Abstract
Intracerebral hemorrhage (ICH) is a common and often fatal stroke subtype for which specific therapies and treatments remain elusive. To address this, many recent experimental and translational studies of ICH have been conducted, and these have led to several ongoing clinical trials. This review focuses on the progress of translational studies of ICH including those of the underlying causes and natural history of ICH, animal models of the condition, and effects of ICH on the immune and cardiac systems, among others. Current and potential clinical trials also are discussed for both ICH alone and with intraventricular extension.
1. Introduction
Intracerebral hemorrhage (ICH) is a particularly devastating form of stroke with high mortality and morbidity (Keep et al 2012; Qureshi et al 2009). Relative to ischemic stroke, there have been few preclinical studies and clinical trials for the development of treatments for ICH. However, increased interest in ICH over the past decade has improved our knowledge of the underlying mechanisms of ICH-induced brain injury, which have been found to differ from those of ischemic stroke (Xi et al 2006). These findings have led to the initiation of several ongoing clinical trials investigating ICH treatment.
This review aims to describe the underlying causes and natural history of ICH, as well as the animal models employed in its study. This is followed by a discussion of the systemic effects of ICH, focusing on immune and cardiac effects, areas that have been largely neglected in research on ICH research. Current and potential clinical trials in ICH alone and with intraventricular extension are also discussed, of which the latter is particularly difficult to treat and is associated with higher mortality (Hanley 2009).
2. Causes of bleeding
Spontaneous ICH, i.e., ICH that is not related to trauma, most frequently occurs secondary to hypertension, with up to 70% of patients with ICH having a history of hypertension (Mendelow et al 2005). However, ICH may also result from bleeding associated with amyloid angiopathy, tumors, hemorrhagic conversion of ischemic stroke, dural venous sinus thrombosis, vasculitis and vascular malformations such as cavernous angiomas, arteriovenous fistulae, arteriovenous malformations, venous angiomas, and aneurysms (Qureshi et al 2001b; Ruiz-Sandoval et al 1999). ICH is considered primary if there is not an identifiable underlying structural lesion that is likely to be responsible for the hemorrhage. It is most commonly associated with arteriosclerosis as a result of hypertension and amyloid angiopathy (Ritter et al 2005; Tuhrim et al 1999).
Hypertension is a significant contributory factor for ICH and is associated with morbidity and mortality in all age groups (Ruiz-Sandoval et al 1999). Chronic hypertension induces degenerative changes in small arterioles, making them prone to rupture. Treatment of hypertension therefore reduces the annual risk of hemorrhage in hypertensive patients. In the elderly, amyloid angiopathy is a significant cause of bleeding. The presence of either the e2 or the e4 allele of the apolipoprotien E gene also increases the risk of ICH through β-amyloid deposition and fibrinoid necrosis in the vessel wall, rendering it more likely to rupture (O'Donnell et al 2000).
Vascular lesions are prone to rupture, which can result in ICH, subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), or any combination thereof, with each subtype having a distinct natural history. For untreated aneurysms, the natural history varies by size, location, and shape, with large and daughter dome-containing aneurysms having higher rates of rupture. Of aneurysms in the anterior circulation, those in the anterior and posterior communicating arteries have the highest rates of rupture (Gross et al 2013). The natural history of AVMs varies, with annual rates of rupture between 0.9 and 34%. Furthermore, depending on the study, the rate of rupture increases for hemorrhagic lesions, deeper locations, older age, larger lesions, and pregnancy (Gross and Du 2012b; Halim et al 2004; Hernesniemi et al 2008; Stapf et al 2006). Asymptomatic cavernous malformations are generally benign with annual rates of ruptures of 0 to 0.6%. However, if a patient is symptomatic with a prior hemorrhage, the re-bleed rate is 5 to 6% with the risk of re-bleeding decreasing over time. Pregnancy is not a durable risk factor for hemorrhage of cavernous malformations (Al-Holou et al 2012; Flemming et al 2012; Gross et al 2013). The annual risk of hemorrhage from dural AV fistulas is dependent on the presence of leptomeningeal venous drainage, which is 0, 2, and 46% for no drainage, asymptomatic lesions with leptomeningeal venous drainage, and symptomatic lesions with leptomeningeal venous drainage, respectively (Gross and Du 2012a).
Post-partum ICH is a rare, but increasingly recognized, cause of hemorrhage in young women and is thought to be due to angiopathy in the post-partum period (Bateman et al 2006). The overall incidence of ICH in pregnancy and the post-partum period is 4.6-53/100,000 and is associated with significant maternal mortality (Bateman et al 2006; Khan and Wasay 2013).
Risk of ICH also is increased by the use of anticoagulants. In the United States, approximately 20% of patients with ICH use anticoagulants. Additional risk factors include greater age, male sex, cigarette smoking, and heavy use of alcohol (Ariesen et al 2003), whereas high cholesterol is associated with a decreased risk of ICH (Ariesen et al 2003).
It is still controversial whether statin therapy is a potential risk factor of intracerebral hemorrhage. Evidence suggests cholesterol lowering drugs result in hemorrhagic stroke(Goldstein et al 2009). However, recent analyses of randomized controlled trials showed statin therapy are not associated with brain hemorrhage(Hackam et al 2011; McKinney and Kostis 2012).
3. Natural history of ICH
3.1. Hematoma enlargement
Post-ICH hematoma enlargement occurs in about one third of patients (Broderick et al 1990; Brott et al 1997; Fujii et al 1994; Fujii et al 1998; Kazui et al 1996; Kazui et al 1997). For example, in a study of hematoma enlargement in patients with ICH (n=103) 38% of patients experienced a hematoma expansion within 20 hours (Brott et al 1997). Post-ICH hematoma enlargement causes a midline shift and accelerates neurological deterioration (Broderick et al 1990; Zazulia et al 1999). A spot sign on computerized tomography angiography or contrast extravasation is highly correlated with hematoma enlargement (Hallevi et al 2010), which mostly occurs within the first 24 hours after ICH (Brott et al 1997; Kazui et al 1996). Several ongoing clinical trials are focused on lowering blood pressure quickly after ICH so as to prevent hematoma enlargement and minimize ICH-induced brain damage (Anderson et al 2010; Butcher et al 2010; Qureshi and Palesch 2011). However, the recently announced results of one such trial (INTERACT2, NCT00716079) failed to find a significant reduction in hematoma volume with rapid intensive reductions in blood pressure (Anderson et al 2013).
3.2. Brain Edema
Brain edema is an increase in the water content of brain tissue and results in increased brain volume and intracranial pressure (ICP) (Bodmer et al 2012; Xi et al 2002). Perihematomal edema is commonly observed during the acute and subacute post-ICH stages, appearing as a hypodensity surrounding the clot on CT scan (Figure 1) and as a hyperintensity on T2-weighted or flair MRI (Figure 2). In patients with ICH, edema develops within hours of symptom onset and peaks 1 to 2 weeks later (Broderick et al 1995; Suzuki et al 1985; Zazulia et al 1999). In animal models, perihematomal brain edema develops within hours and peaks several days post-ICH (Suzuki and Ebina 1980; Wagner et al 1996; Xi et al 2002). For example, in a rat model of ICH brain edema peaks at day 3 or 4 post-ICH before decreasing slowly (Enzmann et al 1981; Tomita et al 1994; Xi et al 1998a). In large animal models (e.g., pig), perihematomal edema is located mainly within the white matter (Garcia et al 1994; Tomita et al 1994).
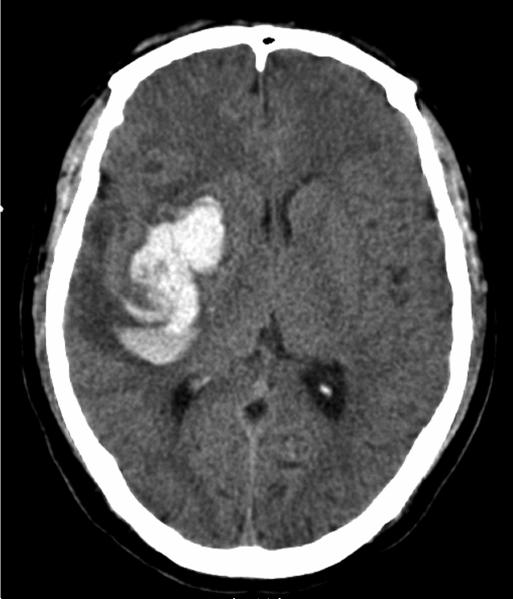
Figure 1.
CT scan showing a patient with an intracerebral hemorrhage.
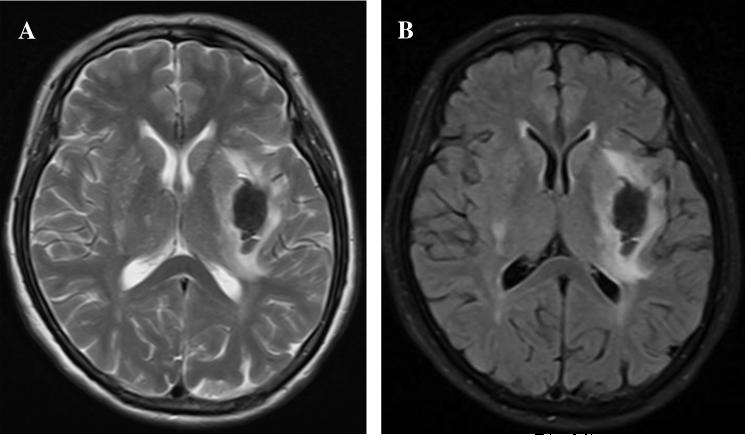
Figure 2.
T2 MRI (A) and Flair MRI (B) showing brain edema around hematoma at the first day in a patient with an intracerebral hemorrhage.
Edema formation post-ICH elevates intracranial pressure and may result in herniation (Ropper 1986). The amount of brain edema around the hematoma has been shown to correlate with poor functional outcome in patients with ICH (Ropper and King 1984; Ropper 1986; Zazulia et al 1999).
Blood components have been shown to contribute to perihematomal edema formation. In the first hours following ICH, perihematomal edema results from clot retraction with movement of serum from the hematoma into surrounding tissue (Wagner et al 1996). The coagulation cascade and thrombin production also have a role in edema in acute edema development, particularly in the first 24 hours post-ICH. Thrombin is an essential component in the coagulation cascade and thrombin inhibition abolishes early brain edema in animal models (Xi et al 1998b; Xi et al 2006). Whereas red blood cell lysis causes delayed brain edema; it has been shown that hemoglobin and its degradation products, as well as carbonic anhydrase-1 (another erythrocyte component), can cause brain edema (Guo et al 2012; Huang et al 2002).
Although multiple forms of edema can occur as a result of ICH, vasogenic edema is the principal form. The blood-brain barrier (BBB) is a physical barrier to the movement of many molecules between blood and brain (Betz et al 1989) and disruption of the BBB following ICH contributes to the development of brain edema (Keep et al 2012; Liu and Sharp 2012). Although the BBB remains intact to large molecules for several hours post-ICH (Wagner et al 1996), by 8-12 hours perihematomal BBB permeability is increased (Yang et al 1994). Animal data has implicated the blood components thrombin and lysed red blood cells in disruption of the BBB following ICH (Lee et al 1997; Xi et al 2001a).
3.3. Neuronal death and brain atrophy
ICH causes significant death of brain cells, including necrosis, apoptosis and autophagy (Keep et al 2012). Necrotic brain tissue has been found around the clot (Suzuki and Ebina 1980), and likely results from either mechanical forces during hematoma formation or components of blood clots and degradation products. Marked perihematomal necrotic cell death has been found in the perihematomal area in a rat model of ICH (Figure 3) (Jin et al 2013). In that study, increased propidium iodide permeability is used as a marker of necrosis. Propidium iodide is a 668 Da membrane impermeable nucleic acid stain that emits bright red fluorescence when bound to RNA or DNA. Another recent study also indicates that programmed necrosis and plasmalemma damage may be useful therapeutic targets for ICH (Zhu et al 2012).
Figure 3. Marked perihematomal necrotic cell death in a rat model of ICH.
Alexa Fluro 488-labeled dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa (DARPP-32) (green) and positive staining for propidium iodide (PI; red) in the ipsilateral basal ganglia at day 3 post- intracerebral hemorrhage. DARPP-32 is a cytosolic protein highly enriched in medium-sized spiny neurons of the striatum. Plasmalemma permeability to PI is associated with markers of cell death. Scale bar = 500 μm (upper panel) and 100 μm (lower panel). The DARPP-32 negative area superimposes the PI-positive area (Jin et al 2013).
Cell death also occurs in brain adjacent to the hematoma (Gong et al 2001; Hickenbottom et al 1999; Matsushita et al 2000; Qureshi et al 2001a; Xue and Del Bigio 2000). Although apoptosis has been implicated in perihematomal brain cell death (Matsushita et al 2000), how important a role apoptosis has in ICH-induced brain damage remains unclear.
Autophagy is a cellular degradation process by which cellular proteins and organelles are sequestered in double membrane vesicles, transported to lysosomes, and digested by lysosomal hydrolases (Wang and Klionsky 2003). It has recently been demonstrated that ICH is able to induce the autophagy-form of programmed cell death, and that iron has an important role in the induction of autophagy. As in other neurological diseases, more research work is needed to determine whether or not autophagy is protective (i.e., it removes dying cells) or harmful (i.e., it induces death in potentially viable cells) (He et al 2008).
Mechanisms that are thought to play a role in neural cell death are described later in the text. However, broadly, they are thought to include the initial physical distortion of brain cells and their connections by the hemorrhage, clot-derived toxic factors (such as iron and hemoglobin) and the brain response to the ICH (e.g. inflammation). The relative role of these factors likely varies with hematoma size. The role of the ischemic cell death in ICH has been the subject of much debate, particularly in relation to whether blood flow changes reach the level necessary to cause brain injury and whether changes in flow reflect rather than cause changes in neuronal function (Xi et al 2006).
The death of brain cells in animal models of ICH results in brain atrophy, which is also characteristic of patients with ICH (Skriver and Olsen 1986). In fact, brain atrophy has been used as an endpoint for experimental ICH studies (Okauchi et al 2009; Okauchi et al 2010). In rats, delayed brain atrophy occurs following ICH induced by infusion of 100 μl autologous whole blood (Felberg et al 2002; Xi et al 2004). Significant caudate atrophy with enlargement of the ipsilateral lateral ventricle was identified 4 weeks post-ICH, and the ipsilateral caudate area was approximately 70 % of that of the contralateral by weeks 8 to 12 (Hua et al 2006).
3.4. Inflammation
Inflammation exacerbates ICH-mediated brain injury. An inflammatory response in the perihematomal area occurs soon after ICH and peaks several days later in humans and in animals (Enzmann et al 1981; Gong et al 2000; Jenkins et al 1989; Xue and Del Bigio 2000). Neutrophil infiltration develops within two days in rats and activated microglial cells persist for long time (Gong et al 2004; Jenkins et al 1989). Inhibition of microglia activation reduces brain damage after ICH in mice (Wang et al 2003; Wang and Tsirka 2005). Recent studies have demonstrated that toll-like receptor 4 has an important role in brain injury following ICH (Lively and Schlichter 2012; Sansing et al 2011; Wang et al 2013).
