Abstract
Primary cell culture provides an experimental platform in which morphology, physiology, and cell-cell communication pathways can be studied under a well-controlled environment. Primary cell cultures of peripheral and central glia offer unique possibilities to clarify responses and pathways to different stimuli. Peripheral glia, satellite glial cells (SGCs), which surround neuronal cell bodies within sensory ganglia, have recently been known as key players in inflammation and neuronal sensitization. The objectives of this study were 1) to establish a cell-based platform of cultured trigeminal SGCs to study glial marker expression and functions under control conditions; 2) to validate the cell-based platform by prostaglandin E2 (PGE2) release response following administration of Cisplatin; and 3) to investigate inhibition of PGE2 release by glial modulators, Ibudilast and SKF86002. Primary cell cultures of SGCs from rat trigeminal ganglia were established following enzymatically and mechanically dissociation of the ganglia. Cultures were characterized in vitro for up to 21 days post isolation for morphological and immunocytochemical characteristics. PGE2 release, determined by ELISA, was used as a pro-inflammatory marker to characterize SGCs response to chemotherapeutic agent, Cisplatin, known to contribute in chemotherapy-induced peripheral neuropathy. Our results indicate that 1) isolated SGCs maintained their characteristics in vitro for up to 21 days; 2) Cisplatin enhanced PGE2 release from the SGCs, which was attenuated by Ibudilast and SKF86002. These findings confirm the utility and validity of the cultured trigeminal SGCs platform for glial activation and modulation; and suggest further investigation on Ibudilast and SKF86002 in prevention of chemotherapy-induced pain.
Keywords: Satellite glial cells, primary culture, trigeminal ganglia, Cisplatin, Ibudilast, SKF86002
Introduction
Satellite glial cells (SGCs) are specialized cells enveloping neurons in the sensory ganglia where they communicate with neurons. It is believed that SGCs are able to modulate neuronal microenvironment and sensory transmission within the sensory ganglia [1-5]. These cells are capable of expressing different receptors; respond to pro-inflammatory substances, and release gliotransmitters that may play a role in the pathogenesis of several disorders [1,4-8]. Accumulating evidence suggests a critical role of SGCs in chemotherapy-induced pain (CIP) and other chronic pain conditions [4,6,7,9,10]. Cisplatin is a chemotherapeutic agent that has been known to induce peripheral sensory disturbances including pain among other side effects [11,12]. Cisplatin and other chemotherapeutic agents are believed to accumulate in dorsal root ganglia (DRG) and trigeminal ganglia (TG) when administered for different types of cancers including orofacial cancers. The accompanying pain and sensory disturbances are dependent on the cumulative dose of the given agent [11,13]. To date, a number of pathological mechanisms are associated with the pathogenesis of chemotherapy-induced peripheral neuropathy (CIPN) including neurotoxicity, apoptosis [14], mitochondrial dysfunction, and abruption of axonal transport [12,13]. However, novel evidence suggests a potential role of glial cells in association with CIPN [10].
One avenue to gain a better insight into the role of SGCs in the sensory ganglia under control and pathological conditions is to establish primary cultures of these cells to test their characteristics and responsiveness. Primary cultures of central glial cells such as astrocytes and microglia have been essential in clarifying responses and intracellular pathways following a range of different stimuli [15-17]. Primary cultures of isolated SGCs have been less studied [4,6], while those may also provide an experimental platform in which morphology, physiology, and cell-cell communications or cellular pathways can be investigated. Hence, in the current study, a cell-based platform of trigeminal SGCs was established to investigate glial marker expression and substance release functions from these cells at different culture times and in response to Cisplatin. Subsequently, Ibudilast and SKF86002 (SKF) were selected as pharmacological tools to examine the modulatory effect of these agents on Cisplatin-activated glial cells. Ibudilast is a non-selective phosphodiesterase inhibitor, conventionally used in the treatment of bronchial asthma [18]. Ibudilast has been linked with suppression of glial cells activity, both in vivo and in vitro [18,19]. The exact mechanism through which Ibudilast suppresses glial activity remains unclear, however, a decreased cytokine expression has been suggested to play a role [18,19]. SKF is an inhibitor of P38 mitogen activated protein kinase (p38 MAPK) pathway. Phosphorylation of P38 MAPK is associated with neuronal activation and sensitization [20,21]. SKF might also act on glial cells and block the release of pro-inflammatory substances from SGCs in the sensory ganglia.
In short, the present study purposes were to establish and characterize a working SGCs-based platform, to validate its responses to Cisplatin-evoked PGE2 release and whether this response can be modulated by agents with glial modulatory effect. Results from this study would shed light on SGC role in CIP and potential application of glial modulatory agents to prevent it.
