Abstract
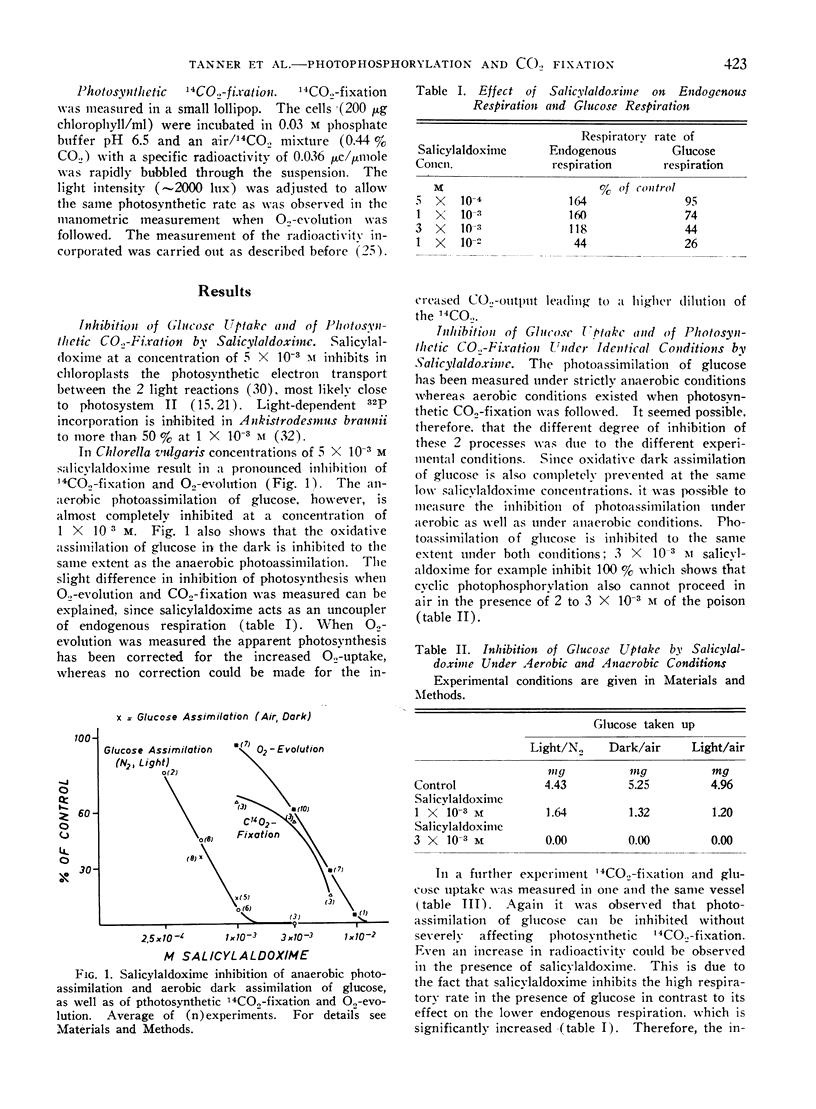
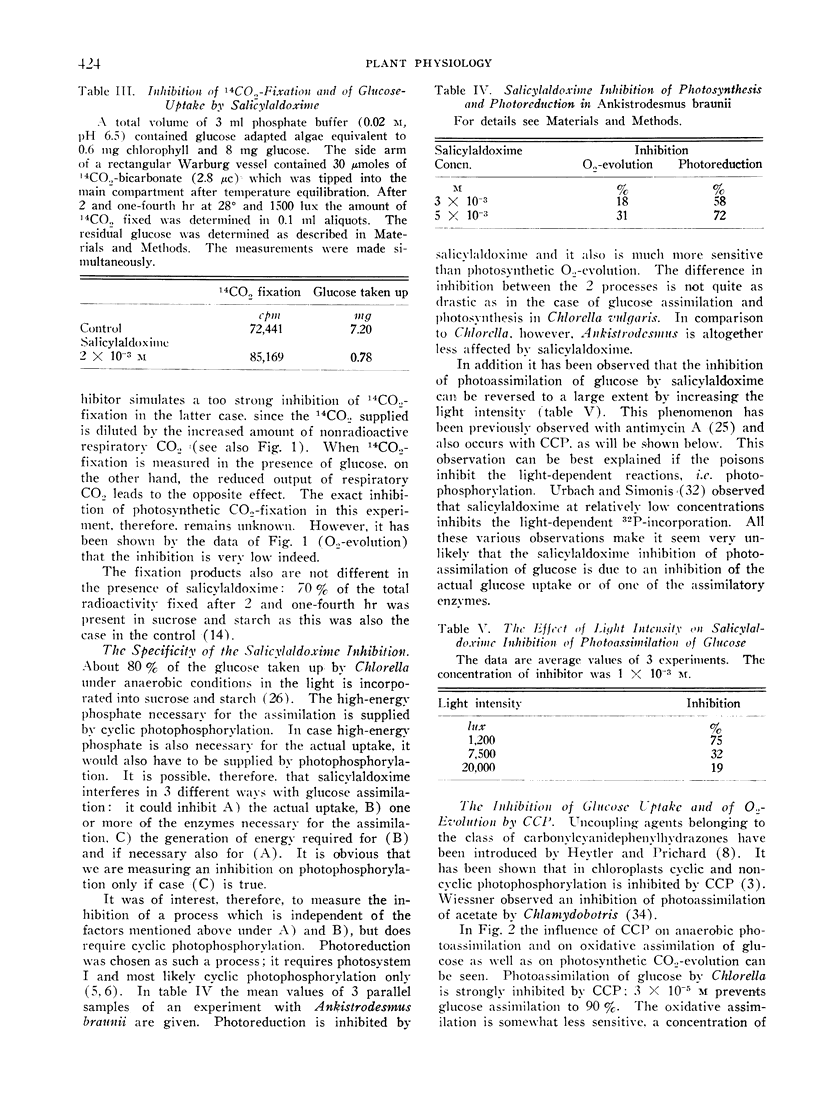
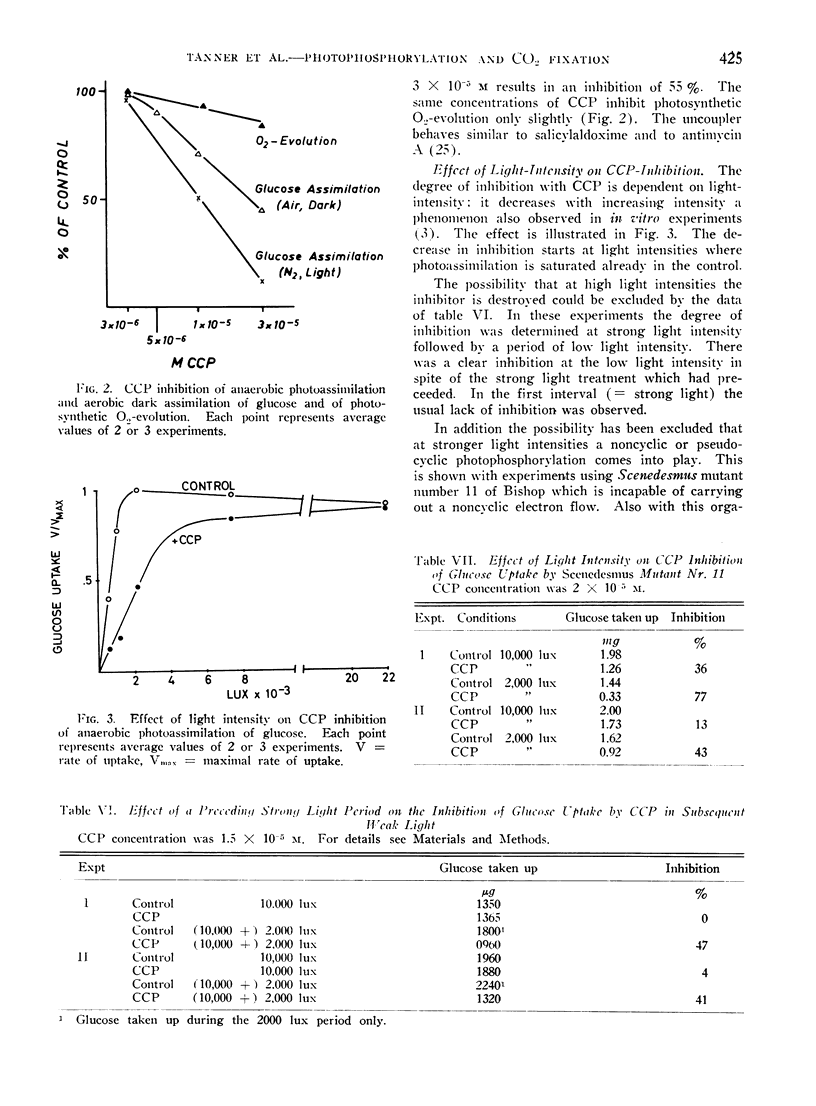
Salicylaldoxime (2 × 10−3m and less) inhibits cyclic photophosphorylation in intact Chlorella cells severely whereas photosynthetic O2-evolution and 14CO2-fixation is hardly affected. Cyclic photophosphorylation in vivo was measured by following anaerobic light dependent glucose uptake. A similar difference in susceptibility has been observed with carbonylcyanide-p-trifluoromethoxyphenylhydrazone. Various controls exclude the possibility that the difference in inhibition was caused by differing experimental conditions or, in the case of glucose assimilation, by an inhibition of a reaction other than photophosphorylation.