3.5. Brain recovery
Although usually incomplete, brain recovery is expected in patients surviving ICH. In such cases, hematoma resolution, reduced edema, neuronal plasticity, and neurogenesis are potential contributors to improved functional recovery. Using doublecortin as a marker in a rat model of ICH, ipsilateral basal ganglia neurogenesis increased as early as 7 days post-ICH, peaked at day 14, and then gradually decreased by 1 month post-ICH. Immunohistochemistry also demonstrated increased doublecortin immunoreactivity in the ipsilateral subventricular zone and basal ganglia at 2 weeks post-ICH. Thrombin also increased doublecortin levels and the thrombin inhibitor hirudin blocked ICH-induced upregulation of doublecortin, thus suggesting a role for thrombin in ICH-induced neurogenesis (Yang et al 2008).
Phagocytosis by microglia and macrophages is involved in hematoma clearance and can be enhanced by administering the peroxisome proliferator activated receptor (PPAR)-γ agonists rosiglitazone or pioglitazone. PPAR-γ agonists accelerate hematoma resolution and reduce ICH-induced deficits in a mouse model of ICH (Zhao et al 2007b).
Rehabilitation has been shown to enhance neurological recovery following ICH. In a rat model of ICH, rehabilitation with enriched environment and skilled reach training improved functional outcome but had no effects on neurogenesis but was associated increased dendritic complexity (plasticity) (Auriat et al 2010).
Studies have demonstrated a functional improvement with the administration of stem cells after ICH (Keep et al 2012). For example, intravenously injected bone marrow stromal cells have been shown to migrate to the site of the hematoma and reduce ICH-induced neurological deficits in rats (Seyfried et al 2010).
4. Animal models
4.1. Overview
Experimental models of ICHs have been available since the 1960's and commonly involve the intracerebral injection of autologous blood, which is a straightforward and effective technique for producing ICH. This type of model has been developed in large animals (e.g., dogs, cats, pigs and monkeys) (Sussman et al 1974; Takasugi et al 1985; Wagner et al 1996; Whisnant et al 1963), by injecting blood into the frontal lobe. For small animals (e.g., rats and mice) blood is injected into the caudate (Belayev et al 2003; Hua et al 2000; Nakamura et al 2004b; Xi et al 1998a; Xi et al 1998b; Xi et al 2001b; Yang et al 1994). This method does not reproduce the arterial vessel rupture present in human spontaneous ICH, but it does control the volume of blood injected and has been shown to be useful for the study of pathophysiological and biochemical consequences of ICH.
Another model of ICH was developed by Rosenberg and colleagues and involves the injection of bacterial collagenase into brain (Rosenberg et al 1990; Rosenberg and Navratil 1997). Originally developed in rats, this approach has also been used extensively in mice (Choudhri et al 1997; Clark et al 1998). Collagenase dissolves the extracellular matrix, ultimately leading to blood vessel rupture and ICH. This model mimics the vascular disruption in spontaneous human ICH, but also induces widespread disruption of the extracellular matrix (including the endothelial basement membrane), which is not found in spontaneous cases of human ICH. Collagenase also appears to induce areas of ischemia that are not generally found after blood injection and are not considered a major component of ICH-induced injury (Zazulia et al 2001).
4.2. Small animal models
Small animal models of ICH have advantages of lower cost, relative homogeneity within strains, cerebrovascular anatomy and physiology similar to that of higher species, and a small brain that is suited to immunohistochemical and biochemical studies. Based on reproducibility, the basal ganglia is often chosen as the site of blood infusion rather areas near the brain surface. Considerable research indicates that this approach can be used for sensitive and reliable assessment of chronic behavioral deficits and treatment effects (Hua et al 2002; Hua et al 2006; Nakamura et al 2004a; Nakamura et al 2004b). The hippocampus has also been used as an injection site because of the relative ease of determining neuronal death (Song et al 2007).
In the mid-to late 1980's, a rat model of ICH was used to examine the relationships between mass effect, perihematomal blood flow, and ICP (Bullock et al 1984; Kingman et al 1988; Mendelow et al 1984; Nath et al 1986; Nath et al 1987). More recently, a method involving [14C]iodoantipyrine has been developed to measure perihematoma blood flow. It also has been used to investigate the mechanisms of brain edema formation following ICH (Keep et al 2012; Xi et al 2001a; Xi et al 2001c; Xi et al 2004; Xi et al 2006), as well as to assess the related neurological deficits and long term brain injury (Hua et al 2002). The effects of age (Gong et al 2004), gender (Nakamura et al 2005a), hypertension (Wu et al 2011a) and low capacity for exercise have also been investigated using this model (He et al 2012). Other uses of the [14C]iodoantipyrine-model of ICH include combining it with MRI to determine extent of brain damage (Wu et al 2010), test potential treatments (Nakamura et al 2004a; Okauchi et al 2009; Okauchi et al 2010; Wu et al 2008; Zhao et al 2011a), and examine hemorrhagic transformation after ischemia-reperfusion (Xing et al 2009).
Mouse models of ICH can also be used to investigate secondary inflammatory responses, intracellular signaling, and molecular events. Models based on the injection of donated blood into the mouse striatum (Belayev et al 2003) and autologous blood injected into the basal ganglia (Nakamura et al 2004b) have also been established. The latter has been used to test the effect of genetic deletions on ICH-induced brain injury (Nakamura et al 2004b; Yang et al 2006). Ischemia-reperfusion can also induce hemorrhagic transformation in mice (Campos et al 2013; del Zoppo et al 2012).
Collagenase rodent models of ICH can differ from blood injection models in their mechanisms of injury and repair, and in the response to therapeutic interventions (MacLellan et al 2008; MacLellan et al 2010). Although collagenase models have been used to examine mechanisms of hematoma enlargement and develop prospective treatments affecting hemostasis, this approach may amplify inflammatory responses and cause neurotoxic effects at high doses (Del Bigio et al 1996; Del Bigio et al 1999; Xue and Del Bigio 2000). Furthermore, extensive bleeding following intracerebral collagenase injection may produce an ischemic cerebral injury that is not representative of human ICH pathology (Weiler et al 1992).
4.3. Large animal models
In large animals, studies of ICH have been performed in cat (Kobari et al 1988), rabbit (Kaufman et al 1985; Narayan et al 1985), dog (Enzmann et al 1981; Qureshi et al 1999; Takasugi et al 1985), monkey (Bullock et al 1988), and pig (Wagner et al 1996), and these enable the examination of surgical treatments with and without pharmacological interventions. The pig is a useful model of ICH because of its large gyrate brain and large content of hemispheric white matter. A pig model of ICH with autologous blood infusion has been used to investigate ICP, blood flow, edema development, metabolism, transcription factor activation and inflammatory gene expression (Wagner and Broderick 2001; Wagner et al 1998; Wagner et al 2002; Wagner et al 2003; Wagner 2007). It has also been used to study surgical clot evacuation (Wagner et al 1999; Zuccarello et al 2002), clot lysis induced by tissue plasminogen activator (tPA) followed by aspiration (Wagner et al 1999), and the optimal time for surgical intervention (Yin et al 2006). It has also been used to test the effects on ICH-induced brain injury of inhibiting heme oxygenase (Wagner et al 2000) and iron chelation with deferoxamine (Gu et al 2009). The pig has also been used to generate a collagenase injection model of ICH (Mun-Bryce et al 2001; Mun-Bryce et al 2004; Mun-Bryce et al 2006) in which collagenase is injected into the primary sensory cortex to examine the alterations in somatosensory-evoked potentials elicited by electrical stimulation and changes in the somatosensory region after ICH.
5. Systemic responses post-ICH
5.1. Inflammatory and immune responses
ICH results in systemic inflammatory response. For example, in patients with hemorrhagic stroke, interleukin-2 levels in peripheral blood T lymphocytes were lower than in healthy controls, but no significant difference was observed between ICH and SAH groups (Zou et al 1997). Experimental data from a murine model of ICH has shown that total neutrophils and lymphocytes are reduced but that monocytes are increased, and that this is related to hematoma size (Illanes et al 2011). Immune cells were reduced in spleen, thymus and lymph nodes 3 days after the injection of 50 μl of blood, but not after an injection of 10 or 30 μl of blood.
5.2. Cardiac responses
The association between brain damage and cardiac death has been well documented (Cheung and Hachinski 2000; Tung et al 2004). Clinical and experimental evidence suggests that traumatic brain injury leads to change in electrocardiogram (ECG), an elevation in cardiac enzymes, myocardial dysfunction, and arrhythmias (Dujardin et al 2001; Elrifai et al 1996; Hurst 2003; Jung et al 2001).
Many patients with SAH or ICH have mild to moderate ECG abnormalities without coronary events during the acute phase of hemorrhagic stroke, suggesting a probability of heart injury caused by brain damage (Cheung and Hachinski 2000; Dujardin et al 2001; Elrifai et al 1996; Hurst 2003; Jung et al 2001; Khechinashvili and Asplund 2002). ECG abnormalities related to lesion location and outcome, but not to the level of the cerebral lesion, frequently occur in patients with ICH (Liu et al 2011b). Elevated troponin levels in SAH patients has long been recognized (Deibert et al 2000; Espiner et al 2002), and elevated cardiac troponin I values also occur in ICH and are independently associated with higher rates of in-hospital mortality (Hays and Diringer 2006). Elevated troponin levels may represent cardiac toxicity mediated by sympathetic activation in response to acute neurologic insults (Cheung and Hachinski 2000; Tung et al 2004).
Experiments in rats have demonstrated that ICH initiates cardiomyocyte contractile (Fang et al 2006). Female rats have higher levels of cardiac HSP-32 than do males, and 17β-estradiol treatment induces higher HSP-32 levels in male rats after ICH, thus suggesting that gender differences in myocardial HSP-32 may be related to estrogen (Ye et al 2011). Also, ICH reduced cardiac HSP-27 and HSP-32 in aged rats, which may be associated with heart injury caused by ICH (Hu et al 2011).
5.3. Others
Iron has been shown to play an important role in ICH-induced brain injury in experimental animals (Gu et al 2009; Hua et al 2006; Nakamura et al 2004a; Okauchi et al 2010; Song et al 2007; Wu et al 2003) and patients (Wu et al 2006). Recent studies show that high serum ferritin levels, an iron storage protein, are independently associated with severe brain edema and poor outcomes in patients with ICH (Mehdiratta et al 2008; Perez de la Ossa et al 2010). Total serum iron in rats is increased after ICH, and this is reduced by minocycline (Zhao et al 2011a). ICH is also associated with significant oxidative damage to DNA in the perihematomal area, as assessed by 8-OHdG immunoreactivity at a number of AP sites and a marked increase in DNA single strand breaks. The iron-chelator deferoxamine reduced 8-OHdG levels following ICH (Nakamura et al 2005b). Acute ICH is associated with increased leukocyte 8-OHdG levels and decreased glutathione peroxidase activity and vitamin E levels, but only 8-OHdG is associated with ICH and the outcome at one month (Chen et al 2011b).
Leptin has recently been discussed as a novel biomarker for clinical outcomes in critical illness. Leptin levels in peripheral blood are highly associated with cerebral hemorrhagic or ischemic stroke (Soderberg et al 1999; Soderberg et al 2003) and independently predict in hospital and 1-week mortality rates of patients with ICH, as well as 6-month clinical outcomes in pediatric traumatic brain injury (Dong et al 2010; Lin et al 2012; Zhao et al 2012). Higher plasma leptin levels correlate with disease severity and markers of systemic inflammation and thus represent a novel biomarker for predicting 6-month clinical outcomes in patients with ICH (Zhao et al 2012).
6. Therapeutic targets and ongoing clinical trials
All ongoing clinical trials have been summarized in a recent review paper(Keep et al 2012). Below are major therapeutic targets and clinical trials.
6.1. Mass effect-surgical clot removal
6.1.1 Preclinical data
ICH results in a hematoma that ruptures or distorts brain connections and – depending on size – increases ICP, with the latter potentially affecting cerebral blood flow. These physical effects are generally termed the ‘mass effect’ (Keep et al 2012). While the initial mass effect occurs at the time of ICH, in some patients the hematoma continues to expand during the first 24 hours (Demchuk et al 2012). In addition, formation of perihematomal edema can further exacerbate increases in ICP and the initial mass effect may trigger other secondary mechanisms of injury (Keep et al 2012).
Multiple preclinical models have been used to examine the mass effect, including the insertions of balloons and injections of ‘inert’ masses. These have demonstrated that physical disruption alone can cause some brain injury (Mendelow 1993).
For over 50 years (McKissock et al 1961; Prasad et al 2008) there have been clinical trials of surgical clot evacuation aimed at reducing the mass effect. Evacuation may also reduce the effects of hematoma-derived factors deleterious to brain (e.g., hemoglobin/iron). There have been very few preclinical trials of clot evacuation because of the difficulties of performing surgical clot-removal studies in animals. However, in a pig model of ICH the effects of ultra-early (3.5 hr) hematoma evacuation have been examined using tPA and aspiration (Wagner et al 1999). Reduced edema formation and BBB disruption were associated with evacuation. Similarly, in a rabbit model, evacuation using urokinase (u-PA) and aspiration after 6 hours reduced perihematomal edema, disruption of the BBB, and glutamate content (Wu et al 2011b).
There are concerns over the extravascular effects of exogenous tPA in relation to ischemic (Yepes et al 2009) and hemorrhagic stroke. The latter has been studied extensively in a pig model of ICH in which tPA liquified the clot for aspiration, but there was evidence of delayed edema formation (Rohde et al 2002) and an increased inflammatory response (Thiex et al 2003). In that model, the hematoma could be removed surgically without the use of tPA, reducing inflammation but having no effect on ICH-induced edema (Thiex et al 2005). The same group of researchers has examined methods to reduce thrombolytic-related injury and found that the glutamate antagonist MK801 reduced injury, as did the use of desmoteplase, an alternate thrombolytic (Rohde et al 2008; Thiex et al 2007).
However, it should be noted that the adverse effects of tPA are debated. As noted above, Wagner et al. found reduced brain edema after tPA thrombolysis in a pig model of ICH (Wagner et al 1999) and recently, Mould et al. reported reduced perihematomal edema with tPA thrombolysis and aspiration in human patients with ICH (Mould et al 2013).
6.1.2 Past and current clinical trials of clot evacuation
Since the first clinical trial of surgical ICH evacuation (McKissock et al 1961) there have been multiple small trials with conflicting results (reviewed in Prasad et al. 2008). These trials have been based on either the use of surgery alone or surgery with a thrombolytic agent to help dissolve the clot prior to aspiration. Doubts over the utility of clot evacuation led to a large surgical trial (the Surgical Trial in Intercerebral Hemorrhage (STICH) trial, n=1033) that failed to demonstrate any benefit of clot evacuation (Mendelow et al 2005). However, questions remained over the utility of clot evacuation. For example, it was not clear whether certain patient subsets could benefit from clot evacuation. It should also be noted that ICH location is important in determining outcome. For example, hindbrain hemorrhages are particularly devastating and it is generally accepted that surgical decompression is potentially life-saving in such cases (Adeoye and Broderick 2010; Anderson et al 2010).
