Background: M2-type macrophages are proangiogenic and protumorigenic, whereas M1-type macrophages are antiangiogenic.
Results: Doxycycline is a potent inhibitor of M2-type macrophage polarization in both human and mouse macrophages in vitro and in vivo.
Conclusion: Preventing M2-type macrophage polarization correlates with inhibition of pathological angiogenesis.
Significance: Doxycycline may be used to enhance current antiangiogenic treatment approaches in neovascular age-related macular degeneration and in certain cancers.
Keywords: Angiogenesis, Antibiotics, Inflammation, Innate Immunity, Macrophages, Wound Healing, Macular Degeneration
Abstract
Macrophages occur along a continuum of functional states between M1-type polarized macrophages with antiangiogenic and antitumor activity and M2-type polarized macrophages, which have been implicated to promote angiogenesis and tumor growth. Proangiogenic M2-type macrophages promote various pathologic conditions, including choroidal neovascularization in models of neovascular age-related macular degeneration, or certain cancers, such as glioblastoma multiforme. Thus, a potential novel therapeutic approach to target pathological angiogenesis in these conditions would be to inhibit the polarization of macrophages toward the proangiogenic M2-type. However, no pharmacological inhibitors of M2-type macrophage polarization have been identified yet. Here we performed an unbiased pharmacological and small chemical screen to identify drugs that inhibit proangiogenic M2-type macrophage polarization and block pathologic macrophage-driven neovascularization. We identified the well tolerated and commonly used antibiotic doxycycline as a potent inhibitor of M2-type polarization of macrophages. Doxycycline inhibited, in a dose-dependent manner, M2-type polarization of human and bone marrow-derived mouse macrophages without affecting cell viability. Furthermore, doxycycline inhibited M2-type macrophage polarization and subsequent neovascularization in vivo in a laser injury model of choroidal neovascularization. Thus, doxycycline could be used to enhance current antiangiogenic treatment approaches in various conditions that are promoted by proangiogenic M2-type macrophages, including neovascular age-related macular degeneration and certain cancers.
Introduction
Macrophage populations occur along a continuum between two functional states of polarization. M1-type (“classically activated”) macrophage polarization is triggered by bacterial lipopolysaccharides and interferon γ, and those macrophages have been attributed to have antiangiogenic and antitumorigenic activities. M2-type (“alternatively activated”) macrophage polarization occurs particularly in response to IL-4/IL-13 and has been associated with protumorigenic and proangiogenic features and increased tissue remodeling (1).
Proangiogenic M2-type macrophages have been shown to play a significant role in promoting pathological angiogenesis in various conditions, such as in models of neovascular age-related macular degeneration (AMD)2 or in some cancers (1, 2). For example, we have shown recently, in a laser injury model of neovascular AMD, that choroidal neovascularization (CNV) is stimulated by macrophages that express the prototypical M2-type macrophage markers arginase 1 or YM1 (3). Laser injury to the RPE/choroid causes infiltration of macrophages within 2 days, which promotes CNV that forms within 4 days after laser injury in this model (2). Expression profiling revealed that macrophages that infiltrated into evolving CNV lesions are characterized by expression of M2-type markers, such as arginase 1, YM1, or IL-1RA, whereas M1-type markers were not induced after laser injury (2). Immunolabeling for M2-type markers revealed that almost all macrophages at sites of laser injury were activated M2-type macrophages (F4/80+Arg1+YM1+), whereas the quiescent resident non-lesional choroidal macrophages were not expressing M2-type markers (F4/80+Arg1−YM1−) (2, 3).
Infiltration of M2-type macrophages into sites of laser injury correlated with increased transcript levels of the potent proangiogenic cytokine IL-1β and subsequent neovascularization (2). The highest levels of both M2-type markers (e.g. Arg1) and IL-1β were noticed at day 3 after laser injury, a time point when onset of neovascularization is stimulated by IL-1β, whereas lower levels occurred for both at day 5 after laser injury when neovascular lesions had already fully formed (2). These findings suggest that infiltration of M2-type proangiogenic macrophages into lasered lesions results in stimulation of CNV, possibly in part through increased levels of the proangiogenic cytokine IL-1β. Consistent with this hypothesis, the ablation of macrophages in this model inhibited CNV formation (2, 3). Similarly, targeting IL-1β signaling inhibited CNV lesion formation in this laser injury model of neovascular AMD as well (4). Notably, infiltrating M2-type macrophages may promote neovascularization by inducing retinal glia cells to express proangiogenic factors, including VEGF-A and IL-1β (3). The ablation of macrophages inhibited both glia cell activation and subsequent neovascularization in this CNV model (3).