Materials and methods
Animals
All experiments were performed using adult male Wistar rats (2-4 months old) provided by the Animal Research Facility, Pathological institute, Aalborg University Hospital (North). Animals were housed in groups of 3 rats per cage, in a temperature-controlled room, on a 12 h light/dark cycle with access to food and water ad libitum. Animals were deeply anesthetized with a mixture of hypnorm (Vm21757/4000, Vetapharma, UK), midazolam (Hameln pharmaceuticals, UK), and isotonic saline (25%, 25%, 50% v/v. 0.3 ml/100 g) and then euthanized by cervical dislocation. Three animals were used for immunohistochemistry. These animals were deeply anesthetized and euthanatized by transcardial perfusion with isotonic saline followed by 10% formalin buffer solution (BAF-0010-03A CellPath, UK). Subsequent to the cervical dislocation or the transcardial perfusion, both ganglia were surgically removed from the skull.
All procedures were conducted according to the ethical guidelines delineated by the Danish Animal Experiments Inspectorate in accordance with the guidelines set by the International Association for the Study of Pain (IASP) for use of laboratory animals in medical research.
Characterization of SGCs: immunohistochemistry
The isolated TG, were placed in 10% formalin buffer solution overnight at 4°C. The tissue was washed in phosphate buffered saline (PBS; 14190-094 Gibco life technologies, Invitrogen, CA, USA) and then incubated in 20% sucrose in PBS for 48 h at 4°C. The tissue was treated with a 40% sucrose solution overnight (4°C) before it was embedded in Tissue-Tek (4583, Sakura Finetek, NL) in a cryo-mold (4565 Sakura Finetek, NL) and then frozen separately. Each ganglion was serially sectioned in 10 μm slices on a cryostat (MICROM, Thermo Fisher Scientific, DE). The slices were mounted on poly lysine coated glass slides (1510.1260 Hounisen Laboratorieudstyr, DK). Slides were incubated in 5% bovine serum albumin (BSA; EQBAH62 Europa Bioproducts, UK) and 0.2% Triton X100 (Sigma-Aldrich, MO, USA) for 1 h at room temperature (RT) before they were washed in PBS. Primary antibodies against glutamine synthetase (GS: G2781 Sigma-Aldrich, MO, USA) and glial fibrillary acidic protein (GFAP: G3893 Sigma-Aldrich, MO, USA) were added in dilutions of 1:4000 and 1:400, respectively, in 1% BSA solution at 4°C overnight. Slides were washed in PBS prior to incubation with secondary antibody Alexa fluor 555 (donkey anti-rabbit) conjugate (ab150074, Abcam, Cambridge, UK) and Alexa fluor 488 (donkey anti-mouse) conjugate (ab150105, Abcam, Cambridge, UK) diluted 1:500, respectively in 1% BSA, for 1 h at RT. The fluorescent nuclear dye Hoechst was added for 10 min at RT, in a 1:2000 dilution in 1% BSA, before the slides were washed in PBS and then in Milli-Q water. Slides were mounted (Fluorescent mounting medium; S3023 Dako, DK) and images were obtained using a Nikon microscope (Az100, Nikon, Tokey, Japan) equipped with a fluorescent illuminator (L200/D, Prior Scientific, Rockland, MA, USA) and a digital camera (DS-Vi1 Nikon, Tokey, JP) connected to a personal computer. Image J (public domain software) was used for further analysis and noise-to-signal ratio adjustments.
Characterization of SGCs: immunocytochemistry
The isolated ganglia were sectioned and incubated in 5 mg/ml collagenase (Collagenase; C9891 Sigma-Aldrich, MO, USA) in Ham’s F12 growth medium (21765-029 Gibco life technologies, Invitrogen, CA, USA) supplemented with 1% penicillin/streptomycin for 15 min at 37°C in a humidified 5% CO2 incubator (Pen/Strep; 15140 Gibco life technologies, Invitrogen, CA, USA). The collagenase solution was centrifuged (5 min, 1300RPM) and the pellet was resuspended in 1 ml 0.125% trypsin (15090-046 Gibco life technologies, Invitrogen, CA, USA) and incubated for 5-10 min before the suspension was centrifuged once more (5 min, 1300RPM). The sectioned and enzymatically digested ganglia were then mechanically dissociated to a homogenous solution by repeated pipetting against the bottom of the tube. The solution was transferred to uncoated culture flasks and cultures were maintained in a humidified 5% CO2 incubator (37°C) for up to 21 days while changing the medium twice the first day, at 3 h and 24 h, and then continuously every second day.