Sub-analysis of the STICH I trial results indicated that a subset of patients with ICH with superficial (< 1cm from cortical surface) lobar hemorrhages might benefit from clot evacuation, perhaps because of reduced surgical trauma in such patients relative to deep-seated hemorrhages. This resulted in the STICH II trial (Mendelow et al 2011), the results of which have just been reported with again no evidence of significantly improved outcome compared to medical treatment (Mendelow et al 2013).
Another question regarding clot evacuation is whether technical developments might reduce surgical trauma and produce discernible benefits. Thus, other approaches currently being tested use minimally invasive surgery in combination with hematoma lysis methods. In the Minimally Invasive Surgery plus rtPA for Intercerebral Hemorrhage Evacuation (MISTIE) trial (NCT00224770), a minimally invasive approach is being used with t-PA to assist evacuation (Morgan et al 2008) and this has recently been reported to reduce perihematomal edema (Mould et al 2013). There has also been interest in using a combination of t-PA and sonothrombolysis to increase the speed of hematoma lysis in patients (Newell et al 2011).
6.1.3 Future directions
Although on-going clinical trials are expected to advance our understanding of the potential benefits of clot evacuation in patients with ICH, few studies have yet to address the timing of clot evacuation. Although it could be argued that the earliest possible evacuation of hematomas might be best, a substantial portion of patients (~20-40%) undergo hematoma expansion during the first 24 hours (Delgado Almandoz et al 2010; Dowlatshahi et al 2011) and attempting to remove a hematoma where there is continued bleeding is potentially dangerous, particularly if a thrombolytic is being used. A trial using ultra-early clot evacuation in patients was unsuccessful (Morgenstern et al 1998).
A reliable method of identifying patients that are experiencing hematoma expansion would assist in determining when patients might more safely undergo evacuation. The ‘spot-sign’ on CT angiography has been proposed as a marker for the identification of patients that may benefit from the use of a pro-coagulant to stop hematoma expansion (SPOTLIGHT and STOP-IT trials, NCT01359202 and NCT00810888 respectively) (Chen-Roetling et al 2009b). There is also some evidence that administration of the Factor VIIa pro-coagulant in combination with clot evacuation limits rebleeding (Imberti et al 2012; Sutherland et al 2008).
As noted above, t-PA can be used to induce thrombolysis and allow the aspiration of hematomas, but there are some concerns over the potential extravascular effects of t-PA. A recent meta-analysis has suggested that u-PA, an alternate thrombolytic, is superior to t-PA for IVH clot evacuation (Gaberel et al 2011).
It should be noted that surgical clot evacuation – with or without a thrombolytic – is incomplete. For example, Dye et al. (Dye et al 2012) reported that an average of 21% of a hematoma remains after endoscopic surgery using t-PA for clot lysis prior to aspiration. This, together with the required delay before evacuation, suggests that even if current clinical trials of evacuation are successful in reducing ICH-induced brain injury and mortality, use in combination with another therapeutic may be beneficial. For example, this might involve promotion of the phagocytosis of the remaining hematoma or administration of agents that reduce hematoma-induced neurotoxicity (see sections below).
6.2. Mass-erythrocyte phagocytosis
6.2.1 Preclinical data
Enhancing the endogenous mechanisms involved in hematoma resolution is an alternative strategy in the treatment of ICH that may be able to overcome the brain trauma associated with surgical clot removal. Although there is relatively little known about the mechanisms involved in hematoma resolution and how they are regulated, resolution may involve the lysis of red blood cell (RBC), in which energy depletion and complement are thought to play a role (Ducruet et al 2009). Phagocytosis of RBCs by microglia and/or infiltrating macrophages has also been suggested as having roles in resolution (Zhao 2009). It will be important to delineate the mechanisms involved for improved assessment of ICH-induced injury, e.g., RBC lysis (but not phagocytosis) will release intracellular contents into the brain extracellular space with possible neurotoxic effects. Indeed, injection of lysed RBCs into rat brain causes extensive brain damage (Wu et al 2002; Xi et al 1998a).
The time course of hematoma resolution differs is slower in human ICH than in commonly used animal models (Xi et al 2006). As such, the relative importance of different clearance mechanisms may differ between species.
One potential method of enhancing clot resolution is through the use of PPARγ agonists such as pioglitazone. These have been shown to enhance phagocytosis by microglia/macrophages and accelerate the rate of clot resolution in a rodent model of ICH (Zhao 2009; Zhao et al 2006; Zhao et al 2007a).
However, PPARγ agonists are pleiotropic agents and may have actions beyond accelerating clot resolution. For example, PPARγ agonists have anti-inflammatory effects, induce anti-oxidant defense mechanisms (e.g., catalase), reduce excitotoxicity, and upregulate anti-apoptotic genes (Zhao et al 2007a). Inflammation, oxidative stress, excitotoxicity, and apoptosis have all been proposed as having a role in secondary brain injury after ICH (Keep et al 2012).
6.2.2 SHRINC clinical trial
The preclinical data described above led to the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC; NCT00827892) clinical trial. Although principally aimed at determining the safety of pioglitazone, this dose escalation study also examined the effect of pioglitazone on hematoma/edema resolution in patients with ICH (Gonzales et al 2012). Although recently completed, the results of this study have yet to be reported.
6.2.3 Alternate Approaches
There is a growing interest in determining how to effect hematoma resolution in terms of duration and mechanism. Systemically, phagocytosis is essential for removing old or damaged RBCs. Cell-surface molecules act as ‘eat-me’ (e.g., phosphatidylserine) or ‘don't eat me’ (e.g., CD47) signals to potential phagocytes (Brown and Neher 2012). These signals interact with specific macrophage receptors (e.g., CD47 interacts with SIRPα) regulating macrophage function (Brown and Neher 2012). After ICH, the CD36 receptor on microglia/macrophages is important in regulating RBC phagocytosis and the effects of PPARγ agonists on phagocytosis is mediated by CD36 upregulation (Zhao 2009). As we gain a greater understanding of how hematoma resolution occurs, it should be possible to develop new methods to regulate this process , for example by enhancing hematoma clearance by upregulating ‘eat-me’ or down-regulating ‘don't eat me’ signals.
Another potential approach is to prevent RBC lysis and the associated release of hemoglobin/iron into the brain extracellular space. Activation of the complement system, and insertion of the membrane attack complex, can cause RBC lysis. The complement system is activated after ICH and complement inhibition is reduced after ICH-induced brain injury in rat (Ducruet et al 2009; Hua et al 2000; Xi et al 2001a). However, complement activation may have effects on brain injury that are not related to hematoma resolution (Ducruet et al 2009; Hua et al 2000; Xi et al 2001a). Moreover, components of the complement system (C1q and C3b) induce RBC phagocytosis (Brown and Neher 2012). Further research is therefore required to identify how best to manipulate the complement system in the treatment of ICH.
6.3. Brain iron overload and deferoxamine
6.3.1. Preclinical data
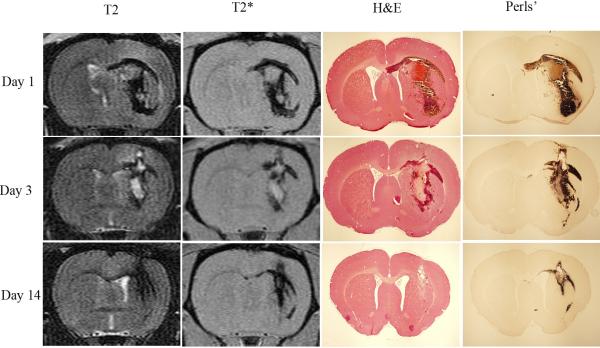
Iron has a major role in brain damage following ICH (Wagner et al 2003; Xi et al 2006). Brain injury after ICH appears to involve several phases (Xi et al 2006), including an early phase involving the clotting cascade activation and thrombin production (Gebel et al 1998; Lee et al 1996; Lee et al 1997; Wagner et al 1996; Xi et al 1998b) and a later phase involving erythrocyte lysis and iron toxicity (Huang et al 2002; Nakamura et al 2004a; Wagner et al 2003; Wu et al 2006; Wu et al 2003; Xi et al 1998a). After erythrocyte lysis within the hematoma, iron concentrations in the surrounding brain can dramatically increase (Figure 4). A 3-fold increase in brain non-heme iron follows ICH in rats with levels remaining high for at least one month (Wu et al 2003). Brain iron overload causes brain edema in the acute phase of ICH and brain atrophy at later phases.
Figure 4.
T2 and T2* MRI, H&E, and Perls’ staining in a rat model of intracerebral hemorrhage at 1, 3 and 14 days post-hemorrhage (Wu et al 2010).
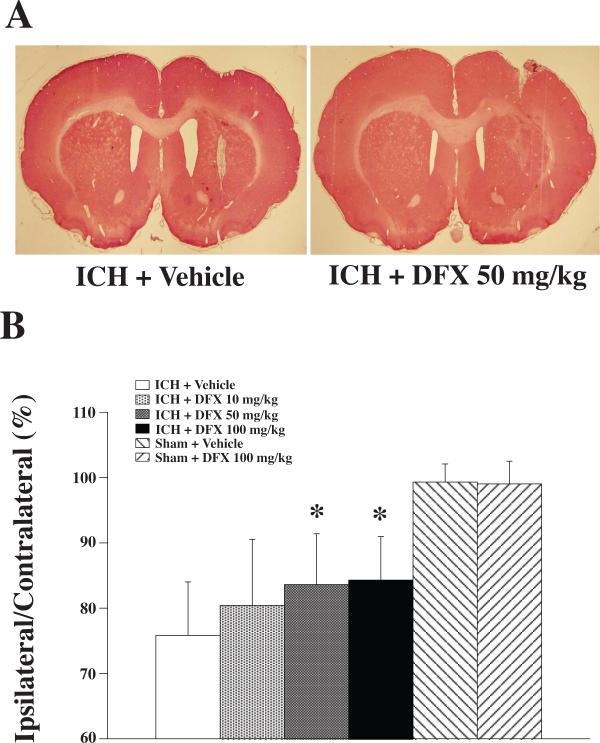
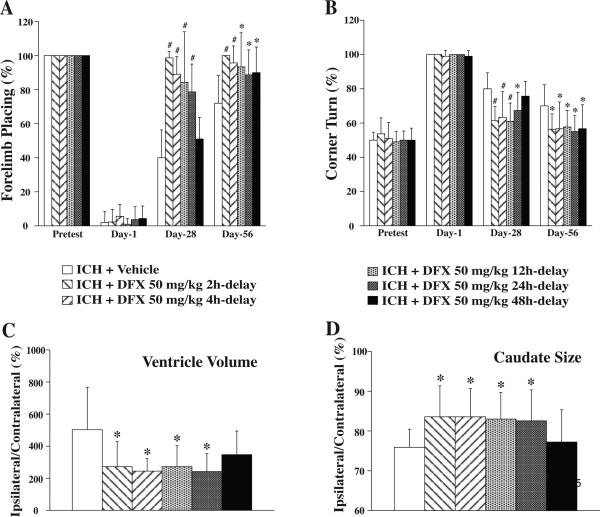
The iron chelator deferoxamine has been shown to reduce ICH-induced brain edema, neuronal death, brain atrophy, and neurological deficits in young rats (Hua et al 2006; Nakamura et al 2004a; Song et al 2007). Clinical data also suggest a role for iron in ICH-induced brain injury, with clot lysis shown to be associated with the development of perihematomal edema (Wu et al 2006). Recent studies show that high levels of serum ferritin, an iron storage protein, are independently associated with poor outcomes and severe brain edema in patients with ICH (Mehdiratta et al 2008; Perez de la Ossa et al 2010). A translational project was therefore conducted to determine an optimal dose, a therapeutic time window, and optimal treatment durations for deferoxamine (Okauchi et al 2009). Male Fischer 344 rats (18-months old) had an intracaudate injection of 100 μL autologous whole blood and were treated with varying doses of deferoxamine (10, 50 and 100 mg/kg) or vehicle at 2 and 6 hours post-ICH, and then every 12 hours for up to 7 days. Behavioral tests were performed throughout the experiments and rats were sacrificed at days 3 and 56 for brain edema determination and brain atrophy measurement respectively. All tested doses of deferoxamine attenuated perihematomal brain edema at 3 days post-ICH, whereas 50 and 100 mg/kg deferoxamine also reduced ICH-induced ventricle enlargement, caudate atrophy, and ICH-induced neurological deficits in aged rats. Although 10 mg/kg deferoxamine reduced ventricle enlargement and forelimb placing deficits, this concentration did not reduce caudate atrophy or corner turn deficits (Figure 5). These results indicate that deferoxamine can reduce ICH-induced brain injury in aged as well as young rats and that a dose of greater than 10 mg/kg is optimal in this model.
Figure 5.
A: Coronal gross H&E sections eight weeks after intracerebral hemorrhage (ICH) and treatment with vehicle or deferoxamine (DFX; 50 mg/kg). B: Caudate size expressed as a percentage of the contralateral side. Values are expressed as the means ± SD. *p<0.05, #p<0.01 vs. ICH + Vehicle group (Okauchi et al 2009).
Potential therapeutic time windows and durations for deferoxamine administration have also been investigated in rats (Okauchi et al 2010). Aged male Fischer 344 rats (18-month old) received an intra-caudate injection of 100 μL autologous whole blood, followed by intramuscular deferoxamine or vehicle with varying start times and duration periods. Subgroups of rats were sacrificed at post-ICH day 3 and 56 for brain edema measurement and brain atrophy determination respectively, and behavioral tests were conducted on days 1, 28 and 56. If started within the first 12 hours following ICH, systemic administration of deferoxamine was shown to reduce brain edema. If started within 2 hours of ICH and administered for 7 days or more, deferoxamine treatment attenuated ICH-induced ventricle enlargement, caudate atrophy, and neurological deficits. When deferoxamine treatment started within 24 hours and administered for 7 days ICH-induced brain atrophy and neurological deficits were attenuated without detectable side effects (Figure 6).
Figure 6.
Therapeutic time window of deferoxamine (DFX) for use in treating brain atrophy and improving functional outcome. A: Forelimb placing test; B: Corner turn test; C: Ventricle volume expressed as a percentage of the contralateral side at eight weeks post-intracerebral hemorrhage (ICH); D: Caudate size expressed as a percentage of the contralateral side at eight weeks post-ICH. Values are expressed as the means ±SD. *p<0.05, #p<0.01 vs. ICH+Vehicle group, respectively (Okauchi et al 2010).
