Abstract
Isoglobotrihexosylceramide (iGb3) has been identified as a potent CD1d-presented self-antigen for mouse iNKT cells. The role of iGb3 in humans remains unresolved, however, as there have been conflicting reports about iGb3-dependent human iNKT-cell activation, and humans lack iGb3 synthase, a key enzyme for iGb3 synthesis. Given the importance of human immune responses, we conducted a human-mouse cross-species analysis of iNKT-cell activation by iGb3-CD1d. Here we show that human and mouse iNKT cells were both able to recognise iGb3 presented by mouse CD1d (mCD1d), but not human CD1d (hCD1d), as iGb3-hCD1d was unable to support cognate interactions with the iNKT-cell TCR. The structural basis for this discrepancy was identified as a single amino acid variation between hCD1d and mCD1d, a glycine-to-tryptophan modification within the alpha2-helix that prevents flattening of the iGb3 headgroup upon TCR ligation. Mutation of the human residue, Trp153, to the mouse ortholog, Gly155, therefore allowed iGb3-hCD1d to stimulate human iNKT cells. In conclusion, our data indicate that iGb3 is unlikely to be a major antigen in human iNKT-cell biology.
Keywords: antigen presentation, CD1d, iNKT, isogloboside 3, species differences
Introduction
The nonpolymorphic MHC-like protein CD1d presents lipid antigens to a highly conserved T-cell subset known as invariant Natural Killer T (iNKT) cells. These iNKT cells exert critical roles in host defence and immune tolerance, and manipulating iNKT cells in mice has been shown to provide protection from cancer, infections and autoimmunity [1].
iNKT cells are characterised by their co-expression of NK-cell receptors and a highly conserved, semi-invariant TCR. All iNKT TCRs use a specific Vα-Jα rearrangement – Vα24-Jα18 in humans and Vα14-Jα18 in mice – coupled with a single Vβ family in humans, i.e. Vβ11, and a restricted Vβ repertoire in mice. The iNKT-CD1d system is highly conserved amongst mammals. Indeed, human and mouse iNKT cells exhibit cross-species reactivity for CD1d [2] due to conserved contacts between CD1d and Vα-Jα18 [3]. However, recent studies have identified species-specific differences in the ability of iNKT cells to respond to certain lipid antigens [4, 5], indicating that structural differences between human and mouse CD1d proteins may impact on the validity of preclinical assessments of iNKT-cell-targeting therapies.
A key property of human and mouse iNKT cells that is critical for their activation under inflammatory conditions is their auto-reactivity to CD1d [6]. This is dependent on both inflammation-induced synthesis of endogenous glycolipid antigens [7–10] and interactions between the CDR3β loop of the semi-invariant iNKT-cell TCR and the CD1d protein [11, 12]. Over the past decade, the search for CD1d-presented iNKT-cell stimulatory glycolipid antigens has led to the identification of several endogenous and microbial glycolipids. Isoglobotrihexosylceramide (iGb3) was one of the first identified endogenous iNKT-cell antigens [7, 13], and several studies have since confirmed that iGb3 can activate mouse iNKT cells via CD1d [14–18]. However, the importance of this glycolipid for iNKT-cell development and its biological role in mice remains uncertain, as iGb3 synthase gene-deleted mice exhibit no apparent defects in either iNKT-cell repertoire or function despite their inability to produce this glycolipid [19]. Whether iGb3 plays a role for human iNKT-cell biology is even more uncertain. Arguing against a key role for iGb3 as an important self-antigen for iNKT cells in humans are studies showing that the human gene for iGb3 synthase is neither expressed nor functional [20], and that iGb3 could not be detected in human tissue [21]. Furthermore, while it is well established that iGb3 can functionally activate mouse iNKT cells in vitro, published studies on the antigenicity of iGb3 for human iNKT cells have been inconsistent [10, 13, 22, 23].
Based on the above we reasoned that the lack of a clear understanding of the role of iGb3 as an iNKT-cell antigen was the lack of systematic studies directly comparing iGb3 antigenicity for mouse and human iNKT cells. Therefore we cross-examined the potential of iNKT cells from both species to respond to iGb3 in the context of either human CD1d (hCD1d) or mouse CD1d (mCD1d), and investigated possible molecular mechanisms underlying species-selective differences in iGb3 antigenicity.
Results
iGb3 in the context of human CD1d (hCD1d) does not activate human iNKT cells
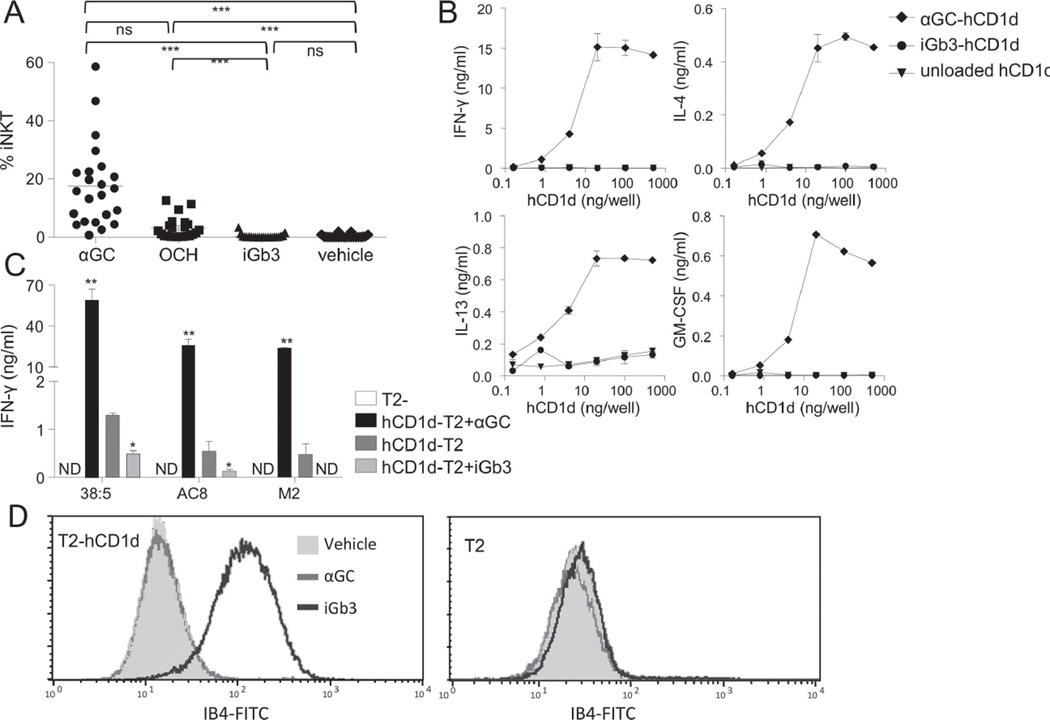
In order to investigate the controversial area of iGb3 antigenicity in humans, we employed a panel of tests to examine human iNKT activation in response to iGb3. We first compared the in vitro expansion of human iNKT cells in PBMCs from 23 healthy subjects in response to iGb3, as well as to the potent iNKT-cell antigen α-galactosylceramide KRN7000 (hereafter termed αGC), the weaker αGC analogue OCH (all at 100 ng/ml), and lipid vehicle (0.5% polysorbate 20 in 150 mM NaCl). After two weeks, the proportion of iNKT cells in the iGb3-treated PBMC cultures was not higher than in the vehicle-treated cultures, while a clear expansion could be seen for cultures incubated with the alpha-galactosylceramide antigens αGC and its lower affinity analogue, OCH (Figure 1A).