Thus, M2-type macrophages are critical for laser-induced CNV and are likely also important for promoting angiogenesis in various other pathological conditions, such as in some cancers (5). For example, the reduction of M2-type markers in tumor-associated macrophages correlated with reduced glioblastoma multiforme progression and enhanced survival (6). These findings suggest that the inhibition of the polarization of macrophages toward the proangiogenic M2-type may provide a novel therapeutic approach to inhibit macrophage-driven neovascularization in various pathologic conditions, such as in neovascular AMD or glioblastoma multiforme.
Current approaches to target CNV in neovascular AMD or tumor angiogenesis are mainly on the basis of anti-VEGF-A therapies (7). However, anti-VEGF-A therapies are not always effective, and disease progression can occur despite long-term anti-VEGF-A treatment (8). Thus, alternative or additional therapeutic approaches to target pathologic blood vessel growth are highly desired.
Here we performed an unbiased small chemical and pharmacological screen to identify inhibitors of M2-type macrophage polarization. We found that the antibiotic doxycycline inhibits M2-type macrophage polarization in a dose-dependent manner in both human and mouse macrophages in vitro. Furthermore, doxycycline inhibited M2-type macrophage polarization, IL-1β expression, and subsequent neovascularization in the laser-induced CNV model in vivo. Thus, the M2-type inhibitory activity of doxycycline may contribute to its antiangiogenesis effects that have been reported in this laser CNV assay or in tumor growth assays (9, 10).
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
THP-1 cells were obtained from the ATCC and cultured in RPMI 1640 medium with 10% FBS and 1% Anti-Anti (Invitrogen, catalog no. 15240). BMDMs were isolated from both femurs and tibias of adult C57Bl/6j mice. Bone marrow cells were cultured in DMEM (Invitrogen, catalog no. 11995) containing 10% FBS and 1% Anti-Anti and supplemented with macrophage colony-stimulating factor (M-CSF) (10 ng/ml, Peprotech).
Human recombinant IL-4 and IL-13 were obtained from R&D Systems. Human IFN-γ, murine IL-4, and murine M-CSF were obtained from Peprotech. Prostaglandin E2 (PGE2) was obtained from Santa Cruz Biotechnology Inc. Doxycycline (Dox) was obtained from APP Pharmaceuticals. Phorbol myristate acetate (PMA), minocycline, and tetracycline were obtained from Sigma.
Chemical Screen Using a Cell-based MRC1 Promoter Luciferase Reporter Assay
Sequences containing the human MRC1 promoter (comprising sequences of 1.0 kb, 1.5 kb, and 2.5 kb upstream of the start codon) were amplified by PCR using a bacterial artificial chromosome (BAC) clone, RP11-16O1, as a template. The 5′ primer was flanked with a KpnI restriction endonuclease sequence and the 3′ primer with a XhoI restriction endonuclease enzyme sequence. MRC1 promoter-containing sequences were cloned into the pGL4.20 (puro) luciferase reporter plasmid (Promega) between the KpnI and XhoI cloning sites. Primer sequences are listed in supplemental Table S1.
To establish stable cell lines for a cell-based chemical screen, pGL4.20/MRC1 promoter plasmids were transfected into THP-1 cells using Nucleofector II (Lonza) according to the protocols of the manufacturer. Transfected cells were selected with puromycin (0.3 μg/ml) treatment for 3 weeks, starting on day 3 after electroporation. Single spheres were selected from surviving THP-1 cells and expanded. All of these single spheres were tested by a luciferase reporter assay after they were primed with PMA (100 nm for 24 h) and subsequently induced to polarize to M2-type macrophages that highly express MRC1 by human IL-4 (20 ng/ml for an additional 3 days). Several cell clones that stably expressed the MRC1 luciferase constructs over several passages were obtained, and a 1.0-kb MRC1 luciferase-expressing cell clone was used for subsequent experiments that showed consistently high luciferase activity after IL-4 treatment.
Cells from this clone were seeded into 384-well plates at a density of 105 cells/well and treated with PMA (100 nm for 24 h), followed by treatment with IL-4 (20 ng/ml) and with small chemical compounds each at 20 μm (Spectrum Collection, 2320 compounds, Microsource) for an additional 3 days. Luciferase activity was determined using the Steady-Glo luciferase assay system (Promega, catalog no. E2520). Data were analyzed by Tibco Spotfire software v. 5.0. Positive hits were defined by Z scores of <−5.
