Opioid receptors and enkephalinergic nerve terminals are widely distributed throughout respiratory-related regions of the brainstem and in the phrenic motor nucleus of the spinal cord (Xia & Haddad, 1991; Laferrière et al. 1999; Wang et al. 2002; Haji et al. 2003a; Lonergan et al. 2003a,b; Strornetta et al. 2003). Since opiate drugs given systemically will act on opioid receptors with conjoint selectivity in all respiratory regions, respiratory depression is unlikely to be dependent on actions at a single site.
Therapeutic doses of opioids given to most mammalian species depress respiratory rate, minute ventilation, alveolar–arterial gas exchange and respiratory responsiveness to hypoxia and hypercapnia (Jaffe & Martin, 1990). Opioid-mediated depression of respiration is due at least in part to direct effects on the brainstem respiratory network, which includes several sites of action in medullary and pontine regions (reviewed by Pattison, 2008; Lalley, 2008). The degree of opioid-mediated respiratory depression depends on agonist dose, opioid receptor density and the subtypes of opioid receptor in various respiratory regions. Species variability and stage of development are also factors (Santiago & Edelman, 1985).
In the paragraphs to follow, we review results of studies that indicate that the pre-Bötzinger complex (preBötC) is not essential for respiratory depression by systemically administered opioid analgesics.
Medullary neurons distributed throughout the bulbar respiratory network are depressed by local or systemic administration of opioids
Immunolabelling and intracellular recording have shown that ventral respiratory column (VRC) bulbospinal neurons, propriobulbar neurons and laryngeal motoneurons express μ-and Δ-opioid receptors (Fig. 1, and Haji et al. 2003a). Functional studies in cats reveal an even wider bulbar distribution of opioid receptors. For example, opioids given i.v. or juxtacellularly by microiontophoresis depress respiratory neuron discharges in the dorsolateral pons, nucleus tractus solitarii and VRC through pre-and postsynaptic actions (Denavit-Saubié et al. 1978; Tabatabai et al. 1989). Juxtacellular microiontophoresis of morphine evokes postsynaptic depression, whereas i.v. morphine in analgesic doses evokes both pre-and postsynaptic depression (Haji et al. 2003b, Fig. 8). Fentanyl given i.v. also has dose-dependent, pre-and postsynaptic depressant actions that slow respiratory rhythm in lowest doses and depress motor output in higher doses (Lalley, 2003, Fig. 4). Juxtacellular picolitre pressure ejection of DAMGO or morphine on canine bulbospinal inspiratory and expiratory VRC neurons depresses their activity, which can be reversed by picoejected naloxone. However, depression produced by clinical i.v. doses of remifentanil cannot be reversed by picoejected naloxone, suggesting that the i.v. effects are presynaptically exerted (Stucke et al. 2008).
Figure 1.
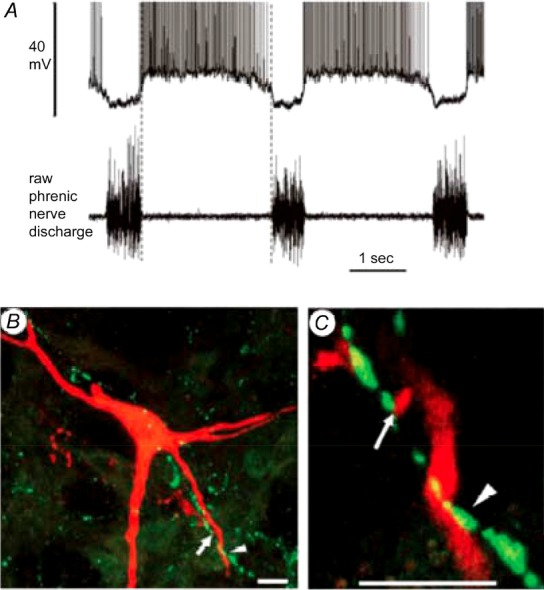
A, membrane potential and discharge trajectories of a decrementing expiratory (E-Dec) neuron in the VRC (upper trace) with corresponding phrenic nerve activity (lower trace). B and C, merged single slice confocal scans (1.8 μm thick) showing close appositions between DOR-immuno-reactive presynaptic terminal boutons and the dendrites (arrowhead) and labelled boutons (arrow) of the E-Dec neuron shown in A. Scale bars, 10 μm. Figure adapted with permission from Lonergan et al. 2003b, Fig. 2, panels O–Q.