Figure 1.
iGb3 does not activate human iNKT cells when presented by hCD1d. (A) Expansion of human iNKT cells from human PBMC cultures (n=23) after 12–16 days culture in the presence of either αGC, OCH, iGb3 (all at 100 ng/ml) or vehicle. The percentage of iNKT cells among T cells is shown. Each symbol represents a single sample and the data are representative of XXX experiments performed. NS: not significant; ***p<0.001, Friedman’s test with Dunn’s Multiple Comparison post-test. (B) Cytokine release of purified (>99%) polyclonal human iNKT cells in response to plate-bound unloaded hCD1d, hCD1d-αGC or hCD1d-iGb3. Data are shown as mean ± SEM and are representative of two independent experiments. (C) IFN-γ secretion of three autoreactive human iNKT clones (38:5, AC8 and M2) in response to hCD1d-T2 cells that were pulsed with αGC (black columns), vehicle (dark grey columns) or iGb3 (light grey columns). Data are shown as mean ± SEM of XXX samples/replicates and are representative of/pooled from XXX experiments performed. *p<0.05, **p<0.01, as compared with vehicle-pulsed hCD1d-T2, paired t-test. ND: Not detected. (D) FITC-conjugated IB4-lectin binding to T2-hCD1d cells (left) or CD1d-negative T2 cells (right) that were pulsed with either iGb3 (black line histogram), αGC (grey line histogram) or vehicle (grey-filled histogram) was determined by flow cytometry. Data shown are representative of XXX experiments performed.
To more directly assess functional activation iNKT cells by the iGb3-hCD1d complex, a pure human iNKT line (>99% iNKT) was stimulated with plate-bound recombinant hCD1d that was either loaded with iGb3 (iGb3-hCD1d) or αGC (αGC-hCD1d), or unloaded. Whereas plate-bound αGC-hCD1d (at 4 ng/well or greater) effectively stimulated the release of GM-CSF, IL-13, IL-4 and IFN-γ from the human iNKT line (Figure 1B), no cytokine release was detectable for any tested concentration of iGb3-loaded or unloaded hCD1d. Furthermore, we tested the response of three high-affinity human iNKT clones to hCD1d expressing cell lines (hCD1d-T2) that were pulsed with iGb3, αGC or lipid vehicle. As expected, all these high-affinity clones displayed marked self-reactivity to hCD1d-T2 in the absence of additional lipid and no response to hCD1d-deficient T2 cells (T2). Interestingly, all three clones exhibited clearly weaker responses towards iGb3-pulsed than vehicle-pulsed hCD1d-T2 (Figure 1C), consistent with iGb3 loading onto CD1d and displacement of more stimulatory endogenous lipids. Further confirmation of iGb3 loading onto hCD1d-T2 cells during pulsing was supplied by bright staining of iGb3-pulsed hCD1d-T2 with the Griffonia simplicifolia lectin IB4 which binds the terminal α-linked sugar of iGb3. In contrast, neither αGC- nor vehicle pulsed hCD1d-T2, nor iGb3 pulsed CD1d-negative T2 cells stained with FITC-IB4 (Figure 1D). These results collectively supported the concept that human iNKT cells are not stimulated by hCD1d-presented iGb3.
