Abstract
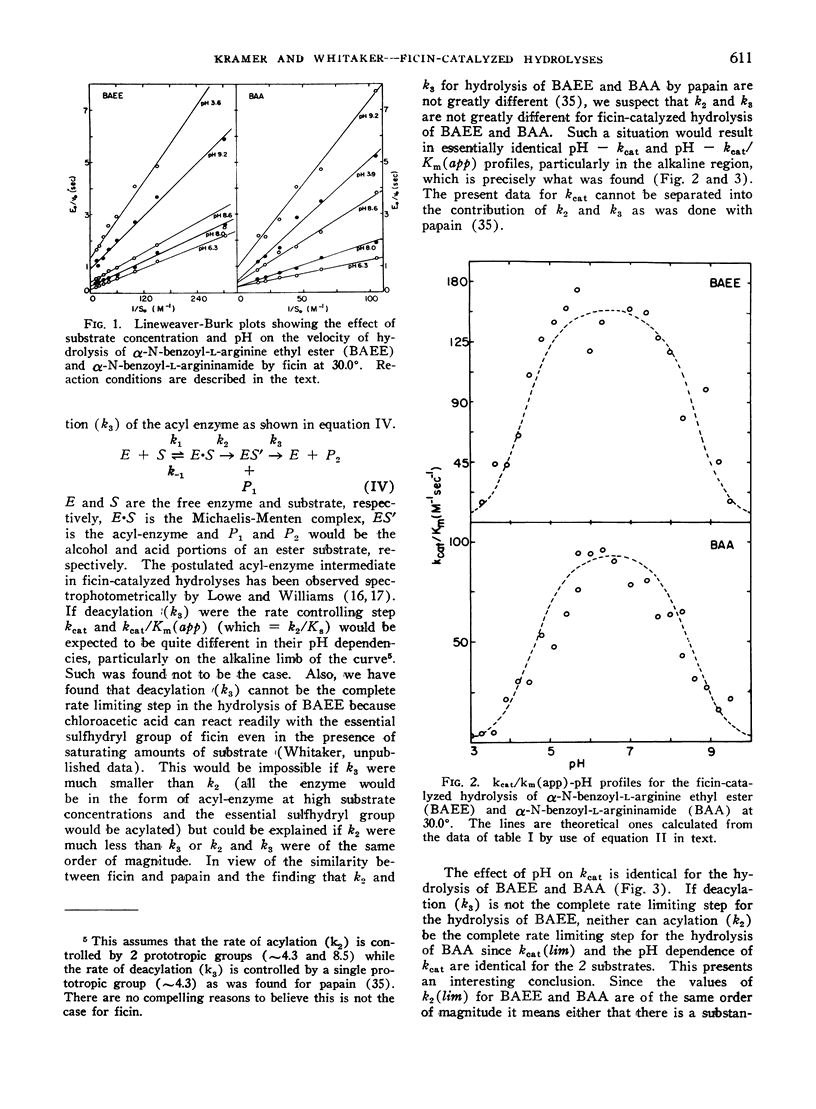
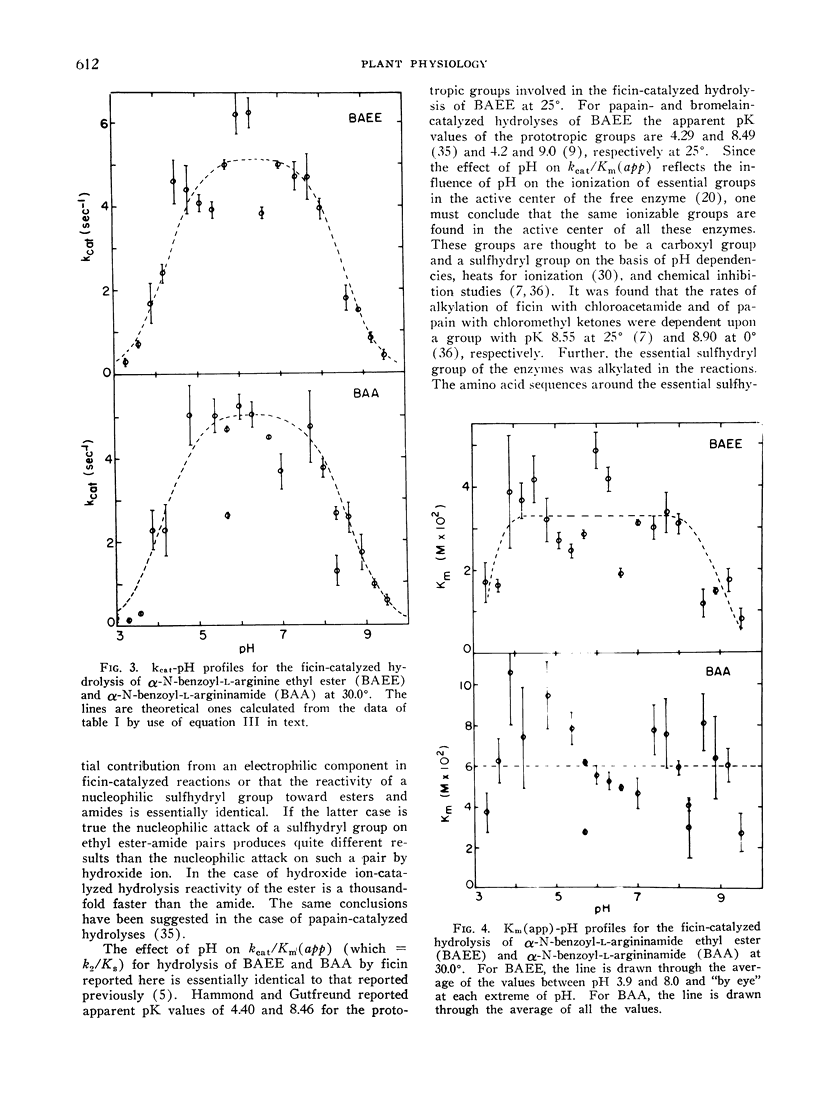
The effect of pH on the hydrolysis of α-N-benzoyl-l-arginine ethyl ester (BAEE) and α-N-benzoyl-l-argininamide (BAA) by a proteolytic enzyme component purified from Ficus carica var. Kadota latex has been studied in detail over the pH range of 3 to 9.5. kcat (lim) values for the hydrolysis of BAEE and BAA were essentially identical (5.20 and 5.01 sec−1, respectively at 30°). kcat values for hydrolysis of BAEE and BAA were dependent on prototropic groups with apparent pK values of 4.24 and 8.53 and 4.10 and 8.59, respectively. kcat (lim) values for tht hydrolysis of BAEE and BAA were essentially identical (5.20 and groups of pK 4.33 and 8.60 and 4.55 and 8.51, respectively. Thus the pH optimum is 6.5 for both substrates. Km (app) values for BAEE and BAA were 3.32 × 10−2m and 6.03 × 10−2m respectively over the pH range of 3.9 to 8.0. These data are interpreted in terms of the involvement of a carboxyl and a sulfhydryl group in the active center of the enzyme. The data do not support the concept that deacylation of the acyl-enzyme is completely the rate controlling step in the hydrolyses. Rather, it appears that the magnitude of k2 and k3 are not greatly different.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD S. A., GUTFREUND H. Ficincatalysed reactions: the affinity of ficin for some arginine derivatives. Biochem J. 1956 May;63(1):61–64. doi: 10.1042/bj0630061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubacher L. J., Bender M. L. The preparation and properties of trans-cinnamoyl-papain. J Am Chem Soc. 1966 Dec 20;88(24):5871–5880. doi: 10.1021/ja00976a032. [DOI] [PubMed] [Google Scholar]
- Brubacher L. J., Bender M. L. The preparation and properties of trans-cinnamoyl-papain. J Am Chem Soc. 1966 Dec 20;88(24):5871–5880. doi: 10.1021/ja00976a032. [DOI] [PubMed] [Google Scholar]
- FINKLE B. J., SMITH E. L. Crystalline papain; number and reactivity of thiol groups; chromatographic behavior. J Biol Chem. 1958 Feb;230(2):669–690. [PubMed] [Google Scholar]