Prior to Immunofluorescence staining, the cultures were washed in PBS and fixed in a 10% formalin buffer solution for 20 min. After washing, the morphology of each culture was assessed using phase contrast microscopy. PBS was replaced with 0.2% Triton X-100 (X100 Sigma-Aldrich, MO, USA) in 1% BSA for 15 min. The cells were washed twice in PBS before they were incubated in 5% BSA at RT for 1 h. Primary antibodies against GS (G2781 Sigma-Aldrich, MO, USA) and GFAP (G3893 Sigma-Aldrich, MO, USA) were diluted 1:4000 and 1:400, respectively, in 1% BSA solution and placed overnight at 4°C. The following day, cells were washed in PBS before incubated with the secondary antibody Alexa fluor 555 (donkey anti-rabbit) conjugate (ab150074, Abcam, Cambridge, UK) and Alexa fluor 488 (donkey anti-mouse) conjugate (ab150105, Abcam, Cambridge, UK) diluted 1:500, respectively in 1% BSA, for 1.5 h at RT. The cells were then incubated with the nuclear dye Hoechst in dilution 1:5000 in 1% BSA for 20 min. Finally; cells were washed in PBS and then in Milli-Q water before they were mounted with glass cover slips (Fluorescent mounting medium; S3023 Dako, DK). Images were obtained using a Nikon microscope (Az100, Nikon, Tokey, Japan) equipped with a fluorescent illuminator (L200/D, Prior Scientific, Rockland, MA, USA) and a digital camera (DS-Vi1 Nikon, Tokey, JP) connected to a computer. Image J (public domain software) was used for further analysis and noise-to-signal ratio adjustments.
Cultured SGCs stimulation and inhibition
Cisplatin was provided as a 1 mg/mL stock solution (3.32 mM). The stock solution was diluted in Ham’s F12 growth medium to a working dilution of 66 μM. Ibudilast (I0157 Sigma-Aldrich, MO, USA) was dissolved in Ham’s F12 growth medium and made into 10 mM aliquots. Before administration, the aliquots were diluted to a final concentration of 100 μM.
SKF (S0193 Sigma-Aldrich, MO, USA), was dissolved in DMSO and made into 10 mM aliquots. Prior to the experimental administrations, aliquots were diluted to final concentrations of 1, 10, or 100 μM. Aliquots were stored at -20°C. All working dilutions were prepared using Ham’s F12 growth medium. No cross reactivity was observed.
Immunoassay for the determination of prostaglandin E2 release
For each experiment, cells were plated in 24-well culture plates (growth area 1.9 cm2) at a density of ≈105,263 pr. cm2. After two days incubation in a 37°C, humidified, 5% CO2 incubator, the cell confluence reached to 80-90%. The cells were washed and either control- or treatment medium was applied to appropriated wells and incubated at 37°C for 8 h. For the characterization of Cisplatin-activated SGCs, the cells were plated at various culture times of 7, 14, and 21 days, and treated with different Cisplatin concentrations (0, 16.5, 33, 66 μM). Prior to the termination of each experiment, samples of condition medium were collected and stored at -20°C for later analysis. Each sample was analyzed with a competitive, highly sensitive enzyme-linked immunosorbent assay (ELISA) for the determination of prostaglandin E2 in biological fluids (ADI-930-001 Enzo life sciences, NY, USA).
Statistical analysis
Statistical comparisons were conducted using GraphPad Prism v5.0 statistical software (GraphPad software, CA, USA). For multiple comparisons of different Cisplatin concentrations a repeated measure analysis of variance (ANOVA) was used followed by a Dunnett’s post-hoc. For multiple comparisons of different time points or treatments, one-way ANOVA was used followed by a Bonferroni post-hoc test. The difference between two samples was assessed using a two-tailed paired t-test. Data are shown as mean ± standard error of the mean (SEM). P≤0.05 was considered statistically significant.
Results
Characterization of the SGCs in TG tissue
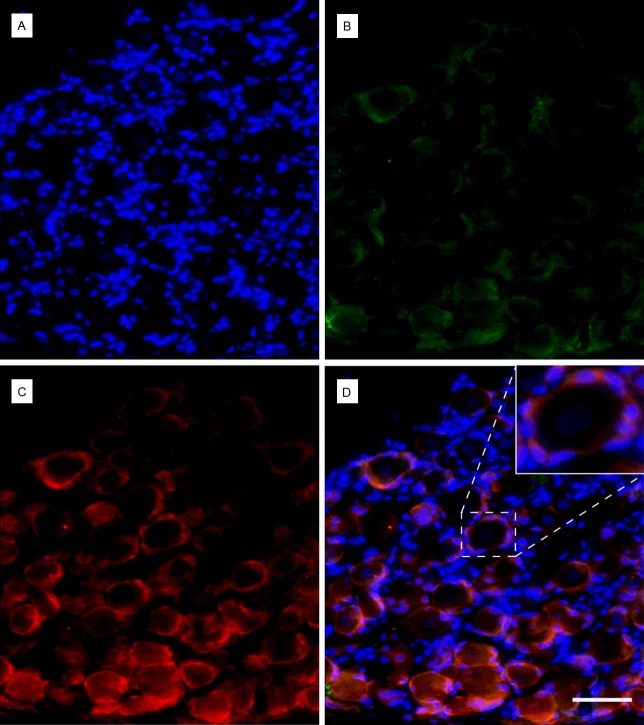
In order to evaluate the presence of the SGC in the isolated TG, immunohistochemistry was carried out. Using fluorescent microscopy, the TG sections showed larger neuronal cell bodies, with a central nucleus identified by Hoechst staining (Figure 1). Enveloping the larger nerve cell bodies, the smaller SGCs were easily identified through the positive immunostaining against GS and to a lesser extent by GFAP, which were faintly stained (Figure 1).