THP-1 Cell-based MRC1 Promoter Luciferase Reporter Assay
THP-1 cells from the clone used in the chemical screen assay were seeded into 96-well plates at a density of 105 cells/well and treated with PMA (100 nm for 24 h), followed by pretreatment with doxycycline for 1 day, and then treated with doxycycline and IL-4 (20 ng/ml) for an additional 3 days. Doxycycline was used in concentrations between 5 and 20 μm. Alternatively, instead of doxycycline, we used minocycline or tetracycline at the indicated concentrations. In the experiments using different inducers of M2-type macrophage polarization, IL-13 was used at 20 ng/ml, PGE2 at 0.1 μm, or PMA at 200 nm. Luciferase activity was determined using the Steady-Glo luciferase assay system (Promega, catalog no. E2520). Each group was assayed three times, and each experiment was performed in triplicate.
Doxycycline Inhibition of M2-type Macrophage Polarization in THP-1 and BMDMs
THP-1 cells were first primed with PMA (100 nm for 24 h). Cells were then pretreated with doxycycline for 24 h and subsequently treated with human IL-4 (20 ng/ml) and doxycycline for an additional 3 days. BMDMs from C57Bl/6j mice were split once before use. Two days after splitting, cells were pretreated for 24 h with doxycycline in DMEM containing M-CSF (10 ng/ml) and 1% FBS and then subjected to murine IL-4 (20 ng/ml) and doxycycline treatment for an additional 3 days. For all experimental groups, triplicates were used, and experiments were repeated three times.
Cytotoxicity Assay
A cytotoxicity assay kit (Promega, catalog no. G1782) was used according to the protocol of the manufacturer to assess the cytotoxic effects of all treatments used in these experiments. Lactate dehydrogenase activity was measured by reading A490 nm absorbance on a PerkinElmer Life Sciences Victor X3 plate reader. Luciferase activity was normalized to cell viability (A490). All experiments were performed in triplicate.
Experimental CNV Model
Experimental induction of CNV lesions was performed as described previously (2). Briefly, the eyes of age- and gender-matched mice were exposed to laser photocoagulation for induction of experimental CNV after the eyes were dilated with 1% tropicamide. Laser photocoagulation was performed using a 532-nm laser (Visulas 532S, Carl Zeiss Meditec, Dublin, Ireland). Lesions were induced using a power of 200 milliwatt, a spot size of 50 μm, and a duration of 100 ms. The eyes were fixed and examined either 3 days or 5 days after laser treatment.
Five days after laser injury, the size of CNV lesions was measured in choroidal flat mounts from C57Bl/6j female mice (n = 7 mice/group). The eyes were enucleated and fixed in 4% paraformaldehyde. The RPE/choroid tissue (posterior eye) was used for immunolabeling with anti-CD31 antibodies to detect blood vessels (Alexa Fluor 488 secondary antibody). Choroidal flat mounts were analyzed by epifluorescence microscopy using a Zeiss microscope (Carl Zeiss Microscopy, Jena, Germany). Images were obtained with a ×10 objective. CNV lesions were measured using Zeiss AxioVision software version 4.8.2. Average CNV size was determined for each mouse, and differences between mice treated with Dox or H2O were assessed by Student's t test. For semiquantitative RT-PCR and Western blotting experiments, 14 laser spots were induced in each eye. These experiments were confirmed independently in separate experimental mouse groups (three times), revealing similar results.
Doxycycline Treatment of Mice in Laser Injury or Skin Wound Healing Experiments
Doxycycline at 0.5 mg/30 g of BW (low) or at 1.8 mg/30 g BW (high) was injected intraperitoneally into C57Bl/6j mice 1 day before laser-induced CNV injury and before four 6-mm punch wounds were induced on the backs of the mice. Thereafter, doxycycline was injected daily until the mice were sacrificed. The results of these experiments were confirmed in three independent sets of mice. All animal studies were approved by Massachusetts General Hospital Subcommittee on Research Animal Care.
