect of adenosine on input resistance. The hyperpolarization of the cell caused by the application of adenosine appears to be the most easily saturated measure of adenosine's actions. After an initial hyperpolarization upon application of 20 μM adenosine, membrane potential did not hyperpolarize any further upon increasing adenosine concentration to 100 and then 150 μM (Fig 8B). Because evoked activity was not blocked in these experiments, the membrane potential expressed stronger fluctuations (as indicated by larger standard error of these measurements) as compared to experiments conducted under TTX (see Fig 7). The possibility of a small effect size being masked by large variability cannot be excluded.
2.6. Functional A2A receptors are present in layer 2/3 of neocortex
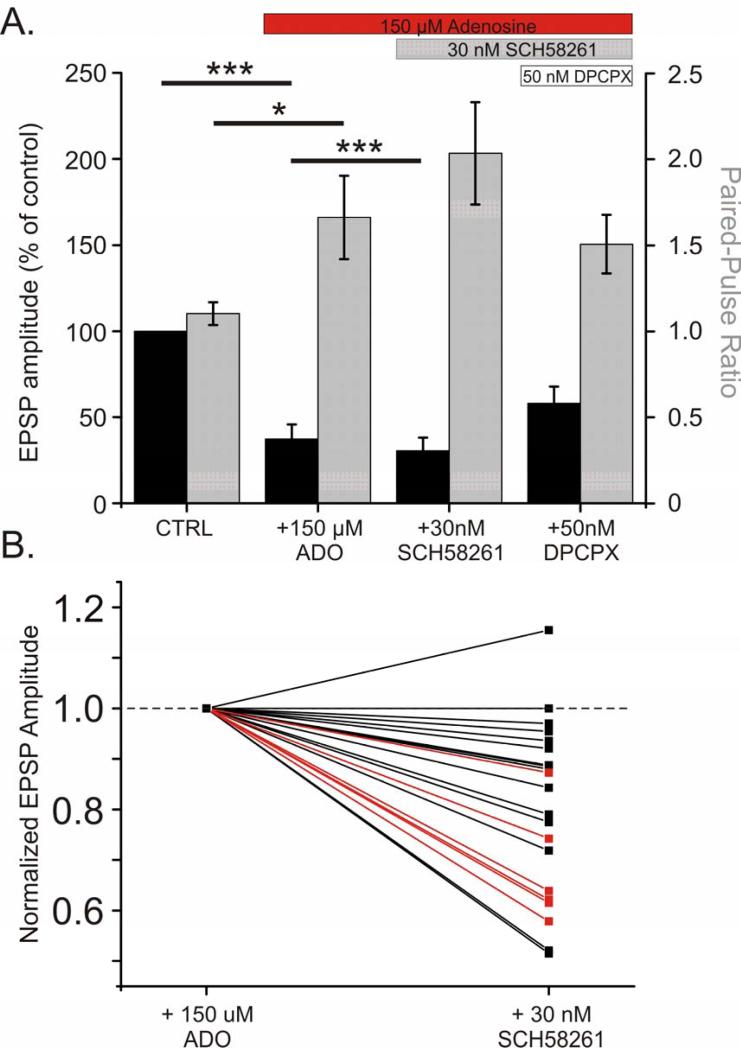
Results presented thus far show that A1R activation plays a major role in mediating suppressive effects of adenosine on excitatory synaptic transmission in our preparation. Evidence obtained using immunochemical methods and binding of A2AR ligands in synaptosomes suggests the presence of A2ARs in the cerebral cortex albeit at much lower levels than for example, in the striatum (Lopes et al., 1999; Marchi et al., 2002; Lopes et al., 2004). The activation of A2ARs is associated with a facilitation of excitatory transmission (namely an increase in glutamate release), which is in opposition to A1R effects. Because A1Rs have higher affinity to adenosine, and their expression in the cortex is much higher than of other subtypes (Fredholm et al., 2001b; Ciruela et al., 2006; Ciruela et al., 2011), effects of adenosine at low concentrations might be dominated by A1R activation. High concentrations (100-150 μM) of adenosine may activate both A1 and A2A receptors in our preparation. We questioned if at such high concentrations adenosine has saturated the A1Rs and also activates facilitatory A2ARs. In this scenario the maximal reduction in EPSP amplitude elicited by A1R activation would be curbed by A2AR activation. The effect of adenosine on EPSP amplitude described in figure 8 would then be a combination of an inhibitory effect of A1R activation with a smaller facilitatory component produced by A2AR activation. If this is the case, then under high concentrations of adenosine the blockade of A2ARs should further reduce the amplitude of the EPSP. To test this hypothesis, we conducted a series of experiments in which application of 150 μM adenosine was followed by application of the selective A2AR antagonist SCH-58261 (30 nM). As described above, application of 150 