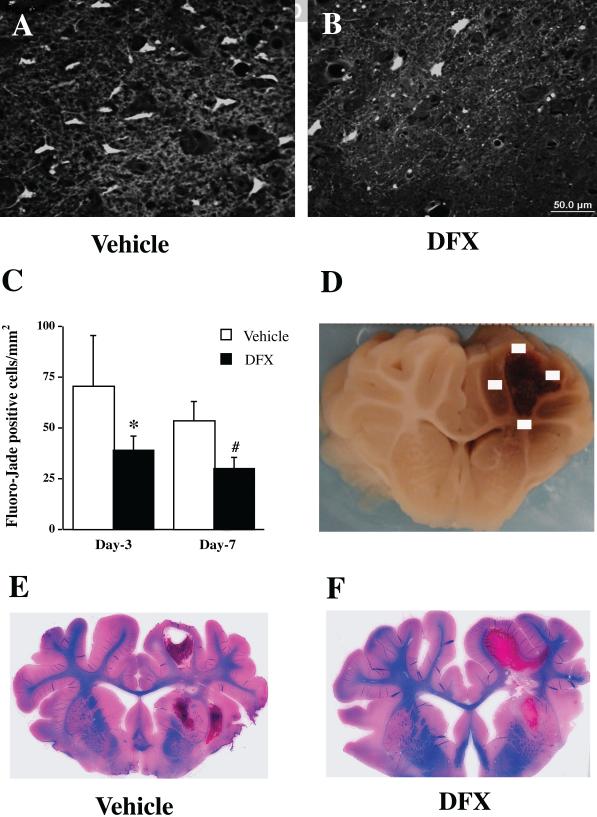
Similar deferoxamine studies have also been performed in large animals, and these are critical for translational research. In a pig model of ICH, autologous blood was injected into the right frontal lobe and either deferoxamine (50 mg/kg, IM) or vehicle were administered 2 hours post-ICH and then every 12 hours up to 7 days (Gu et al 2009). Animals were sacrificed at post-ICH day 3 or 7 and iron accumulation, white matter injury, and neuronal death were examined. A reddish zone developed around the hematoma in all ICH pigs (n=16) and deferoxamine treatment significantly reduced this zone at post-ICH days 3 and 7. Enhanced Perls’ reaction revealed good spatial correlation between iron accumulation and the reddish zone. Deferoxamine also reduced the number of perihematomal Perls’ positive cells, ferritin positive cells, neuronal death, and white matter damage (Figure 7) (Gu et al 2009).
Figure 7.
Fluoro-Jade C positive cells in the perihematomal area (A-C) and Luxol fast blue staining (E & F) post- intracerebral hemorrhage (ICH). Fluoro-Jade C staining was used to detect neuronal degeneration. Luxol fast blue-stained was used to measure white matter. Part D shows four sampled fields for Fluoro-Jade C cell counting. Pigs had ICH and were treated with either vehicle or deferoxamine. Values are means ±SD. *p<0.05, #p<0.01 vs. vehicle, respectively. n=4. Scale bar = 50 μm (A & B) (Gu et al 2009).
The effects of deferoxamine on post-ICH brain injury were also tested in other animal models, with mixed results (Warkentin et al 2010; Wu et al 2011c). In a mouse model of ICH induced by collagenase injection, systemic use of deferoxamine reduced brain iron levels, neuronal death, inflammation, and neurological deficits. However, deferoxamine did not reduce brain edema in this model (Wu et al 2011c). In contrast, deferoxamine failed to reduce brain edema and neurological deficits in collagenase-induced ICH in rats (Warkentin et al 2010).
It should be noted that, however, although deferoxamine is an iron chelator, it also can activate hypoxia inducible factor-1α and inhibit Prolyl 4-hydroxylase activity which may lead to protection from oxidative-stress induced cell death (Aminova et al 2005; Siddiq et al 2008).
6.3.2. Deferoxamine-ICH phase I and phase II
A NIH-funded phase I trial of deferoxamine has been recently performed to test the safety and tolerability of deferoxamine in patients with ICH (Selim et al 2011). This multicenter, dose-determining, phase I trial applied the Continual Reassessment Method with deferoxamine given by intravenous infusion for 3 days and treatment started within 18 hours of ICH onset. Twenty patients with ICH were enrolled with 7 mg/kg/day as the starting dose and 62 mg/kg/day as the maximum tolerated dose. Their results demonstrated that consecutive daily intravenous infusion of deferoxamine in Patients with ICH is safe and well tolerated.
A phase II trial (NCT01662895) of high-dose deferoxamine in intracerebral hemorrhage (HI-DEF) is currently underway. Its aim is determine whether treatment with deferoxamine mesylate is sufficiently promising in regard to to improving functional outcomes before pursuing a phase III clinical trial to examine its effectiveness as a treatment for ICH.
6.3.3. Alternate approaches
A recent study has shown that minocycline reduces brain iron overload following ICH and attenuates iron-induced brain edema (Zhao et al 2011b). Minocycline is a potent inhibitor of microglia activation and has been reported to provide neurovascular protection by inhibiting microglia or reducing matrix metalloproteinase (Murata et al 2008; Tikka et al 2001). Moreover, minocycline is also an iron chelator (Grenier et al 2000) and has been recently shown to attenuate iron neurotoxicity in cortical neuronal culture by chelating iron (Chen-Roetling et al 2009a). Thus, minocycline may show greater protection than an agent targeting iron or inflammation alone.
6.4. Lowering blood pressure after hematoma enlargement
6.4.1. Preclinical data
Collagenase-induced models of ICH have been used for preclinical studies of hematoma enlargement, but the results on the effects of blood pressure on hematoma expansion have been inconsistent. In a rat model of ICH, hypertension is associated with larger hematoma (Bhatia et al 2012). However, Wu et al. (Wu et al 2011a) found no difference in hemorrhage volume between spontaneously hypertensive rats compared to normotensive controls after collagenase injection. It is possible that acute changes in blood pressure rather than chronic hypertension may be more important in hematoma expansion. For example, Benveniste et al. (Benveniste et al 2000) examined ICH after biopsy and found no difference in hemorrhage volume between spontaneously hypertensive rats and normotensive controls rats, but did observe increased hemorrhage in normotensive rats subjected to acute increases in blood pressure.
6.4.2. INTERACT, ICH ADAPT, and ATACH-II
Several recently completed or ongoing trials have addressed the potential of lowering blood pressure in hematoma expansion, The Intensive Blood Pressure Reduction In Acute Cerebral Hemorrhage Trial (INTERACT 1/2)(Anderson et al 2010; Anderson et al 2013), The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH ADAPT) (Butcher et al 2010) and Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH-II) trial (Qureshi and Palesch 2011).
In INTERACT, patients with ICH were randomly assigned to an intensive (target systolic blood pressure to 140 mmHg) or standard guideline-based management of blood pressure (target systolic blood pressure to 180 mm Hg) using routine intravenous agents. INTERACT2 found that intensive lowering of blood pressure did not reduce mortality or severe disability, nor did it reduce hematoma expansion. However, an ordinal analysis of modified Rankin scores showed improved functional outcomes (Anderson et al 2013). The final interpretation of these results will likely await the results of the other blood pressure trials.
The hypothesis of ICH ADAPT is that the reduction of blood pressure does not result in significant or harmful changes in cerebral blood flow in patients with acute ICH. Two hours after randomization to a systolic blood pressure target of <150 or <180 mmHg, cerebral blood flow is measured using computed tomography perfusion (Butcher et al 2010). ATACH-II is a multi-center, randomized Phase III trial in which intravenous nicardipine will be used within 3 hours of the onset of ICH to reduce systemic blood pressure to ≤ 140 mmHg. It is yet unknown whether this will show long-term therapeutic benefits but evidence of reduced hematoma expansion has been reported (Qureshi et al 2010).
6.4.3. Alternate approaches
Factor VIIa has been shown to reduce early hematoma enlargement in a rat model of ICH (Kawai et al 2006), while several onging studies are investigating collagenase-induced ICH in warfarin treated animals (Illanes et al 2011; Lauer et al 2013). Illanes et al. (Illanes et al 2011) have examined the effects of a variety of methods of reversing warfarin anti-coagulation on ICH in mice. They found smaller hemorrhages with concentrated pro-thrombin complex and frozen plasma. FVIIa and tranexamic acid had less of an effect. The inhibition of plasma kallikrein is an alternative approach to reducing hematoma expansion. A recent study has found that it inhibits platelet aggregation and hematoma expansion (Liu et al 2011a).
6.5. Hemorrhage intraventricular extension and hydrocephalus
Non-traumatic, spontaneous ICH is associated with IVH in 42-55% of cases. IVH is an independent predictor of worse outcomes with mortality rates of 29-78%, compared to 5-29% for ICH without IVH (Hanley 2009). Moreover, the volume of blood within the ventricle is associated with outcome in IVH, and >20 ml of blood in the ventricle is independently associated with a worse outcome (Sumer et al 2002; Tuhrim et al 1999). Intraventricular hemorrhagic extension is more common when the focus of hemorrhage is adjacent to the ventricle, such as in the thalamus or caudate (Hallevi et al 2008; Sykora et al 2012). One study reported a 100% incidence of IVH in caudate ICH (Hallevi et al 2008). Large volume ICH and hypertension are also associated with intraventricular hemorrhagic extension (Pang et al 1986; Steiner et al 2006). In addition, the location of the hematoma in the 3rd and 4th ventricle may contribute to poor outcome by causing autonomic dysfunction (Hallevi et al 2012; Sykora et al 2012).
There are several IVH grading scales based on the percentage of the ventricular system that is filled with blood and the distension of the ventricles (Graeb et al 1982; Hallevi et al 2009; Hwang et al 2012; LeRoux et al 1992; Morgan et al 2013), some of which are used to help predict prognosis.
IVH can result in acute hydrocephalus by obstruction of the ventricular system or extraventricular compression from ICH (Lodhia et al 2006; Zazulia 2008). Acute hydrocephalus is associated with increased ICP, reduced cerebral perfusion, and death, and is an independent predictor of mortality in ICH with IVH extension (Mayfrank et al 1997; Pang et al 1986; Stein et al 2010). Clots localized adjacent to the ventricle are associated with hydrocephalus (Mayfrank et al 2000; Pang et al 1986; Sumer et al 2002). Chronically, IVH may also result in hydrocephalus, and although the mechanism is still unclear it probably involves an inflammatory-mediated pathway and scarring of the CSF outflow pathways.
The fact that spontaneous decompression of ICH into the ventricular system does not improve outcome (Hallevi et al 2008), combined with increased morbidity and mortality of IVH in the setting of ICH, highlights the potential deleterious effects of blood within the ventricular system. As a result, the majority of recent clinical research on IVH has focused on hastening removal of intraventricular blood through primarily catheter-directed thrombolysis with u-PA, and more recently intraventricular recombinant t-PA , ultrasound, and endoscopic removal.
6.5.1. Preclinical data
There are several models of adult IVH, all of which are variations on injection of blood products into the ventricle. Pang et al. (Pang et al 1986) studied IVH in a canine model in which 9 ml of preclotted autologous blood was injected. The thrombolytic u-PA hastened clot resolution, decreased ventricular size, and reduced the amount of periventricular injury. Mayfrank et al, (1997) developed a porcine model of IVH by injecting 10 ml of autologous blood along with thrombin into the ventricle. Treatment with rt-PA decreased ventricle size and accelerated clearance of the hemorrhage (Mayfrank et al 1997; Ment et al 1982). However, this model is limited in its applications as IVH was induced by co-injection of blood along with thrombin, which alone is sufficient for ventricular enlargement. A rodent model of IVH, created by injection of 200 μl autologous blood into the ventricle (Lodhia et al 2006), has been used to study the potentially deleterious role of iron in post-IVH brain injury (Chen et al 2011c). The iron-handling proteins heme-oxygenase-1 and ferritin are up-regulated after IVH and treatment with the iron chelator deferoxamine decreases post-IVH ventricular enlargement. Spontaneous IVH has been induced in neonatal animal models after hypertension in the newborn beagle (Goddard et al 1980; Litrico et al 2013) and after hypotension followed by volume expansion in a beagle puppy model (Ment et al 1982). Spontaneous IVH has also been induced in premature rabbits (Chua et al 2009; Lorenzo et al 1982).
Multiple animal models have evaluated treatment with intraventricular rt-PA post-IVH (Mayfrank et al 1997; Pang et al 1986). Enhanced finbrinolysis is thought to clear blood from the ventricular more quickly and decrease the time in which the ependymal and subarachnoid spaces are in contact with blood. In these models, u-PA/t-PA reduced damage to the ependymal surface (Mayfrank et al 2000; Pang et al 1986; Qing et al 2009). A recent clinical study evaluating inflammation after intraventricular treatment with t-PA found reduced leukocyte numbers in the CSF of patients that received rt-PA (Hallevi et al 2012).
It remains unclear how IVH results in hydrocephalus. Although older theories attribute hydrocephalus to fibrosis of CSF outflow pathways, there is no robust preclinical data supporting this. Pang's often cited study concluded that only “minimal fibrosis” of the arachnoid villi was noted (Pang et al 1986). Fibrosis of the subarachnoid space leading to delayed hydrocephalus is another possibility but this remains to be substantiated.
6.5.2. CLEAR clinical trial
Current treatment recommendations for ICH with IVH or hydrocephalus are for ICP monitoring when Glasgow Coma Scale (GCS) is less than 8 and for ventricular drainage if there is a decreased level of consciousness. A CPP goal of 50-70 mmHg also is recommended. Although such management strategies have tended to be largely supportive in nature, there is a push now for active removal of intraventricular clot, as the presence and volume of clot has repeatedly been shown to be independently related to outcome (Hanley 2009). The Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR-IVH) is a series of clinical trials evaluating the safety, optimal dosing, dosing interval, and efficacy of rt-PA for treatment of IVH (Naff et al 2011; Ziai et al 2012b). This phase II trial was designed to evaluate the safety of intraventricular injection of 3 ml rt-PA (1 mg/ml) versus 3 ml saline placebo for the treatment of IVH in the setting of small (<30 ml) supratentorial ICH (Naff et al 2011). Primary safety outcome measures included death at 30 days, symptomatic bleeding, and ventriculitis. The rate of ventricular clot lysis was a secondary outcome measure. Although there were no significant differences between the treatment groups in regard to any of the safety outcomes, symptomatic bleeding occurred in 23% of patients after treatment with rt-PA compared to 5% for the placebo group. Patient characteristics were well matched between the treatment groups with the exception of having 73% males in the rt-PA group. Clot resolution occurred at a faster rate with intraventricular rt-PA (18% per day versus 8% per day for placebo), and required fewer days with an external ventricular drain (EVD) and a shorter duration of treatment. Although there was a trend for the rt-PA group to have improved outcome at 30 days on a variety of scales (i.e., Glosgow Outcome Scale, modified Rankin scale, NIH stroke scale, and Barthel index score) this result was not significantly different from that of the placebo group. A number of additional analyses of the CLEAR-IVH data have also been published (Zacharia et al 2012; Ziai et al 2012a). Clot lysis was found to be dose dependent and occurred most quickly in the midline ventricles (Adams and Diringer 1998; Webb et al 2012). In addition, for the rt-PA group, higher baseline levels of plasminogen and lower baseline platelet counts are associated with faster initial clot lysis (Sumer et al 2002; Ziai et al 2012b). A phase III CLEAR-IVH clinical trial to assess long-term outcome is currently underway (clinicaltrials.gov NCT00784134).