Figure 1.
Trigeminal ganglion slices stained with Hoechst (A) Satellite glial cells (SGCs) nuclei are small and brightly stained whereas the nuclei of neurons are larger and faintly stained. (B) Glial fibrillary acidic protein (GFAP), low expression of GFAP in the SGCs cytoplasm. (C) Glutamine synthetase (GS), clear staining of SGCs cytoplasm. (D) Overlay of the different channels, with zoom insert of a neuron enveloped by SGCs. (A-D) Scale bar: 50 μm; Magnification:200x.
Characterization of the isolated SGCs in culture
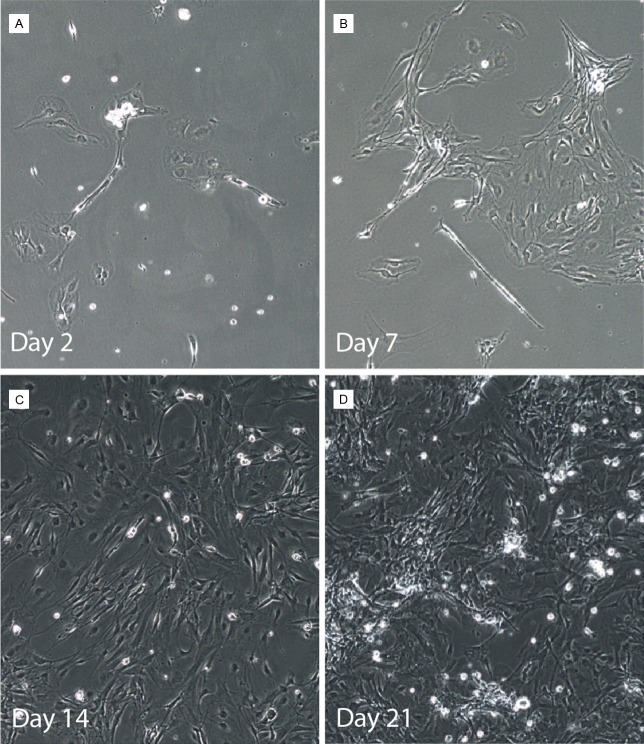
Phase microscopy was used to observe the morphology of isolated SGCs after 2, 7, 14, and 21 days in culture (Figure 2). These observations allowed interpretation of the morphological development as a consequence of time in culture (up to 21 days). Exploiting SGCs ability to adhere to uncoated culture flasks, SGCs were isolated from neurons and other component of TG section. After 2 days in culture, cells were scattered over the bottom of the flasks, as individual cells or in small groups, with thin or larger-flat-fan-like patterns. Depending on the homogeneity of the cell suspension and the isolation step after 3 h and 24 h in culture, single neurons were sometimes observed at this step. After 7 days in culture, a clear proliferation of cells was seen, where cells gathered in clusters across the bottom of the flasks. Several of these cells had lost their larger-flat-fan-like processes in favor of the long thinner processes. At 14 days in culture most of the cells had gained the spindle shaped appearance and covered the bottom of the culture flasks. After 21 days in culture, the cells were multilayered and had in some areas begun to form clusters and some of the cells had processes that were aligned and orientated in the same direction (Figure 2).
Figure 2.
Phase contrast microscopy of isolated satellite glial cells (SGCs) at different culture times. (A) Cells 2 days, (B) 7 days, (C) 14 days, and (D) 21 days after isolation. Magnification: 100x.
Immunofluorescence staining was used to examine the phenotypical expression of the markers GS and GFAP in the isolated cultures over time. Expression of the markers was examined after 2, 7, 14, and 21 days in culture. Majority of the isolated cells were immunopositive for GS and had the morphology of cultured SGCs (Figure 3). Most cells were also immunopositive for GFAP. No clear tendency was observed for increase or decrease in the immunoreactivity for either GS or GFAP in response to the culture time (Figure 3).
Figure 3.
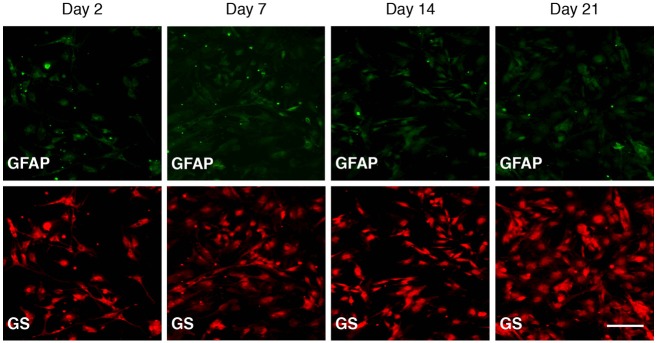
Immunofluorescent expression of the markers glial fibrillary acidic protein (GFAP) and glutamine synthetase (GS) by isolated satellite glial cells (SGCs). Columns 1-4 represent 2, 7, 14, and 21 days in culture, respectively. The upper row staining against GFAP shows low expression of GFAP in the cytoplasm of SGCs. In the lower row staining against GS, shows fine staining of cytoplasm of SGCs. No clear difference was observed in the immunoexpression of either marker as a consequence of time in culture. Scale bar: 100 μm; Magnification: 100x.