μM adenosine reduced the EPSP amplitude (Fig 9A; average of 37.3 ± 8.5% of baseline; N = 24, p <0.001). Addition of SCH-58261 elicited a small yet significant further reduction in the EPSP amplitude (to an average of 30.5 ± 7.7% of baseline; p <0.001). In the 21 inputs that were not abolished by 150 μM adenosine, 19 showed a decrease in EPSP amplitude upon application of SCH-58261, and in 6 of these inputs, the decrease in EPSP amplitude was found to be significant (Fig 9B). This suggests functional A2ARs are present in the neocortex, where they act in opposition to A1Rs to modulate excitatory transmission. Subsequent addition of A1R antagonist DPCPX, and thus blockade of both A1 and A2A receptors was expected to relieve EPSPs from suppression. The EPSP amplitude increased after addition of 50 nM DPCPX. However, the recovery was not complete, (only to 59.4 ± 9.8% of baseline). We attribute the incomplete recovery to the inability of the competitive A1R antagonist DPCPX to completely block the activation of A1Rs by the saturating concentration (150 μM) of adenosine.
Fig 9. On the background of a saturating concentration of adenosine, blockade of facilitatory A2A receptors by SCH-58261 leads to further reduction of EPSP amplitude.
A. Average reduction in evoked EPSP amplitude as percent of baseline (n = 24 inputs from 20 cells; black bars). PPR shown in grey. After application of 150 μM adenosine, 30nM SCH-58261 reduced the EPSP amplitude further, suggesting that under a high concentration of adenosine, the suppressive effect of inhibitory A1Rs is curbed by the activation of facilitatory A2A receptors.
B. Changes in normalized EPSP amplitude after the application of 30 nM SCH-58261 for individual inputs. For each input, normalized EPSP amplitudes during 150 μM adenosine and after subsequent addition of 30 nM SCH-58261 are connected with a line. Inputs which demonstrated a significant change (within subjects comparison of 20 individual EPSPs from each condition; p < 0.05, paired t-test) in EPSP amplitude after SCH-58261 application are highlighted in red.
In the next series of experiments 100 μM adenosine and then 30 nM SCH-58261 were applied sequentially but this time on the background of A1R blockade with 50 nM DPCPX. On the background of 50 nM DPCPX, 100 μM adenosine moderately reduced the EPSP amplitude to 66.3 ± 5.2% of control, but SCH-58261 was unable to reduce the amplitude of the EPSP further (63.7 ± 7.9% of control; n = 16, p = 0.63; data not shown). These results suggest that A2ARs may facilitate transmission in a manner that requires A1R activation.
