Abstract
Synthetic bone graft substitutes based on composites consisting of a polymer and a calcium-phosphate (CaP) ceramic are developed with the aim to satisfy both mechanical and bioactivity requirements for successful bone regeneration. In the present study, we have employed extrusion to produce a composite consisting of 50 wt.% poly(D,L-lactic acid) (PLA) and 50 wt.% nano-sized hydroxyapatite (HA) powder, achieving homogeneous distribution of the ceramic within the polymeric phase. In vitro, in both a simulated physiological saline (SPS) and a simulated body fluid (SBF), a greater weight loss was observed for PLA/HA than for PLA particles upon 12-week immersion. Furthermore, in SPS, a continuous release of calcium and phosphate from the composite was measured, whereas in SBF, decrease of the amount of the two ions in the solution was observed both for PLA and PLA/HA accompanied with the formation of a CaP layer on the surface. In vitro characterization of the composite bioactivity was performed by culturing human mesenchymal stromal cells (hMSCs) and assessing proliferation and osteogenic differentiation, with PLA as a control. Both PLA/HA composite and PLA control were shown to support hMSCs proliferation over a period of two weeks. In addition, the composite significantly enhanced alkaline phosphatase (ALP) activity of hMSCs in osteogenic medium as compared with the polymer control. A novel implant design was employed to develop implants from dense, extruded materials, suitable for testing osteoinductivity in vivo. In a preliminary study in dogs, PLA/HA composite implants induced heterotopic bone formation upon 12-week intramuscular implantation in all animals, in contrast to PLA control, which was not osteoinductive. Unlike in vitro, a more pronounced degradation of PLA was observed in vivo as compared with PLA/HA composite.
Keywords: composite, hydroxyapatite, polylactic acid, osteoinduction, bone regeneration
Introduction
Synthetic biomaterials have been developed and improved over more than five decades as an alternative to natural bone grafts. Among other benefits, synthetic bone graft substitutes could overcome the limited availability of autologous bone grafts, an issue that is becoming increasingly important with continuous population aging. To be accepted as a comprehensive alternative to natural bone, synthetic bone graft substitutes need to meet a number of requirements, including provision of mechanical support, and bioactivity in terms of osteoconductivity and osteoinductivity. While the existing synthetic bone graft substitutes are generally accepted as being osteoconductive, i.e., able to promote “the recruitment and migration of osteogenic cells into the wound site,”1 recent research efforts have strongly focused on osteoinductivity of materials, defined by Friedenstein as “the induction of undifferentiated inducible osteoprogenitor cells that are not yet committed to the osteogenic lineage to form osteoprogenitor cells,”2 because osteoinductivity is considered to be essential for successful healing of large, critically sized bone defects. The exact mechanism of osteoinduction by synthetic biomaterials has not yet been fully deciphered; however, a growing number of materials has shown osteoinductive potential, demonstrated by the induction of bone formation upon implantation in heterotopic sites such as muscle or subcutis in various animal models. While osteoinduction has occasionally been shown for polymers3 and porous metals,4 biomaterials with osteoinductive potential are mainly calcium-phosphate (CaP) ceramics. With chemical compositions close to that of bone mineral, CaP ceramics have logically raised considerable interest in bone regeneration. Previous studies have recorded heterotopic bone formation in, among others, hydroxyapatite (HA),5 β-tricalcium phosphate (β-TCP),6 biphasic calcium phosphate (BCP) (a mixture of HA and β-TCP),7 brushite8 and octacalcium phosphate9 in various animal models.
While CaP ceramics have shown the strongest osteoinductive potential among synthetic materials, intrinsic brittleness limits their use as bone graft substitutes to non-load bearing sites. This could possibly be overcome by the development of composite materials. Combinations of polymers and ceramics offer numerous opportunities for improving the mechanical properties of ceramics while retaining their bioactivity.10 For example, composites based on polylactic acid (PLA), a biocompatible and biodegradable aliphatic polyester and HA have been investigated as potential bone graft substitutes in a number of studies. Addition of HA microparticles to PLA was shown to increase the compressive and bending strengths in a dose-dependent manner.11 Similarly, diametral tensile strength of PLA improved upon incorporation of HA.12 In the study by Nejati et al. it was furthermore shown that the size of ceramic particles can be used to tailor mechanical properties of the composites: HA nanoparticles increased the compressive strength of the composite in comparison to HA microparticles, making it comparable to cancellous bone.13 In vitro, support of attachment and growth of MSCs and (pre)osteoblasts has been described in a number of studies.13-16 In vivo, porous composites made of PLA and HA microparticles were shown osteoconductive when implanted in a 6 mm defect in a rabbit femoral intercondylar notch model.17 Similarly, porous PLA/HA composite materials produced using gas foaming showed successful healing of a critical-sized cranial defect in rats.18 Osteoinductivity of a PLA/HA porous composite produced using a solvent-based method was demonstrated upon intramuscular implantation in dogs.19,20
Besides chemistry, structural properties of biomaterials have also been suggested to affect level of osteoinductivity.21 Microporosity, for instance, contributes to this phenomenon: an increase in microporosity and specific surface area of a ceramic has been shown to lead to a higher osteoinductive potential.22,23 Geometrical features of the materials at the macroscopic level have also been shown to strongly affect material-induced bone formation. Indeed, heterotopic bone formation has been shown to preferentially form inside pores, channels and concavities and rarely on the implant periphery,8,24 suggesting that “protected” environment inside the material is required for osteoinduction to occur and/or be maintained.
In the current study, a PLA/HA composite was developed using extrusion, a manufacturing technique that does not require the use of solvents. In vitro, human mesenchymal stromal cells (hMSCs) were cultured on the composite material and proliferation and osteogenic differentiation were assessed, with PLA as a control. Furthermore, the osteoinductive potential of the composite was investigated in a preliminary in vivo study in a canine intramuscular model. Since extruded materials are dense, not containing “natural” protected areas like pores or channels, we designed the implants such that an “artificial pore” is created between plates of extruded materials in order to test the ability of these extruded materials to induce heterotopic bone formation (Fig. 1).
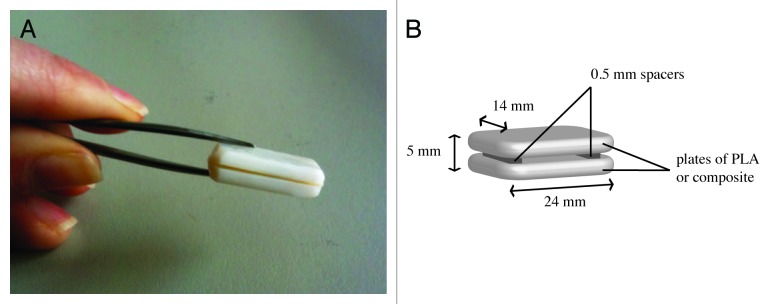
Figure 1. Implant design. Digital photograph (A) and schematic representation with dimensions (B) of implants used for intramuscular implantation in a canine model to assess osteoinductivity.