Semiquantitative RT-PCR
Choroidal tissue lysates from C57Bl/6j mice that were subjected to laser-induced CNV experiments (14 laser spots/eye) were used for RNA isolation either 3 or 5 days after laser injury. RPE/choroid tissues were used after removal of retinas and lysed in 0.5 ml TRIzol (Invitrogen) with a Qiagen TissueLyser II. RNA was then extracted following standard protocols. For in vitro experiments, RNA was isolated with TRIzol as well. 0.5 μg of RNA was used to obtain cDNA using the Transcriptor first-strand cDNA synthesis kit (Roche). Semiquantitative RT-PCR was performed using a LightCycler 480 SYBR Green I master mix (Roche) on a LightCycler 480 system (Roche). Primers used are listed in supplemental Table S2. For all experimental groups, triplicates were used, and experiments were independently confirmed three times.
Western Blotting
Cells lysates were obtained using Nonidet P-40 lysis buffer. Similarly, choroidal/RPE tissues obtained from each mouse were lysed in 100 μl of Nonidet P-40 lysis buffer containing 1 mm PMSF and protease inhibitor mixture (cOmplete, Roche) using a Qiagen TissueLyser II. 20–30 μg of total protein was loaded onto NuPage 4–12% BisTris gradient gels (Invitrogen) and transferred to nitrocellulose membranes (GE Lifesciences). Antibodies used detected MRC1 (SCBT, catalog no. SC-376108, 1:200), CD68 (SCBT, catalog no. SC-9139, 1:1000), Arg-1 (SCBT, catalog no. SC-25830, 1:1000), F4/80 (SCBT, catalog no. SC-18345, 1:200), β-actin (Neomarkers, catalog no. Rb-9421-P1, 1:1000), and tubulin (Sigma, catalog no. T9026, 1:2000). HRP-conjugated secondary antibodies were used, and the chemiluminescence signal was determined with SuperSignal WestPico chemiluminescent substrate (Pierce).
Flow Cytometry
For THP-1 cell staining, cells were first stained with a live/dead cell viability kit (Invitrogen, catalog no. L23102). Then, cells were blocked with human IgG (10 μg/ml), diluted in staining buffer (0.5% BSA + 1 mm EDTA + 0.01% sodium azide) on ice for 10 min, and subsequently stained with anti-human MRC1-PE-Cy7 antibodies (eBioscience, catalog no. 25-2069, 1:50) on ice for 1 h. Subsequently, cells were fixed in 2% paraformaldehyde on ice for 15 min. Next, cells were stained with anti-human CD68-FITC antibodies (eBioscience, catalog no. 11-0689, 1:50) in staining buffer containing 0.5% saponin on ice for 45 min. After washing with staining buffer, cells were analyzed on a FACSCanto flow cytometer (BD Biosciences). Flow cytometry data were analyzed by Flowjo software (Tree Star, version 9.6.2). All experiments were performed in triplicate.
Statistical Analyses
Two-tailed Student's t test was used for statistical analyses. p < 0.05 was considered to be statistically significant.
RESULTS
Generation of an in Vitro Cell-based Assay for Quantitation of M2-type Macrophage Polarization
To identify small chemical or pharmacologic inhibitors of M2-type macrophage polarization, we developed an in vitro cell-based assay to accurately quantify M2-type polarization of human macrophages. We generated expression constructs in which a luciferase reporter is driven by the macrophage mannose receptor 1 (MRC1) promoter because MRC1 is a prototypical M2-type marker in human macrophages. We generated three different constructs that included either 2.5 kb, 1.5 kb, or 1.0 kb of the genomic sequence upstream of the start codon, and all contain the MRC1 promoter (supplemental Fig. S1A). We established THP-1 cell clones that stably expressed these expression constructs and tested the induction of luciferase expression using IL-4 and/or IL-13 treatment, which induces M2-type polarization and MRC1 expression in macrophages. We found that IL-4 and IL-13 strongly induce luciferase activity in these stably transfected THP-1 cell clones (supplemental Fig. S1B). We selected a clone with a 1.0-kb genomic sequence upstream of the start codon for subsequent experiments, which showed consistent luciferase induction over several passages. THP-1 cells derived from this clone were induced to differentiate with 24-hour PMA treatment, followed by 3-day incubation with 20 ng/ml IL-4, showing a >5-fold induction of luciferase activity. This luciferase induction is consistent with a M2-type polarization of macrophages induced by IL-4. Luciferase activation was not further increased by adding IL-13 or doubling the dose of IL-4 or IL-13 to 40 ng/ml (supplemental Fig. S1B). Thus, for all subsequent experiments, we used 20 ng/ml IL-4 for the induction of luciferase in these cells. Of note, when treating the PMA-treated THP-1 MRC1-luciferase clone first with IL-4 to induce M2-polarization, subsequent treatment with the macrophage M1-type inducers IFN-γ and LPS reduced luciferase activity, consistent with a shift from M2- to M1-type polarization (supplemental Fig. S1C). Conversely, when cells were first treated with IFN-γ/LPS and subsequently treated with IL-4, an increase of luciferase activity suggested an M1- to M2-type repolarization (supplemental Fig. S1C). These findings suggest a reversible plasticity of differentiated macrophages to undergo either M1- or M2-type polarization in vitro. Western blotting confirmed that the increased MRC1-luciferase activity after IL-4 treatment in these cells correlates with increased MRC1 protein levels, whereas IFN-γ/LPS treatment reduced MRC1 protein levels (supplemental Fig. S1D).