- HAMMOND B. R., GUTFREUND H. The mechanism of ficin-catalysed reactions. Biochem J. 1959 Jun;72(2):349–357. doi: 10.1042/bj0720349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAWAY M. R., MATHIAS A. P., RABIN B. R. THE CHEMICAL REACTIVITY OF THE THIOL GROUP IN THE ACTIVE CENTRE OF FICIN. Biochim Biophys Acta. 1964 Oct 23;92:111–124. doi: 10.1016/0926-6569(64)90275-5. [DOI] [PubMed] [Google Scholar]
- Henry A. C., Kirsch J. F. Papain-catalyzed reactions of esters with alcohols. The nature of the rate-determining step. Biochemistry. 1967 Nov;6(11):3536–3544. doi: 10.1021/bi00863a027. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- KIRSCH J. F., KATCHALSKI E. REACTION OF PAPAIN WITH ETHYL (CARBONYL-18O)HIPPURATE. Biochemistry. 1965 May;4:884–890. doi: 10.1021/bi00881a014. [DOI] [PubMed] [Google Scholar]
- KRAMER D. E., WHITAKER J. R. FICUS ENZYMES. II. PROPERTIES OF THE PROTEOLYTIC ENZYMES FROM THE LATEX OF FICUS CARICA VARIETY KADOTA. J Biol Chem. 1964 Jul;239:2178–2183. [PubMed] [Google Scholar]
- Kirsch J. F., Igelström M. The kinetics of the papain-catalyzed hydrolysis of esters of carbobenzoxyglycine. Evidence for an acyl-enzyme intermediate. Biochemistry. 1966 Feb;5(2):783–791. doi: 10.1021/bi00866a053. [DOI] [PubMed] [Google Scholar]
- LIGHT A., FRATER R., KIMMEL J. R., SMITH E. L. CURRENT STATUS OF THE STRUCTURE OF PAPAIN: THE LINEAR SEQUENCE, ACTIVE SULFHYDRYL GROUP, AND THE DISULFIDE BRIDGES. Proc Natl Acad Sci U S A. 1964 Nov;52:1276–1283. doi: 10.1073/pnas.52.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. A STUDY OF SOME THIOL ESTER HYDROLYSES AS MODELS FOR THE DEACYLATION STEP OF PAPAIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:194–198. doi: 10.1042/bj0960194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. DIRECT EVIDENCE FOR AN ACYLATED THIOL AS AN INTERMEDIATE IN PAPAIN- AND FICIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:189–193. doi: 10.1042/bj0960189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- SGARBIERI V. C., GUPTE S. M., KRAMER D. E., WHITAKER J. R. FICUS ENZYMES. I. SEPARATION OF THE PROTEOLYTIC ENZYMES OF FICUS CARICA AND FICUS GLABRATA LATICES. J Biol Chem. 1964 Jul;239:2170–2177. [PubMed] [Google Scholar]