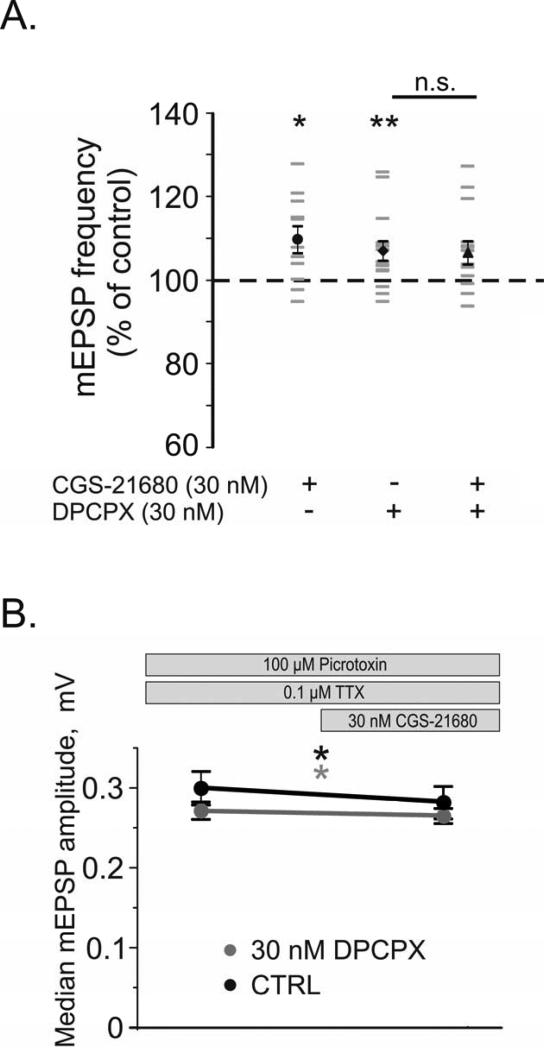
To further investigate the involvement of A2ARs in regulation of excitatory synaptic transmission in the neocortex, mEPSPs were recorded on the background of 0.1 μM TTX and 100 μM picrotoxin (Fig 10). The addition of the A2A agonist CGS-21680 (30 nM) significantly increased the frequency of mEPSPs to 109.8 ± 3.2% of control (from 5.8 ± 1.0 to 6.3 ± 1.0 events/s; Fig 10A black circle, n = 11, p < 0.05). In a separate group of cells, application of 30nM DPCPX similarly increased the mEPSP frequency to 107.0 ± 2.3% of control (from 6.2 ± 0.8 to 6.5 ± 0.8 events/s; black diamond, n = 15, p < 0.01). Interestingly, application of CGS-21680 on the background of DPCPX in these cells yielded no further change in the frequency of events (black triangle, 106.6 ± 2.8% of control, n = 14; p=0.692 comparison to DPCPX, p=0.061 comparison to control). A between subjects comparison reveals that the change in mEPSP frequency when CGS-21680 is added on the background of DPCPX is significantly different from the change induced by CGS-21680 in the absence of DPCPX (p < 0.05). The blockade of A1Rs by DPCPX removed the A1R mediated adenosine tone and abolished the ability of CGS-21680 to increase the frequency of mEPSPs. Thus A1R activation is necessary for the A2AR to exert its facilitatory effects on mEPSP frequency. These data suggest that A2ARs facilitate excitatory transmission through an A1R dependent mechanism.
Fig 10. A2AR Agonist CGS-21680 Increases mEPSP frequency and decreased median mEPSP amplitude.
A. Changes in the frequency of mEPSPs after application of selective adenosine receptor ligands (shown as % of control). Individual neurons are shown in grey, with averages denoted by black symbols. In N = 11 neurons, the A2AR agonist CGS-21680 (30 nM) increased the frequency of mEPSPs (black circle). In another N = 15 neurons, the A1R antagonist DPCPX (30nM) also increased the frequency of mEPSPs (black diamond). After application of DPCPX, subsequent addition of 30 nM CGS-21680 to these same neurons did not lead to further increase of mEPSP frequency (black triangle).
B. Changes in the amplitude of the median mEPSP after CGS-21680 application. In N = 11 neurons, the A2AR agonist CGS-21680 (30 nM) decreased the median mEPSP amplitude (black line). In N = 14 neurons, on the background of 30 nM DPCPX (grey line), 30nM CGS-21680 decreased the median mEPSP amplitude (grey line).
Bath application of 30 nM CGS-21680 also revealed a small yet significant decrease of the median amplitude of mEPSPs from an average of 0.30 ± 0.02 mV to 0.28 ± 0.02 mV (Fig 10B, black line, n = 11, p < 0.05). On the background of 30 nM DPCPX (grey line), the median mEPSP amplitude significantly changed from an average of 0.273 ± 0.005 mV to 0.266 ± 0.004 mV after addition of 30nM CGS-2168 (n = 14, p < 0.05). The skew of the amplitude distributions was unchanged by CGS-21680 application in either group (data not shown). Whereas CGS-21680's enhancement of mEPSP frequency is facilitatory, the reduction in mEPSP amplitude serves to inhibit excitatory transmission. In both groups, no significant changes in input resistance or membrane potential were observed after CGS-21680 application (data not shown).