Functional characteristics of SGCs
In order to look at the functional characteristics of the SGCs, PGE2 release was used as a pro-inflammatory marker to characterize SGCs response to Cisplatin. To determine the appropriate concentration of Cisplatin, a concentration-response experiment was preformed initially (Figure 4). Cells from 21-day-old cultures were incubated for 8 h with 0, 16.5, 33, or 66 μM Cisplatin. A concentration-dependent elevation in PGE2 release was observed with a significant difference between 66 μM Cisplatin and the control. In order to assess if culture age had any pro-inflammatory effect on PGE2 release, the release was determined in the conditioned medium obtained from 7, 14, and 21 day-old-cells incubated with either the control or 66 μM Cisplatin media. No significant difference was observed between the three time points for either control or 66 μM Cisplatin (Figure 5). The 2nd day-time-point was not included due to a low cell yield at this time point.
Figure 4.
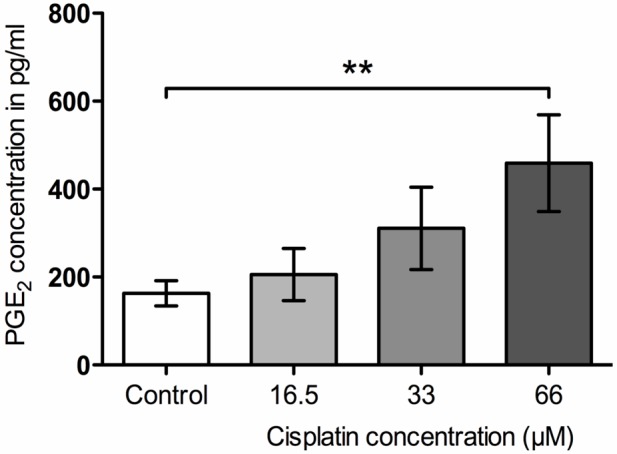
Increased prostaglandin E2 (PGE2) concentrations in the medium in response to the applied Cisplatin concentrations. **p<0.01 compared with control.
Figure 5.
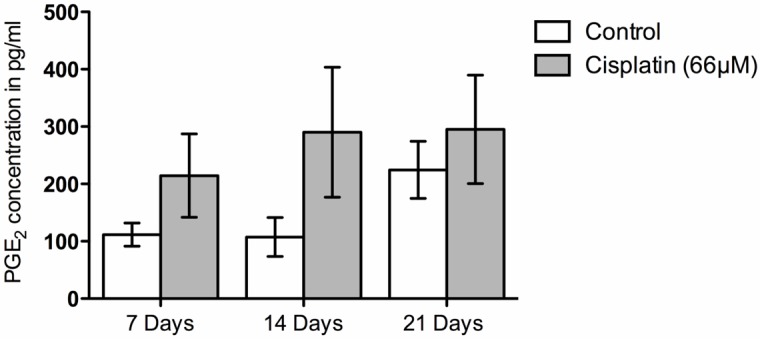
Comparison of prostaglandin E2 (PGE2) concentrations across different culture times. No significant differences were observed for either control or Cisplatin-treated cultures between the different time points (7, 14, and 21 days).
Inhibition of Cisplatin-evoked PGE2 release by Ibudilast and SKF
In order to validate isolated SGCs as a platform for studying pharmacological agents, Ibudilast and SKF were examined for their modulatory effects on SGCs activated by Cisplatin. SGCs with a culture age of 21 days were incubated with 66 μM Cisplatin or control and 100 μM Ibudilast (the concentration of Ibudilast was based on previous results from our laboratory). Cisplatin (66 μM) induced a significant release of PGE2 compared with control. Although, Ibudilast (100 μM) seemed to reduce the PGE2 concentration compared with Cisplatin (66 μM), no significant difference was fond between Cisplatin- and Ibudilast-treated samples (Figure 6). Three different concentrations of SKF were tested for its modulatory effects on Cisplatin-induced PGE2 release (1, 10, or 100 μM). All 3 concentrations of SKF were able to significantly block PGE2 release from SGCs activated by Cisplatin (66 μM) (Figure 7).
Figure 6.
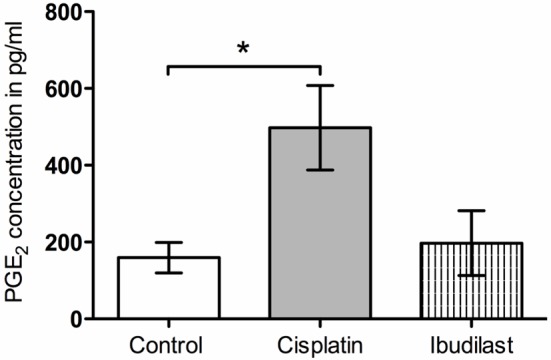
Modulation of Cisplatin-evoked prostaglandin E2 (PGE2) release by 100 μM Ibudilast. *p<0.05 compared with control.
