Abstract
The change in polyphenol content in the primed leaves of burley, flue-cured, and Turkish tobaccos during air-curing was related to the activities and isozymes of polyphenol oxidase and peroxidase. The quantity of chlorogenic acid was rapidly reduced during the first week of curing. The decrease in rutin content during curing was less significant, especially when the concentration of chlorogenic acid was high in leaf tissues. This result was further confirmed by in vitro assays with partially purified tobacco polyphenol oxidase.
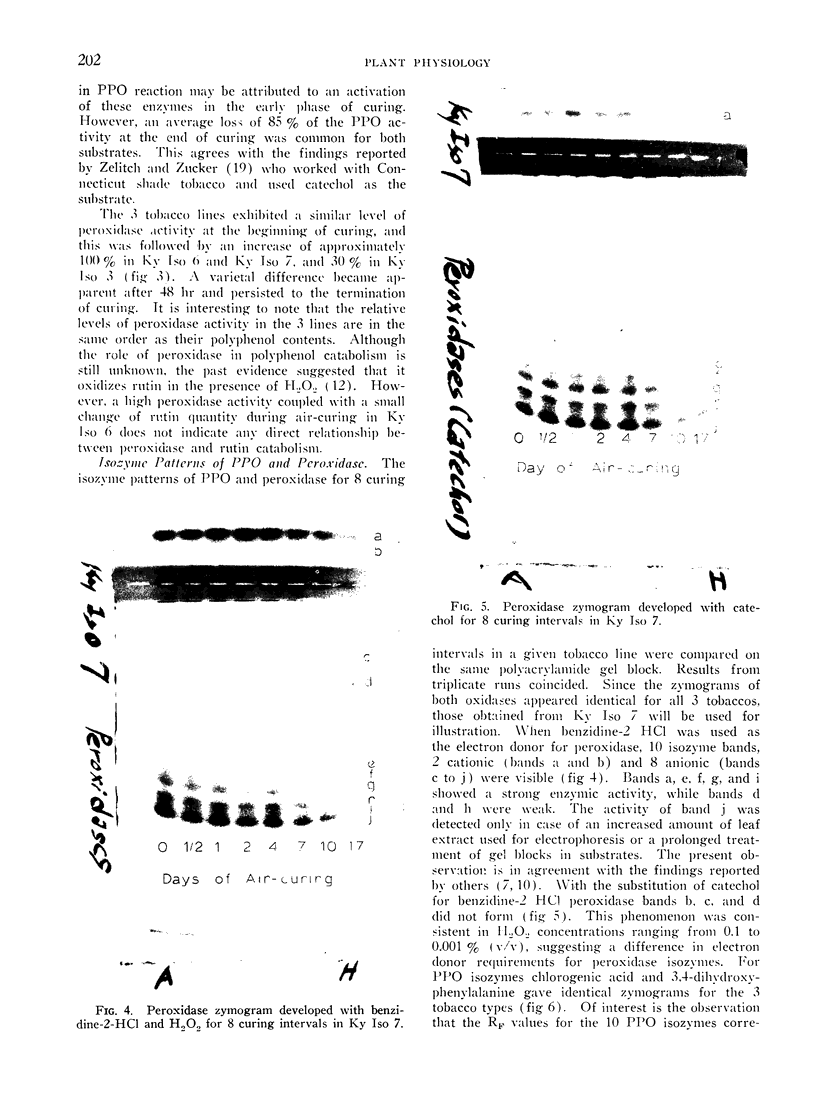
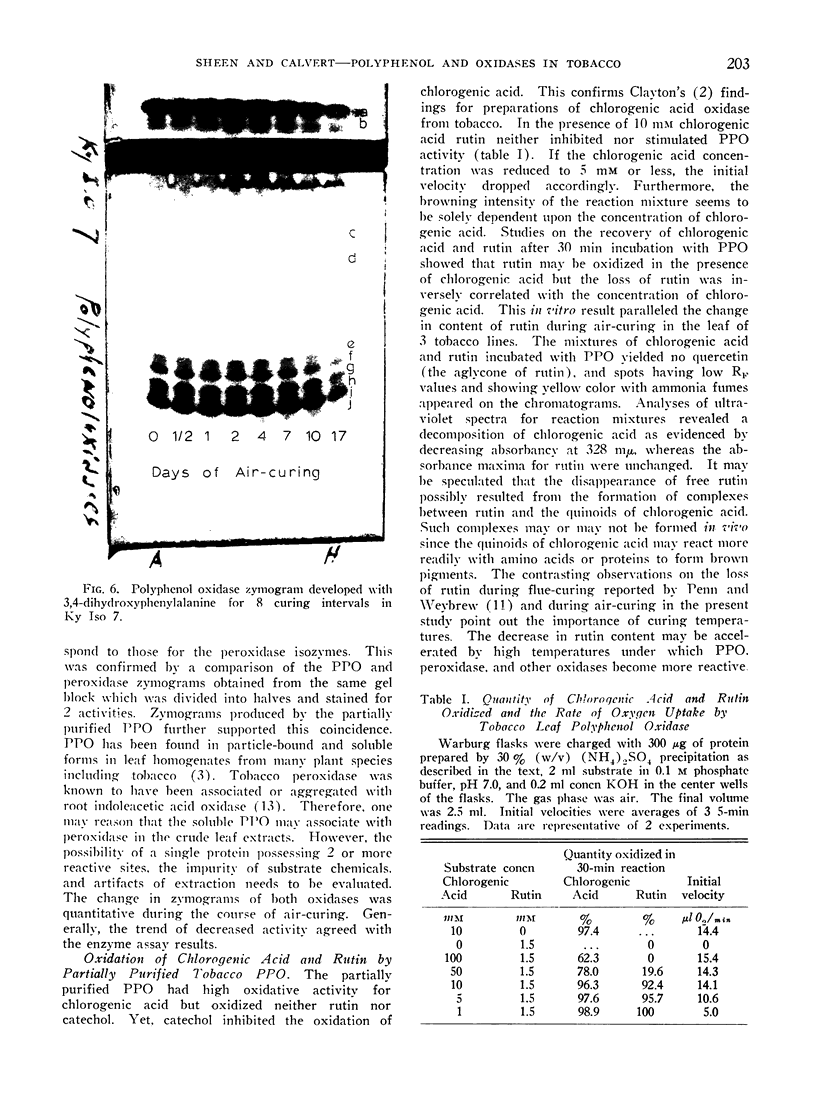
The polyphenol oxidase activity did not differ at any stage of curing in the 3 tobaccos. When the activity was measured by the oxidation of 3,4-dihydroxyphenylalanine it rose rapidly during the first day of curing and then decreased sharply so that in the fully cured leaf only 15% activity remained. The increase in activity was not observed when chlorogenic acid was used as the substrate. A similar level of peroxidase activity was found in the 3 tobaccos before curing. Peroxidase activities increased rapidly during the first 24 hr of curing, declined thereafter, and remained highest in the flue-cured tobacco, less in the Turkish line, and least in the burley at the end of curing process.