Results
Materials characterization
The HA powder was phase-pure as determined by XRD analysis (Fig. 2A) and had a particle size of 69.9 ± 12.8 nm in width and 308.7 ± 61.2 nm in length (Fig. 2B), as determined by SEM imaging. FTIR spectra of PLA and PLA/HA composite powders are shown in Figure 2C. In contrast to PLA control, PLA/HA composite spectrum contained phosphate (604 and 562 cm−1) and hydroxyl (632 cm−1) bands, characteristic of HA, confirming incorporation of the ceramic in the polymer phase.25 A modification of the main PLA peak (symmetric and asymmetric deformational vibrations of C–H in CH3 groups), was observed in the composite spectrum around 1000 cm−1 due to the HA phosphate band situated at the same wavelength. The pronounced intensities of HA-specific bands in the composite spectrum are in line with the quantity of HA incorporated.

Figure 2. Characterization of PLA, PLA/HA and HA. XRD spectrum of nano-HA powder (A), SEM micrograph of nano-HA powder (B), and FTIR spectra of nano-HA powder, PLA and PLA/HA composite (C).
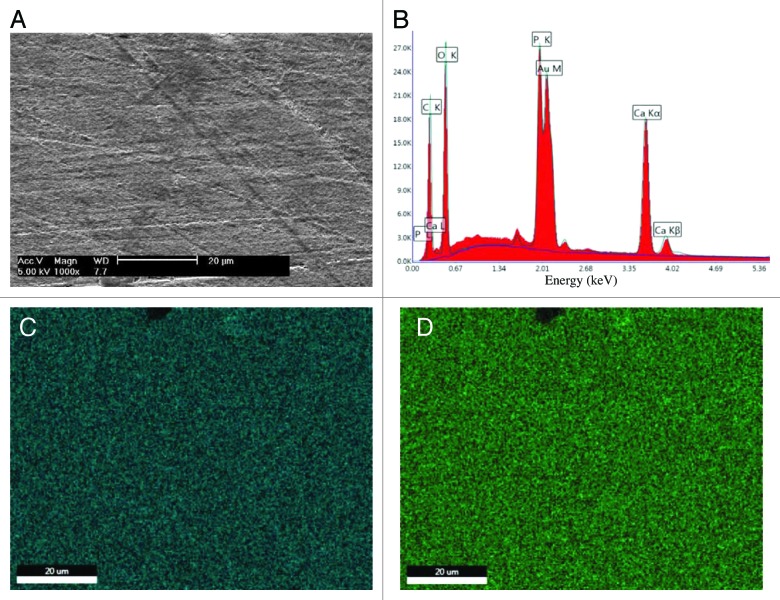
EDAX analysis of a gold-sputtered PLA/HA pellet, after polishing and sterilization, confirmed presence of HA by the pronounced calcium and phosphorus peaks in the EDAX spectrum (Fig. 3A and B), while no calcium or phosphorus was observed in the spectrum of the PLA control (data not shown). Phosphorous and calcium mapping of PLA/HA composite showed homogeneous distribution of both elements (Fig. 3C and D).
Figure 3. Composite homogeneity. SEM micrograph (A), EDAX spectrum (B) and elemental maps of calcium (C) and phosphorous (D) atoms of PLA/HA pellet after polishing and sterilization. (scale bars: 20 μm).
Calcium and phosphate release and degradation study
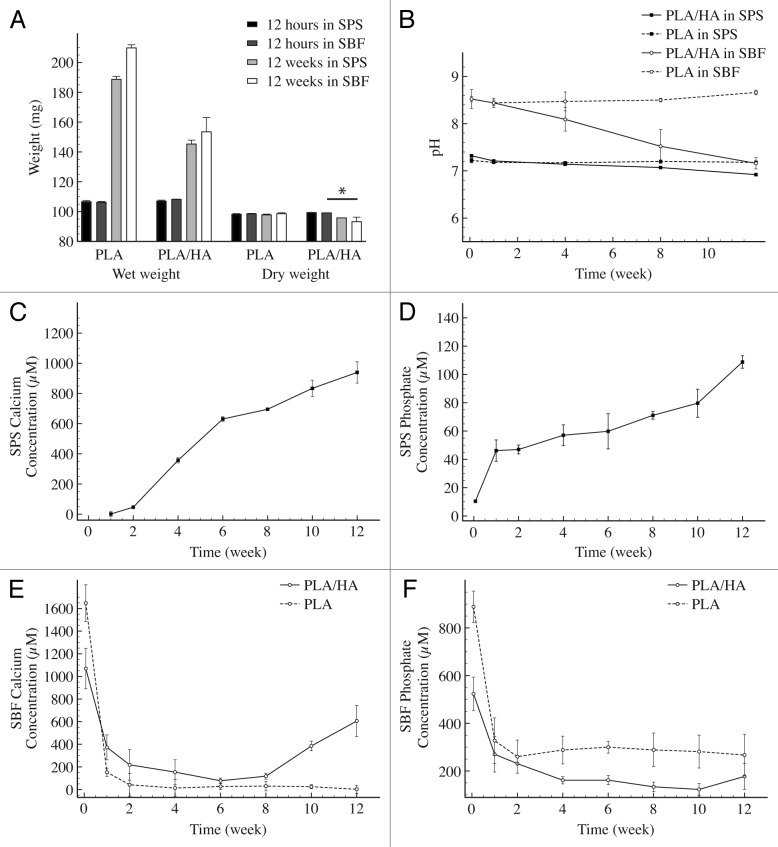
Degradation and ion release tests were performed on material particles with a size of 0.5–1 mm. The behavior in an aqueous environment of PLA/HA composite and PLA control materials was tested over three months in SPS, a buffered solution with an ionic strength similar to that of blood plasma, and in SBF, which mimics the mineral composition of blood plasma and is saturated toward dicalcium phosphate dihydrate (Fig. 4). Measurements of the wet weight of the immersed particles showed that the PLA water uptake approximately doubled the initial dry weight of the particles after 12 wk of immersion in both solutions. The swelling of PLA/HA particles appeared to be less pronounced with a wet weight increase of approximately 1.5 times the initial weight (Fig. 4A). After drying, particles were weighed again to assess the mass loss. While no measurable decrease could be detected in the case of PLA particles in either solution, the dry weight of PLA/HA particles decreased with about 4% in SPS and with about 7% in SBF over the three months of the experiment. The pH of SPS and SBF solutions was stable over time in the case of PLA particles, whereas for PLA/HA particles a decrease from 7.32 to 6.92 was observed in SPS and from 8.53 to 7.16 in SBF between 12 h and 12 wk of immersion (Fig. 4B).