A Small Chemical Screen Identifies Doxycycline as an Inhibitor of M2-type Macrophage Polarization
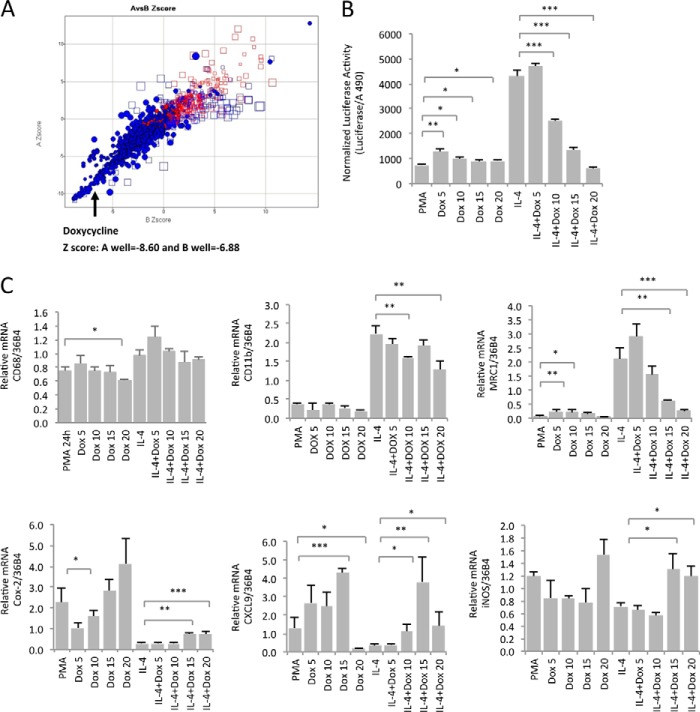
Next, we used the THP-1 cell clone stably transfected with the MRC1 luciferase construct for a small chemical screen using the Spectrum collection. Positive hits were defined as having Z scores of <−5. Among the most significant positive hits in this assay was the antibiotic doxycycline (A Z score, −8.6; B Z score, −6.9) (Fig. 1A). We chose to further investigate the role of doxycycline as a potential inhibitor of M2-type macrophage polarization because of its well known anti-inflammatory effects. For example, doxycycline inhibits the inflammatory skin condition rosacea, but it is not known what the mechanistic basis of this anti-inflammatory role is.
FIGURE 1.
Doxycycline inhibits MRC1 expression in human macrophages. A, a small chemical screen identifies doxycycline as an inhibitor of MRC1 expression in THP1 cell-derived macrophages. Positive hits were identified that inhibited MRC1 promoter-driven luciferase expression in a stably transfected THP-1 cell clone. Z scores of <−5 are indicated as blue ● in the bottom left square. Red ● indicate control wells. □ are excluded edge wells. The Z scores for doxycycline were −8.60 and −6.88. B, doxycycline inhibits MRC1 promoter-driven luciferase activity in a dose-dependent manner. The THP-1 cell clone used for the chemical screen was treated with PMA for 24 h, then pretreated with doxycycline for 24 h, and then with or without 20 ng/ml IL-4 for 3 days. Concomitant with IL-4 treatment, doxycycline was added at doses up to 20 μm. Luciferase activity was normalized to viable cells (using a lactate dehydrogenase release cytotoxicity assay). C, semiquantitative RT-PCR in naive THP-1 cells for the macrophage differentiation markers CD11b and CD68; the M2-type marker MRC1; and the M1-type markers COX-2, CXCL9, and inducible nitric oxide synthase (iNOS). Doxycycline dose-dependently inhibits MRC1 expression but not expression of CD11b or CD68. An increase in M1-type markers was noticed with doxycycline at 15 and 20 μm. Values were normalized to 36B4. Experiments were performed in triplicate. Concentrations are micromolar. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Thus, we tested whether doxycycline inhibited IL-4-induced luciferase activity in a dose-dependent manner in the stably expressing MRC1 luciferase THP-1 cell clone used in the screen without inducing cell toxicity. We could reliably confirm that doxycycline inhibits IL-4-induced luciferase activity (and, therefore, MRC1 expression) in a dose-dependent manner at doses between 10 and 20 μm (Fig. 1B). On the basis of the inhibitory activity of doxycycline on luciferase activation in this assay, the IC50 of doxycycline was ∼12 μm.