Intravenous opioids produce dose-dependent rhythm slowing at numerous brainstem sites of action
In anaesthetized adult rats with intact nervous systems, i.v. injections of μ-opioid-receptor-selective agonists have dose-dependent depressant effects on respiration. Lowest doses produce bradypnoea by prolonging the inspiratory phase and decreasing peak inspiratory flow rate. These effects are accompanied by prolongation of discharges in inspiratory VRC neurons and prolongation of diaphragmatic EMG activity. The prolongation of inspiration is linked to effects on respiratory phase-terminating neurons (Fone & Wilson, 1986), which are widely distributed in the bulbar respiratory network (Ezure, 1990).
The rostrolateral pons is an important site for opioid-mediated slowing of respiratory rhythm
In unanaesthetized midcollicular decerebrate dogs, slowing of phrenic nerve (PN) respiratory rhythm is not affected by opioid actions in preBötC, rather the more likely site of action is in the parabrachial/Kölliker–Fuse complex of the pons. Opioids applied locally in preBötC or administered systemically have opposite effects on respiratory phase duration. Whereas i.v. infusion of remifentanil in clinical doses reduces PN burst rate, nanolitre microinjection of DAMGO (100 μm) in preBötC increases burst rate and decreases peak PN. Furthermore, naloxone given i.v. reverses remifentanil depression of PN burst rate but has no effect when injected into preBötC (Mustapic et al. 2010). The rostrolateral pons seems the more likely site of slowing, because microinjection of DAMGO into the parabrachial/Kölliker–Fuse complex slows PN burst rate, which is antagonized by naloxone microinjection. Naloxone microinjection also reverses slowing of PN burst rate by i.v. clinical doses of remifentanil (Prkic et al. 2012). These findings are consistent with the study of Hurlé et al. 1985 in cats, which found that opioid application to the dorsolateral surface of the pons in decerebrate cats depresses breathing frequency but not tidal volume.
PreBötC is not solely responsible for depression of eupnoeic ventilation and responsiveness to hypoxia and hypercapnia
Intravenous administration of opioids to unanaesthetized goats decreases breathing rate and increases 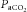 (Meyer et al. 2006). The sites of opioid-mediated respiratory depression are at present unknown. Eupnoeic ventilation is not depressed by opioid actions in preBötC of awake goats (Krause et al. 2009), but the ventilatory responses to hypercapnia and hypoxia are attenuated by DAMGO microinjection into the preBötC. However, opioids depress chemosensitivity in other areas of the respiratory controller (Hurlé et al. 1985; Kirby & McQueen, 1986; Zhang et al. 2011, 2012; Dias et al. 2012).
(Meyer et al. 2006). The sites of opioid-mediated respiratory depression are at present unknown. Eupnoeic ventilation is not depressed by opioid actions in preBötC of awake goats (Krause et al. 2009), but the ventilatory responses to hypercapnia and hypoxia are attenuated by DAMGO microinjection into the preBötC. However, opioids depress chemosensitivity in other areas of the respiratory controller (Hurlé et al. 1985; Kirby & McQueen, 1986; Zhang et al. 2011, 2012; Dias et al. 2012).
Recently, Montandon and colleagues (2011) reported that they identified in rats ‘the critical site of the medulla, the preBötC, that mediates opioid-induced respiratory depression in vivo’. They also claim that neurokinin-1-receptor-expressing preBötC neurons are critical for respiratory rate depression. This conclusion is based on (1) similar depressant effects of opioids given i.v. and applied with microdialysis perfusion probes (200 μm diameter) dorsal to, but not directly in the pre-BötC, and (2) antagonism of i.v. opioid-mediated depression by microdialysis of naloxone dorsal to preBötC. The probe concentrations of fentanyl and naloxone were markedly higher (>2000 nM) than plasma concentrations (<100 nm) that depress (Yassen et al. 2006) and reverse (Yeadon & Kitchen, 1990) ventilation, respectively. To block i.v. fentanyl-induced depression, 300 μm naloxone was perfused for ∼45 min prior to i.v. fentanyl injection, which would have allowed it to diffuse and block μ-opioid receptors at great distances from the probe. Analysis of the method using the relationship between probe distances and response latencies does not provide a unique solution, rather it implicates multiple sites with similar high correlation values outside the preBötC region. Thus our concern is that the microdialysis probably affected respiratory neurons in the vicinity of preBötC and possibly well beyond. Indeed, other groups have failed to reverse opioid respiratory depression by i.v. injection when naloxone is microinjected into pre-BötC (e.g. Mustapic et al. 2010; Zhang et al. 2012). Moreover, Lonergan et al. (2003a) showed that microinjection of the μ-opioid receptor agonist endomorphin-1 in preBötC of the adult rat at sites where inspiratory and expiratory discharges were recorded increased PN discharge frequency.