Figure 7.
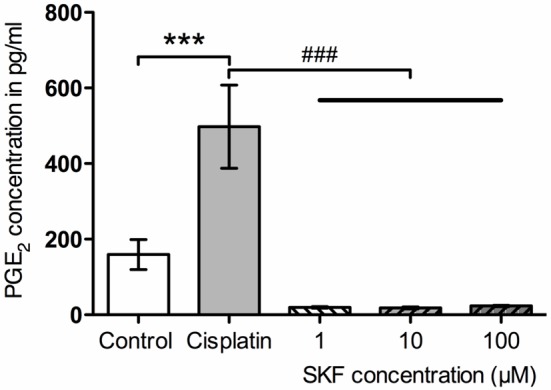
Modulation of Cisplatin-evoked prostaglandin E2 (PGE2) release by different concentrations of SKF. ***p<0.001 compared with control; ###p<0.001 compared with Cisplatin.
Discussion
In the current study we characterized and validated a cell-based platform from the isolated trigeminal SGCs. We found that SGCs could be maintained in primary cultures for up to 21 days without evident changes in the immunoreactivity of the glial markers GS and GFAP. In addition, we characterized the cell-based platform under basal conditions and after stimulation with Cisplatin. This chemotherapeutic agent is believed to activate ion channels (including potassium, sodium, and calcium channels) on dorsal root ganglia neurons and stimulate glial cells to release pro-inflammatory substances, e.g. PGE2. It has been suggested that pro-inflammatory substances might play a role in peripheral sensory disturbances and neuropathic pain [4,19,22]. Our results support that the cell-based platform has a potential for investigation of substance release and studying of pharmacological responses under the controlled environment for up to 21 days.
Characterization of the trigeminal SGCs
The descriptions of SGCs provided in this study and the immunoreactivity against the glial cell markers GFAP and GS are in accordance with previous descriptions of SGCs [1,4,7,23,24]. In vivo GFAP has a natural low expression in naive animals but is up regulated in response to various stimuli. This up regulation has generally been associated with “glial cell activation” [5,25]. Although, expressed in a variety of other cell types, GFAP is generally expressed by non-myelinating glial cells in the nervous system and thus considered a reliable glial marker [5,26]. In addition, GS, is selectively expressed by SGCs in sensory ganglia, making it a useful marker for study and identification of SGCs [5].
In the present study, primary cultured SGCs maintained their basic morphology with a small cell body with 2-3 processes giving them a spindle like appearance. The most abundant change in the culture was the increased density of cells in response to prolonged culture time. We found that the isolated cells were GFAP and GS positive with no evident changes in expression for either marker up to 21 days in culture. Belzer et al. have investigated the phenotypical changes in SGCs in mice trigeminal cultures up to 14 days. They report a change in morphology and declined GS expression in response to a culture time above 2 days with no recovery at 14 days in culture [27]. The discrepancy could be due to different animal species as well as difference in cell cultures. Belzer et al used trigeminal cultures e.g. mixed neuron and SGC cultures, and the reported morphological changes are to a large extent in relation to neurons and thus their results are not directly comparable with our findings. Other groups have reported a stable expression of GS in isolated SGCs at 7 or 14 days in culture using methods similar to the one used in this study [4,7]. Thus, we propose that isolated SGCs maintain vital- and keep several important characteristics in vitro up to 21 days. Hence, isolated SGCs offer a platform to investigate substance release in responses to various stimuli and inhibitors.
Cisplatin-evoked PGE2 release
Cisplatin is still among the widely used anticancer agents [28]. However, sensory neuropathy caused by Cisplatin and the initial complaints of pain and paresthesias predominantly in the distal extremities are among common reasons for early discontinuation of Cisplatin [19,22]. Often sensory disturbances are dependent on the cumulative dose and thus delayed in onset, and may appear weeks after initiation of Cisplatin [22]. The underlying mechanisms of Cisplatin-induced sensory neuropathy are not fully elucidated. However, cellular responses ranging from apoptosis to pathways controlling cellular growth, differentiation, and stress have been suggested [22]. Inflammation and the release of different pro-inflammatory molecules from both neuronal and non-neuronal cells such as SGCs have also been suggested to play a role in the induction and maintenance of CIP [13,19,22]. For instance, PGE2 might be involved in CIP or CIPN through activation of prostaglandin E receptors on neurons and activation of second messenger molecules such as protein kinase A and C, which are downstream products of the kinases associated with phosphorylation of transient receptor potential vanilloid -1 channels (TRPV1) involved in sensitization of peripheral neurons [29,30]. In the percent study, we examined the functionality of the cultured SGCs under basal conditions and after incubation with Cisplatin. We found that incubating SGC cultures with Cisplatin led to a significant release of PGE2 in the cultured medium. This observation suggests that release of pro-inflammatory mediator PGE2 within the sensory ganglia may interfere with neuronal activity and signal transmission at the ganglion level and take part in CIP.