Doxycycline Inhibits Transcript and Protein Levels of M2-type Markers in Human Macrophages
Next, we tested whether doxycycline had the same effect on MRC1 transcript levels in naive THP-1 cells that were differentiated into macrophages and induced to undergo M2-type polarization with IL-4 treatment to ensure that doxycycline inhibits MRC1 expression rather than affecting only luciferase activity. Thus, we tested the effect of doxycycline on the expression of several prototypical markers of differentiated macrophages, of M1-type macrophages, and of M2-type macrophages. Importantly, although the macrophage differentiation markers CD68 and CD11b did not show a consistent dose-dependent reduction of expression with doxycycline treatment (CD11b showed a moderate decrease, whereas CD68 did not), MRC1 expression in differentiated THP-1 cells was potently and dose-dependently inhibited by doxycycline (IC50 of ∼12 μm) (Fig. 1C). Treatment with doxycycline at 15 and 20 μm concentrations resulted in a significant and progressive reduction of MRC1 expression, strongly resembling the results of the MRC1-luciferase experiments (Fig. 1C). In contrast, M1-type macrophage markers such as Cox-2, CXCL9, or inducible nitric oxide synthase (iNOS) showed a dose-dependent increase with doxycycline treatment, suggesting that doxycycline inhibits IL-4-induced M2-type macrophage polarization and promotes a shift toward an M1-type macrophage cell type (Fig. 1C).
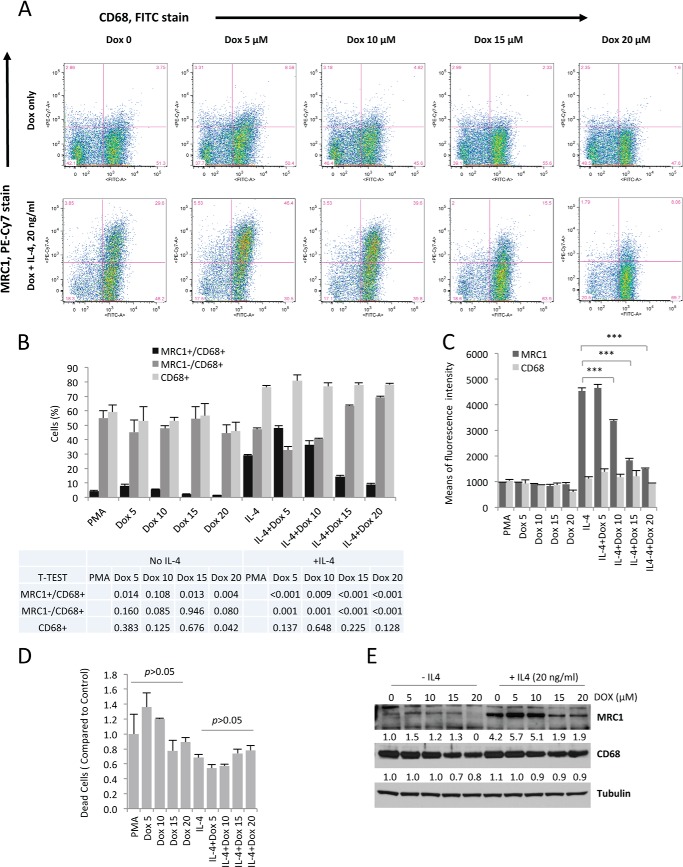
To confirm that the observed reduction of MRC1 transcript levels with doxycycline treatment also results in reduced MRC1 protein levels, we tested whether doxycycline inhibits MRC1 protein levels in differentiated macrophages that were induced to undergo M2-type polarization. PMA-treated THP-1 cells were induced to undergo M2-type polarization with IL-4 and were treated with doxycycline at various concentrations for 3 days before being used for FACS analysis. We found a dose-dependent decrease of the MRC1 signal in these FACS experiments (using the mean fluorescence intensity of the MRC1 signal revealed an IC50 of ∼12 μm), whereas the CD68 signal was unchanged (Fig. 2, A–C). A significant reduction of MRC1 protein levels was noticed in cells that were treated with 10, 15, or 20 μm of doxycycline (Fig. 2C), consistent with the observation of a potent inhibitory effect of doxycycline at these concentrations in the luciferase assays and the MRC1 semiquantitative RT-PCR experiments. Notably, no increased cell death was noticed at these concentrations (Fig. 2D).