3. DISCUSSION
Results of the present study demonstrate that in rat visual cortex, adenosine suppresses synaptic transmission to layer 2/3 pyramidal neurons, decreases their input resistance and leads to membrane hyperpolarization. These pre- and postsynaptic effects of adenosine are mediated predominantly by A1 receptors. We provide, to our knowledge, the first evidence for functional A2A receptors in the neocortex which are activated at high adenosine concentrations and may oppose the presynaptic actions of A1Rs. Moreover, our results suggest interaction between A1 and A2A receptors, whereby A2ARs exert their facilitatory effect on synaptic transmission inhibiting the A1R mediated suppression.
3.1. Presynaptic action of adenosine: activation of A1 receptors leads to a reduction in release probability
The most pronounced effect of adenosine application in layer 2/3 of the rat visual cortex was the suppression of synaptic transmission. Adenosine reduced the evoked EPSP amplitude in a concentration dependent and reversible manner. Concurrent with the reduction in the EPSP amplitude was an increase in the paired-pulse ratio. Because the paired-pulse ratio is inversely related to release probability (Voronin, 1993; Murthy et al., 1997; Dobrunz and Stevens, 1997; Oleskevich et al., 2000), these results suggest that an inhibition of transmission was due to a reduction in release probability. This conclusion is further supported by the finding that 20 μM adenosine reduces the frequency of mEPSPs, which reflects presynaptic properties. These effects of adenosine were mediated by the A1R, as they were abolished by application of the selective A1R antagonist DPCPX (30 nM). These results for layer 2/3 pyramidal neurons are consistent with prior data showing that adenosine and A1R agonists can increase the paired- pulse ratio in the hippocampus and in layer 1 and 5 of the visual cortex (Dunwiddie and Haas, 1985; Murakoshi et al., 2001; Kirmse et al., 2008). It has been also reported that adenosine application reduced the frequency of mEPSPs in the hippocampus (Scanziani et al., 1992) and layers 1 and 5 of the neocortex (Murakoshi et al., 2001; Kirmse et al., 2008). In layer 2/3 of the somatosensory cortex, removal of tonic A1R activation resulted in an increase in mEPSC frequency (Deng et al., 2011). In hippocampus, presynaptic A1 receptors mediate inhibitory effects on synaptic transmission by reducing the influx of calcium through voltage-dependent calcium channels (Wu and Saggau, 1994, 1997; Gundlfinger, 2007), and by modulation of release machinery downstream from Ca2+ influx (Scanziani et al., 1992; Wu and Saggau, 1997). In both cases, the result of this presynaptic inhibition would be a reduction of release probability.
Our results on the presynaptic effects of adenosine at layer 2/3 synapses most closely correspond to the findings reported for layer 5 pyramidal neurons from rat visual cortex (Murakoshi et al., 2001). These authors found that adenosine application leads to a reduction in evoked EPSC amplitude, an increase in the paired-pulse ratio, and a decrease in miniature EPSC frequency. However, they report only presynaptic changes, and did not observe any changes in membrane conductance or the mean mEPSC amplitude. The decrease of input resistance and hyperpolarization of layer 2/3 pyramids in our experiments might restrain propagation of synaptic activity through this layer. This may be one of the reasons for the strongest inhibition of activity in layers 2/3 by adenosine observed with voltage-sensitive dye imaging (Kovac et al., 2008). These results stress the layer-specificity of adenosine's effects in the neocortex.
In the hippocampus, a tonic inhibition of synaptic transmission by endogenous adenosine acting on the A1R is documented both in vivo and in vitro (Dunwiddie and Diao, 1994; Wu and Saggau, 1994; Lopes et al., 2002; Manita et al., 2004). Results obtained using transgenic mice with impaired astrocytic vesicle release indicate that adenosine ‘tone’ is present also in the neocortex, and is provided by adenosine derived from astrocytic-released ATP (Halassa et al. 2009). An increase in the frequency of mEPSPs upon application of DPCPX in the absence of exogenously applied adenosine, observed in our experiments, lends support to this notion. Presence of the tonic inhibition of synaptic transmission by endogenous adenosine in the neocortex substantiates the role of adenosine as a neuromodulator capable of bi-directional modulation of synaptic transmission.