Additional studies with u-PA, which has now been withdrawn from the market in the United States, have added to our knowledge of how thrombolysis acts during ICH associated IVH. A small randomized trial investigating intraventricular u-PA found that u-PA was safe and increased the rate of clot resolution. However, there was no demonstrable difference in outcome for those treated with u-PA versus those in the placebo group (Huttner et al 2007; King et al 2012). An earlier study of 20 patients evaluated in a combined open label (12 patients) and randomized (8 patients) fashion found improved survival at 30 days with EVD plus u-PA, compared with EVD alone (Naff et al 2000). This was followed by a randomized double blind placebo controlled trial to evaluate the safety of intraventricular u-PA. However, enrollment was terminated when u-PA was withdrawn from the U.S. market and the study was limited to 12 patients (Naff et al 2004). A recent meta-analysis evaluating EVD and EVD + u-PA or rt-PA included 4 randomized and 8 observational studies. Overall mortality was reduced in the EVD + fibrinolytic group. However, when outcome by fibrinolytic type was evaluated, only u-PA was associated with decreased mortality (Gaberel et al 2011; Staykov et al 2009).
Treatment with rt-PA is not without risk,and given the generally larger numbers of symptomatic bleeding events in those treated with intraventricular thrombolysis/EVD compared with EVD alone, the risk of hemorrhaging should be viewed as substantial. The safety of rt-PA appears to be increased when all ventricular catheter fenestrations are within the ventricle, as reported in a retrospective review of 27 patients treated with intraventricular rt-PA (Jackson et al 2012). The association between neuronal degeneration and t-PA in animal models (Tsirka et al 1995) has led to the evaluation of edema surrounding the hematoma after treatment with t-PA. Two retrospective studies have not identified a significant difference in edema to hematoma volume ratio in patients treated with intraventricular rt-PA versus external ventricular drainage (EVD) alone (Volbers et al 2013; Ziai et al 2013). However, another retrospective study found a significant increase in peri-hematoma edema at 3 and 4 days post admission after treatment with intraventricular rt-PA (Ducruet et al 2010). Current AHA/ASA guidelines for management of spontaneous ICH state that intraventricular rt-PA treatment is investigational as the efficacy is not yet known (Morgenstern et al 2010).
6.5.3. Alternate approaches
Post-ICH hydrocephalus is common and various studies have attempted to evaluate management strategies for this clinical condition. Trials investigating treatment with intraventricular t-PA have been primarily focused on morbidity and mortality as the primary outcomes. Development of chronic hydrocephalus requiring a shunt occurs in 20-28% of patients with ICH who receive ventriculostomy, yet the need for a shunt is often a secondary outcome measure (Miller et al 2008; Zacharia et al 2012). Although a number of variables are associated with the likelihood of needing a shunt, only thalamic location of hemorrhage and elevated ICP have been found to be independently associated with need for shunt. In such cases, the rates of chronic hydrocephalus after thalamic hemorrhage with IVH were as high as 66-68% (Chen et al 2011a; Miller et al 2008; Zacharia et al 2012).
Acute hydrocephalus is often managed with EVD or lumbar drainage, but more often the former (Sumer et al 2002). In a retrospective case series evaluation, EVD was not associated with improved outcome or reduction in ventricular size. Moreover, when there was a change in ventricular size it did not correspond to a change in level of consciousness (Adams and Diringer 1998). However, in a meta-analysis, EVD alone reduced mortality rate to 58% from 78% for conservative management of SAH or ICH-associated IVH (Nieuwkamp et al 2000). A combination of EVD followed by lumbar drainage after the obstructive component has cleared has been reported and long-term shunt rates in this retrospective study were lower with the addition of lumbar drainage (Huttner et al 2006b; Huttner et al 2007). A prospective non-randomized study has evaluated EVD and intraventricular fibrinolysis with rt-PA until clearance of 3rd and 4th ventricular blood followed by lumbar drain if the patient failed an EVD clamping trial. Rates of shunting were lowest in this group compared with historical controls of EVD alone, EVD combined with lumbar drain, and EVD and intraventricular fibrinolysis with rt-PA (Staykov et al 2009). Treatment of IVH-associated thalamic hemorrhage with EVD alone or EVD followed by endoscopic removal of hemorrhage was evaluated in a prospective randomized study. Although there was no difference in outcome or mortality between groups, there was a decrease in the need for ventricular shunting in the group that underwent endoscopic surgery (48 versus 90%) (Bateman et al 2006; Chen et al 2011a).
Endoscopic third ventriculostomy (ETV) has also been described as a treatment for acute obstructive hydrocephalus secondary to IVH (Ballabh 2010; Oertel et al 2009). A combination of endoscopic evacuation of intraventricular hematoma (through bilateral burr holes) along with ETV and EVD placement was effective in 24 of 25 patients in preventing long term hydrocephalus (Yadav et al 2007). A separate case series of 13 patients treated with endoscopic removal of IVH via a flexible endoscope reported that this procedure appeared safe, with none of the patients in this series developing hydrocephalus (Longatti et al 2004). However, there are concerns over this procedure for the management of IVH in the acute period, as small fragments of clot may cause delayed obstructive hydrocephalus. Similarly, treatment with antifibrinolytic agents has been suggested as potentially increasing the rates of hydrocephalus after hemorrhage (Harrigan et al 2010; Naff et al 2011).
The DITCH (Dutch Intraventricular Thrombolysis after Cerebral Hemorrhage study) trial is currently underway to evaluate ventricular drainage and thrombolysis with t-PA on outcome 3 months post-ICH with intraventricular extension.
Endoscopic removal of IVH is appealing as case series have thus far reported low rates of hydrocephalus. As the presence of an EVD may prolong stay in an ICU (Huttner et al 2006a), initial management to clear intraventricular blood with an endoscope may provide more immediate removal of blood and less reliance on serial rt-PA injections into a catheter. A clinical trial is currently examining evaluate neurologic outcomes in patients with IVH, hydrocephalus, and an opening ICP of at least 20 mmHg treated with intraventricular rt-PA versus endoscopic removal of clot (clinicaltrials.gov-NCT01064011).
Treatment of intraventricular clot with rt-PA has been augmented with catheter-directed thrombolysis with ultrasound (Newell et al 2011). Three patients with IVH were treated in this study and had a reduction in the size of their clot. Although this study is limited by the small sample size and a retrospective control group, catheter-directed and focused ultrasound therapy for clot lysis may present potential areas of future investigation. A phase II/III trial is underway to assess need for permanent VP shunt after ICH (<60 ml) and IVH with casting of the 3rd and 4th ventricles treated with EVD plus rt-PA versus EVD, rt-PA and lumbar drainage after opening on the 3rd and 4th ventricles (clinicaltrials.gov, NCT 01041950).
7. Future Directions
There has also been a marked increase in preclinical research into cerebral hemorrhage in the past decade, resulting in the identification of novel potential therapeutic targets (Keep et al 2012) that are starting to form the basis of clinical trials (Selim et al 2011). As with other neurological conditions, there are concerns over whether animal models recapitulate the human disease and whether preclinical efficacy will translate to the clinic. Determining the validity of preclinical models is a major goal.
There are many approaches to try and modulate hematoma size. Reducing hematoma expansion, promoting endogenous hematoma clearance or clot evacuation. Each method has potential advantages and disadvantages (e.g. potential side-effects) and a greater understanding is needed of relative merits.
While information has been gained into the role of clot-derived factors (e.g. thrombin and iron) in ICH-induced brain injury, it is likely that many other factors in the clot modulate brain function (enhancing or reducing injury). In addition, how these factors interact in the setting of ICH is largely unknown. These are potentially important areas for future research.
Patients with similar hematomas may have very different outcomes. The underlying basis for such differences is largely unknown. Understanding such differences may give insight into how to develop therapies for ICH as well as guiding patient-based therapy.
8. Conclusions
Our understanding of the mechanisms involved in ICH-induced injury have increased in the last two decades and there are now multiple, potentially pivotal, ongoing clinical trials, creating hope that effective treatments for these devastating forms of stroke may be discovered. The end may be in sight. However, it should be noted that others have thought this before and that ICH covers a spectrum of conditions dependent upon location and size of the hematoma. It is, therefore, likely that no one therapeutic approach (if one is discovered) will be best for all patients and, even in a single patient, a combination of approaches may provide the best possibility of a positive outcome (Morgenstern 2012).
Highlights.
Causes of bleeding
Natural history of ICH
ICH animal models
Systemic responses after ICH
Therapeutic targets and ongoing clinical trials
Acknowledgment
This work was supported by grants NS-034709, NS-057539, NS-073595, NS-079157, NS-084049 and NS-007222 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of abbreviations
- BBB
blood-brain barrier
- ECG
electrocardiogram
- ETV
endoscopic third ventriculostomy
- EVD
external ventricular drain
- ICH
Intracerebral hemorrhage
- ICP
intracranial pressure
- IL-2
interleukin-2
- IVH
intraventricular hemorrhage
- PPAR
peroxisome proliferator activated receptor
- RBC
red blood cell
- rt-PA
recombinant t-PA
- SAH
subarachnoid hemorrhage
- tPA
tissue plasminogen activator
- u-PA
urokinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998;50:519–23. doi: 10.1212/wnl.50.2.519. [DOI] [PubMed] [Google Scholar]
- Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol. 2010;6:593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- Al-Holou WN, O'Lynnger TM, Pandey AS, Gemmete JJ, Thompson BG, Muraszko KM, Garton HJ, Maher CO. Natural history and imaging prevalence of cavernous malformations in children and young adults. Journal of neurosurgery Pediatrics. 2012;9:198–205. doi: 10.3171/2011.11.PEDS11390. [DOI] [PubMed] [Google Scholar]
- Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, Lamanna JC, Ratan RR. Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem. 2005;280:3996–4003. doi: 10.1074/jbc.M409223200. [DOI] [PubMed] [Google Scholar]
- Anderson CS, Huang Y, Arima H, Heeley E, Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Morgenstern LB, Chalmers J, Wang JG. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41:307–12. doi: 10.1161/STROKEAHA.109.561795. [DOI] [PubMed] [Google Scholar]
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B, Hata J, Arima H, Parsons M, Li Y, Heritier S, Li Q, Woodward M, Simes RJ, Davis SM, Chalmers J. Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage. N Engl J Med. 2013 [Google Scholar]
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke; a journal of cerebral circulation. 2003;34:2060–5. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- Auriat AM, Wowk S, Colbourne F. Rehabilitation after intracerebral hemorrhage in rats improves recovery with enhanced dendritic complexity but no effect on cell proliferation. Behavioural Brain Research. 2010;214:42–7. doi: 10.1016/j.bbr.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, Berman MF. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006;67:424–9. doi: 10.1212/01.wnl.0000228277.84760.a2. [DOI] [PubMed] [Google Scholar]
- Belayev L, Saul I, Curbelo K, Busto R, Belayev A, Zhang Y, Riyamongkol P, Zhao W, Ginsberg MD. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke; a journal of cerebral circulation. 2003;34:2221–7. doi: 10.1161/01.STR.0000088061.06656.1E. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Kim KR, Hedlund LW, Kim JW, Friedman AH. Cerebral hemorrhage and edema following brain biopsy in rats: significance of mean arterial blood pressure. Journal of Neurosurgery. 2000;92:100–7. doi: 10.3171/jns.2000.92.1.0100. [DOI] [PubMed] [Google Scholar]
- Betz AL, Iannotti F, Hoff JT. Brain edema: A classification based on blood-brain barrier integrity. Cereb Brain Metab Rev. 1989;1:133–54. [PubMed] [Google Scholar]
- Bhatia P, Chamberlain R, Luo X, Hartley E, Divani A. Elevated Blood Pressure Causes Larger Hematoma in a Rat Model of Intracerebral Hemorrhage. Translational Stroke Research. 2012;3:428–34. doi: 10.1007/s12975-012-0199-0. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Vaughan KA, Zacharia BE, Hickman ZL, Connolly ES. The Molecular Mechanisms that Promote Edema After Intracerebral Hemorrhage. Translational Stroke Research. 2012;3:S52–S61. doi: 10.1007/s12975-012-0162-0. [DOI] [PubMed] [Google Scholar]
- Broderick J, Brott T, Kothari R. Very early edema growth with ICH. Stroke. 1995;26:184. [Google Scholar]
- Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J. Ultra-early evaluation of intracerebral hemorrhage. Journal of Neurosurgery. 1990;72:195–9. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends in Biochemical Sciences. 2012;37:325–32. doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: Description of technique, ICP changes and neuropathological findings. Neurological research. 1984;6:184–8. doi: 10.1080/01616412.1984.11739687. [DOI] [PubMed] [Google Scholar]
- Bullock R, Brock-Utne J, van Dellen J, Blake G. Intracerebral hemorrhage in a primate model: effect on regional cerebral blood flow. Surgical neurology. 1988;29:101–7. doi: 10.1016/0090-3019(88)90065-1. [DOI] [PubMed] [Google Scholar]
- Butcher K, Jeerakathil T, Emery D, Dowlatshahi D, Hill MD, Sharma M, Buck B, Findlay M, Lee TY, Demchuk AM. The Intracerebral Haemorrhage Acutely Decreasing Arterial Pressure Trial: ICH ADAPT. International journal of stroke. 2010;5:227–33. doi: 10.1111/j.1747-4949.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, Waeber C. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke; a journal of cerebral circulation. 2013;44:505–11. doi: 10.1161/STROKEAHA.112.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Liu CL, Tung YN, Lee HC, Chuang HC, Lin SZ, Cho DY. Endoscopic surgery for intraventricular hemorrhage (IVH) caused by thalamic hemorrhage: comparisons of endoscopic surgery and external ventricular drainage (EVD) surgery. World Neurosurg. 2011a;75:264–8. doi: 10.1016/j.wneu.2010.07.041. [DOI] [PubMed] [Google Scholar]