Figure 4. In vitro degradation and calcium phosphate release. Wet and dry weights of the PLA and PLA/HA particles upon immersion in SPS and SBF over a period of 12 wk. (A) pH of the solutions. (B) Calcium and phosphate release from PLA/HA particles in SPS (C and Drespectively) and of PLA and PLA/HA in SBF (E and F respectively). Statistical analysis was performed using one way ANOVA with Tukey’s multiple comparison post-hoc test (P < 0.05 and n = 3).
Release of calcium and phosphate from the PLA/HA composite was measured in SPS and SBF solutions over the time of the degradation study (Fig. 4C–F). In SPS, calcium signal was first detected after two weeks of immersion, whereas phosphate was detected as early as one week after immersion in SPS. A continuous release of both calcium and phosphate from the composite was observed during the time frame tested, without reaching a plateau. No calcium or phosphate was observed in SPS upon immersion of PLA particles over the 12-wk period. In the case of samples immersed in SBF, precipitation occurred shortly after mixing of the salts, leading to a milky solution after 12 h. Simultaneously, the concentrations of calcium and phosphate dropped over the first week of immersion for both PLA and PLA/HA, before reaching a plateau. For PLA/HA samples, an increase in concentration of calcium and phosphate was observed at 8 and 10 wk, respectively.
The SEM micrographs of cross-sections of PLA and PLA/HA particles, immersed for 12 wk in SPS, showed a dense surface of both PLA and PLA/HA. Underneath the surface, the material was porous in the case of PLA particles, whereas in the PLA/HA particles a dense “core” could still be observed (Fig. S1A and B). For samples immersed in SBF, the SEM micrographs of both PLA and PLA/HA particles surfaces, showed the formation of globular precipitates, which were identified as CaP by EDAX analysis (data not shown). The deposition of this layer was observed as early as 12 h upon immersion, and at all later time points. After 12 wk in SBF, PLA particle surface exhibited holes suggesting degradation, and deposits of CaP. Similar CaP deposits were observed on PLA/HA particles with porous bulk material underneath the surface (Fig. S1C and D).
HMSCs proliferation and osteogenic differentiation
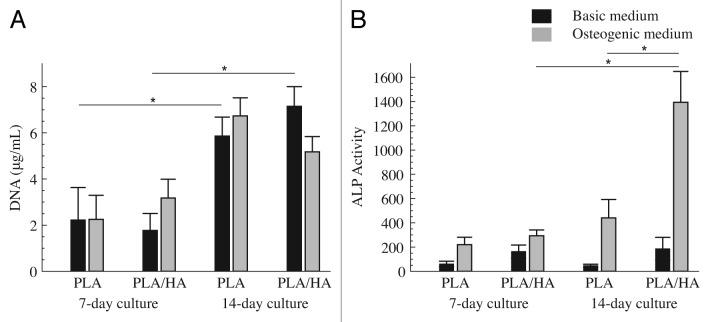
For in vitro cell culture experiments, PLA and PLA/HA pellets with a diameter of 10 mm and a thickness of 2 mm were seeded with hMSCs. DNA was quantified after 7 and 14 d of culture in basic and osteogenic medium as an indirect measure of cell proliferation (Fig. 5A). Both PLA/HA composite and PLA control sustained hMSCs proliferation over the 2-wk culture period. A significant increase in DNA amounts between day 7 and day 14 was observed for cells cultured in basic medium on both PLA and PLA/HA. An increase in time was also observed for cells cultured in osteogenic medium on both material types, however, the differences were not statistically significant. No differences between PLA and PLA/HA were observed at either time point, independent of the medium used. Similarly, no effect of the medium type on cell proliferation was observed on either material type at days 7 and 14.
Figure 5. HMSCs proliferation and osteogenic differentiation. DNA content (A) and ALP activity corrected for DNA content (B) of hMSCs cultured on PLA and PLA/HA pellets in basic and osteogenic medium for 7 and 14 d. Statistical analysis was performed using one way ANOVA with Tukey’s multiple comparison post-hoc test (P < 0.05 and n = 3).
ALP activity of hMSCs was quantified and corrected for DNA content after 7 and 14 d of culture in basic and osteogenic medium (Fig. 5B). In osteogenic medium, ALP level increased between 7 and 14 d on both PLA/HA composite and PLA control, although a statistically significant increase was only observed for PLA/HA. No temporal increase was observed when cells were cultured in basic medium. Regarding the effect of the material type, the trend observed was that, independent of the medium used, ALP level of hMSCs cultured on PLA/HA composite was higher than that of cells cultured on PLA control at both 7 and 14 d. The difference in ALP expression between cells cultured on PLA/HA and PLA was, however, only statistically significant after 14 d in osteogenic medium.
Osteoinductive potential
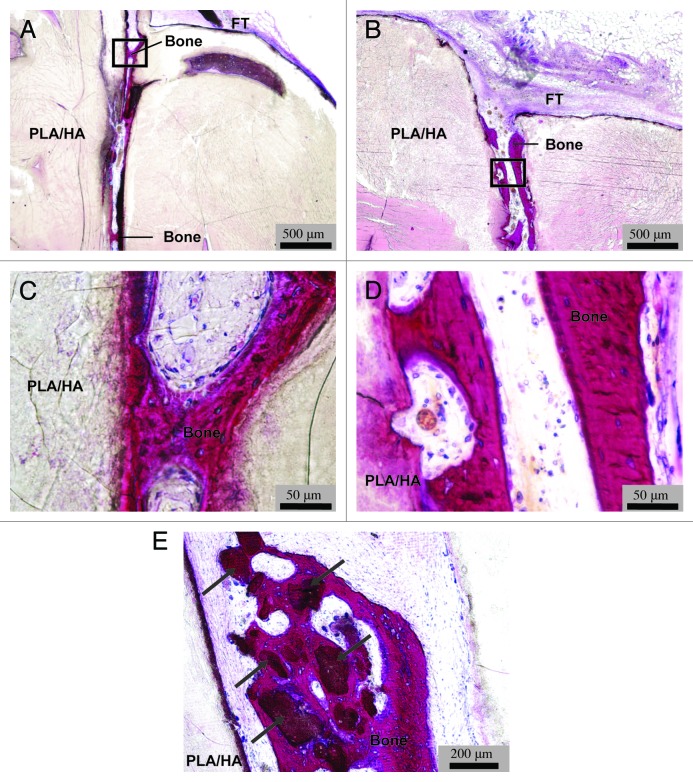
For the in vivo study, implants were used that consisted of two paired identical plates of PLA or PLA/HA composite with a size of 24 × 14 × 5 mm3 with two 0.5 mm thick PEOT/PBT spacers between them (Fig. 1). There were no surgical complications and all animals experienced an uneventful recovery from the surgery. At implant retrieval, no clinical signs of inflammation or infection (i.e., swelling or redness) were observed upon visual inspection of the implantation area. Only two out of five implanted PLA samples were found and explanted. In the areas where the remaining three PLA samples were implanted, no material remnants or signs of tissue other than muscle were observed. In contrast, all 12 implanted PLA/HA samples were retrieved, and while 2 were lost during processing (dissolved in MMA monomer), 10 could be histologically analyzed. Macroscopic and microscopic observation showed that a fibrous capsule with a thickness varying between 50 and 200 µm had formed around all samples of both PLA and PLA/HA (Fig. 6A-–D). The fibrous capsule seemed somewhat thicker around the PLA than around the PLA/HA implants, but as only two PLA samples were retrieved, a good comparison was difficult to make. The PLA samples were largely degraded (Fig. 6A–C) which may be an explanation why the remaining three samples were impossible to trace at explantation. Degradation of composite samples appeared to be less pronounced than that of PLA control (Fig. 6B). Analysis of the samples showed that in all cases, the PEOT/PBT spacers used to create the gap between the two biomaterial plates were completely degraded. As a result, the two plates were in close contact with each other, limiting ingrowth of tissue inside the implant. The unexpected degradation of spacers, and thus loss of the gap were attributed to the improper choice of PEOT/PBT copolymer composition. Microscopic analysis of tissue formed around the implants showed no signs of bone formation or mineralization in PLA controls (Fig. 6C). Fibrous tissue surrounding PLA/HA implants contained no mineral or bone either, however, in all 10 analyzed PLA/HA samples, heterotopic bone formation was observed inside the implants, particularly in the areas where the original entrance of the gap between the two material plates was located (Fig. 7A and B). Based on qualitative observations, the amount of bone formation was relatively limited. Nevertheless, the maximum depth of ingrowth of bone into the implant was observed to be about 4 mm. Newly formed bone was normal in appearance, showing mineralized matrix aligned with a layer of osteoblasts (Fig. 7C and D.). Another qualitative observation suggested that heterotopic bone formation was most pronounced in the highly degraded areas of implant (Fig. 7E).