3.2. Postsynaptic actions of adenosine on layer 2/3 pyramidal neurons
Adenosine hyperpolarized layer 2/3 pyramidal neurons and decreased their input resistance, suggesting functional postsynaptic receptors. None of these effects were observed during application of adenosine on the background of A1R blockade by DPCPX, suggesting that they were mediated by A1 receptors. Both the decrease in input resistance and hyperpolarization of the membrane potential can be explained by the increase of K+ conductance. Indeed, evidence from hippocampal and striatal neurons shows that A1Rs enhance potassium currents through the activation of G-Protein coupled inwardly rectifying potassium channels (Trussel and Jackson, 1985, 1987; Greene and Haas, 1991; Thompson et al., 1992). Studies of neocortical neurons report unequivocal results. A1R mediated enhancement of potassium currents was found in dissociated neurons from the neocortex (Takigawa and Alzheimer 1999), but no postsynaptic effects of adenosine were reported in layer 5 neurons from slices of rat visual or associative frontal cortex (Murakoshi et al., 2001; Brand et al., 2001). Our demonstration of postsynaptic actions of adenosine in layer 2/3 may underlie the layer- specific increase of inhibition of activity propagation in neocortical slices (Kovac et al., 2008).
Changes of potassium currents and associated changes of the membrane properties may underlie a small decrease in median mEPSP amplitude observed in our experiments. It is important to note however that the main effect of A1R activation on synaptic transmission to layer 2/3 pyramids involves presynaptic mechanisms. In experiments with Cs-based pipette solution that blocked the contribution of potassium channel modulation to adenosine effects, we still observed a clear decrease of the amplitude and an increase of the paired-pulse ratio of evoked EPSCs during adenosine application.
3.3. A role for A2A receptors in neocortex? Insight from other brain areas
Our results provide evidence for functional A2A receptors in the neocortex. Increasing adenosine concentration from 100 to 150 μM failed to induce further significant reduction of EPSP amplitude. However, application of a selective A2AR antagonist SCH-58261 on the background of 150 μM adenosine did lead to a further reduction of EPSP amplitude. We interpret these results as suggesting that high concentrations of adenosine activate A2ARs which facilitate synaptic transmission, and that SCH-58261 application inhibits this facilitation thus leading to a decrease of the EPSP amplitude. Notably, this effect was found to be significant in only a proportion (25%, 6 out of 24) of inputs. These results agree with prior work in the hippocampus, that suggested that A2ARs are present at much fewer synapses than the A1Rs (Rebola et al. 2005a,b). It remains to be elucidated what governs the expression of A2ARs only at some specific types of inputs; perhaps they are strategically located and activated only under specific circumstances.
Known interactions between A1Rs and A2ARs suggest a role for the A2AR as a modulator of the A1R. At glutamatergic striatal synapses, presynaptic A1Rs and A2ARs can form heteromers in the membrane in which A2AR activation decreases the affinity of the A1R for its agonists through direct receptor-receptor interactions (Ciruela et al., 2006; Ferré et al. 2007a,b; Ciruela et al., 2011). Additionally, interaction between A1Rs and A2ARs has been demonstrated in synaptosomes extracted from cortical and hippocampal regions from young adult rats. In this preparation, activation of A2ARs led to a protein kinase C dependent decrease of the binding affinity for ligands of A1Rs (Lopes et al., 1999). In the hippocampus, Lopes et al. (2002) found that a facilitation of transmission by A2AR agonist CGS-21680 was dependent upon an inhibitory A1R tone, and was likely presynaptic. Our results suggest a similar interaction between A2A and A1 receptors at neocortical synapses. Application of CGS-21680 led to an increase of mEPSP frequency and thus facilitation of excitatory transmission. However, no facilitation was observed when CGS-21680 was applied on the background of DPCPX that blocked A1R mediated inhibitory tone. These results suggest that A2ARs exert their effect on presynaptic release mechanisms via modulation of A1Rs rather than by affecting the release directly.