It is hypothesized that SGCs are activated during CIP, and that such activation might promote or maintain neuronal activation. Furthermore, PGE2 might act on the prostaglandin E receptors expressed on the ganglion neurons to modulate neuronal activity via second messenger molecules associated with the sensitization of peripheral neurons [29-31].
Pharmacological modulation of Cisplatin-evoked PGE2 release
The modulatory effects of Ibudilast and SKF were also investigated in the present study in order to validate the established platform for the investigation of pharmacological agents on SGCs functional characteristics.
Ibudilast has a limited target profile aside from its effect as a phosphodiesterase inhibitor and has shown promising results as a modulator for glial cell activity [18,19,32]. Ibudilast inhibitory effect on glial activation has been suggested through inhibition of the release of pro-inflammatory molecules like PGE2 and cytokines [18,19,32]. This is in line with our findings that there was a tendency towards lower PGE2 release from cultured SGCs activated by Cisplatin after incubation with Ibudilast. Hence, Ibudilast might have therapeutic utility targeting glial cells as a novel treatment strategy for pain. Ibudilast has previously been tested in rat models of neuropathic pain following peripheral nerve injury and CIPN [18,32-34]. In these models, administration of Ibudilast resulted in sustained to transient relief of allodynic-symptoms [18,32-34]. No direct interactions have been identified between Ibudilast and neurons/glial cells that may account for the entire anti-allodynic effect of Ibudilast. Thus, it remains unclear if the phosphodiesterase inhibition directly contributes to the decrease in allodynia observed in the different animal models [18,32]. In animal models of CIPN induced by paclitaxel and vincristine, mechanical allodynia was slightly reduced after administration of phosphodiesterase inhibitors, Propentofylline and Ibudilast [32,35]. Thus, the analgesic effect of Ibudilast might stem from both the inhibitory effect on phosphodiesterase as well as the attenuation of glial activation through the inhibition of pro-inflammatory molecules such as cytokines [18].
SKF is a pharmacological agent that inhibits p38 MAPK, a key regulator of inflammatory response. This agent was used in the present study to serve as an anti-inflammatory cytokine-suppressive agent and was found to significantly decrease the PGE2 concentrations in the Cisplatin-treated medium. These results indicate that PGE2 release by isolated SGCs depends on the phosphorylation of p38 MAPK and that Cisplatin promotes this phosphorylation. The involvement or activation of p38 MAPK in glial cells and CIP is still largely unknown and additional studies are needed to clarify the role of p38 MAPK. However, results from Losa et al., Mizukoshi et al. and our laboratory suggest that p38 MAPK is most likely involved in glial cell activation and CIP [21,36]. Based on these results inhibition of p38 MAPK might serve as a beneficial strategy for management of chemotherapy-induced, inflammatory, and neuropathic pain.
Methodological considerations
We characterized isolated SGCs after 2, 7, 14 and 21 days in culture. However, cultures younger than 7 days did not yield enough cells for functional characterization under normal conditions and following exposure to Cisplatin. Therefore, it was not possible to examine the response of SGCs upon exposure to Cisplatin after 2 days in culture. Thus, using the current method we cannot discharge the possibility that SGCs might be more or less sensitive/active during the first days in culture. However, the immunoreactivity against GFAP and GS at 2 days in culture does not seem different from the immunoreactivity at 7, 14 and 21 days in culture. The stable GFAP expression might still suggest that the PGE2 secretion after 2 days in culture would mimic the concentrations at 7, 14 and 21 days. GFAP is a commonly used marker for glial cell activation, and an increased GFAP expression has been observed in a number pain models [10,23,37]. In contrast, Liu et al. demonstrated increased GFAP expression in SGCs in the absence of mechanical allodynia following sham operation in an animal model of spinal nerve ligation [38]. These findings indicate that GFAP expression is not a specific marker for SGC activation in relation to pain.
A key phenotypical characteristic of SGCs in the intact ganglion is their close relation with neuron [27]. Very little is known about the complex relation between SGCs and neurons in the intact ganglion and thus it is difficult to speculate how and to what extent SGCs are affected by the isolation or to what extend this model might mimic the response by SGCs in vivo. Whole organ culture can address some of these questions as the relationship between SGCs and neurons is preserved. This method is currently under investigation in our lab.
Conclusion
The present study showed that 1) primary cultures of trigeminal SGCs maintain the glial cell morphology and immunoreactivity against GFAP and GS in vitro; 2) cultured SGCs were stimulated by Cisplatin and release PGE2, which confirmed the sustained functionality of these cells in vitro; and 3) PGE2 release was attenuated by Ibudilast and SKF. Together these findings support that the established SGC platform is valid and functions for investigating substance release and pharmacological modulation. Inhibitory effects of Ibudilast and SKF on Cisplatin-evoked PGE2 release from SGCs suggest a potential role of these agents for prevention of CIP.