Figure 6. In vivo degradation of implants and formation of fibrous capsule. Images of methylene blue and basic fuchsin stained histological sections of implants after 12-wk intramuscular implantation in dogs. Digital photographs of a PLA (A) and a PLA/HA (B) implant section (scale bars: 5 mm), showing a more pronounced degradation of the polymer as compared with the composite implant. High magnification micrographs of a PLA (C) and a PLA/HA (D) implant section, showing fibrous capsule (FC) that had formed around both implant types and the surrounding muscle tissue (M) (scale bars: 100 µm).
Figure 7. In vivo bone formation in composite implants. Low magnification micrographs (AandB) and enlargements of black square areas in (A and B), respectively (CandD) of representative methylene blue and basic fuchsin stained sections of PLA/HA composite implants from two dogs. Images (A and B) show the entrance of the protective gap between the two composite plates surrounded with fibrous tissue (FT). Bone formation can be observed inside the gap (Bone). The newly formed bone was normal in appearance, with osteoid containing osteocytes and aligned with a layer of osteoblasts (CandD). Bone formation was also observed surrounding composite particles formed upon degradation (indicated by arrows, scale bars: A and B, 500 µm; C and D, 50 µm; E, 200 µm).
Discussion
Synthesis of ceramic/polymer composite materials by extrusion does not require use of organic solvents, which is an important advantage when aiming for implantable materials, as remnants of solvents are difficult to completely remove from the material.26 In the present study, we have confirmed that up to 50 wt% nano-sized HA particles can homogeneously be incorporated into PLA by using extrusion. Previous studies performed on PLA and HA blends have shown that presence of ceramic in the polymeric matrix has a dose dependent effect on various properties of the polymer, such as mechanical behavior, degradation rate, and bioactivity.11,20,27,28 Verheyen et al. have demonstrated that a 50 wt% content of HA mixed with PLA improved the osteoconductivity as compared with the polymeric control in a transcortical implantation model in goats.27 Hasegawa and colleagues provided first evidence for osteoinduction by porous scaffolds made of PDLLA/HA composite with a weight ratio 70/30, upon intramuscular implantation in dogs.19 In contrast, no heterotopic bone formation was observed in PDLLA scaffolds without HA. In a study by Barbieri et al., in which porous PDLLA/nano apatite composites with a ceramic content of 10 wt%, 20 wt% and 40 wt% produced using a solvent-based method were tested, only the composite with 40 wt% apatite led to heterotopic bone formation upon intramuscular implantation in dogs for 12 wk.20 In a more recent study by Barbieri et al., a similar PDLLA/nano apatite blend with a ceramic content of 50 wt% produced by extrusion was shown to trigger osteoinduction in a sheep intramuscular model.29 Regarding the extrusion process, it could be argued that a temperature of 150 °C may affect the molecular weight of the polymer, as PLA is known to be temperature sensitive.30 Analysis of polymer before and after extrusion using Ubbelohde viscosimeter, however showed a minor effect of the extrusion process on molecular weight of the polymer (data not shown). It has previously been suggested that ceramic fillers limit the thermal degradation of the polymer during extrusion,30 while the amount of ceramic incorporated correlates with frictional degradation.28
Degradation is a critical parameter of biomaterials. In the case of bone graft substitutes, degradation rate of the material should be comparable to the rate of new bone formation, in order for the material to provide sufficient support while leaving space for tissue growth. Degradation must therefore be controlled. PLA is known to undergo autocatalytic hydrolysis, producing lactic acid, and its degradation rate is strongly influenced by the molecular weight: the lower the molecular weight is, the faster PLA degrades.29,31 Indeed, strong degradation of PLA occurred upon implantation of the polymer in paraspinal muscles of dogs, to the extent that a number of implants could not be retrieved from the animals 12 wk after implantation. This level of degradation is not surprising, considering the low molecular weight of the polymer used. Although degradation of PLA/HA implants was also observed, its extent was significantly lower than that of PLA, which could be explained by the buffering effect of the ceramic phase of the composite that hampered the autocatalytic degradation of the polymer. The observed decrease in degradation of PLA/HA composite as compared with the PLA control was in accordance with earlier studies.32,33 Degradation of the polymeric phase of the composite is also crucial for the release of calcium and phosphate ions from the ceramic phase, which is suggested to be the origin of bioactivity of CaPs.34,35 For example, in vitro effects of free calcium ions have been extensively studied on various cell types, including hMSCs,36 osteoblasts,37 macrophages,38 human periosteum-derived cells (hPDCs),39 etc. Calcium ions have shown to influence the proliferation, morphology and osteogenic differentiation of hMSCs.36 Similarly, in vitro studies on the effect of inorganic phosphate ions have shown a concentration-dependent effect on osteogenic differentiation of hPDCs,39 whereas osteoblast-like cells were shown to undergo apoptosis when treated with high levels of inorganic phosphate.40 On the other hand, hypophosphatemia has been shown to inhibit osteoclast formation and osteoblast differentiation to mature osteocytes.41 Despite this body of evidence for the effect of free calcium and phosphate ions on cell proliferation and osteogenic differentiation, as we recently reviewed, it is difficult to pinpoint a single parameter that is responsible for biological response to a CaP material in vitro or in vivo.21 Release of calcium from a CaP ceramic is accompanied by other events, such as phosphate release, reprecipitation of a biological apatite layer, possibly containing endogenous proteins and other factors, and change of surface topography, all of which can affect bioactivity of the material as well. In our study, we have demonstrated a continuous release of calcium and phosphate ions from the composite over 12 wk in SPS in vitro. In addition, a small but more pronounced weight loss was observed for PLA/HA composite particles as compared with PLA control particles along with a stronger decrease of pH of the solution. SPS is a solution with ionic strength similar to the blood plasma, but it is, unlike blood plasma not saturated toward dicalcium phosphate dihydrate. Upon immersion in SBF, which simulates the mineral composition of blood plasma, weight loss of PLA/HA particles was again more pronounced than that of PLA particles, and so was the pH decrease. However, a decrease of calcium and phosphate ions measured was probably a consequence of the formation of a CaP layer on the surface of both PLA and PLA/HA. In the case of PLA/HA, an increase in calcium and phosphate concentrations in SBF at the latest time points may be attributed to the drop in pH observed. Although SBF is theoretically supposed to better mimic the mineral composition of body fluids,42 in vivo conditions of fluid refreshment regimes, local pH, temperature and presence of endogenous factors are difficult, if not impossible to mimic in vitro, which is why release data as described here can at best be considered an indication of whether or not ions release from the composite material takes place, and to which extent this release is dependent on the degradation of the polymer phase. Nevertheless, considering clear signs of PLA/HA degradation in vivo, and differences in the extent of degradation between the polymer and the composite, it is plausible that release of calcium and phosphate from the ceramic phase occurred in parallel.