Interestingly, recent work in the hippocampus (Dias et al. 2012) suggests that application of CGS-21680 leads also to a slow-developing facilitation of AMPA-evoked currents (Dias et al. 2012). This postsynaptic action of A2ARs was protein-kinase A-dependent but was not disturbed by A1R blockade. In contrast to prior work in hippocampus (Lopes et al., 2002) and our results from neocortical neurons, Dias et al. (2012) did not observe evidence of presynaptic changes (as measured by mEPSC frequency) but report an increase in mEPSC amplitude after CGS-21680 application. In our experiments with CGS-21680, the agonist was permitted to wash into the bath for 30 to 40 minutes, but no long timescale changes were observed. These contrasting results may reflect differential sub-cellular distribution of A2A receptors in the neocortex and hippocampus.
What role, then, could the bidirectional modulation of excitatory transmission by adenosine serve? Under normal physiological conditions both neurons and astrocytes release adenosine and ATP into the extracellular space in an activity-dependent manner (Pascual et al., 2005; Wall and Dale, 2008; Halassa et al., 2009; Lovatt et al., 2012). At moderate activity levels this extracellular adenosine activates a portion of high-affinity A1Rs, setting a global inhibitory tone, on the background of which synaptic activity can be regulated both upwards and downwards. High frequency activation of synapses can cause ATP or adenosine to be co-released with transmitter, resulting in a transient and local increase in adenosine concentration restricted to the active synapses. This high local concentration of adenosine may activate A2ARs located at these synapses (Cunha, 2008; Costenla et al., 2010). These A2ARs may locally suppress the function of A1Rs by decreasing their affinity to adenosine, or by downregulating the receptor. The result is a facilitation (or at least reduced suppression) of transmission at the active synapses, and a more diffuse, A1R mediated inhibition of transmission at surrounding synapses. This antagonistic interaction between A2A and A1 receptors has been proposed to increase signal salience and mediate heterosynaptic depression (Pascual et al., 2005; Cunha, 2008). This example provides some indication of a possible functional relevance of two adenosine receptors with opposing effects, and of sparse A2A receptors. Further studies are necessary to elucidate exactly how, and under what circumstances, these two receptors interact in the neocortex, and the implications of such an interaction for cortical processing and plasticity.
3.4. Conclusions and outlook
We studied modulation of synaptic transmission by adenosine in layer 2/3 pyramidal neurons, which mediate long-range interaction between cortical areas and multisensory integration. We show that adenosine, via activation of A1 receptors, acts presynaptically by suppressing release at excitatory synapses, and postsynaptically by decreasing input resistance and hyperpolarizing the membrane of layer 2/3 pyramids. Moreover, facilitatory A2ARs that oppose the A1R-mediated suppression are present at a subset of presynaptic terminals. This presents the first, to our knowledge, physiological evidence for functional A2ARs in the neocortex. Each of these results is in general agreement with reported actions of adenosine in other brain structures. The specific picture of adenosine effects in layer 2/3 pyramids suggests the following possible scenario of the regulation of their activity by adenosine.
Because the release of adenosine and/or ATP from neurons and glia is activity dependent, adenosine has a stabilizing effect on neuronal activity, mediated by high-affinity A1 receptors. Increasing activity raises extracellular adenosine concentration which suppresses neurotransmitter release and neuronal excitability, while decreasing activity has the opposite effects. Notably, adenosine can play a role both in local regulation of activity on the time scale of seconds and minutes, but also in global, slow processes, such as regulation of slow sleep oscillations and sleep homeostasis. Indeed, adenosine levels rise during prolonged wakefulness, and adenosine antagonists such as caffeine promote wakefulness.
Synapses at which A2A receptors are expressed may be subject to an additional level of regulation. With strong activity adenosine levels would increase high enough to activate lower- affinity A2ARs which oppose the suppressive A1R action. Synaptic transmission at the very focus of activity will be suppressed less than in the broader surrounding area of activated A1Rs. Possible functions of the resulting activity profile may include increasing signal salience and regulation of synaptic plasticity. Future experiments will reveal which factors govern sparse A2AR expression at a subpopulation of neocortical synapses, and which functions these receptors may play in the operation of neocortical networks.