iGb3 does not support binding of human iNKT TCRs to hCD1d
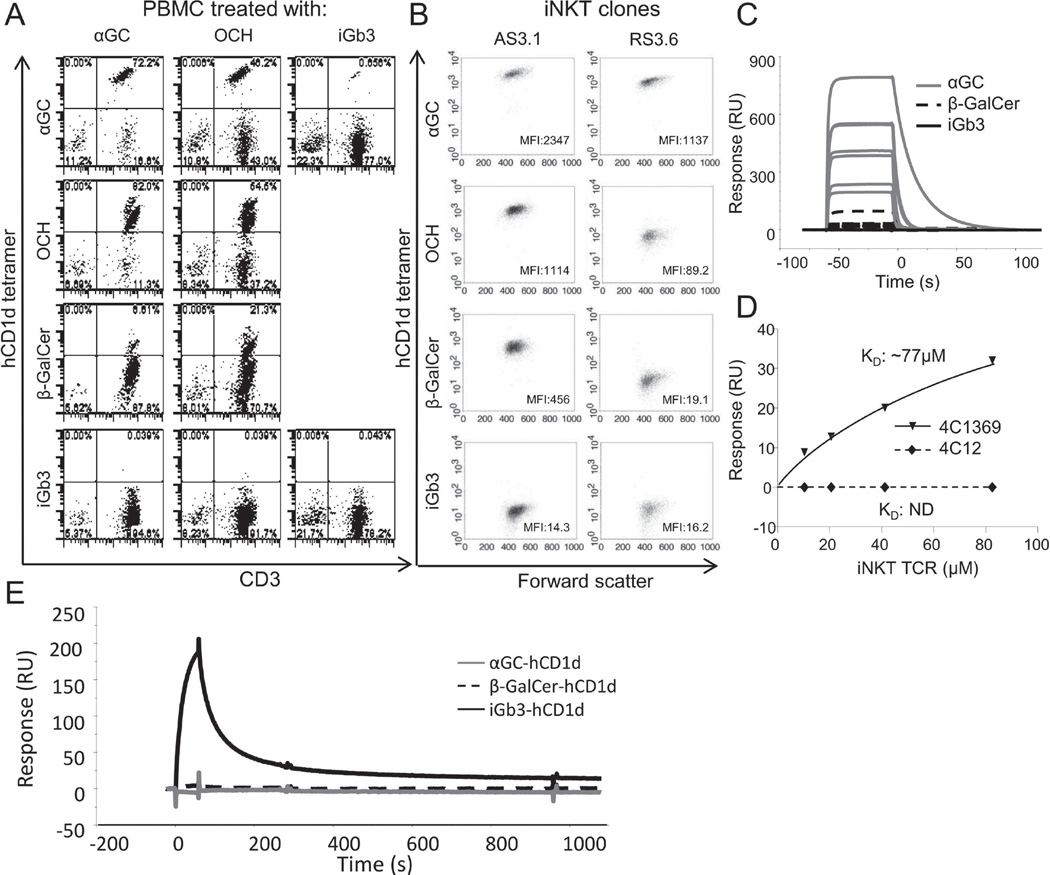
A prerequisite for CD1d-mediated iNKT-cell activation is the direct binding of the iNKT TCR to the lipid-CD1d complex. To examine whether iGb3 can promote the interaction between hCD1d and human iNKT TCRs we compared the binding of a panel of hCD1d tetramers with that of αGC- and OCH-expanded human iNKT cell cultures. As expected, iNKT cells were most brightly stained by αGC-hCD1d tetramers, followed by medium staining with OCH-hCD1d tetramers and weak but detectable staining with β-galactosylceramide (β-GalCer)-hCD1d tetramers. In contrast, hCD1d-iGb3 tetramers failed to detectably stain iNKT cells in these lines, nor in separate, iGb3-stimulated T-cell lines, indicating that iGb3 did not selectively expand a population of iGb3-specific human iNKT cells (Figure 2A). Furthermore, we compared the binding of the hCD1d-iGb3 tetramers with that of a panel of human iNKT clones with differing iNKT TCR affinity for CD1d, which had previously been expanded from healthy donors’ PBMC in an antigen unbiased way [11]. As expected, higher affinity iNKT clones were consistently stained more brightly by all hCD1d-tetramers, and each clone displayed an identical hierarchy of binding towards tetramers loaded with the different lipid antigens. Amongst the hCD1d-lipid antigen complexes tested, hCD1d-iGb3 was clearly the least antigenic, as it failed to stain even the highest affinity iNKT clones (Figure 2B).
Figure 2.
Human iNKT TCRs do not bind to the hCD1d-iGb3 complex. (A) Binding of fluorescent-conjugated hCD1d tetramers generated by in vitro refolding using either αGC, OCH or β-GalCer (rows 1–3) to (A) human iNKT cells in different lipid antigen-treated PBMC lines (columns 1–3), and (B) to two human iNKT clones of different TCR affinity (AS3.1, high affinity TCR; RS3.6, low affinity TCR). (C) Binding of seven different recombinant human iNKT TCRs at 1 µM concentration to chip-immobilised recombinant hCD1d-αGC (solid grey line), hCD1d- β-GalCer (dotted black line) and hCD1d-iGb3 (solid black line). Figure shows overlaid sensograms from surface plasmon resonance. (D) Binding of a high affinity iNKT TCR (4C1369) and a low affinity iNKT TCR (4C12) to hCD1d-iGb3. Concentration response curves display binding of both TCRs to chip-bound hCD1d-iGb3. (E) Overlaid surface plasmon resonance sensograms showing binding of IB4 lectin to chip-immobilised recombinant hCD1d-iGb3 (black line), hCD1d-β-GalCer (dotted black line) or hCD1d-αGC complexes (grey line). All data shown are representative of two or more independent experiments performed.
To confirm that the recombinant in vitro refolded hCD1d molecules used for these experiments were loaded with iGb3, direct binding of IB4 lectin to hCD1d-iGb3 complexes was assessed by surface plasmon resonance. IB4 was found to bind strongly to chip-immobilised hCD1d-iGb3 monomers, but did not bind to either αGC- or β-GalCer-loaded hCD1d monomers (Figure 2E).
To further corroborate the apparent lack of cognate interaction between the human iNKT TCR and hCD1d-iGb3, we directly examined the binding of a panel of 7 human iNKT TCRs at equimolar concentration (1 µM) to hCD1d-iGb3-, -αGC and -β-GalCer using surface plasmon resonance. All TCRs bound well to hCD1d-αGC, and, as expected, the highest affinity TCRs also bound appreciably to hCD1d-β-GalCer [11]. However, at this analyte concentration, no detectable binding was observed for any of the iNKT TCRs to hCD1d-iGb3 (Figure 2C).The lowest and the highest affinity iNKT TCRs (4C12 and 4C1369 respectively) were then tested at different concentrations for binding to hCD1d-iGb3. No detectable binding of the low affinity iNKT to hCD1d-iGb3 was observed, and although the high affinity iNKT TCR exhibited some binding to hCD1d-iGb3 (KD: 77µM; Figure 2D), it was approximately 350× weaker than its affinity towards hCD1d-αGC, and 35× weaker than its affinity towards either hCD1d-β-GalCer-or hCD1d-LacCer [11].
These findings strongly indicate that the failure of iGb3 to functionally activate human iNKT cells in vitro is due to the failure of iGb3-loaded hCD1d to support successful interactions with the great majority of the human iNKT TCR repertoire.
iNKT cells respond to iGb3 when presented by mouse CD1d (mCD1d) but not hCD1d
In contrast to the above results, mouse iNKT cells are activated via binding of their TCRs to iGb3 presented by mouse CD1d (mCD1d) [14–18]. In order to explore this apparent species difference in iGb3 antigenicity, a cross-species analysis of iNKT activation was carried out. Polyclonal human and mouse iNKT lines (both >99% pure) were challenged with plate-bound iGb3-loaded, αGC-loaded, or unloaded hCD1d or mCD1d. As expected, both mouse and human lines responded well to αGC-loaded hCD1d or mCD1d. Both mouse and human lines were also weakly activated by iGb3 when presented by mCD1d, but neither line was activated by hCD1d-iGb3 (Figure 3A). Similarly, in contrast to their lack of response to iGb3-pulsed hCD1d-T2, the three high-affinity human iNKT clones described responded strongly to iGb3-pulsed mCD1d-T2 (Figure 3B).