Both PLA/HA composite and PLA control sustained proliferation of hMSCs over a period of 14 d. In general PLA is considered a biocompatible material, and indeed in our study no detrimental effects on cell proliferation were observed within the time frame cultured, which was in accordance with a study in which MC3T3-E1 osteoblast were cultured on electrospun PLA and PLA/HA fibers.14 While no effect of material or medium type was observed, cell numbers on both PLA/HA and PLA increased between days 7 and 14, independent of the cell culture medium used. This increase was, however, less pronounced for PLA/HA in osteogenic medium than for the other conditions, which may be explained by an increase in osteogenic differentiation, as was demonstrated by a significant increase in ALP levels of cells cultured on PLA/HA in osteogenic medium, between days 7 and 14. A trend of ALP levels of cells cultured on PLA/HA being higher than those on PLA control was observed at both time points and in both basic and osteogenic medium, although the difference was only significant at 14 d in osteogenic medium. These data suggest that PLA/HA composite improved osteogenic differentiation of hMSCs as compared with PLA without the ceramic and are in accordance with earlier studies using fetal bone cells.43
In vivo, although some cases of inflammatory response were reported upon PLA implantation, this polymer is considered to have an overall satisfactory biocompatibility.44 In our in vivo study, the thin dense fibrous capsule observed around both PLA and PLA/HA implants after 12 wk of intramuscular implantation in dogs, suggested a mild tissue response.45 Presence of bone in all PLA/HA samples implanted intramuscularly was the proof of osteoinductivity of the composite. Previous studies already reported osteoconductivity27 and osteoinductivity19,20,29 of PLA/HA blends. While use of solvents may be a disadvantage, solvent-based methods typically result in porous materials, in contrast to extruded material particles, which are dense. As stated earlier, protective areas in an implant, in the form of concavities, channels or pores are considered essential for osteoinduction to occur.21 For this reason, implants for the in vivo part of this study were designed such that a protective area was artificially created between two dense composite plates. With improvement of the choice of spacer material, such as Teflon, the implant design with the protective gap is expected to retain its shape to allow formation of bone even deeper inside the gap. The observation that bone formation was primarily observed in the areas close to the original openings of the gap and in the areas of pronounced degradation of the composite were in accordance with results from an earlier study in which calcium phosphate cement blocks with open and closed channels were tested for osteoinductivity.8 This observation could be related to a better oxygen and nutrient supply in the areas close to gap openings as well as to better availability of calcium phosphate ceramic in the areas of strong degradation of the composite. Indeed, considering that no bone was found in PLA implants, despite extensive degradation, it is suggested that bone formation observed in PLA/HA implants was related to increased availability of the HA ceramic and ionic dissolution/reprecipitation processes occurring in the vicinity of HA particles. Despite the unexpectedly fast degradation of the material used for spacer in the 3D implants, we have shown that osteoinduction by the PLA/HA composite produced by extrusion is possible, when dense materials are shaped into an implant that allows bone induction. In general, such dense materials may be interesting in studies aiming to describe the effect of individual material parameters in phenomena such as osteoinduction, for example, by allowing the change of a single parameter, such as ceramic phase without variability in macro- or microporosity.
Materials and Methods
Materials production and characterization
The composite used in this study consisted of 50 wt.% amorphous poly(D,L-lactic acid) (PLA), (ANaBior or Purac), and 50 wt.% in-house made nano-sized HA powder. HA was produced using a wet precipitation method as described earlier.20 In short, aqueous solutions of (NH4)2HPO4 and Ca(NO3)2·4H2O were mixed at a pH above 10, and the resulting powder was allowed to age, washed and finally resuspended in acetone. A low molecular weight of 55,000 – 59,000 Da was chosen for PLA in order to build a rapidly degradable composite. Characterization of the HA powder was performed using X-ray diffraction (XRD, Rigaku Miniflex) and an environmental scanning electron microscope (SEM; XL30, ESEM-FEG, Philips) in the secondary electron mode with an acceleration voltage of 20 kV (Fig. 2A and B). Composite was produced by extrusion using a twin screw extruder with conical non-converging screws (Artecs BV, Enschede, the Netherlands). The extruder was set at 150 °C and the screw rotation speed at 100 rpm, which allowed the composite to flow out of the extruder after a mixing time of 5 min. Controls made of PLA were prepared using the same process. Following extrusion, PLA/HA composite and PLA control were shaped into particles, pellets and complex three-dimensional implants to be used for the degradation study, in vitro evaluation and in vivo implantation respectively.
Chemical characterization was performed using transmission mode Fourier Transform Infrared spectroscopy with KBr pellets (FTIR; Spectrum100, Perkin Elmer Analytical Instruments). Surface morphology and homogeneity of the ceramic distribution in the polymeric phase were assessed for sterilized PLA/HA and PLA pellets using SEM with an acceleration voltage of 5 kV, coupled to energy dispersive X-ray analyzer (EDAX; Apollo X, Ametek).