Acknowledgements
This study was partly supported by a research grant from Obel family foundation, Aalborg, Denmark. The authors are grateful to the staff at the Animal Research Facility for excellent cooperation. Jens Christian Laursen, PhD-student at the center for Sensory-Motor Interaction, Aalborg University, Denmark is acknowledged for laboratory assistance.
Disclosure of conflict of interest
None.
References
- 1.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Gu Y, Chen Y, Zhang X, Li GW, Wang C, Huang LY. Neuronal soma-satellite glial cell interactions in sensory ganglia and the participation of purinergic receptors. Neuron Glia Biol. 2010;6:53–62. doi: 10.1017/S1740925X10000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010;64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, Reuter U. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One. 2011;6:e17360. doi: 10.1371/journal.pone.0017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laursen JC, Cairns BE, Dong XD, Kumar U, Somvanshi RK, Arendt-Nielsen L, Gazerani P. Glutamate dysregulation in the trigeminal ganglion: A novel mechanism for peripheral sensitization of the craniofacial region. Neuroscience. 2014;256:23–35. doi: 10.1016/j.neuroscience.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res. 2012;1487:183–191. doi: 10.1016/j.brainres.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 9.Takeda M, Tsuboi Y, Kitagawa J, Nakagawa K, Iwata K, Matsumoto S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol Pain. 2011;7:5. doi: 10.1186/1744-8069-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain. 2013;17:571–580. doi: 10.1002/j.1532-2149.2012.00219.x. [DOI] [PubMed] [Google Scholar]
- 11.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 12.Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:1–5. doi: 10.1155/2011/843019. Article ID 843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- 14.Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998;101:2842–2850. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Ohtaki H, Tsumuraya T, Song D, Sato A, Ohara K, Miyamoto K, Nakano H, Kiriyama K, Dohi K, Hiraizumi Y, Matsunaga M, Shioda S. Establishment and characterization of primary adult microglial culture in mice. Acta Neurochir Suppl. 2013;118:49–54. doi: 10.1007/978-3-7091-1434-6_8. [DOI] [PubMed] [Google Scholar]
- 18.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- 19.Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59:3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizukoshi K, Sasaki M, Izumi Y, Miura M, Watanabe M, Amaya F. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience. 2013;234:77–87. doi: 10.1016/j.neuroscience.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006;2:247–257. doi: 10.1017/s1740925x07000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010;6:3–10. doi: 10.1017/S1740925X10000037. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, McLachlan EM. Inflammation in sympathetic ganglia proximal to sciatic nerve transection in rats. Neurosci Lett. 2004;365:39–42. doi: 10.1016/j.neulet.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 26.de Almeida-Leite CM, Arantes RM. Primary culture of glial cells from mouse sympathetic cervical ganglion: a valuable tool for studying glial cell biology. J Neurosci Methods. 2010;194:81–86. doi: 10.1016/j.jneumeth.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Belzer V, Shraer N, Hanani M. Phenotypic changes in satellite glial cells in cultured trigeminal ganglia. Neuron Glia Biol. 2010;6:237–243. doi: 10.1017/S1740925X1100007X. [DOI] [PubMed] [Google Scholar]
- 28.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 29.Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34:1170–1173. doi: 10.1248/bpb.34.1170. [DOI] [PubMed] [Google Scholar]
- 30.Patwardhan AM, Vela J, Farugia J, Vela K, Hargreaves KM. Trigeminal Nociceptors Express Prostaglandin Receptors. J Dent Res. 2008;87:262–266. doi: 10.1177/154405910808700306. [DOI] [PubMed] [Google Scholar]
- 31.Kadoi J, Takeda M, Matsumoto S. Prostaglandin E2 potentiates the excitability of small diameter trigeminal root ganglion neurons projecting onto the superficial layer of the cervical dorsal horn in rats. Exp Brain Res. 2007;176:227–236. doi: 10.1007/s00221-006-0608-2. [DOI] [PubMed] [Google Scholar]
- 32.Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 34.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 35.Sweitzer SM, Pahl JL, DeLeo JA. Propentofylline attenuates vincristine-induced peripheral neuropathy in the rat. Neurosci Lett. 2006;400:258–261. doi: 10.1016/j.neulet.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez Losa J, Parada Cobo C, Guinea Viniegra J, Sanchez-Arevalo Lobo VJ, Ramon y Cajal S, Sanchez-Prieto R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene. 2003;22:3998–4006. doi: 10.1038/sj.onc.1206608. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SY, Robinson CR, Zhang H, Dougherty PM. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J Pain. 2013;14:205–214. doi: 10.1016/j.jpain.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu FY, Sun YN, Wang FT, Li Q, Su L, Zhao ZF, Meng XL, Zhao H, Wu X, Sun Q, Xing GG, Wan Y. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 2012;1427:65–77. doi: 10.1016/j.brainres.2011.10.016. [DOI] [PubMed] [Google Scholar]