Conclusions
Respiratory depression by opioids involves an array of dose-dependent responses: bradypnoea, reduced tidal volume, impaired pulmonary gas exchange and blunting of respiratory responsiveness to hypoxia and hypercapnia. The studies cited above show that all of these symptoms of depression can be elicited by local opioid actions at various locations in the bulbar respiratory network. Opioids postsynaptically depress bulbospinal neurons downstream from the preBötC and in the dorsolateral pons where neurons projecting to the spinal cord are located. In addition, the presence of enkephalinergic nerve terminals in the phrenic motor nucleus indicates that opioid depressant effects can bypass the preBötC. We do not dispute an indirect role of preBötC in opioid-mediated respiratory depression, but we believe that preBötC μ-opioid receptors are not essential for respiratory depression by systemic administration of opioid analgesics.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;592/6/1163
Biographies
Peter Lalley is Professor Emeritus and Faculty/Staff member in theDepartment ofNeuroscience,University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin. His research interests are primarily directed at neurotransmitter modulation of the central respiratory network and breathing. Paul Pilowsky is Professor and Group Leader at the Heart Research Institute, Sydney, Australia. His work focuses on deep brain networks that control airways, breathing and blood pressure.
Hubert Foster is Professor of Physiology at the Medical College of Wisconsin, Milwaukee, Wisconsin. His research is primarily focused on studies of ventilatory control under physiological conditions in awake as well as sleeping mammals. Edward Zuperku is Professor of Biomedical Engineering in the Anesthesiology Department of the Medical College of Wisconsin and the Zablocki VA Medical Center, Milwaukee, Wisconsin. His specific areas of investigation involve the functional organization of the brainstem respiratory control system. Pharmacological studies include the effects of anaesthetics and opioids on respiratory neurons and breathing patterns. 
Additional information
Competing interests
None declared.
References
- Denavit-Saubié M, Champagnat J, Zieglgänsberger W. Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res. 1978;155:55–67. doi: 10.1016/0006-8993(78)90305-0. [DOI] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Branco LG, Gargaglioni LH. Opioid μ-receptors in the rostral medullary raphe modulate hypoxia-induced hyperpnea in unanesthetized rats. Acta Physiol (Oxf) 2012;204:435–442. doi: 10.1111/j.1748-1716.2011.02345.x. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Fone KC, Wilson H. The effects of alfentanil and selected narcotic analgesics on the rate of action potential discharge of medullary respiratory neurones in anaesthetized rats. Br J Pharmacol. 1986;89:67–76. doi: 10.1111/j.1476-5381.1986.tb11121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Ohi Y, Yamazaki H, Takeda R. Biphasic effects of morphine on bulbar respiratory neuronal activities in decerebrate cats. Neuropharmacology. 2003b;45:368–379. doi: 10.1016/s0028-3908(03)00154-0. [DOI] [PubMed] [Google Scholar]
- Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of μ receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett. 2003a;351:37–40. doi: 10.1016/s0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- Hurlé MA, Mediavilla A, Flórez J. Differential respiratory patterns induced by opioids applied to ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology. 1985;24:597–606. doi: 10.1016/0028-3908(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Martin WR. Opioid agonists and antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York: Pergamon; 1990. pp. 485–521. 8th edn pp. . [Google Scholar]
- Kirby GC, McQueen DS. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Br J Pharmacol. 1986;88:889–898. doi: 10.1111/j.1476-5381.1986.tb16263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. μ-Opioid receptor agonist injections into the presumed pre-Bötzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol. 2009;107:1591–1599. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolo M, Tonkovic-Capin V, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Subtype composition and responses of respiratory neurons in the pre-Bötzinger region to pulmonary afferent inputs in dogs. J Neurophysiol. 2005;93:2674–2687. doi: 10.1152/jn.01206.2003. [DOI] [PubMed] [Google Scholar]