- Chen YC, Chen CM, Liu JL, Chen ST, Cheng ML, Chiu DT. Oxidative markers in spontaneous intracerebral hemorrhage: leukocyte 8-hydroxy-2'-deoxyguanosine as an independent predictor of the 30-day outcome. Journal of neurosurgery. 2011b;115:1184–90. doi: 10.3171/2011.7.JNS11718. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke; a journal of cerebral circulation. 2011c;42:465–70. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun. 2009a;386:322–6. doi: 10.1016/j.bbrc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Li Z, Chen M, Awe OO, Regan RF. Heme oxygenase activity and hemoglobin neurotoxicity are attenuated by inhibitors of the MEK/ERK pathway. Neuropharmacology. 2009b;56:922–8. doi: 10.1016/j.neuropharm.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung RT, Hachinski V. The insula and cerebrogenic sudden death. Arch Neurol. 2000;57:1685–8. doi: 10.1001/archneur.57.12.1685. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr., Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke; a journal of cerebral circulation. 1997;28:2296–302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke; a journal of cerebral circulation. 2009;40:3369–77. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke; a journal of cerebral circulation. 1998;29:2136–40. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- Deibert E, Aiyagari V, Diringer MN. Reversible left ventricular dysfunction associated with raised troponin I after subarachnoid haemorrhage does not preclude successful heart transplantation. Heart. 2000;84:205–7. doi: 10.1136/heart.84.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR, Yan HJ, Buist R, Peeling J. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke. 1996;27:2312–9. doi: 10.1161/01.str.27.12.2312. discussion 9-20. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Yan HJ, Campbell TM, Peeling J. Effect of fucoidan treatment on collagenase-induced intracerebral hemorrhage in rats. Neurological research. 1999;21:415–9. doi: 10.1080/01616412.1999.11740953. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, Wang X, Hosomi N, Mabuchi T, Koziol JA. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:919–32. doi: 10.1038/jcbfm.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, Goldstein JN, Rosand J, Lev MH, Gonzalez RG, Romero JM. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, Kobayashi A, Boulanger JM, Lum C, Gubitz G, Padma V, Roy J, Kase CS, Kosior J, Bhatia R, Tymchuk S, Subramaniam S, Gladstone DJ, Hill MD, Aviv RI. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–14. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- Dong XQ, Huang M, Hu YY, Yu WH, Zhang ZY. Time course of plasma leptin concentrations after acute spontaneous basal ganglia hemorrhage. World Neurosurg. 2010;74:286–93. doi: 10.1016/j.wneu.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–44. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducruet AF, Zacharia BE, Hickman ZL, Grobelny BT, Yeh ML, Sosunov SA, Connolly ES., Jr. The complement cascade as a therapeutic target in intracerebral hemorrhage. Experimental Neurology. 2009;219:398–403. doi: 10.1016/j.expneurol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducruet AF, Hickman ZL, Zacharia BE, Grobelny BT, Narula R, Guo KH, Claassen J, Lee K, Badjatia N, Mayer SA, Connolly ES., Jr. Exacerbation of perihematomal edema and sterile meningitis with intraventricular administration of tissue plasminogen activator in patients with intracerebral hemorrhage. Neurosurgery. 2010;66:648–55. doi: 10.1227/01.NEU.0000360374.59435.60. [DOI] [PubMed] [Google Scholar]
- Dujardin KS, McCully RB, Wijdicks EF, Tazelaar HD, Seward JB, McGregor CG, Olson LJ. Myocardial dysfunction associated with brain death: clinical, echocardiographic, and pathologic features. J Heart Lung Transplant. 2001;20:350–7. doi: 10.1016/s1053-2498(00)00193-5. [DOI] [PubMed] [Google Scholar]
- Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Journal of Neurosurgery. 2012;117:767–73. doi: 10.3171/2012.7.JNS111567. [DOI] [PubMed] [Google Scholar]
- Elrifai AM, Bailes JE, Shih SR, Dianzumba S, Brillman J. Characterization of the cardiac effects of acute subarachnoid hemorrhage in dogs. Stroke; a journal of cerebral circulation. 1996;27:737–41. doi: 10.1161/01.str.27.4.737. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- Enzmann DR, Britt RH, Lyons BE, Buxton JL, Wilson DA. Natural history of experimental intracerebral hemorrhage: sonography, computed tomography and neuropathology. Ajnr: Am J Neuroradiol. 1981;2:517–26. [PMC free article] [PubMed] [Google Scholar]
- Espiner EA, Leikis R, Ferch RD, MacFarlane MR, Bonkowski JA, Frampton CM, Richards AM. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clinical endocrinology. 2002;56:629–35. doi: 10.1046/j.1365-2265.2002.01285.x. [DOI] [PubMed] [Google Scholar]
- Fang CX, Wu S, Ren J. Intracerebral hemorrhage elicits aberration in cardiomyocyte contractile function and intracellular Ca2+ transients. Stroke; a journal of cerebral circulation. 2006;37:1875–82. doi: 10.1161/01.STR.0000227232.39582.66. [DOI] [PubMed] [Google Scholar]
- Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Annals of Neurology. 2002;51:517–24. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- Flemming KD, Link MJ, Christianson TJ, Brown RD., Jr. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012;78:632–6. doi: 10.1212/WNL.0b013e318248de9b. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage [see comments]. Journal of Neurosurgery. 1994;80:51–7. doi: 10.3171/jns.1994.80.1.0051. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–6. doi: 10.1161/01.str.29.6.1160. [DOI] [PubMed] [Google Scholar]
- Gaberel T, Magheru C, Parienti JJ, Huttner HB, Vivien D, Emery E. Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage: a meta-analysis. Stroke; a journal of cerebral circulation. 2011;42:2776–81. doi: 10.1161/STROKEAHA.111.615724. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Ho KL, Caccamo D. In: Intracerebral hemorrhage: Pathology of selected topics. In: Intracerebral Hemorrhage. Kase CS, Caplan LR, editors. Butterworths; Boston: 1994. pp. 45–72. [Google Scholar]
- Gebel JM, Sila CA, Sloan MA, Granger CB, Mahaffey KW, Weisenberger J, Green CL, White HD, Gore JM, Weaver WD, Califf RM, Topol EJ. Thrombolysis-related intracranial hemorrhage: a radiographic analysis of 244 cases from the GUSTO-1 trial with clinical correlation. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. Stroke. 1998;29:563–9. doi: 10.1161/01.str.29.3.563. [DOI] [PubMed] [Google Scholar]
- Goddard J, Lewis RM, Armstrong DL, Zeller RS. Moderate, rapidly induced hypertension as a cause of intraventricular hemorrhage in the newborn beagle model. The Journal of pediatrics. 1980;96:1057–60. doi: 10.1016/s0022-3476(80)80641-x. [DOI] [PubMed] [Google Scholar]
- Goldstein MR, Mascitelli L, Pezzetta F. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2009;72:1448. doi: 10.1212/01.wnl.0000346751.39886.01. author reply -9. [DOI] [PubMed] [Google Scholar]
- Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage. Brain Res. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–83. doi: 10.1097/00006123-200104000-00037. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–5. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Gonzales NR, Shah J, Sangha N, Sosa L, Martinez R, Shen L, Kasam M, Morales MM, Hossain MM, Barreto AD, Savitz SI, Lopez G, Misra V, Wu TC, El Khoury R, Sarraj A, Sahota P, Hicks W, Acosta I, Sline MR, Rahbar MH, Zhao X, Aronowski J, Grotta JC. Design of a prospective, dose-escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC). Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–6. doi: 10.1148/radiology.143.1.6977795. [DOI] [PubMed] [Google Scholar]
- Grenier D, Huot MP, Mayrand D. Iron-chelating activity of tetracyclines and its impact on the susceptibility of Actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob Agents Chemother. 2000;44:763–6. doi: 10.1128/aac.44.3.763-766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross BA, Du R. The natural history of cerebral dural arteriovenous fistulae. Neurosurgery. 2012a;71:594–602. doi: 10.1227/NEU.0b013e31825eabdb. discussion -3. [DOI] [PubMed] [Google Scholar]
- Gross BA, Du R. Hemorrhage from arteriovenous malformations during pregnancy. Neurosurgery. 2012b;71:349–55. doi: 10.1227/NEU.0b013e318256c34b. discussion 55-6. [DOI] [PubMed] [Google Scholar]
- Gross BA, Thomas AJ, Frerichs KU, Du R. Cerebrovascular neurosurgery in 2012. J Clin Neurosci. 2013;20:776–82. doi: 10.1016/j.jocn.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–3. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Hua Y, Wang J, Keep RF, Xi G. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Translational Stroke Research. 2012;3:130–7. doi: 10.1007/s12975-011-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, Donner A, Mamdani M, Douketis JD, Arima H, Chalmers J, MacMahon S, Tirschwell DL, Psaty BM, Bushnell CD, Aguilar MI, Capampangan DJ, Werring DJ, De Rango P, Viswanathan A, Danchin N, Cheng CL, Yang YH, Verdel BM, Lai MS, Kennedy J, Uchiyama S, Yamaguchi T, Ikeda Y, Mrkobrada M. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124:2233–42. doi: 10.1161/CIRCULATIONAHA.111.055269. [DOI] [PubMed] [Google Scholar]
- Halim AX, Johnston SC, Singh V, McCulloch CE, Bennett JP, Achrol AS, Sidney S, Young WL. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke; a journal of cerebral circulation. 2004;35:1697–702. doi: 10.1161/01.STR.0000130988.44824.29. [DOI] [PubMed] [Google Scholar]
- Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, Gonzales NR, Illoh K, Noser EA, Grotta JC. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008;70:848–52. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallevi H, Dar NS, Barreto AD, Morales MM, Martin-Schild S, Abraham AT, Walker KC, Gonzales NR, Illoh K, Grotta JC, Savitz SI. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Critical care medicine. 2009;37:969–74. e1. doi: 10.1097/CCM.0b013e318198683a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallevi H, Abraham AT, Barreto AD, Grotta JC, Savitz SI. The Spot Sign in Intracerebral Hemorrhage: The Importance of Looking for Contrast Extravasation. Cerebrovascular Diseases. 2010;29:217–20. doi: 10.1159/000267842. [DOI] [PubMed] [Google Scholar]
- Hallevi H, Walker KC, Kasam M, Bornstein N, Grotta JC, Savitz SI. Inflammatory response to intraventricular hemorrhage: time course, magnitude and effect of t-PA. Journal of the neurological sciences. 2012;315:93–5. doi: 10.1016/j.jns.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–8. doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan MR, Rajneesh KF, Ardelt AA, Fisher WS., 3rd Short-term antifibrinolytic therapy before early aneurysm treatment in subarachnoid hemorrhage: effects on rehemorrhage, cerebral ischemia, and hydrocephalus. Neurosurgery. 2010;67:935–9. doi: 10.1227/NEU.0b013e3181ebaa36. discussion 9-40. [DOI] [PubMed] [Google Scholar]
- Hays A, Diringer MN. Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology. 2006;66:1330–4. doi: 10.1212/01.wnl.0000210523.22944.9b. [DOI] [PubMed] [Google Scholar]
- He Y, Wan S, Hua Y, Keep RF, Xi G. Autophagy after experimental intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:897–905. doi: 10.1038/sj.jcbfm.9600578. [DOI] [PubMed] [Google Scholar]
- He Y, Liu W, Koch LG, Britton SL, Keep RF, Xi G, Hua Y. Susceptibility to intracerebral hemorrhage-induced brain injury segregates with low aerobic capacity in rats. Neurobiology of disease. 2012;49C:22–8. doi: 10.1016/j.nbd.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823–9. doi: 10.1227/01.NEU.0000330401.82582.5E. discussion 9-31. [DOI] [PubMed] [Google Scholar]
- Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472–7. doi: 10.1161/01.str.30.11.2472. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- Hu H, Wang L, Okauchi M, Keep RF, Xi G, Hua Y. Deferoxamine affects heat shock protein expression in heart after intracerebral hemorrhage in aged rats. Acta neurochirurgica Supplement. 2011;111:197–200. doi: 10.1007/978-3-7091-0693-8_33. [DOI] [PubMed] [Google Scholar]
- Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92:1016–22. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–84. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep R, Wu J, Schallert T, Hoff J, G X. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–12. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- Huang F, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–93. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Hurst JW. Electrocardiographic changes in intracranial hemorrhage mimicking myocardial infarction. N Engl J Med. 2003;349:1874–5. doi: 10.1056/NEJM200311063491922. author reply -5. [DOI] [PubMed] [Google Scholar]
- Huttner HB, Kohrmann M, Berger C, Georgiadis D, Schwab S. Influence of intraventricular hemorrhage and occlusive hydrocephalus on the long-term outcome of treated patients with basal ganglia hemorrhage: a case-control study. Journal of neurosurgery. 2006a;105:412–7. doi: 10.3171/jns.2006.105.3.412. [DOI] [PubMed] [Google Scholar]
- Huttner HB, Schwab S, Bardutzky J. Lumbar drainage for communicating hydrocephalus after ICH with ventricular hemorrhage. Neurocritical care. 2006b;5:193–6. doi: 10.1385/NCC:5:3:193. [DOI] [PubMed] [Google Scholar]
- Huttner HB, Nagel S, Tognoni E, Kohrmann M, Juttler E, Orakcioglu B, Schellinger PD, Schwab S, Bardutzky J. Intracerebral hemorrhage with severe ventricular involvement: lumbar drainage for communicating hydrocephalus. Stroke; a journal of cerebral circulation. 2007;38:183–7. doi: 10.1161/01.STR.0000251795.02560.62. [DOI] [PubMed] [Google Scholar]
- Hwang BY, Bruce SS, Appelboom G, Piazza MA, Carpenter AM, Gigante PR, Kellner CP, Ducruet AF, Kellner MA, Deb-Sen R, Vaughan KA, Meyers PM, Connolly ES., Jr. Evaluation of intraventricular hemorrhage assessment methods for predicting outcome following intracerebral hemorrhage. Journal of neurosurgery. 2012;116:185–92. doi: 10.3171/2011.9.JNS10850. [DOI] [PubMed] [Google Scholar]
- Illanes S, Liesz A, Sun L, Dalpke A, Zorn M, Veltkamp R. Hematoma size as major modulator of the cellular immune system after experimental intracerebral hemorrhage. Neuroscience letters. 2011;490:170–4. doi: 10.1016/j.neulet.2010.11.065. [DOI] [PubMed] [Google Scholar]
- Imberti R, Pietrobono L, Klersy C, Gamba G, Iotti G, Cornara G. Intraoperative intravenous administration of rFVIIa and hematoma volume after early surgery for spontaneous intracerebral hemorrhage: a randomized prospective phase II study. Minerva Anestesiologica. 2012;78:168–75. [PubMed] [Google Scholar]
- Jackson DA, Patel AV, Darracott RM, Hanel RA, Freeman WD, Hanley DF. Safety of Intraventricular Hemorrhage (IVH) Thrombolysis Based on CT Localization of External Ventricular Drain (EVD) Fenestrations and Analysis of EVD Tract Hemorrhage. Neurocritical care. 2012 doi: 10.1007/s12028-012-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Maxwell W, Graham D. Experimental intracerebral hematoma in the rat: sequential light microscopic changes. Neuropathol Appl Neurobiol. 1989;15:477–86. doi: 10.1111/j.1365-2990.1989.tb01247.x. [DOI] [PubMed] [Google Scholar]
- Jin H, Xi G, Keep RF, Wu J, Hua Y. DARPP-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Translational Stroke Research. 2013;4:130–4. doi: 10.1007/s12975-012-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung F, Setzer M, Hohnloser SH. Severe intracranial bleeding mimicking acute inferior myocardial infarction with right ventricular involvement. Cardiology. 2001;95:48–50. doi: 10.1159/000047343. [DOI] [PubMed] [Google Scholar]
- Kaufman HH, Pruessner JL, Bernstein DP, Borit A, Ostrow PT, Cahall DL. A rabbit model of intracerebral hematoma. Acta neuropathologica. 1985;65:318–21. doi: 10.1007/BF00687015. [DOI] [PubMed] [Google Scholar]
- Kawai N, Nakamura T, Nagao S. Early hemostatic therapy using recombinant factor VIIa in a collagenase-induced intracerebral hemorrhage model in rats. Acta neurochirurgica Supplement. 2006;96:212–7. doi: 10.1007/3-211-30714-1_46. [DOI] [PubMed] [Google Scholar]
- Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–7. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–5. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Wasay M. Haemorrhagic strokes in pregnancy and puerperium. International journal of stroke : official journal of the International Stroke Society. 2013;8:265–72. doi: 10.1111/j.1747-4949.2012.00853.x. [DOI] [PubMed] [Google Scholar]
- Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovascular diseases. 2002;14:67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- King NK, Lai JL, Tan LB, Lee KK, Pang BC, Ng I, Wang E. A randomized, placebo-controlled pilot study of patients with spontaneous intraventricular haemorrhage treated with intraventricular thrombolysis. J Clin Neurosci. 2012;19:961–4. doi: 10.1016/j.jocn.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Kingman TA, Mendelow AD, Graham DI, Teasdale GM. Experimental intracerebral mass: description of model, intracranial pressure changes and neuropathology. J Neuropathol Exp Neurol. 1988;47:128–37. doi: 10.1097/00005072-198803000-00005. [DOI] [PubMed] [Google Scholar]
- Kobari M, Gotoh F, Tomita M, Tanahashi N, Shinohara T, Terayama Y, Mihara B. Bilateral hemispheric reduction of cerebral blood volume and blood flow immediately after experimental cerebral hemorrhage in cats. Stroke; a journal of cerebral circulation. 1988;19:991–6. doi: 10.1161/01.str.19.8.991. [DOI] [PubMed] [Google Scholar]
- Lauer A, Pfeilschifter W, Schaffer CB, Lo EH, Foerch C. Intracerebral haemorrhage associated with antithrombotic treatment: translational insights from experimental studies. Lancet neurology. 2013;12:394–405. doi: 10.1016/S1474-4422(13)70049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84:91–6. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–8. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- LeRoux PD, Haglund MM, Newell DW, Grady MS, Winn HR. Intraventricular hemorrhage in blunt head trauma: an analysis of 43 cases. Neurosurgery. 1992;31:678–84. doi: 10.1227/00006123-199210000-00010. discussion 84-5. [DOI] [PubMed] [Google Scholar]
- Lin C, Huang SJ, Wang N, Shen ZP. Relationship between plasma leptin levels and clinical outcomes of pediatric traumatic brain injury. Peptides. 2012;35:166–71. doi: 10.1016/j.peptides.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Litrico S, Almairac F, Gaberel T, Ramakrishna R, Fontaine D, Sedat J, Lonjon M, Paquis P. Intraventricular fibrinolysis for severe aneurysmal intraventricular hemorrhage: a randomized controlled trial and meta-analysis. Neurosurgical review. 2013 doi: 10.1007/s10143-013-0469-7. [DOI] [PubMed] [Google Scholar]
- Liu DZ, Sharp FR. Excitatory and Mitogenic Signaling in Cell Death, Blood-brain Barrier Breakdown, and BBB Repair after Intracerebral Hemorrhage. Translational Stroke Research. 2012;3:S62–S9. doi: 10.1007/s12975-012-0147-z. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao BB, Clermont AC, Blair P, Chilcote TJ, Sinha S, Flaumenhaft R, Feener EP. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nature Medicine. 2011a;17:206–10. doi: 10.1038/nm.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ding Y, Yan P, Zhang JH, Lei H. Electrocardiographic abnormalities in patients with intracerebral hemorrhage. Acta neurochirurgica Supplement. 2011b;111:353–6. doi: 10.1007/978-3-7091-0693-8_59. [DOI] [PubMed] [Google Scholar]
- Lively S, Schlichter LC. Age-Related Comparisons of Evolution of the Inflammatory Response After Intracerebral Hemorrhage in Rats. Translational Stroke Research. 2012;3:132–46. doi: 10.1007/s12975-012-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhia KR, Shakui P, Keep RF. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta neurochirurgica Supplement. 2006;96:207–11. doi: 10.1007/3-211-30714-1_45. [DOI] [PubMed] [Google Scholar]
- Longatti PL, Martinuzzi A, Fiorindi A, Maistrello L, Carteri A. Neuroendoscopic management of intraventricular hemorrhage. Stroke; a journal of cerebral circulation. 2004;35:e35–8. doi: 10.1161/01.STR.0000113736.73632.F6. [DOI] [PubMed] [Google Scholar]
- Lorenzo AV, Welch K, Conner S. Spontaneous germinal matrix and intraventricular hemorrhage in prematurely born rabbits. Journal of neurosurgery. 1982;56:404–10. doi: 10.3171/jns.1982.56.3.0404. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:516–25. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2010;41:S95–8. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, Lo EH. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. Journal of Cerebral Blood Flow & Metabolism. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Kuker W, Gilsbach JM. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke; a journal of cerebral circulation. 1997;28:141–8. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Kim Y, Kissler J, Delsing P, Gilsbach JM, Schroder JM, Weis J. Morphological changes following experimental intraventricular haemorrhage and intraventricular fibrinolytic treatment with recombinant tissue plasminogen activator. Acta neuropathologica. 2000;100:561–7. doi: 10.1007/s004010000219. [DOI] [PubMed] [Google Scholar]
- McKinney JS, Kostis WJ. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke; a journal of cerebral circulation. 2012;43:2149–56. doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- McKissock W, Richardson A, Taylor J. Primary intracerebral haemorrhage: a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;2:221–6. [Google Scholar]
- Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2008;39:1165–70. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, Bullock R, Teasdale GM, Graham DI, McCulloch J. Intracranial haemorrhage induced at arterial pressure in the rat. Part 2: Short term changes in local cerebral blood flow measured by autoradiography. Neurol Res. 1984;6:189–93. doi: 10.1080/01616412.1984.11739688. [DOI] [PubMed] [Google Scholar]
- Mendelow AD. Mechanisms of ischemic brain damage with intracerebral hemorrhage. Stroke. 1993;24:I115–7. discussion I8-9. [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Mitchell PM, Murray GD, Rowan EN, Gholkar AR, Investigators SI. Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials [Electronic Resource] 2011;12:124. doi: 10.1186/1745-6215-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Stewart WB, Duncan CC, Lambrecht R. Beagle puppy model of intraventricular hemorrhage. Journal of neurosurgery. 1982;57:219–23. doi: 10.3171/jns.1982.57.2.0219. [DOI] [PubMed] [Google Scholar]
- Miller C, Tsivgoulis G, Nakaji P. Predictors of ventriculoperitoneal shunting after spontaneous intraparenchymal hemorrhage. Neurocritical care. 2008;8:235–40. doi: 10.1007/s12028-007-9018-y. [DOI] [PubMed] [Google Scholar]
- Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochirurgica - Supplement. 2008;105:147–51. doi: 10.1007/978-3-211-09469-3_30. [DOI] [PubMed] [Google Scholar]
- Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK, Lane K, Quinn TJ, Diener-West M, Weir CJ, Higgins P, Rafferty M, Kinsley K, Ziai W, Awad I, Walters MR, Hanley D. The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke; a journal of cerebral circulation. 2013;44:635–41. doi: 10.1161/STROKEAHA.112.670653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern LB, Frankowski RF, Shedden P, Pasterur W, Grotta JC. Surgical treatment for intracerebral hemorrhage(STICH): A single-center, randomized clinical trial. Neurology. 1998;51:1359–63. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr., Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–29. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern LB. Treatment of Intracerebral Hemorrhage-Is the Glass Half Full or Half Empty? Translational Stroke Research. 2012;3:S4–S5. doi: 10.1007/s12975-012-0179-4. [DOI] [PubMed] [Google Scholar]
- Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, Bistran-Hall AJ, Ullman NL, Vespa P, Martin NA, Awad I, Zuccarello M, Hanley DF. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013;44:627–34. doi: 10.1161/STROKEAHA.111.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun-Bryce S, Wilkerson AC, Papuashvili N, Okada YC. Recurring episodes of spreading depression are spontaneously elicited by an intracerebral hemorrhage in the swine. Brain research. 2001;888:248–55. doi: 10.1016/s0006-8993(00)03068-7. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Roberts LJ, Hunt WC, Bartolo A, Okada Y. Acute changes in cortical excitability in the cortex contralateral to focal intracerebral hemorrhage in the swine. Brain research. 2004;1026:218–26. doi: 10.1016/j.brainres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Roberts L, Bartolo A, Okada Y. Transhemispheric depolarizations persist in the intracerebral hemorrhage swine brain following corpus callosal transection. Brain research. 2006;1073-1074:481–90. doi: 10.1016/j.brainres.2005.12.071. [DOI] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–7. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, Coplin W, Narayan R, Haines S, Cruz-Flores S, Zuccarello M, Brock D, Awad I, Ziai WC, Marmarou A, Rhoney D, McBee N, Lane K, Hanley DF., Jr. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke; a journal of cerebral circulation. 2011;42:3009–16. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA, Bederson J, Bullock R, Schmutzhard E, Pfausler B, Keyl PM, Tuhrim S, Hanley DF. Treatment of intraventricular hemorrhage with urokinase : effects on 30-Day survival. Stroke; a journal of cerebral circulation. 2000;31:841–7. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- Naff NJ, Hanley DF, Keyl PM, Tuhrim S, Kraut M, Bederson J, Bullock R, Mayer SA, Schmutzhard E. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–83. doi: 10.1227/01.neu.0000108422.10842.60. discussion 83-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. Journal of Neurosurgery. 2004a;100:672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff J, Keep R. Intracerebral hemorrhage in mice: Model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004b;24:487–95. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hua Y, Keep R, Park J, Xi G, Hoff J. Estrogen therapy for experimental intracerebral hemorrhage. J Neurosurg. 2005a;103:97–103. doi: 10.3171/jns.2005.103.1.0097. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005b;1039:30–6. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Narayan RK, Narayan TM, Katz DA, Kornblith PL, Murano G. Lysis of intracranial hematomas with urokinase in a rabbit model. Journal of neurosurgery. 1985;62:580–6. doi: 10.3171/jns.1985.62.4.0580. [DOI] [PubMed] [Google Scholar]
- Nath FP, Jenkins A, Mendelow AD, Graham DI, Teasdale GM. Early hemodynamic changes in experimental intracerebral hemorrhage. Journal of neurosurgery. 1986;65:697–703. doi: 10.3171/jns.1986.65.5.0697. [DOI] [PubMed] [Google Scholar]
- Nath FP, Kelly PT, Jenkins A, Mendelow AD, Graham DI, Teasdale GM. Effects of experimental intracerebral hemorrhage on blood flow, capillary permeability, and histochemistry. J Neurosurg. 1987;66:555–62. doi: 10.3171/jns.1987.66.4.0555. [DOI] [PubMed] [Google Scholar]
- Newell DW, Shah MM, Wilcox R, Hansmann DR, Melnychuk E, Muschelli J, Hanley DF. Minimally invasive evacuation of spontaneous intracerebral hemorrhage using sonothrombolysis. Journal of neurosurgery. 2011;115:592–601. doi: 10.3171/2011.5.JNS10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247:117–21. doi: 10.1007/pl00007792. [DOI] [PubMed] [Google Scholar]
- O'Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, Ikeda D, Greenberg SM. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240–5. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- Oertel JM, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR. Endoscopic third ventriculostomy for obstructive hydrocephalus due to intracranial hemorrhage with intraventricular extension. Journal of neurosurgery. 2009;111:1119–26. doi: 10.3171/2009.4.JNS081149. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–63. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41:375–82. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1. Canine intraventricular blood cast model. Neurosurgery. 1986;19:540–6. doi: 10.1227/00006123-198610000-00008. [DOI] [PubMed] [Google Scholar]
- Perez de la Ossa N, Sobrino T, Silva Y, Blanco M, Millan M, Gomis M, Agulla J, Araya P, Reverte S, Serena J, Davalos A. Iron-related brain damage in patients with intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2010;41:810–3. doi: 10.1161/STROKEAHA.109.570168. [DOI] [PubMed] [Google Scholar]
- Prasad K, Mendelow AD, Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD000200.pub2. CD000200. [DOI] [PubMed] [Google Scholar]
- Qing WG, Dong YQ, Ping TQ, Lai LG, Fang LD, Min HW, Xia L, Heng PY. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. Journal of neurosurgery. 2009;110:462–8. doi: 10.3171/2008.4.JNS17512. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Wilson DA, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266–72. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Ling GS, Khan J, Suri MF, Miskolczi L, Guterman LR, Hopkins LN. Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Critical Care Medicine. 2001a;29:152–7. doi: 10.1097/00003246-200101000-00030. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001b;344:1450–60. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham A, Ezzeddine MA, Goldstein JN, Hussein HM, Suri MF, Tariq N, Antihypertensive Treatment of Acute Cerebral Hemorrhage Study I Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67:570–6. doi: 10.1001/archneurol.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocritical Care. 2011;15:559–76. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter MA, Droste DW, Hegedus K, Szepesi R, Nabavi DG, Csiba L, Ringelstein EB. Role of cerebral amyloid angiopathy in intracerebral hemorrhage in hypertensive patients. Neurology. 2005;64:1233–7. doi: 10.1212/01.WNL.0000156522.93403.C3. [DOI] [PubMed] [Google Scholar]
- Rohde V, Rohde I, Thiex R, Ince A, Jung A, Duckers G, Groschel K, Rottger C, Kuker W, Muller HD, Gilsbach JM. Fibrinolysis therapy achieved with tissue plasminogen activator and aspiration of the liquefied clot after experimental intracerebral hemorrhage: rapid reduction in hematoma volume but intensification of delayed edema formation. J Neurosurg. 2002;97:954–62. doi: 10.3171/jns.2002.97.4.0954. [DOI] [PubMed] [Google Scholar]
- Rohde V, Uzma N, Thiex R, Samadani U. Management of delayed edema formation after fibrinolytic therapy for intracerebral hematomas: preliminary experimental data. Acta Neurochir Suppl. 2008;105:101–4. doi: 10.1007/978-3-211-09469-3_21. [DOI] [PubMed] [Google Scholar]
- Ropper AH, King RB. Intracranial pressure monitoring in comatose patients with cerebral hemorrhage. Arch Neurol. 1984;41:725–8. doi: 10.1001/archneur.1984.04050180047016. [DOI] [PubMed] [Google Scholar]
- Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. New England Journal of Medicine. 1986;314:953–8. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–7. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–6. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sandoval JL, Cantu C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke; a journal of cerebral circulation. 1999;30:537–41. doi: 10.1161/01.str.30.3.537. [DOI] [PubMed] [Google Scholar]
- Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Annals of neurology. 2011;70:646–56. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M, Yeatts S, Goldstein JN, Gomes J, Greenberg S, Morgenstern LB, Schlaug G, Torbey M, Waldman B, Xi G, Palesch Y. Safety and Tolerability of Deferoxamine Mesylate in Patients With Acute Intracerebral Hemorrhage. Stroke. 2011;42:3067–74. doi: 10.1161/STROKEAHA.111.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried DM, Han YX, Yang DM, Ding J, Shen LH, Savant-Bhonsale S, Chopp M. Localization of bone marrow stromal cells to the injury site after intracerebral hemorrhage in rats Laboratory investigation. Journal of Neurosurgery. 2010;112:329–35. doi: 10.3171/2009.2.JNS08907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Ratan RR. Prolyl 4-hydroxylase activity-responsive transcription factors: from hydroxylation to gene expression and neuroprotection. Front Biosci. 2008;13:2875–87. doi: 10.2741/2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver EB, Olsen TS. Tissue damage at computed tomography following resolution of intracerebral hematomas. Acta Radiologica: Diagnosis. 1986;27:495–500. doi: 10.1177/028418518602700502. [DOI] [PubMed] [Google Scholar]
- Soderberg S, Ahren B, Stegmayr B, Johnson O, Wiklund PG, Weinehall L, Hallmans G, Olsson T. Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke; a journal of cerebral circulation. 1999;30:328–37. doi: 10.1161/01.str.30.2.328. [DOI] [PubMed] [Google Scholar]
- Soderberg S, Stegmayr B, Ahlbeck-Glader C, Slunga-Birgander L, Ahren B, Olsson T. High leptin levels are associated with stroke. Cerebrovascular diseases. 2003;15:63–9. doi: 10.1159/000067128. [DOI] [PubMed] [Google Scholar]
- Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–3. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, Pile-Spellman J, Mohr JP. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–5. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- Staykov D, Huttner HB, Struffert T, Ganslandt O, Doerfler A, Schwab S, Bardutzky J. Intraventricular fibrinolysis and lumbar drainage for ventricular hemorrhage. Stroke; a journal of cerebral circulation. 2009;40:3275–80. doi: 10.1161/STROKEAHA.109.551945. [DOI] [PubMed] [Google Scholar]
- Stein M, Luecke M, Preuss M, Boeker DK, Joedicke A, Oertel MF. Spontaneous intracerebral hemorrhage with ventricular extension and the grading of obstructive hydrocephalus: the prediction of outcome of a special life-threatening entity. Neurosurgery. 2010;67:1243–51. doi: 10.1227/NEU.0b013e3181ef25de. discussion 52. [DOI] [PubMed] [Google Scholar]
- Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, Skolnick BE, Davis SM. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–73. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 73-4. [DOI] [PubMed] [Google Scholar]
- Sumer MM, Acikgoz B, Akpinar G. External ventricular drainage for acute obstructive hydrocephalus developing following spontaneous intracerebral haemorrhages. Neurol Sci. 2002;23:29–33. doi: 10.1007/s100720200020. [DOI] [PubMed] [Google Scholar]
- Sussman BJ, Barber JB, Goald H. Experimental intracerebral hematoma. Reduction of oxygen tension in brain and cerebrospinal fluid. J Neurosurg. 1974;41:177–86. doi: 10.3171/jns.1974.41.2.0177. [DOI] [PubMed] [Google Scholar]
- Sutherland CS, Hill MD, Kaufmann AM, Silvaggio JA, Demchuk AM, Sutherland GR. Recombinant factor VIIa plus surgery for intracerebral hemorrhage. Canadian Journal of Neurological Sciences. 2008;35:567–72. doi: 10.1017/s0317167100009343. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Ebina T. Sequential changes in tissue surrounding ICH. In: Pia HW, Longmaid C, Zierski J, editors. Spontaneous Intracerebral Hematomas. Springer; Berlin: 1980. pp. 121–8. [Google Scholar]
- Suzuki R, Ohno K, Hiratsuka H, Inaba Y. Chronological changes in brain edema in hypertensive intracerebral hemorrhage observed by CT and xenon-enhanced CT. In: Inaba Y, Klatzo I, Spatz M, editors. Brain Edema. Springer; Berlin Heidelbery, New York, Tokyo: 1985. pp. 613–20. [Google Scholar]
- Sykora M, Steiner T, Poli S, Rocco A, Turcani P, Diedler J. Autonomic effects of intraventricular extension in intracerebral hemorrhage. Neurocritical care. 2012;16:102–8. doi: 10.1007/s12028-011-9637-1. [DOI] [PubMed] [Google Scholar]
- Takasugi S, Ueda S, Matsumoto K. Chronological changes in spontaneous intracerebral hematoma--an experimental and clinical study. Stroke. 1985;16:651–8. doi: 10.1161/01.str.16.4.651. [DOI] [PubMed] [Google Scholar]
- Thiex R, Kuker W, Muller HD, Rohde I, Schroder JM, Gilsbach JM, Rohde V. The long-term effect of recombinant tissue-plasminogen-activator (rt-PA) on edema formation in a large-animal model of intracerebral hemorrhage. Neurol Res. 2003;25:254–62. doi: 10.1179/016164103101201463. [DOI] [PubMed] [Google Scholar]
- Thiex R, Kuker W, Jungbluth P, Kayser C, Muller HD, Rohde I, Gilsbach JM, Rohde V. Minor inflammation after surgical evacuation compared with fibrinolytic therapy of experimental intracerebral hemorrhages. Neurol Res. 2005;27:493–8. doi: 10.1179/016164105X17369. [DOI] [PubMed] [Google Scholar]
- Thiex R, Weis J, Krings T, Barreiro S, Yakisikli-Alemi F, Gilsbach JM, Rohde V. Addition of intravenous N-methyl-D-aspartate receptor antagonists to local fibrinolytic therapy for the optimal treatment of experimental intracerebral hemorrhages. J Neurosurg. 2007;106:314–20. doi: 10.3171/jns.2007.106.2.314. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. Journal of Neuroscience. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Ito U, Ohno K, Hirakawa K. Chronological changes in brain edema induced by experimental intracerebral hematoma in cats. Acta Neurochir - Suppl. 1994;60:558–60. doi: 10.1007/978-3-7091-9334-1_154. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–4. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical care medicine. 1999;27:617–21. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, Zaroff J. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2004;35:548–51. doi: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- Volbers B, Wagner I, Willfarth W, Doerfler A, Schwab S, Staykov D. Intraventricular fibrinolysis does not increase perihemorrhagic edema after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2013;44:362–6. doi: 10.1161/STROKEAHA.112.673228. [DOI] [PubMed] [Google Scholar]
- Wagner K, Broderick J. Lo EH, Marwah J, editors. Hemorrhagic Stroke: Pathophysiologicl Mechanisms and Neuroprotective Treatments. Neurprotection. 2001:471–508. [Google Scholar]
- Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27:490–7. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88:1058–65. doi: 10.3171/jns.1998.88.6.1058. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, Brott TG. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–8. doi: 10.3171/jns.1999.90.3.0491. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Hua Y, de Courten-Myers GM, Broderick JP, Nishimura RN, Lu SY, Dwyer BE. Tin-mesoporphyrin, a potent heme oxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies. Cellular & Molecular Biology. 2000;46:597–608. [PubMed] [Google Scholar]
- Wagner KR, Packard BA, Hall CL, Smulian AG, Linke MJ, De Courten-Myers GM, Packard LM, Hall NC. Protein oxidation and heme oxygenase-1 induction in porcine white matter following intracerebral infusions of whole blood or plasma. Dev Neurosci. 2002;24:154–60. doi: 10.1159/000065703. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2003;23:629–52. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wagner KR. Modeling intracerebral hemorrhage: glutamate, nuclear factor-kappa B signaling and cytokines. Stroke. 2007;38:753–8. doi: 10.1161/01.STR.0000255033.02904.db. [DOI] [PubMed] [Google Scholar]
- Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Annals of neurology. 2003;54:655–64. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Tuftsin fragment 1-3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005;36:613–8. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke. 2013;44:2545–52. doi: 10.1161/STROKEAHA.113.001038. [DOI] [PubMed] [Google Scholar]
- Warkentin LM, Auriat AM, Wowk S, Colbourne F. Failure of deferoxamine, an iron chelator, to improve outcome after collagenase-induced intracerebral hemorrhage in rats. Brain Research. 2010;1309:95–103. doi: 10.1016/j.brainres.2009.10.058. [DOI] [PubMed] [Google Scholar]
- Webb AJ, Ullman NL, Mann S, Muschelli J, Awad IA, Hanley DF. Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventricular thrombolytic: the Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR IVH) program. Stroke; a journal of cerebral circulation. 2012;43:1666–8. doi: 10.1161/STROKEAHA.112.650523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JM, Edens RE, Linhardt RJ, Kapelanski DP. Heparin and modified heparin inhibit complement activation in vivo. Journal of immunology. 1992;148:3210–5. [PubMed] [Google Scholar]
- Whisnant JP, Sayer GP, Millikan CH. Experimental intracerebral hematoma. Arch Neurol. 1963;9:586–92. [Google Scholar]
- Wu G, Xi G, Huang F. Spontaneous intracerebral hemorrhage in humans: hematoma enlargement, clot lysis, and brain edema. Acta neurochirurgica Supplement. 2006;96:78–80. doi: 10.1007/3-211-30714-1_19. [DOI] [PubMed] [Google Scholar]
- Wu G, Xi G, Hua Y, Sagher O. T2* magnetic resonance imaging sequences reflect brain iron deposition folowing intracerebral hemorrhage. Translational Stroke Research. 2010;1:31–4. doi: 10.1007/s12975-009-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bao X, Xi G, Keep RF, Thompson BG, Hua Y. Brain injury after intracerebral hemorrhage in spontaneously hypertensive rats. Journal of neurosurgery. 2011a;114:1805–11. doi: 10.3171/2011.1.JNS101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Li C, Wang L, Mao Y, Hong Z. Minimally invasive procedures for evacuation of intracerebral hemorrhage reduces perihematomal glutamate content, blood-brain barrier permeability and brain edema in rabbits. Neurocritical Care. 2011b;14:118–26. doi: 10.1007/s12028-010-9473-8. [DOI] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011c;31:1243–50. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep R, Schallert T, Hoff J, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Research. 2002;953:45. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–9. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, Hua Y. Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:59–65. doi: 10.1007/978-3-211-09469-3_13. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998a;89:991–6. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, Brott TG, Hoff JT. The role of blood clot formation on early edema development following experimental intracerebral hemorrhage. Stroke. 1998b;29:2580–6. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: Effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001a;32:2932–8. doi: 10.1161/hs1201.099820. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001b;32:162–7. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Bhasin RR, Duong HK, Hoff JT. Mechanisms of edema formation following intracerebral hemorrhage: Does hemolysate cause ischemia in intracerebral hemorrhage? J Neurosurg. 2001c;94:162A. [Google Scholar]
- Xi G, Keep RF, Hoff JT. Rasmussen P, editor. Pathophysiology of brain edema formation. Neurosurgery clinics of North America. 2002;13:371–83. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Xi G, Fewel M, Hua Y, Thompson B, Hoff J, Keep R. Intracerebral hemorrhage: pathophysiology and therapy. Neurocritical Care. 2004;1:5–18. doi: 10.1385/ncc:1:1:5. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xing Y, Hua Y, Keep RF, Xi G. Effects of deferoxamine on brain injury after transient focal cerebral ischemia in rats with hyperglycemia. Brain Res. 2009;1291:113–21. doi: 10.1016/j.brainres.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230–2. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A. Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC neurology. 2007;7:1. doi: 10.1186/1471-2377-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Yang S, Nakamura T, Hua Y, Keep RF, Younger JG, Hoff JT, Xi G. Intracerebral hemorrhage in complement C3-deficient mice. Acta neurochirurgica Supplement. 2006;96:227–31. doi: 10.1007/3-211-30714-1_49. [DOI] [PubMed] [Google Scholar]
- Yang S, Song S, Hua Y, Nakamura T, Keep RF, Xi G. Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2008;39:2079–84. doi: 10.1161/STROKEAHA.107.508911. [DOI] [PubMed] [Google Scholar]
- Ye Z, Xie Q, Xi G, Keep RF, Hua Y. Effects of gender on heart injury after intracerebral hemorrhage in rats. Acta neurochirurgica Supplement. 2011;111:119–22. doi: 10.1007/978-3-7091-0693-8_19. [DOI] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Yin X, Zhang X, Wang W, Chang L, Jiang Y, Zhang S. Perihematoma damage at different time points in experimental intracerebral hemorrhage. J Huazhong Univ Sci Technolog Med Sci. 2006;26:59–62. doi: 10.1007/BF02828039. [DOI] [PubMed] [Google Scholar]
- Zacharia BE, Vaughan KA, Hickman ZL, Bruce SS, Carpenter AM, Petersen NH, Deiner S, Badjatia N, Connolly ES., Jr. Predictors of long-term shunt-dependent hydrocephalus in patients with intracerebral hemorrhage requiring emergency cerebrospinal fluid diversion. Neurosurgical focus. 2012;32:E5. doi: 10.3171/2012.2.FOCUS11372. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–73. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Diringer MN, Videen TO, Adams RE, Tundt K, Aiyagari V, Grubb Jr RL, Powers WJ. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–10. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Zazulia AR. Hydrocephalus in ICH: what do we really know? Neurocritical care. 2008;8:233–4. doi: 10.1007/s12028-008-9055-1. [DOI] [PubMed] [Google Scholar]
- Zhao F, Hua Y, He Y, Keep RF, Xi G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2011a;42:3587–93. doi: 10.1161/STROKEAHA.111.623926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Hua Y, He Y, Keep RF, Xi G. Minocycline-induced attenuation of iron overload and brain injury following experimental intracerebral hemorrhage. Stroke. 2011b;42:3587–93. doi: 10.1161/STROKEAHA.111.623926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–85. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QJ, Sun M, Zhang XG, Wang LX. Relationship between serum leptin levels and clinical outcomes of hypertensive intracerebral hemorrhage. Clin Exp Hypertens. 2012;34:161–4. doi: 10.3109/10641963.2011.561902. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26:811–20. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007a;38:3280–6. doi: 10.1161/STROKEAHA.107.486506. Epub 2007 Oct 25. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007b;61:352–62. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, Ericsson M, Lo EH, Whalen MJ. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012;43:524–31. doi: 10.1161/STROKEAHA.111.635672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai W, Moullaali T, Nekoovaght-Tak S, Ullman N, Brooks JS, Morgan TC, Hanley DF. No exacerbation of perihematomal edema with intraventricular tissue plasminogen activator in patients with spontaneous intraventricular hemorrhage. Neurocritical care. 2013;18:354–61. doi: 10.1007/s12028-013-9826-1. [DOI] [PubMed] [Google Scholar]
- Ziai WC, Melnychuk E, Thompson CB, Awad I, Lane K, Hanley DF. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Critical care medicine. 2012a;40:1601–8. doi: 10.1097/CCM.0b013e318241e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai WC, Muschelli J, Thompson CB, Keyl PM, Lane K, Shao S, Hanley DF. Factors affecting clot lysis rates in patients with spontaneous intraventricular hemorrhage. Stroke; a journal of cerebral circulation. 2012b;43:1234–9. doi: 10.1161/STROKEAHA.111.641050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Fang S, Yuan G, Shen G. [Investigation of potency to produce interleukin-2 of the peripheral blood T lymphocytes in patients with hemorrhagic stroke]. Hua Xi Yi Ke Da Xue Xue Bao. 1997;28:304–6. [PubMed] [Google Scholar]
- Zuccarello M, Andaluz N, Wagner KR. Minimally invasive therapy for intracerebral hematomas. Neurosurgery clinics of North America. 2002;13:349–54. doi: 10.1016/s1042-3680(02)00008-6. [DOI] [PubMed] [Google Scholar]