Highlights.
Distribution of adenosine receptors and their actions are brain-region specific
We study actions of adenosine on layer 2/3 pyramids in rat visual cortex in slices
A1R activation decreases input resistance and hyperpolarizes layer 2/3 pyramids
Presynaptically, A1R activation decreases release probability
Neocortical A2ARs facilitate transmission in an A1R dependent manner
ACKNOWLEDGEMENTS
We are grateful to Patrick Randall and Eric Nunes for their advice and assistance. Supported by the grant R01MH087631 from the NIH and Startup funds from the University of Connecticut to MV.
ABBREVIATIONS
- A1R
Adenosine Receptor type 1
- A2AR
Adenosine Receptor type 2A
- Ado
Adenosine
- APV
D-(-)-2-Amino-5-phosphonopentanoic acid
- ATP, AMP
Adenosine triphosphate, Adenosine monophosphate
- CGS-21680
4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2- yl]amino]ethyl]benzenepropanoic acid hydrochloride (A2AR agonist)
- DPCPX
8-Cyclopentyl-1,3-dipropylxanthine (A1R antagonist)
- EPSC
Excitatory Postsynaptic Current
- EPSP
Excitatory Postsynaptic Potential
- mEPSP
miniature Excitatory Postsynaptic Potential
- SCH-58261
2-(2-Furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin- 5-a-mine (A2AR antagonist)
- TTX
Tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bjorness TE, Greene RW. Adenosine and sleep. Curr. Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A1 and A3 receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Cohen RZ, Larocque S. Distribution of adenosine A1 receptors in primary visual cortex of developing and adult monkeys. Exp. Brain Res. 1998;123:351–354. doi: 10.1007/s002210050579. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26(7):2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Gómez-Soler M, Guidolin D, Borroto-Escuela D, Agnati L, Fuxe K, Fernández-Dueñas V. Adenosine receptor containing oligomers: Their role in the control of dopamine and glutamate neurotransmission in the brain. Biochim. Biophys. Acta. 2011;1808:1245–1255. doi: 10.1016/j.bbamem.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Costenla AR, Cunha RA, Mendonça A. Caffeine, adenosine receptors, and synaptic plasticity. J. Alzheimer's Dis. 2010;20:S25–S34. doi: 10.3233/JAD-2010-091384. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signalling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem. Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr. Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a src kinase dependent pathway. Glia. 2011;59:1084–1093. doi: 10.1002/glia.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RB, Ribeiro JA, Sebastião AM. Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A2A receptors. Hippocampus. 2012;22:276–291. doi: 10.1002/hipo.20894. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118(6):1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J. Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J. Pharmacol. Exp. Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 1997;17(20):7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fastbom J, Pazos A, Palacios JM. The distribution of adenosine A1 receptors and 5’-nucleotidase in the brain of some commonly used experimental animals. Neuroscience. 1987;22:813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Quiroz C, Luján R, Popoli P, Cunha R, Agnati L, Fuxe K, Woods A, Lluis C, Franco R. Adenosine receptor heteromers and their integrative role in striatal function. Sci. World J. 2007a;7(S2):74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007b;30(9):440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001a;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001b;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog. Neurobiol. 1991;36:329–341. doi: 10.1016/0301-0082(91)90005-l. [DOI] [PubMed] [Google Scholar]
- Gundlfinger A, Bischofberger J, Johenning FW, Torvinen M, Schmitz D, Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of Presynaptic calcium channels. J. Physiol. 2007;582(1):263–277. doi: 10.1113/jphysiol.2007.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM. Thalamocortical dynamics of sleep: Roles of purinergic neuromodulation. Semin. Cell Dev. Biol. 2011;22:245–251. doi: 10.1016/j.semcdb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmse K, Dvorzhak A, Grantyn R, Kirischuk S. Developmental downregulation of excitatory GABAergic transmission in neocortical layer I via presynaptic adenosine A1 receptors. Cereb. Cortex. 2008;18:424–432. doi: 10.1093/cercor/bhm077. [DOI] [PubMed] [Google Scholar]