- Laferrière A, Liu JK, Moss IR. μ-and δ-opioid receptor densities in respiratory-related brainstem regions of neonatal swine. Brain Res Dev Brain Res. 1999;112:1–9. doi: 10.1016/s0165-3806(98)00149-7. [DOI] [PubMed] [Google Scholar]
- Lalley PM. μ-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1287–R1304. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Opioidergic and dopaminergic modulation of respiration. Resp Physiol Neurobiol. 2008;164:160–167. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Mu opioid receptors in rat ventral medulla: effects of endomorphin–1 on phrenic nerve activity. Respir Physiol Neurobiol. 2003a;138:165–178. doi: 10.1016/s1569-9048(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Presynaptic Δ opioid receptors differentially modulate rhythm and pattern generation in the ventral respiratory group of the rat. Neuroscience. 2003b;121:959–973. doi: 10.1016/s0306-4522(03)00591-8. [DOI] [PubMed] [Google Scholar]
- Meyer LC, Fuller A, Mitchell D. Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R405–R413. doi: 10.1152/ajpregu.00440.2005. [DOI] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBötzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of μ–opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol. 2010;103:409–418. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100:747–758. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- Prkic I, Mustapic S, Radocaj T, Stucke EA, Hopp FA, Dean C, Zuperku EJ. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol. 2012;108:2430–2441. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago TV, Edelman NH. Opioids and breathing. J Appl Physiol. 1985;59:1675–1685. doi: 10.1152/jappl.1985.59.6.1675. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol. 2003;455:113–124. doi: 10.1002/cne.10486. [DOI] [PubMed] [Google Scholar]
- Stucke AG, Zuperku EJ, Sanchez A, Tonkovic-Capin M, Tonkovic-Capin V, Mustapic S, Stuth EA. Opioid receptors on bulbospinal respiratory neurons are not activated during neuronal depression by clinically relevant opioid concentrations. J Neurophysiol. 2008;100:2878–2888. doi: 10.1152/jn.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Tabatabai M, Kitahata LM, Collins JG. Disruption of the rhythmic activity of the medullary inspiratory neurons and phrenic nerve by fentanyl and reversal with nalbuphine. Anesthesiology. 1989;70:489–495. doi: 10.1097/00000542-198903000-00020. [DOI] [PubMed] [Google Scholar]
- Wang QP, Zadina JE, Guan JL, Kastin AJ, Funahashi H, Shioda S. Endomorphin–2 immunoreactivity in the cervical dorsal horn of the rat spinal cord at the electron microscopic level. Neuroscience. 2002;113:593–605. doi: 10.1016/s0306-4522(02)00153-7. [DOI] [PubMed] [Google Scholar]
- Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991;549:181–193. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M. Mechanism-based pharmacokinetic-pharmacodynamic modelling of the respiratory-depressant effect of buprenorphine and fentanyl in rats. J Pharmacol Exp Ther. 2006;319:682–692. doi: 10.1124/jpet.106.107953. [DOI] [PubMed] [Google Scholar]
- Yeadon M, Kitchen I. Multiple opioid receptors mediate the respiratory depressant effects of fentanyl-like drugs in the rat. Gen Pharmacol. 1990;21:655–664. doi: 10.1016/0306-3623(90)91013-h. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Opioid μ–receptors in medullary raphe region affect the hypoxic ventilation in anesthetized rats. Respir Physiol Neurobiol. 2012;168:281–288. doi: 10.1016/j.resp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhuang J, Zhang C, Xu F. Activation of μ–receptors in the commissural subdivision of the nucleus tractus solitarius abolishes the ventilatory response to hypoxia in anesthetized rats. Anesthesiology. 2011;115:353–363. doi: 10.1097/ALN.0b013e318224cc1f. [DOI] [PubMed] [Google Scholar]