Figure 3.
Polyclonal mouse and human iNKT cells respond to mCD1d-iGb3. (A) Cytokine secretion of polyclonal mouse (top) and human (bottom) iNKT lines in response to plate-bound hCD1d (filled symbols) and mCD1d (empty symbols) that were either unloaded (triangles) or loaded with αGC (diamonds) or iGb3 (cirlces). IFN-γ, IL-4, IL-13 or GM-CSF release was determined by ELISA and shown as mean ± SEM of XXX replicates/samples, data are representative of two independent experiments. (B) IFN-γ secretion of three autoreactive human iNKT clones (38:5, AC8 and M2) in response to mCD1d-T2 cells that were pulsed with either αGC (black columns), vehicle (dark grey columns) or iGb3 (light grey columns). Data are shown as mean ± SEM of XXX samples/replicates and are representative of/pooled from XXX experiments. *p<0.05, **p<0.01, compared with vehicle-pulsed mCD1d-T2, paired t-test. ND: Not detected. (C) Staining of αGC-pulsed (black line histogram), iGb3-pulsed (dotted black line) and vehicle-pulsed (grey-filled histogram) hCD1d-T2 cells (top) and mCD1d-T2 cells (bottom) with fluorescent-conjugated human iNKT TCR tetramer. Data are representative of four independent experiments.
These results strongly suggested that species differences in CD1d structure determine the ability of CD1d-iGb3 to support cognate iNKT-TCR binding. To directly test this hypothesis we examined the binding of fluorescent-conjugated human iNKT TCR tetramers to mCD1d- and hCD1d-expressing T2 lymphoblasts that were pulsed with iGb3, αGC, or vehicle. In line with the functional data presented above, pulsing hCD1d-T2 cells with iGb3 inhibited human iNKT TCR tetramer binding compared with that of vehicle alone, whereas pulsing mCD1d-T2 cells with iGb3 enhanced human iNKT TCR tetramer binding (Figure 3C).
A single amino acid in the hCD1d α2 domain prevents iGb3 presentation to iNKT cells
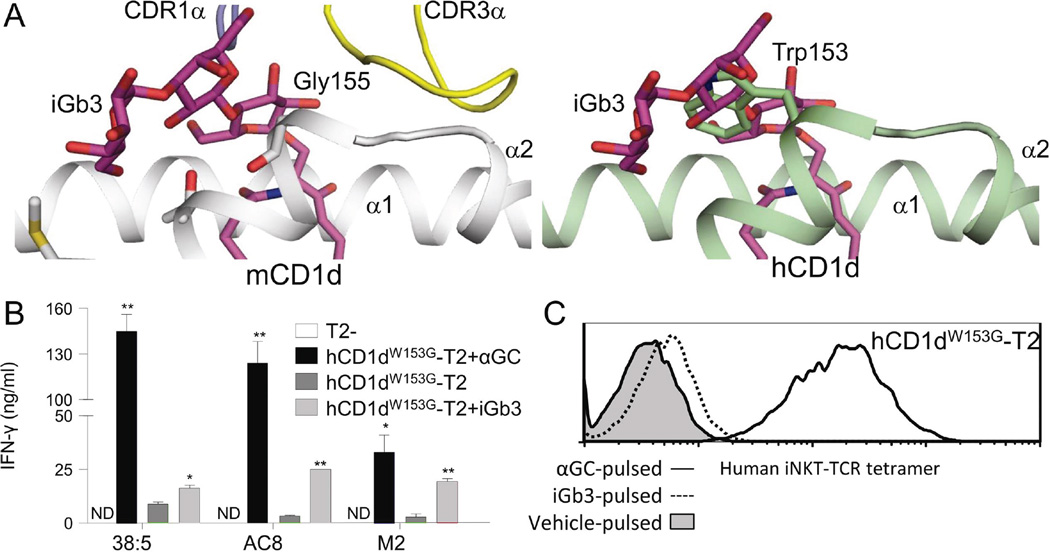
Mouse iNKT TCR binding to mCD1d-iGb3 depends on an induced fit remodelling of the bulky headgroup of iGb3 against the α2 helix of mCD1d upon TCR ligation [24, 25]. In this, the proximal carbohydrate portion of iGb3 is flattened against Glycine 155 (G155) in mCD1d. We hypothesised that the presence in hCD1d of a bulky tryptophan residue at this position (W153) may be the molecular basis for species differences in the presentation of iGb3 by hCD1d and mCD1d (Figure 4A). Based on this hypothesis, we introduced a single tryptophan to glycine mutation at this site (W153G) into hCD1d and expressed this CD1d protein (hCD1dW153G) on T2 lymphoblasts (hCD1dW153G-T2) at a closely matched expression level to the previously used wild-type hCD1d-T2 line (data not shown). In contrast to the results with wild-type hCD1d, and similar to the results with mCD1d, all three human high-affinity iNKT clones exhibited strong responses to iGb3-pulsed T2-hCD1dW153G (Figure 4B). Furthermore, iNKT TCR tetramer staining of T2-hCD1dW153G, unlike T2-hCD1d, was enhanced by iGb3 pulsing (Figure 4C). Therefore, this single amino acid difference (W153G) sufficiently explains the substantial mouse-human species differences in the antigenicity of iGb3.
Figure 4.