In vitro degradation and ion release dynamics
For the degradation study, material particles with a size of 0.5–1 mm were produced by grinding and subsequent sieving of the extruded material. 100 mg of particles were immersed in 5 ml of either simulated physiological saline (SPS) or simulated body fluid (SBF) in triplicate. SPS contained 137 mM Na+, 177 mM Cl- and 50 mM 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid (HEPES). The pH of SPS was adjusted to 7.3 using 1 M NaOH. SBF was prepared using the method proposed by Bohner and Lemaitre42 and contained 142 mM Na+, 109.90 mM Cl-, 34.88 mM HCO3-, 2.31 mM Ca2+ and 1.39 mM HPO42-. The pH of SBF was adjusted to 7.4 using 1 M HCl. Samples were placed in a shaking waterbath at 37 °C for a maximum of three months. Calcium and phosphate concentrations and the pH of the solutions were analyzed after 12 h, 1, 2, 4, 6, 8, 10 and 12 wk. Calcium and phosphate concentrations were determined using a quantitative colorimetric method (QuantiChromTM Calcium assay kit (DICA-500) and QuantiChromTM Phosphate assay kit (DIPI-500), respectively). Optical density of solutions after adding reagents from the kits was read with a microplate spectrophotometer (Thermo Scientific Multiskan GO) at 612 nm and 620 nm for calcium and phosphate release, respectively. The degradation experiment was performed on independent triplicates for the time points 1, 4, 8 and 12 wk. The intermediate measures of calcium and phosphate concentrations after 12 h, 2, 6, and 10 wk were performed by sampling 300 µL of solution, which was not refreshed, from the independent triplicates aforementioned. This procedure was selected to avoid the error induced by solution refreshment. The material particles were collected, rinsed with MilliQ water and air-dried for two days before being characterized by SEM. The wet and the dry weights of the particles were recorded after 12 h and 12 wk of immersion in SPS or SBF.
In vitro cell culture
Bone marrow aspirates were obtained after written informed consent, and hMSCs were isolated and proliferated as described previously.46,47 Briefly, aspirates were resuspended by using 20-gauge needles, plated at a density of 5 × 105 cells/cm2 and cultured in hMSC proliferation medium containing α-minimal essential medium (Gibco), 10% fetal bovine serum (Lonza), 0.2 mM ascorbic acid (Sigma Aldrich), 2 mM L-glutamine (Gibco), 100 units/ml penicillin (Gibco), 10 μg/ml streptomycin (Gibco), and 1 ng/ml basic fibroblast growth factor (FGF) (Fisher Scientific). Cells were grown at 37 °C in a humid atmosphere with 5% CO2. Medium was refreshed twice a week, and cells were used for further sub-culturing or cryopreservation. Cells were trypsinised prior to seeding on materials.
For in vitro cell culture experiments, the extruded rods of PLA and PLA/HA composite were molded into pellets with a diameter of 10 mm and a thickness of 2 mm using a Teflon mold and a heating press at 120 °C and 20 kN for 5 min. Pellets were then polished using sandpaper (grades 500 and 2400) to ensure a smooth surface. After sterilization with 70% isopropanol for two times 3 min and complete evaporation at room temperature, pellets were placed in ultra-low attachment 24 well-plates (Corning) to minimize the adhesion of cells to the plate. Prior to cell seeding, pellets were conditioned overnight in proliferation medium. 4000 hMSCs of passage 3 were seeded on each pellet in proliferation medium. After 24 h, proliferation medium was replaced by either basic (proliferation medium without FGF) or differentiation medium (basic medium supplemented with 10nM dexamethasone). Cells were cultured for 7 and 14 d at 37 °C in a humid atmosphere with 5% CO2, with refreshment of medium every 2 to 3 d. HMSCs proliferation was evaluated by quantifying DNA content (CyQUANT® Cell Proliferation assay) and ALP production was assessed at the enzymatic level (CDP-Star® Reagent) as an early marker for osteogenic differentiation. Expression of ALP by hMSCs cultured on tissue culture plastic under osteogenic conditions has been shown to occur at around day 11,48 whereas on different biomaterials, including polymer/ceramic composites, hMSCs have been shown to express ALP even earlier,49 which is why we selected 7 and 14 d of culture as endpoints for our in vitro experiment. All analyses were performed in triplicate.
In vivo study
Implant preparation and implantation procedure
Implants were produced by molding the extruded rods of PLA and PLA/HA composite into 24 × 14 × 5 mm3 plates by using a poly(dimethylsiloxane) mold with smooth surface, two microscope glass slides and 2-foldback clips, by applying pressure. The setup was left at 120 °C for 5 min. Upon removal from the mold, the plates were paired and glued together with smooth surfaces facing one another using a 0.5 mm thick poly(ethylene oxide terephthalate) / poly(butylene terephtalate) - (PEOT/PBT) (PolyVation BV) spacers and super glue (Pattex) (Fig. 1). Following an aPEOTbPBTc nomenclature, the composition used in this study was 300PEOT55PBT45 where, (a) is the molecular weight in g/mol of the starting poly(ethylene glycol) (PEG) blocks used in the copolymerisation, while (b) and (c) are the weight ratios of the PEOT and PBT blocks, respectively. Prior to implantation, samples were sterilized by ethylene oxide (Isotron Nederland BV).
Twelve PLA-HA composite and 5 PLA control implants were inserted in paraspinal muscles of 8 skeletally mature male mongrel dogs (1–4 y old, weighing 10–15 kg) upon approval by the local animal care committee (Animal Center, Sichuan University, Chengdu, China; protocol P11029). The surgical procedure was performed under general anesthesia of the animals (pentobarbital sodium, Merck; 30 mg/kg body weight). After shaving the lumbar area and disinfection of the skin with iodine, the paraspinal muscles were exposed. Using blunt dissection, intramuscular pockets were created bilaterally, and each pocket was filled with one implant. Distance among pockets was sufficient to avoid effect of individual implants on each other’s behavior. Upon implant placement, the wound was closed in layers using silk sutures. Following the surgeries, animals were given buprenorphine (0.1 mg per animal) intramuscularly for 2 d as analgesics and penicillin (40 mg/kg) by intramuscular injection for 3 consecutive days to prevent infection. Twelve weeks after implantation, the animals were sacrificed with overdose of pentobarbital sodium to harvest the samples.
Histological analysis
After explantation, samples, with surrounding muscle tissue were fixed in 4% buffered paraformaldehyde (pH 7.4) and kept at 4 °C for one week. Fixed samples were rinsed with phosphate buffer solution (PBS), dehydrated by ethanol series (70–100%) and infiltrated with a methylmethacrylate (MMA, LTI Nederland) solution that polymerized at 30 °C within 1 wk. Longitudinal non-decalcified sections with a thickness of 10–15 μm were cut using a diamond saw microtome (Leica Leitz 1600) and stained with 1% methylene blue and 0.3% basic fuchsin after etching with an HCl/ethanol mixture.