- Kovac S, Sirin Y, Speckmann E- J, Gorji A. Different regional neuroinhibitory effects of adenosine on stimulus-induced patterns of bioelectric activity of rat hippocampal and neocortical tissues. Neuroscience. 2008;152:547–557. doi: 10.1016/j.neuroscience.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Stoelzel C, Chistiakova M, Volgushev M. Heterosynaptic plasticity induced by intracellular tetanization in layer 2/3 pyramidal neurons in rat auditory cortex. J. Physiol. 590. 2012;10:2253–2271. doi: 10.1113/jphysiol.2012.228247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Henry JL. Adenosine A2 receptor mediation of pre- and postsynaptic excitatory effects of adenosine in rat hippocampus in vitro. Eur. J. Pharmacol. 1998;347:173–182. doi: 10.1016/s0014-2999(98)00105-8. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J. Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience. 2002;112(2):319–329. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. Binding of the prototypical adenosine A2A receptor agonist CGS21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br. J. Pharmacol. 2004;141:1006–1014. doi: 10.1038/sj.bjp.0705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc. Natl. Acad. Sci. 2012;109:6265–6270. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manita S, Kawamura Y, Sato K, Inoue M, Kudo Y, Miyakawa H. Adenosine A1-receptor-mediated tonic inhibition of glutamate release at rat hippocampal CA3-CA1 synapses is primarily due to inhibition of N-type Ca2+ channels. Eur. J. Pharmacol. 2004;499:265–274. doi: 10.1016/j.ejphar.2004.07.113. [DOI] [PubMed] [Google Scholar]
- Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M. Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br. J. Pharmacol. 2002;136:434–440. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketonergic diet suppresses seizures in mice through adenosine A1 receptors. J. Clin. Invest. 2011;121(7):2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça A, Sebastião AM, Ribeiro JA. Adenosine: Does it have a neuroprotective role after all? Brain Research Reviews. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Song SY, Konishi S, Tanabe T. Multiple G-protein-coupled receptors mediate presynaptic inhibition at single excitatory synapses in the rat visual cortex. Neurosci. Lett. 2001;309:117–120. doi: 10.1016/s0304-3940(01)02051-1. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Clements J, Walmsley B. Release probability modulates short-term plasticity at a rat giant terminal. J. Physiol. 2000;524(2):513–523. doi: 10.1111/j.1469-7793.2000.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú M, Quiroz C, Guitart X, Ferré S. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 2011;61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005a;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Rebola N, Rodrigues RJ, Lopes LV, Richardson PJ, Oliveira CR, Cunha RA. Adenosine A1 and A2A receptors are co-expressed in pyramidal neurons and co-localized in glutamatergic nerve terminals of the rat hippocampus. Neuroscience. 2005b;133:79–83. doi: 10.1016/j.neuroscience.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gähwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Stevens CF. Quantal release of neurotransmitter and long-term potentiation. Neuron. 1993;10:55–63. doi: 10.1016/s0092-8674(05)80028-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997;27(4):322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Variance analysis of current fluctuations of adenosine- and baclofen-activated GIRK channels in dissociated neocortical pyramidal cells. J. Neurophysiol. 1999;82:1647–1650. doi: 10.1152/jn.1999.82.3.1647. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J. Physiol. 539. 2002;1:67–75. doi: 10.1113/jphysiol.2001.012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Haas HL, Gähwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J. Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussel LO, Jackson MB. Adenosine-activated potassium conductance in cultured striatal neurons. Proc. Natl. Acad. Sci. 1985;82:4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussel LO, Jackson MB. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J. Neurosci. 1987;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Eysel UT. Synaptic transmission in the neocortex during reversible cooling. Neuroscience. 2000;98:9–22. doi: 10.1016/s0306-4522(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Voronin LL. On the quantal analysis of hippocampal long-term potentiation and related phenomena of synaptic plasticity. Neuroscience. 1993;56(2):275–304. doi: 10.1016/0306-4522(93)90332-a. [DOI] [PubMed] [Google Scholar]
- Wall M, Dale N. Activity-dependent release of adenosine: a critical re-evaluation of mechanism. Curr. Neuropharmacol. 2008;6:329–337. doi: 10.2174/157015908787386087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20(5):204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]