Tryptophan W153 in hCD1d is responsible for human-mouse species differences in iGb3 presentation. (A) The crystal structure of iGb3-mCD1d complexed with iNKT TCR (left, from Pellicci et al., 2011) and a superposition of the iGb3 headgroup on hCD1d (right) are shown, suggesting that W153 in hCD1d could sterically hinder the iNKT TCR-induced flattening of the iGb3 headgroup. (B) IFN-γ release by three human iNKT clones (38:5, AC8 and M2) in response to hCD1dW153G-T2 cells that were pulsed with either black αGC (black columns), vehicle (dark grey columns) or iGb3 (light grey columns). Data were generated in parallel with Figures 1C and 3B and are shown as mean ± SEM of XXX samples/repliates, representative of/pooled from XXX experiments. *p<0.05, **p<0.01, compared with vehicle-pulsed hCD1dW153G-T2, paired t-test. ND: Not detected. (C) Staining of αGC-pulsed (black line histogram), iGb3-pulsed (dotted line histogram) and vehicle-pulsed (grey-filled histogram) hCD1dW153G-T2 cells with fluorescent-conjugated human iNKT TCR tetramer. Data were generated in parallel with Figure 3C, and are representative of three independent experiments.
Discussion
The identification and characterisation of endogenous CD1d ligands which act as antigens for autoreactive iNKT activation is deemed to be of great importance for our understanding of iNKT biology and the design of iNKT-targeting therapeutics. Similarly, understanding species differences in CD1d lipid antigen presentation to iNKT cells will be of key importance to the successful translation of putative iNKT lipid antigens from preclinical studies to human clinical trials. In this study, we have identified significant species differences in the presentation of the most-studied endogenous iNKT antigen, the glycosylceramide iGb3, as a consequence of structural differences in the mouse and human CD1d proteins.
Initial reports suggested that the endogenous glycolipid iGb3 could potently activate both mouse and human iNKT cells [13], but more recent studies have been unable to fully replicate these findings in humans [10, 22]. Importantly, while some studies have identified weak responses of human iNKT cells to iGb3 [13, 17, 26], these studies have not directly demonstrated a cognate iNKT TCR interaction with human CD1d. Here we show that iGb3 can activate both human and mouse iNKT cells in the context of mouse CD1d (mCD1d), but neither mouse nor human iNKT cells in the context of human CD1d (hCD1d). The absence of any iNKT cell expansion observed after iGb3 challenge of PBMCs from 23 healthy donors strongly suggested that iGb3 was not a major antigen for human iNKT cells. Furthermore, no evidence was found of cytokine release by a purified polyclonal iNKT line in response to plate-bound iGb3-hCD1d. We have previously shown that the human iNKT repertoire contains clones of variable TCR affinity for CD1d and that high affinity clones exhibit greater reactivity towards weak endogenous antigens [11]. While it is known that many relatively weakly antigenic endogenous lipids are able to support iNKT autoreactive activation [12], it is important to note that all three different high-affinity autoreactive iNKT clones used in our study displayed clearly weaker responses to iGb3-pulsed hCD1d-T2 cells than to vehicle-pulsed hCD1d-T2 cells. This indicates that, in the context of hCD1d, iGb3 is not merely permissive towards human iNKT activation, but is significantly less antigenic than the endogenous lipids naturally loaded on hCD1d. This conclusion is strongly supported by the findings that none of a panel of several high-affinity human iNKT cells could be stained with iGb3-loaded hCD1d tetramers, despite their binding to hCD1d tetramers loaded with the endogenous weak antigen β-GalCer, and the very low binding affinity observed between a high affinity natural human iNKT TCR and iGb3-hCD1d. It is interesting to note that iNKT cells expanded in vitro with the partial agonist antigen OCH stained more brightly with β-GalCer-tetramers compared with iNKT cells expanded with the strong antigen KRN7000, suggesting slightly biased expansion of higher-affinity iNKT clones by the weaker antigen OCH.
These data cannot formally rule out the possibility that certain unusual iNKT TCRs may be able to recognise hCD1d-iGb3, and a recent study found weak but appreciable binding of a single human iNKT TCR to iGb3-hCD1d and activation of a mouse iNKT hybridoma by hCD1d in the presence of very high concentrations of iGb3 [25], while another study has described a single human iNKT clone to be similarly activated by very high concentrations of iGb3 [23]. However, the lack of hCD1d-iGb3 tetramer-positive iNKT cells even in iGb3-treated PBMC cultures described here and our inability to identify any human iNKT clones which were functionally activated by iGb3-hCD1d, suggests that if they exist, iGb3-reactive human iNKT cells must be very rare and/or difficult to detect.
Previous reports have suggested that CD1d loading with iGb3 may be difficult to achieve, e.g. requiring either the addition of saposin [13] or solubilisation of the lipid in methanol [26] for efficient loading. Therefore, we carefully assessed iGb3 loading at each stage using the Griffonia simplicifolia lectin IB4, which selectively binds terminal α-galactosyl linkages, such as in iGb3 [27]. This lectin was found to bind both to iGb3-pulsed hCD1d-T2 cells and to in vitro refolded hCD1d-iGb3 complexes, thereby confirming successful loading of iGb3 onto CD1d and its persistent association with CD1d during chromatographic purification of soluble CD1d molecules. While IB4 is not entirely selective towards iGb3, failure of IB4 to bind to either non-iGb3-loaded (i.e. refolded) CD1d molecules or to non-iGb3-pulsed or non-CD1d-expressing T2 cells indicated that it did specifically bind to iGb3 loaded on CD1d molecules in our experiments. In contrast to the above results, mouse iNKT cells are known to be activated by mCD1d-iGb3 via iNKT TCR ligation, and the molecular details of this interaction were recently demonstrated in crystal structures of the ternary iNKT TCR-CD1d-iGb3 complex [24, 25]. These apparent human-mouse species differences in iGb3 antigenicity might be caused by structural differences in CD1d that affect iGb3 presentation to iNKT TCRs or may result from differences in iNKT TCR reactivity towards CD1d-iGb3. To differentiate between these possibilities we carried out a cross-species analysis of iNKT activation by CD1d-iGb3. Interestingly, this revealed that both human and mouse iNKT cells were fully able to respond to iGb3, but only when presented in the context of mCD1d. Furthermore, human iNKT TCR tetramers could effectively stain iGb3-pulsed mCD1d-expressing cells, but not iGb3-pulsed hCD1d-expressing cells. These data clearly indicate that the critical species difference responsible for the failure of iGb3 to activate human iNKT cells resides in the molecular structure of the CD1d molecule itself.