Tissue formation was qualitatively analyzed using a light microscope (Nikon Eclipse E600, DS Cooled Camera Head DS-Fi1c).
Statistical analysis
One way ANOVA with Tukey’s multiple comparison post-hoc test was performed to analyze weight measurements of the materials upon degradation study and DNA and ALP values following cell culture. The level of significance was set at P < 0.05. All data presented are expressed as mean ± standard deviation.
Conclusion
In the present study, extrusion was used as a solvent-free method to synthesize dense composite materials consisting of 50 wt% (poly)D,L, lactic acid and 50 wt% nano-sized hydroxyapatite, for bone regeneration. While proliferation of hMSCs was comparable between the composite and the polymer control not containing ceramic, ALP expression was higher on the composite materials. Furthermore, intramuscular implantation in dogs of composite implants produced using an innovative design, resulted in heterotopic bone formation, in contrast to polymer- based implants, which were not osteoinductive.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge the financial support of the TeRM Smart Mix Program of The Netherlands Ministry of Education, Culture and Science (CD).
Glossary
Abbreviations:
- ALP
alkaline phosphatase
- CaP
calcium-phosphate
- EDAX
energy dispersive x-ray
- FTIR
fourier transform infrared
- HA
hydroxyapatite
- hMSCs
human mesenchymal stromal cells
- PLA
poly(D,L-lactic acid)
- SEM
scanning electron microscope
- SBF
simulated body fluid
- SPS
simulated physiological saline
- XRD
x-ray diffraction
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/27664
References
- 1.Davies JE, Hosseini MM. Histodynamic of endosseous wound healing. In: Davies JE, editor. Toronto, Canada: Em squared Inc.; 2000. p. 1-14. [Google Scholar]
- 2.Friedenstein AY. Induction of bone tissue by transitional epithelium. Clin Orthop Relat Res. 1968;59:21–37. doi: 10.1097/00003086-196807000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Winter GD, Simpson BJ. Heterotopic bone formed in a synthetic sponge in the skin of young pigs. Nature. 1969;223:88–90. doi: 10.1038/223088a0. [DOI] [PubMed] [Google Scholar]
- 4.Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25:443–50. doi: 10.1016/S0142-9612(03)00551-9. [DOI] [PubMed] [Google Scholar]
- 5.Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17:31–5. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 6.Yuan H, De Bruijn JD, Li Y, Feng J, Yang Z, De Groot K, Zhang X. Bone formation induced by calcium phosphate ceramics in soft tissue of dogs: a comparative study between porous alpha-TCP and beta-TCP. J Mater Sci Mater Med. 2001;12:7–13. doi: 10.1023/A:1026792615665. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Yuan H, Tong W, Zou P, Chen W, Zhang X. Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: variability among different kinds of animals. Biomaterials. 1996;17:2131–7. doi: 10.1016/0142-9612(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 8.Habibovic P, Gbureck U, Doillon CJ, Bassett DC, van Blitterswijk CA, Barralet JE. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008;29:944–53. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Barrère F, van der Valk CM, Dalmeijer RAJ, Meijer G, van Blitterswijk CA, de Groot K, Layrolle P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J Biomed Mater Res A. 2003;66:779–88. doi: 10.1002/jbm.a.10454. [DOI] [PubMed] [Google Scholar]
- 10.Tanner KE. Bioactive ceramic-reinforced composites for bone augmentation. J R Soc Interface. 2010;7(Suppl 5):S541–57. doi: 10.1098/rsif.2010.0229.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin PL, Fang HW, Tseng T, Lee WH. Effects of hydroxyapatite dosage on mechanical and biological behaviors of polylactic acid composite materials. Mater Lett. 2007;61:3009–13. doi: 10.1016/j.matlet.2006.10.064. [DOI] [Google Scholar]
- 12.Wang T, Chow LC, Frukhtbeyn SA, Ting AH, Dong Q, Yang M, Mitchell JW. Improve the Strength of PLA/HA Composite Through the Use of Surface Initiated Polymerization and Phosphonic Acid Coupling Agent. J Res Natl Inst Stand Technol. 2011;116:785–96. doi: 10.6028/jres.116.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nejati E, Firouzdor V, Eslaminejad MB, Bagheri F. Needle-like nano hydroxyapatite/poly(l-lactide acid) composite scaffold for bone tissue engineering application. Mater Sci Eng C. 2009;29:942–9. doi: 10.1016/j.msec.2008.07.038. [DOI] [Google Scholar]
- 14.Jeong SI, Ko EK, Yum J, Jung CH, Lee YM, Shin H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol Biosci. 2008;8:328–38. doi: 10.1002/mabi.200700107. [DOI] [PubMed] [Google Scholar]
- 15.Wang XH, Shi S, Guo G, Fu SZ, Fan M, Luo F, Zhao X, Wei YQ, Qian ZY. Preparation and characterization of a porous scaffold based on poly(D,L-lactide) and N-hydroxyapatite by phase separation. J Biomater Sci Polym Ed. 2011;22:1917–29. doi: 10.1163/092050610X529155. [DOI] [PubMed] [Google Scholar]
- 16.Rizzi SC, Heath DJ, Coombes AG, Bock N, Textor M, Downes S. Biodegradable polymer/hydroxyapatite composites: surface analysis and initial attachment of human osteoblasts. J Biomed Mater Res. 2001;55:475–86. doi: 10.1002/1097-4636(20010615)55:4<475::AID-JBM1039>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa S, Tamura J, Neo M, Goto K, Shikinami Y, Saito M, Kita M, Nakamura T. In vivo evaluation of a porous hydroxyapatite/poly-DL-lactide composite for use as a bone substitute. J Biomed Mater Res A. 2005;75:567–79. doi: 10.1002/jbm.a.30460. [DOI] [PubMed] [Google Scholar]
- 18.Montjovent M-O, Mathieu L, Schmoekel H, Mark S, Bourban P-E, Zambelli P-Y, Laurent-Applegate LA, Pioletti DP. Repair of critical size defects in the rat cranium using ceramic-reinforced PLA scaffolds obtained by supercritical gas foaming. J Biomed Mater Res A. 2007;83:41–51. doi: 10.1002/jbm.a.31208. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa S, Neo M, Tamura J, Fujibayashi S, Takemoto M, Shikinami Y, Okazaki K, Nakamura T. In vivo evaluation of a porous hydroxyapatite/poly-DL-lactide composite for bone tissue engineering. J Biomed Mater Res A. 2007;81:930–8. doi: 10.1002/jbm.a.31109. [DOI] [PubMed] [Google Scholar]
- 20.Barbieri D, Renard AJS, de Bruijn JD, Yuan H. Heterotopic bone formation by nano-apatite containing poly(D,L-lactide) composites. Eur Cell Mater. 2010;19:252–61. doi: 10.22203/ecm.v019a24. [DOI] [PubMed] [Google Scholar]