- SLUYTERMAN L. A. KINETICS OF THE HYDROLYSIS OF BENZOYLGLYCINE ETHYL ESTER CATALYZED BY PAPAIN. Biochim Biophys Acta. 1964 May 4;85:305–315. doi: 10.1016/0926-6569(64)90251-2. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., CHAVRE V. J., PARKER M. J. Kinetics of papain action. II. Effect of pH on hydrolysis of three substrates. J Biol Chem. 1958 Jan;230(1):283–293. [PubMed] [Google Scholar]
- SMITH E. L., KIMMEL J. R., BROWN D. M. Crystalline papain. II. Physical studies; the mercury complex. J Biol Chem. 1954 Apr;207(2):533–549. [PubMed] [Google Scholar]
- SMITH E. L., PARKER M. J. Kinetics of papain action. III. Hydrolysis of benzoyl-L-arginine ethyl ester. J Biol Chem. 1958 Dec;233(6):1387–1391. [PubMed] [Google Scholar]
- SMITH E. L., STOCKELL A., KIMMEL J. R. Crystalline papain. III. Amino acid composition. J Biol Chem. 1954 Apr;207(2):551–561. [PubMed] [Google Scholar]
- STOCKELL A., SMITH E. L. Kinetics of papain action. I. Hydrolysis of benzoly-L-argininamide. J Biol Chem. 1957 Jul;227(1):1–26. [PubMed] [Google Scholar]
- Sluyterman L. A. Reversible inactivation of papain by cyanate. Biochim Biophys Acta. 1967 Jul 11;139(2):439–449. doi: 10.1016/0005-2744(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A. Substrate binding by non-activated papain. Biochim Biophys Acta. 1966 Mar 7;113(3):577–586. doi: 10.1016/s0926-6593(66)80015-2. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A. The rate-limiting reaction in papain action as derived from the reaction of the enzyme with chloroacetic acid. Biochim Biophys Acta. 1968 Jan 8;151(1):178–187. doi: 10.1016/0005-2744(68)90172-1. [DOI] [PubMed] [Google Scholar]
- WHITAKER J. R., BENDER M. L. KINETICS OF PAPAIN-CATALYZED HYDROLYSIS OF ALPHA-N-BENZOYL-L-ARGININE ETHYL ESTER AND ALPHA-N-BENZOYL-L-ARGININAMIDE. J Am Chem Soc. 1965 Jun 20;87:2728–2737. doi: 10.1021/ja01090a034. [DOI] [PubMed] [Google Scholar]
- WHITAKER J. R. Ninhydrin assay in the presence of thiol compounds. Nature. 1961 Feb 25;189:662–663. doi: 10.1038/189662a0. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Whitaker J. R. Kinetics of papain-catalyzed hydrolyses of neutral substrates. Biochemistry. 1967 Dec;6(12):3711–3717. doi: 10.1021/bi00864a013. [DOI] [PubMed] [Google Scholar]