The recently solved crystal structures of mouse iNKT TCRs in complex with CD1d-iGb3 [24, 25] suggested to us a potential explanation for the observed species differences in CD1d presentation of iGb3. In these complexes the carbohydrate headgroup of iGb3 is squashed against the α2 helix of CD1d upon iNKT TCR binding, and held in place by interactions between the terminal galactosyl sugar and CD1d. Crucial for this induced fit of TCR binding is the flattening of the proximal carbohydrate portion of iGb3 against Glycine 155 (G155) in mCD1d. However, a bulky tryptophan residue (W153) occupies this position in hCD1d, which we predicted to sterically hinder a similar induced fit remodelling of hCD1d-iGb3 by the iNKT TCR, rendering the interaction energetically unfavourable and greatly reducing the potential binding affinity (Figure 4A). The general mode of interaction between the iNKT TCR and CD1d is highly conserved in all ternary crystal structures solved to date [12, 24, 28, 29], arguing against the possibility of an unorthodox binding mode allowing iNKT recognition of hCD1d-iGb3. By specifically mutating W153 in hCD1d to glycine to mimic the mouse ortholog, we were able to show that this single residue is indeed responsible for the observed species differences in iGb3 antigenicity. In further support of this, a recent report [25] found that mouse iNKT hybridomas were unable to recognise iGb3 presented by mCD1d with a tryptophan in place of Glyl55.
A full analysis of published CD1d sequences reveals that this glycine-tryptophan mutation in CD1d is solely present in old-world monkeys and apes, and that it must therefore have occurred since the divergence with new world monkeys (approximately 35MYA [30]), as the new world monkey Callithrix jacchus and the lesser primates Microcebus murinus and Otolemur garnetti, in common with nearly all other mammals, use glycine at this position (Supporting Information Figure 1). Interestingly, this is in close concordance with the inactivation of iGb3 synthase, along with other closely related glycosyltransferases, in Old World monkeys and apes [31]. Furthermore, these species are the only species known to produce anti-αGal antibodies [32], which may be able to bind to the α-Gal epitope of iGb3 and destroy iGb3 expressing cells [20]. It is believed that these changes in sugar chain synthesis were a result of significant selective pressure, for instance in response to α-Gal-expressing or targeting pathogens [33]. The contemporaneous glycine-tryptophan mutation in CD1d may therefore be secondary to these changes in sugar metabolism, and may have a compensatory effect to support protein-protein iNKT TCR:CD1d interactions in the absence of iGb3 as a significant self-lipid antigen, or may allow for better presentation of alternative, as yet unknown, endogenous glycolipids produced as functional replacements for iGb3 [31].
This striking example of concomitant evolution offers some support to the suggestion that iGb3 plays an important role in non-human mammalian iNKT function. This proposition, first suggested on the basis of IB4-lectin inhibition of mouse iNKT autoreactive function [7, 13] and correlations between iGb3 reactivity and Vβ usage in murine iNKT cells [15], has been challenged by the failure to detect any overt iNKT dysfunction in iGb3-synthase deficient mice [19]. However, this study did not specifically look for pathologically relevant alterations of iNKT function, and so may have missed important consequences of iGb3 deficiency.
The results of our study complement a growing body of evidence that, despite significant conservation, the human and mouse iNKT-CD1d systems often do not recognise the same antigens. In particular, several lipids are now known to display species-selectivity in their iNKT antigenicity, including certain synthetic C-glycoside αGC analogues [5], α-Gal-diacylglycerol lipids from Borrelia burgdorferi [4, 34], endogenous lyso-phospholipids [35, 36], β-Glucosylceramides [5], and iGb3 as characterised here. Moreover, a very recent study demonstrated that human and mouse iNKT cells have different patterns of fine specificity for synthetic analogues of α-GalCer, based on differential interactions between the TCR and sugar headgroup moieties [37]. A full appreciation of these species differences and the molecular mechanisms responsible would be expected to greatly enhance the potential for accurate translation of potential iNKT-targeting therapies from preclinical animals to man.
Materials and Methods
Lipids
Purified iGb3 was kindly supplied by Prof Susann Teneberg (Gothenburg University), and synthetic iGb3 (acyl chain C26:0; sphingosine chain C18:1) was purchased from Enzo Life Sciences. αGC and OCH were purchased from Matreya. Bovine β-GalCer was purchased from Fluka. All lipids were solubilised at 200 ng/ml in lipid vehicle (0.05% polysorbate-20; 150mM NaCl) by sonication at 80°C.
Isolation of iNKT cells
Polyclonal human iNKT cells were prepared from healthy donors’ PBMCs as previously described [10]. This involved expansion in the presence of IL-2 (50 U/ml; Novartis), IL-15 (5 ng/ml; Peprotech) and αGC (10 ng/ml) for 14d, followed by separation using allophycocyanin-labelled hCD1d-αGC tetramers and magnetic anti-allophycocyanin beads (Miltenyi). Lines were rested for an additional 2 weeks and were >99% αGC-hCD1d tetramer positive.
Preparation of polyclonal murine iNKT cell lines has been previously described [38]. Briefly, this involved magnetic sorting of iNKT cells from wild-type C57BL/6 mouse spleens using allophycocyanin-labelled mCD1d-αGC tetramers and anti-allophycocyanin beads (Miltenyi) followed by expansion with αGC-loaded bone marrow derived DC (BMDC) in vitro. After expansion, lines were re-purified with mCD1d-αGC tetramers and anti-fluorochrome beads, and then rested for an addition 14 days. Lines were >99% mCD1d-αGC tetramer positive, and their Vβ chain usage was highly similar to in vivo iNKTs (10).
Human iNKT cell clones were generated as described previously [11], using flow cytometric single-cell sorting of freshly-prepared healthy donors’ PBMCs using antibodies towards Vα24 and Vβ11, and expansion in the presence of PHA (1µg/ml), γ-irradiated autologous PBMCs, IL-2, IL-7 and IL-15.