- 21.Barradas AMC, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407–29, discussion 429. doi: 10.22203/ecm.v021a31. [DOI] [PubMed] [Google Scholar]
- 22.Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26:3565–75. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AMC, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A. 2010;107:13614–9. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripamonti U, Crooks J, Kirkbride AN. Sintered porous hydroxyapatites with intrinsic osteoinductive activity: Geometric induction of bone formation. S Afr J Sci. 1999;95:335–43. [Google Scholar]
- 25.Nejati E, Mirzadeh H, Zandi M. Synthesis and characterization of nano-hydroxyapatite rods/poly(l-lactide acid) composite scaffolds for bone tissue engineering. Composites Part A. 2008;39:1589–96. doi: 10.1016/j.compositesa.2008.05.018. [DOI] [Google Scholar]
- 26.Freier T, Kunze C, Schmitz KP. Solvent removal from solution-cast films of biodegradable polymers. J Mater Sci Lett. 2001;20:1929–31. doi: 10.1023/A:1013174400236. [DOI] [Google Scholar]
- 27.Verheyen CCPM, de Wijn JR, van Blitterswijk CA, de Groot K, Rozing PM. Hydroxylapatite/poly(L-lactide) composites: an animal study on push-out strengths and interface histology. J Biomed Mater Res. 1993;27:433–44. doi: 10.1002/jbm.820270404. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri D, de Bruijn JD, Luo X, Farè S, Grijpma DW, Yuan H. Controlling dynamic mechanical properties and degradation of composites for bone regeneration by means of filler content. J Mech Behav Biomed Mater. 2013;20:162–72. doi: 10.1016/j.jmbbm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Barbieri D, Yuan H, Luo X, Farè S, Grijpma DW, de Bruijn JD. Influence of polymer molecular weight in osteoinductive composites for bone tissue regeneration. Acta Biomater. 2013;9:9401–13. doi: 10.1016/j.actbio.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu L, Bourban P, Manson J. Processing of homogeneous ceramic/polymer blends for bioresorbable composites. Compos Sci Technol. 2006;66:1606–14. doi: 10.1016/j.compscitech.2005.11.012. [DOI] [Google Scholar]
- 31.Henton DE, Gruber P, Lunt J, Randall J. Polylactic acid technology. In: Mohanty AK, Misra M, Drzal LT, editors. Boca Raton, USA: Taylor & Francis; 2005. p. 527-77. [Google Scholar]
- 32.Sui G, Yang X, Mei F, Hu X, Chen G, Deng X, Ryu S. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J Biomed Mater Res A. 2007;82:445–54. doi: 10.1002/jbm.a.31166. [DOI] [PubMed] [Google Scholar]
- 33.Deng XL, Sui G, Zhao ML, Chen GQ, Yang XP. Poly(L-lactic acid)/hydroxyapatite hybrid nanofibrous scaffolds prepared by electrospinning. J Biomater Sci Polym Ed. 2007;18:117–30. doi: 10.1163/156856207779146123. [DOI] [PubMed] [Google Scholar]
- 34.Geesink RG, de Groot K, Klein CP. Bonding of bone to apatite-coated implants. J Bone Joint Surg Br. 1988;70:17–22. doi: 10.1302/0301-620X.70B1.2828374. [DOI] [PubMed] [Google Scholar]
- 35.Hanawa T, Kamiura Y, Yamamoto S, Kohgo T, Amemiya A, Ukai H, Murakami K, Asaoka K. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J Biomed Mater Res. 1997;36:131–6. doi: 10.1002/(SICI)1097-4636(199707)36:1<131::AID-JBM16>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Barradas AMC, Fernandes HAM, Groen N, Chai YC, Schrooten J, van de Peppel J, van Leeuwen JP, van Blitterswijk CA, de Boer J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33:3205–15. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura S, Matsumoto T, Sasaki J, Egusa H, Lee KY, Nakano T, Sohmura T, Nakahira A. Effect of calcium ion concentrations on osteogenic differentiation and hematopoietic stem cell niche-related protein expression in osteoblasts. Tissue Eng Part A. 2010;16:2467–73. doi: 10.1089/ten.tea.2009.0337. [DOI] [PubMed] [Google Scholar]
- 38.Honda Y, Anada T, Kamakura S, Nakamura M, Sugawara S, Suzuki O. Elevated extracellular calcium stimulates secretion of bone morphogenetic protein 2 by a macrophage cell line. Biochem Biophys Res Commun. 2006;345:1155–60. doi: 10.1016/j.bbrc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Chai YC, Roberts SJ, Schrooten J, Luyten FP. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng Part A. 2011;17:1083–97. doi: 10.1089/ten.tea.2010.0160. [DOI] [PubMed] [Google Scholar]
- 40.Meleti Z, Shapiro IM, Adams CS. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone. 2000;27:359–66. doi: 10.1016/S8756-3282(00)00346-X. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2011;26:1047–56. doi: 10.1002/jbmr.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials. 2009;30:2175–9. doi: 10.1016/j.biomaterials.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Montjovent M-O, Mathieu L, Hinz B, Applegate LL, Bourban P-E, Zambelli P-Y, Månson JA, Pioletti DP. Biocompatibility of bioresorbable poly(L-lactic acid) composite scaffolds obtained by supercritical gas foaming with human fetal bone cells. Tissue Eng. 2005;11:1640–9. doi: 10.1089/ten.2005.11.1640. [DOI] [PubMed] [Google Scholar]
- 44.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 45.Gogolewski S, Jovanovic M, Perren SM, Dillon JG, Hughes MK. Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA) J Biomed Mater Res. 1993;27:1135–48. doi: 10.1002/jbm.820270904. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes H, Mentink A, Bank R, Stoop R, van Blitterswijk C, de Boer J. Endogenous collagen influences differentiation of human multipotent mesenchymal stromal cells. Tissue Eng Part A. 2010;16:1693–702. doi: 10.1089/ten.tea.2009.0341. [DOI] [PubMed] [Google Scholar]
- 47.Both SK, van der Muijsenberg AJC, van Blitterswijk CA, de Boer J, de Bruijn JD. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 2007;13:3–9. doi: 10.1089/ten.2005.0513. [DOI] [PubMed] [Google Scholar]
- 48.Karperien M, Roelen B, Poelmann M, Gittenberger-de Groot A, Hierck B, DeRuiter M, Meijer D, Gibbs S. Morphogenesis, generation of tissue in the embryo. In: Tissue Engineering, van Blitterswijk CA et al. editor. Elsevier: 2008. p.28-72. [Google Scholar]
- 49.Barbieri D, de Bruijn JD, Yuan H. Surface structure of nanocomposites and its properties: a practical example. In: Frontiers in Nanobiomedical Research Series. Tissue Regeneration: Where Nano Structure Meets Biology, Liu Q and Wang H editors. World Scientific Publishing and Co. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.