By polyacrylamide gel block electrophoresis, 10 peroxidase isozyme bands, 2 cationic and 8 anionic, appeared identical in all 3 tobaccos. When catechol replaced benzidine-2 HCl as the electron donor, 1 cationic and 2 anionic peroxidase isozymes did not form. Of interest is that the same 10 peroxidase isozyme bands also exhibited polyphenol oxidase activities when treated with 3,4-dihydroxyphenylalanine or chlorogenic acid. Results suggest that in the crude tobacco leaf extract the peroxidase and polyphenol oxidase may associate as protein complexes, and peroxidase isozymes may differ in electron-donor requirements. Isozyme patterns for both oxidases at various curing intervals differed only quantitatively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAYTON R. A. Properties of tobacco polyphenol oxidase. Arch Biochem Biophys. 1959 Apr;81(2):404–417. doi: 10.1016/0003-9861(59)90219-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sequeira L., Mineo L. Partial purification and kinetics of indoleacetic Acid oxidase from tobacco roots. Plant Physiol. 1966 Sep;41(7):1200–1208. doi: 10.1104/pp.41.7.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Zucker M. Changes in Oxidative Enzyme Activity During the Curing of Connecticut Shade Tobacco. Plant Physiol. 1958 Mar;33(2):151–155. doi: 10.1104/pp.33.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]