Generation of stably transduced CD1d-expressing T2 lymphoblast lines
Wild-type human and mouse full-length CD1d was cloned into the third-generation pELNS lentivector (kindly provided by University of Pennsylvania) using the following primers: hCD1d.Fwd: 5'-TAAGCGGCTAGCCGCCACCATGGGGTGCCTGCTGTTTCTGCTGC-3';hCD1d.Rev:5'-GCGTGTCGACTCACAGGACGCCCTGATAGGAAGTTTGCC-3'; mCD1d.Fwd: 5’-TAAGCGGCTAGCCGCCACCATGCGGTACCTACCATGGCTGTTGC-3’; mCD1d.Rev: 5’-GCGTGTCGACTCACCGGATGTCTTGATAAGCGCTT-3’. In addition, full-length hCD1d was cloned into pGEM-T (Promega) using TA cloning, and mutated by means of a Quikchange site-directed mutagenesis kit (Agilent). A single point mutation (T532G) was inserted using the following primers: WG.Fwd: 5'-CTCAACCAGGACAAGGGGACGAGGGAAACAG-3'; WG.Rev: 5'-CTGTTTCCCTCGTCCCCTTGTCCTGGTTGAG-3'. The mutated hCD1d sequence was then cloned into the pELNS lentivector. All prepared plasmids were confirmed by sequencing.
Lentiviral particles were generated from HEK293TN cells after co-transfection with engineered pELNS lentivector (2.5 µg) and three accessory plasmids, pCMV-VSV-G (1.5 µg), pRSV.REV (3 µg) and pMDL.pg.RRE (3 µg) [39]. Lentiviral particles were collected and used without concentration for spin infection of T2 lymphoblasts. Transduced cells were sorted by flow cytometry on a FACSAria and maintained in RPMI media supplemented with 10% FBS.
Production of recombinant soluble CD1d and TCR molecules
Recombinant soluble CD1d molecules were generated as described previously [40]. The extracellular region of CD1d containing an engineered biotinylation tag at the C-terminus was expressed as inclusion bodies in E. Coli BL21.DE3(pLysS), purified, and denatured and reduced in 6 M guanidine and 20 mM DTT. CD1d was then refolded with β2m in the presence of defined lipid species using an oxidative refolding matrix. Correctly refolded CD1d was purified by gel filtration chromatography using FPLC. Targeted C-terminus biotinylation was performed using a BirA biotinylation kit (Avidity) according to the manufacturer’s instructions, and excess biotin was removed by further gel filtration chromatography. Fluorescently-labelled tetramers were produced by coupling biotinylated CD1d molecules to PE-conjugated streptavidin (Molecular Probes) at a 4:1 molar ratio.
Soluble iNKT TCR homodimers were produced by in vitro refolding as described previously [11]. For surface plasmon resonance studies, a panel of 7 TCRs were produced (for details, see [11]). For TCR tetramer staining, a single iNKT TCR [41] was produced, specifically biotinylated at an engineered C-terminus Avi-tag, and coupled to PE-conjugated streptavidin.
In vitro expansion of iNKT cells
Freshly prepared human PBMC were isolated and incubated in RPMI supplemented with 10% FBS with αGC, OCH, iGb3 (all at 100 ng/ml) or lipid vehicle. After day 5, IL-2 (50 U/ml) was added to the culture. After 12–16 days, the percentage of iNKT cells among live T cells was determined by flow cytometry using a FACScalibur flow cytometer (BD Bioscience; see Supporting Information Figure 2 for gating strategy).
CD1d tetramer staining of iNKT cells
CD1d-tetramer stains of iNKT cells were performed by incubating for 45 min at 4°C. iNKT expanded PBMC cultures were additionally stained with FITC-conjugated anti-CD3, and dead cells were excluded using propidium iodide. iNKT clones were stained with tetramers and propidium iodide alone. Bound fluorescence was determined on a FACSCalibur flow cytometer (BD Bioscience).
Functional activation of iNKT cells
Polyclonal mouse and human iNKT lines were stimulated with plate bound lipid-loaded CD1d molecules. Biotinylated CD1d molecules (from NIH Tetramer Facility) were loaded overnight at 37°C with lipids in lipid vehicle (iGb3: 100× molar excess; αGC: 10× molar excess), and bound to a streptavidin-coated plate at the indicated concentration for iNKT stimulation. Supernatants were taken from stimulation cultures after 16 h, and cytokine concentrations determined by ELISA. High-affinity human iNKT clones were stimulated with CD1d-expressing T2 cells after pulsing with defined lipid species (10 µg/ml in RPMI; 3h at 37°C). Supernatants were collected after 16 h and IFN-γ concentrations determined by ELISA.
Surface plasmon resonance
Biotinylated hCD1d-αGC, hCD1d-β-GalCer and hCD1d-iGb3 complexes were conjugated to a streptavidin-loaded Biacore CM-5 chip (~1000 RU CD1d or HLA bound). 1 µM soluble TCR was then flowed over the cells at 50µl/min and binding assessed at 25°C using a Biacore 3000 machine (Biacore, Sweden). Specific binding was calculated by subtraction of TCR binding to a control protein (HLA-A2*01-NY-Eso-1(157–165)). In addition, serial dilutions of 4C1369 and 4C12 TCRs were flowed over the cell at 50µl/min for determination of concentration dependent binding. Equilibrium dissociation constants (KD) were calculated from the concentration response assuming a 1:1 interaction and applying a non-linear least-squares fitting of the Langmuir binding equation.
TCR tetramer staining of T2 lymphoblasts
CD1d-expressing and CD1d-negative T2 cells were pulsed with lipids (10 µg/ml) or lipid vehicle for 3 h at 37°C, followed by staining with fluorescently-conjugated iNKT TCR tetramer for 20 min at room temperature. Dead cells were excluded with propidium iodide, and bound PE staining was determined on a FACSCalibur flow cytometer. Additionally, lipid-pulsed T2 lines were stained with FITC-conjugated IB4-lectin in the presence of 1 mM CaCl2 and FITC fluorescence determined as above.
Supplementary Material
Acknowledgements
We are grateful to Prof Susann Teneberg (Gothenburg University) for kindly providing purified iGb3 and University of Pennsylvania for providing the pELNS vector used in this study. We would also like to thank Dr Anna Tocheva (University of Southampton) for expert technical assistance. SDG, JPS and SM are supported by the Higher Education Funding Council for England (HEFCE). DIG is supported by a National Health and Medical Research Council of Australia (NHMRC) Senior Principal Research Fellowship and JR is supported by an NHMRC Australia Fellowship.
