Abstract
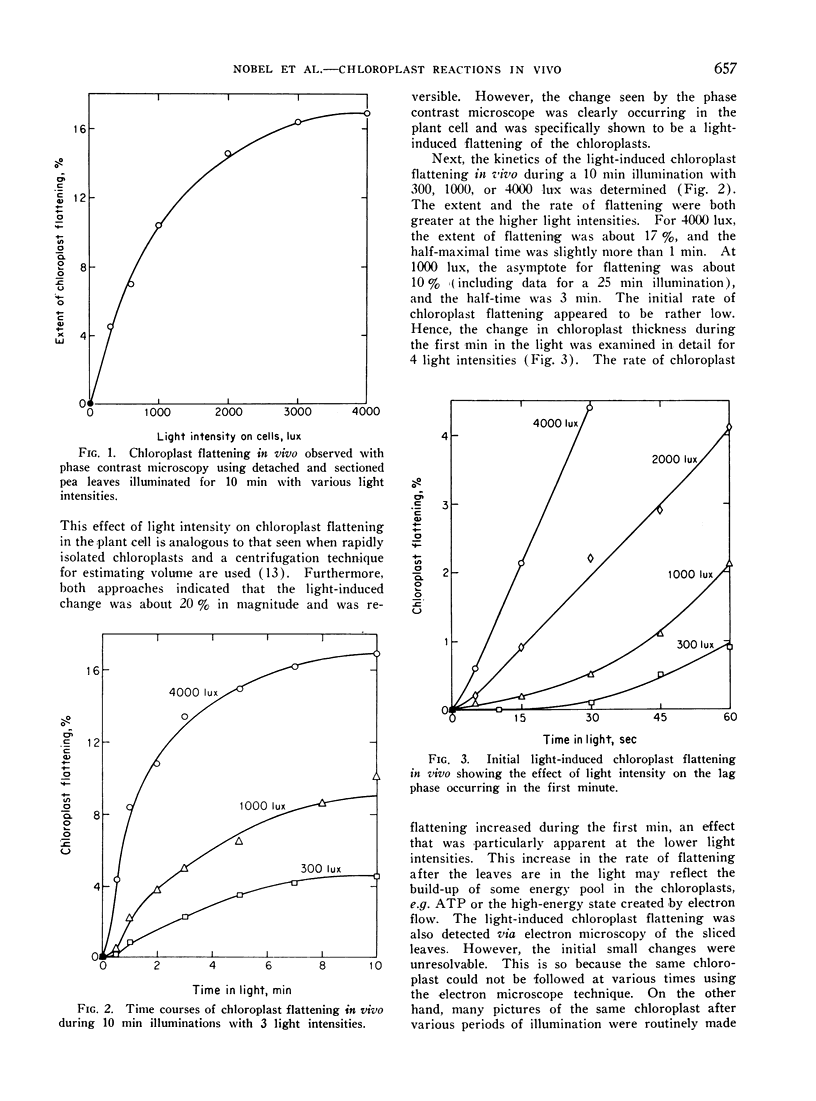
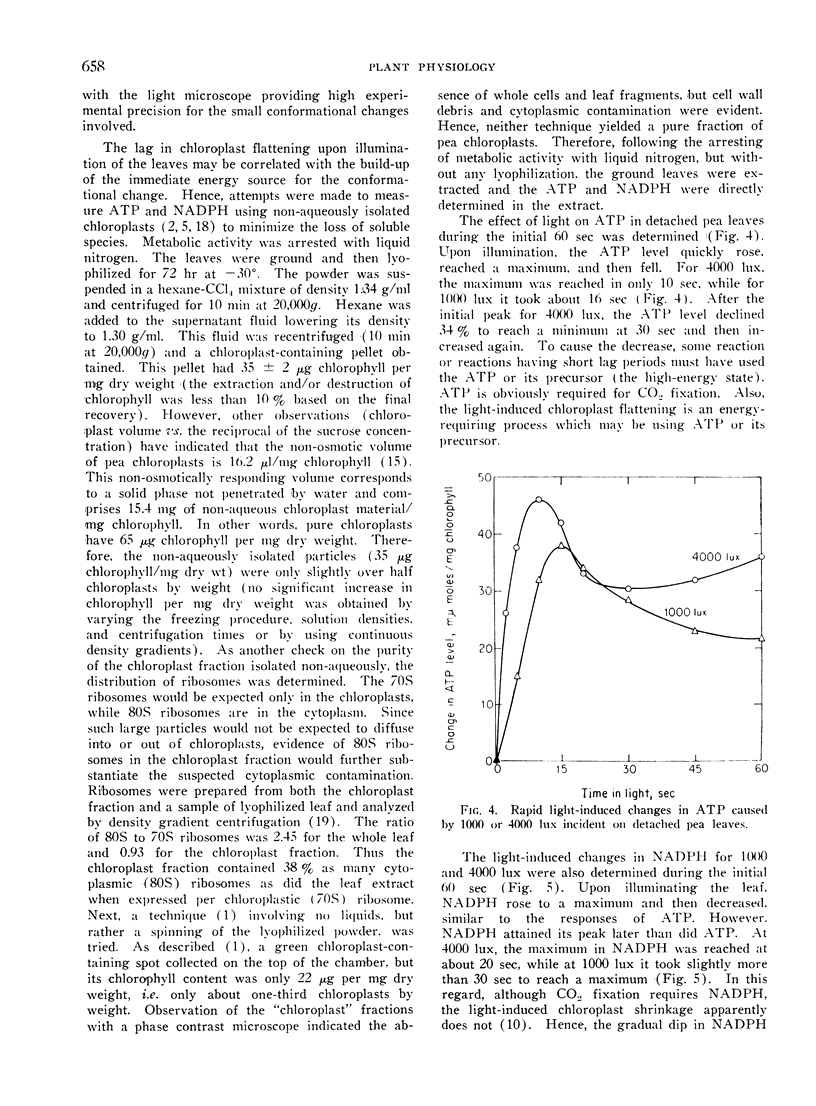
Chloroplasts in living cells of detached and sectioned leaves of Pisum sativum had a thickness of 2.68 ± 0.04 μ in the dark as determined from photographs made using a phase contrast microscope. Upon illumination with 4000 lux for 10 min, the chloroplasts flattened to 2.15 ± 0.04 μ. There was a short lag period of about 11 sec at 1000 lux and 2 sec at 4000 lux before appreciable light-induced flattening occurred. Both ATP and reduced nicotinamide adenine dinucleotide phosphate (NADPH) in detached pea leaves increased upon illumination and then fell during the initial 60 sec. The maximum ATP level was attained in 16 sec at 1000 lux and 10 sec at 4000 lux, while NADPH required about twice as long to reach a maximum. A sustained rate of carbon dioxide fixation occurred after a lag period coinciding in time with the drop in the NADPH level. ATP appeared to be involved not only with carbon dioxide fixation, but also with some reaction beginning sooner, perhaps the light-induced chloroplast flattening. Considering the initial photophosphorylation and the sustained CO2 fixation rates, the ATP formation rate in vivo apparently increased after the leaves had been in the light for a few min.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens M., Neu W., Thalacker R., Thimm H. J. Gewinnung von Chloroplasten ohne Verwendung von Flüssigkeiten. Experientia. 1964 Nov 15;20(11):607–608. doi: 10.1007/BF02144811. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- KUSHIDA H., ITOH M., IZAWA S., SHIBATA K. DEFORMATIONS OF CHLOROPLASTS ON ILLUMINATION IN INTACT SPINACH LEAVES. Biochim Biophys Acta. 1964 Jan 27;79:201–203. doi: 10.1016/0926-6577(64)90051-8. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. A rapid technique for isolating chloroplasts with high rates of endogenous photophosphorylation. Plant Physiol. 1967 Oct;42(10):1389–1394. doi: 10.1104/pp.42.10.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Chloroplast shrinkage and increased photophosphorylation in vitro upon illuminating intact plants of Pisum sativum. Biochim Biophys Acta. 1968 Jan 15;153(1):170–182. doi: 10.1016/0005-2728(68)90157-6. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. Light-Induced Chloroplast Shrinkage in vivo Detectable After Rapid Isolation of Chloroplasts From Pisum sativum. Plant Physiol. 1968 May;43(5):781–787. doi: 10.1104/pp.43.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Light-induced changes in the ionic content of chloroplasts in Pisum sativum. Biochim Biophys Acta. 1969 Jan 14;172(1):134–143. doi: 10.1016/0005-2728(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Stocking C. R. Chloroplast Isolation in Nonaqueous Media. Plant Physiol. 1959 Jan;34(1):56–61. doi: 10.1104/pp.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Function of chloroplast DNA. I. Hybridization studies involving nuclear and chloroplast DNA with RNA from cytoplasmic (80S) and chloroplast (70S) ribosomes. Proc Natl Acad Sci U S A. 1968 Feb;59(2):569–576. doi: 10.1073/pnas.59.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Driessche T. Circadian rhythms in Acetabularia: photosynthetic capacity and chloroplast shape. Exp Cell Res. 1966 Apr;42(1):18–30. doi: 10.1016/0014-4827(66)90315-6. [DOI] [PubMed] [Google Scholar]


