Abstract
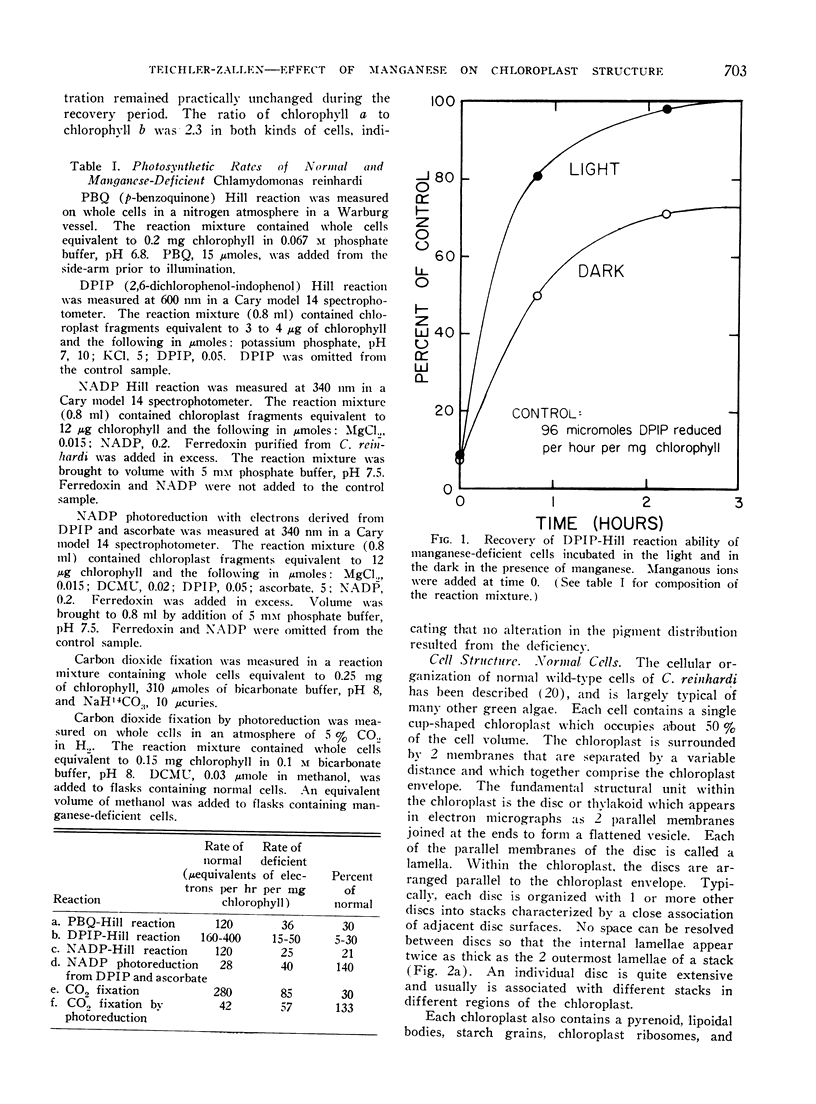
Manganese deficiency was induced in mixotrophically-grown cultures of the green alga Chlamydomonas reinhardi and the effects of this deficiency on photosynthetic activity and cell structure were investigated. Manganese-deficient cells are unable to carry out, at normal rates, photosynthetic reactions involving system II of the photosynthetic electron transport chain. Reactions requiring only system I are not inhibited. Normal system II activity returns within 2 hr in the light following addition of manganous ions to deficient cells.