Abbreviations
- β-GalCer
β-galactosylceramide
- hCD1d
Human CD1d
- iGb3
Isoglobotrihexosylceramide
- iNKT
Invariant Natural Killer T-cell
- mCD1d
Mouse CD1d
Footnotes
Conflicts of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. Structural requirements for galactosylceramide recognition by CD1-restricted NKT cells. J Immunol. 1998;161:5124–5128. [PubMed] [Google Scholar]
- 3.Wun KS, Borg NA, Kjer-Nielsen L, Beddoe T, Koh R, Richardson SK, Thakur M, Howell AR, Scott-Browne JP, Gapin L, Godfrey DI, McCluskey J, Rossjohn J. A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J Exp Med. 2008;205:939–949. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Shiratsuchi T, Chen G, Dellabona P, Casorati G, Franck RW, Tsuji M. Invariant TCR rather than CD1d shapes the preferential activities of C-glycoside analogues against human versus murine invariant NKT cells. J Immunol. 2009;183:4415–4421. doi: 10.4049/jimmunol.0901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gapin L. iNKT cell autoreactivity: what is 'self' and how is it recognized? Nat Rev Immunol. 2010;10:272–277. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 8.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matulis G, Sanderson JP, Lissin NM, Asparuhova MB, Bommineni GR, Schumperli D, Schmidt RR, Villiger PM, Jakobsen BK, Gadola SD. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3beta loop. PLoS Biol. 2010;8:el000402. doi: 10.1371/journal.pbio.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, Godfrey DI, Marrack P, Rossjohn J, Gapin L. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Xia C, Wang J, Thapa P, Li Y, Talukdar A, Nadas J, Zhang W, Zhou D, Wang PG. Synthesis and structure-activity relationship study of isoglobotrihexosylceramide analogues. J Org Chem. 2007;72:9914–9923. doi: 10.1021/jo701539k. [DOI] [PubMed] [Google Scholar]
- 15.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–1207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 17.Xia C, Schumann J, Emmanuel R, Zhang Y, Chen W, Zhang W, De Libero G, Wang PG. Modification of the ceramide moiety of isoglobotrihexosylceramide on its agonist activity in stimulation of invariant natural killer T cells. J Med Chem. 2007;50:3489–3496. doi: 10.1021/jm0701066. [DOI] [PubMed] [Google Scholar]
- 18.Yin N, Long X, Goff RD, Zhou D, Cantu C, 3rd, Mattner J, Mezard PS, Teyton L, Bendelac A, Savage PB. Alpha anomers of iGb3 and Gb3 stimulate cytokine production by natural killer T cells. ACS Chem Biol. 2009;4:199–208. doi: 10.1021/cb800277n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008;6:e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci USA. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balreira A, Cavallari M, Sa Miranda MC, Arosa FA. Uncoupling between CD1d upregulation induced by retinoic acid and conduritol-B-epoxide and iNKT cell responsiveness. Immunobiology. 2010;215:505–513. doi: 10.1016/j.imbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, Reddington F, Illarianov PA, Besra GS, Brenner MB, Gumperz JE. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol. 2006;176:3625–3634. doi: 10.4049/jimmunol.176.6.3625. [DOI] [PubMed] [Google Scholar]
- 24.Pellicci DG, Clarke AJ, Patel O, Mallevaey T, Beddoe T, Le Nours J, Uldrich AP, McCluskey J, Besra GS, Porcelli SA, Gapin L, Godfrey DI, Rossjohn J. Recognition of beta-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12:827–833. doi: 10.1038/ni.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu ED, Girardi E, Wang J, Zajonc DM. Cutting Edge: Structural Basis for the Recognition of {beta}-Linked Glycolipid Antigens by Invariant NKT Cells. J Immunol. 2011;187:2079–2083. doi: 10.4049/jimmunol.1101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia C, Yao Q, Schumann J, Rossy E, Chen W, Zhu L, Zhang W, De Libero G, Wang PG. Synthesis and biological evaluation of alpha-galactosylceramide (KRN7000) and isoglobotrihexosylceramide (iGb3) Bioorg Med Chem Lett. 2006;16:2195–2199. doi: 10.1016/j.bmcl.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU. Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. J Biol Chem. 2000;275:25308–25314. doi: 10.1074/jbc.M002629200. [DOI] [PubMed] [Google Scholar]
- 28.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKTT-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 29.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKTT cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrago CG, Russo CA. Timing the origin of New World monkeys. Mol Biol Evol. 2003;20:1620–1625. doi: 10.1093/molbev/msg172. [DOI] [PubMed] [Google Scholar]
- 31.Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, Goodman M. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci U S A. 2007;104:559–564. doi: 10.1073/pnas.0610012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1—3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macher BA, Galili U. The Galalpha1,3Galbetal,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Li Y, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U S A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei B, Speak AO, Shepherd D, Butters T, Cerundolo V, Platt FM, Kronenberg M. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J Immunol. 2011;186:1348–1360. doi: 10.4049/jimmunol.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wun KS, Ross F, Patel O, Besra GS, Porcelli SA, Richardson SK, Keshipeddy S, Howell AR, Godfrey DI, Rossjohn J. Human and mouse type I Natural Killer T-cell antigen receptors exhibit different fine specificities for CD1d-antigen. J Biol Chem. 2012 doi: 10.1074/jbc.M112.412320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiba A, Cohen N, Brigl M, Brennan PJ, Besra GS, Brenner MB. Rapid and reliable generation of invariant natural killer T-cell lines in vitro. Immunology. 2009;128:324–333. doi: 10.1111/j.1365-2567.2009.03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, Chen JL, Koezuka Y, Roberts IA, Price DA, Dusheiko G, Milstein C, Fersht A, Luzzatto L, Cerundolo V. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98:3294–3298. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gadola SD, Koch M, Maries-Wright J, Lissin NM, Shepherd D, Matulis G, Harlos K, Villiger PM, Stuart DI, Jakobsen BK, Cerundolo V, Jones EY. Structure and binding kinetics of three different human CD1d-alpha-galactosylceramide-specific T cell receptors. J Exp Med. 2006;203:699–710. doi: 10.1084/jem.20052369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.