There also exists a great difference in light saturation of cyclic photophosphorylation and photosynthesis. Evidence is reported that at light saturation of glucose uptake light driven cyclic phosphorylation is indeed the limiting reaction.
The results are considered as evidence that cyclic photophosphorylation does not contribute ATP stoichiometrically to photosynthetic CO2-fixation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., LOSADA M., WHATLEY F. R., TSUJIMOTO H. Y., HALL D. O., HORTON A. A. Photosynthetic phosphorylation and molecular oxygen. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1314–1334. doi: 10.1073/pnas.47.9.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- BISHOP N. I., GAFFRON H. Photoreduction at lambda 705 millimicrons in adapted algae. Biochem Biophys Res Commun. 1962 Aug 31;8:471–476. doi: 10.1016/0006-291x(62)90299-1. [DOI] [PubMed] [Google Scholar]
- Bishop N. I. Comparison of the action spectra and quantum requirements for photosynthesis and photo-reduction of Scenedesmus. Photochem Photobiol. 1967 Sep;6(9):621–628. doi: 10.1111/j.1751-1097.1967.tb08767.x. [DOI] [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Izawa S., Winget G. D., Good N. E. Phlorizin, a specific inhibitor of photophosphorylation and phosphorylation-coupled electron transport in chloroplasts. Biochem Biophys Res Commun. 1966 Jan 24;22(2):223–226. doi: 10.1016/0006-291x(66)90436-0. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Inhibitory effect of salicylaldoxime on chloroplast photooxidation-reduction reactions. Biochem Biophys Res Commun. 1966 Sep 22;24(6):903–908. doi: 10.1016/0006-291x(66)90335-4. [DOI] [PubMed] [Google Scholar]
- Ramírez J. M., Campo F. F., Arnon D. I. Photosynthetic phosphorylation as energy source for protein synthesis and carbon dioxide assimilation by chloroplasts. Proc Natl Acad Sci U S A. 1968 Feb;59(2):606–612. doi: 10.1073/pnas.59.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger G., Vater J., Witt H. T. Effect of salicylaldoxime on the complete electron transport system of photosynthesis and on the isolated reaction cycle II. Biochem Biophys Res Commun. 1967 Feb 21;26(4):477–480. doi: 10.1016/0006-291x(67)90572-4. [DOI] [PubMed] [Google Scholar]
- Vose J. R., Spencer M. Energy sources for photosynthetic carbon dioxide fixation. Biochem Biophys Res Commun. 1967 Nov 30;29(4):532–537. doi: 10.1016/0006-291x(67)90517-7. [DOI] [PubMed] [Google Scholar]
- WIESSNER W. QUANTUM REQUIREMENT FOR ACETATE ASSIMILATION AND ITS SIGNIFICANCE FOR QUANTUM MEASUREMENTS IN PHOTOPHOSPHORYLATION. Nature. 1965 Jan 2;205:56–57. doi: 10.1038/205056a0. [DOI] [PubMed] [Google Scholar]
- Winget G. D., Izawa S., Good N. E. The stoichiometry of photophosphorylation. Biochem Biophys Res Commun. 1965 Dec 9;21(5):438–443. doi: 10.1016/0006-291x(65)90401-8. [DOI] [PubMed] [Google Scholar]