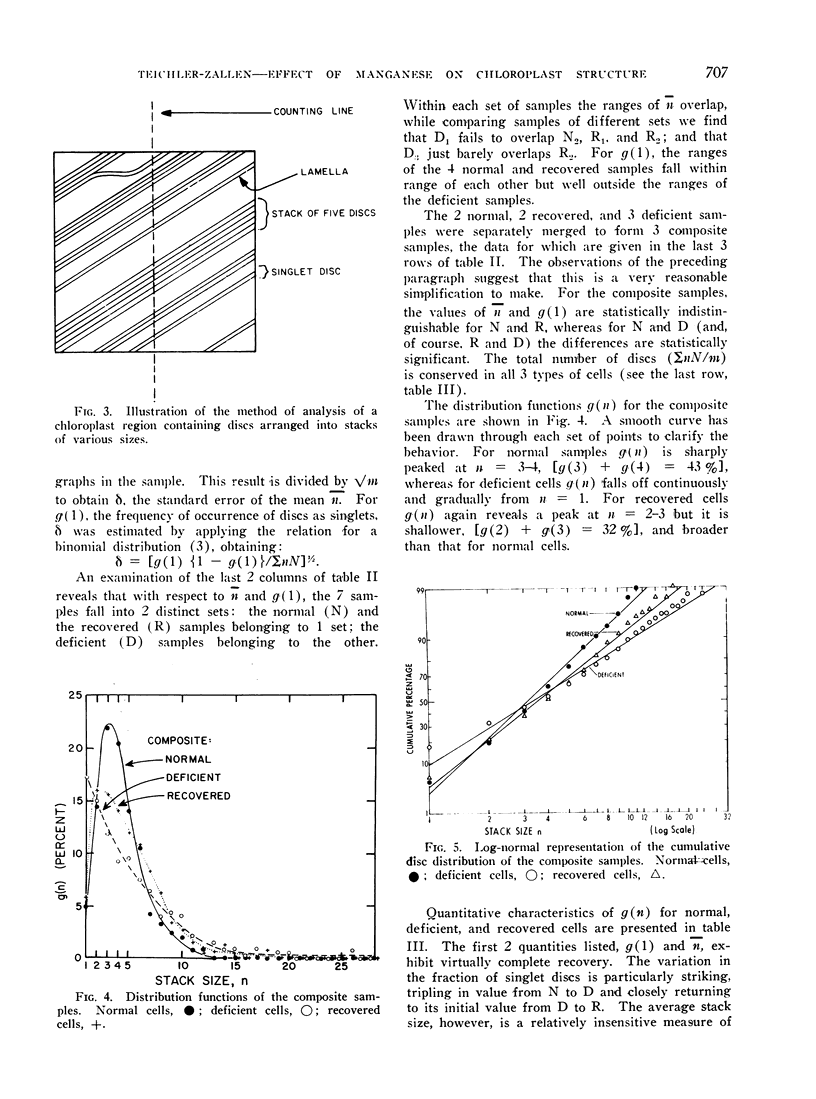
Significant structural alterations for deficient cells were observed in the chloroplast, in the arrangement of discs into stacks. Stacking distributions for normal, deficient, and recovered cells were quantitatively characterized and compared by the introduction of an experimentally-defined distribution function and its attendant parameters. The results provide evidence for a role for manganese in maintaining chloroplast structure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Studies on the function of manganese in photosynthesis. Brookhaven Symp Biol. 1966;19:406–417. [PubMed] [Google Scholar]
- Eyster C., Brown T. E., Tanner H. A., Hood S. L. Manganese Requirement with Respect to Growth, Hill Reaction and Photosynthesis. Plant Physiol. 1958 Jul;33(4):235–241. doi: 10.1104/pp.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R. P., SMILLIE R. M. The pathway of triphosphopyridine nucleotide photoreduction in Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1962 Mar 15;48:417–421. doi: 10.1073/pnas.48.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns D. N., Johnson C. M. Removal of Heavy Metal and Halide Contamination from Macronutrient Salts. Plant Physiol. 1960 Nov;35(6):978–981. doi: 10.1104/pp.35.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G. K., Levine R. P. Photosynthetic Reactions by Chloroplast Fragments of Chlamydomonas reinhardi. Plant Physiol. 1963 Sep;38(5):542–545. doi: 10.1104/pp.38.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., PALADE G. E. Structure and development of the chloroplast in Chlamydomonas. I. The normal green cell. J Biophys Biochem Cytol. 1957 May 25;3(3):463–488. doi: 10.1083/jcb.3.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichler-Zallen D., Levine R. P. An Electron Spin Resonance Study of Manganese in Wild-Type and Mutant Strains of Chlamydomonas reinhardi. Plant Physiol. 1967 Nov;42(11):1643–1647. doi: 10.1104/pp.42.11.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNON L. P., ZAUGG W. S. Photoreductions by fresh and aged chloropasts: requirement for ascorbate and 2, 6-dichlorophenolindophenol with aged chloroplasts. J Biol Chem. 1960 Sep;235:2728–2733. [PubMed] [Google Scholar]