FIGURE 2.
Doxycycline inhibits MRC1 protein levels in human THP-1 cell-derived macrophages without affecting macrophage differentiation. A, FACS analysis shows that doxycycline dose-dependently reduces MRC1 protein levels without affecting levels of the differentiation marker CD68. THP-1 cells were primed for 24 h with PMA and then pretreated with Dox for 24 h. Subsequently, cells were treated with either Dox alone or with Dox and IL-4 for 3 days. Experiments were performed in triplicate, and representative logarithmic FACS data are indicated. The percentage of cells is indicated for each field. B, quantitation of cells (in percent) that are either positive for CD68, MRC1, or both (n = 3/group). Cells were subjected to triple staining with MRC1-PE-Cy7 and CD68-FITC antibodies and a live/dead cell viability kit to exclude dead cells. p values are indicated comparing Dox treatment groups with cells that were not treated with Dox (PMA). C, the levels of MRC1 protein and CD68 protein are represented by the means of fluorescence intensity. D, Dox at doses inhibiting MRC1 protein levels does not cause cell toxicity, as assessed by live/dead cell viability staining. E, Western blotting demonstrates a dose-dependent inhibition of MRC1 protein levels by Dox, whereas CD68 levels are unchanged. Densitometric quantitations of bands normalized to tubulin are indicated (relative to control (first lane)). ***, p < 0.001.
Finally, Western blotting experiments further confirmed a dose-dependent decrease of MRC1 protein levels when IL-4-induced macrophages were treated with doxycycline, without reducing CD68 levels significantly (Fig. 2E). In summary, doxycycline inhibits both transcript and protein levels of MRC1 in a dose-dependent manner in IL-4-treated macrophages without inducing increased cell death.
Doxycycline Inhibits M2-type Macrophage Marker Expression in Bone Marrow-derived Mouse Macrophages
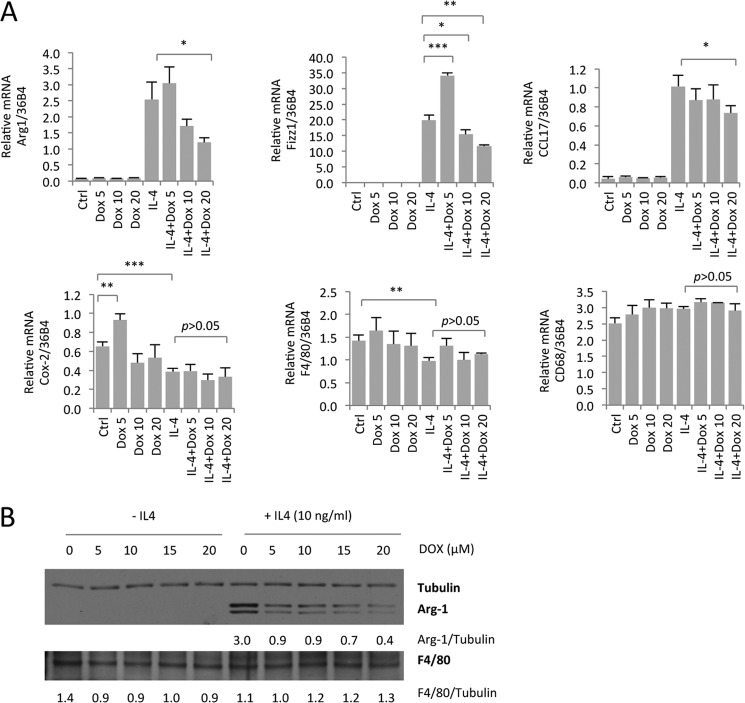
To show that the inhibition of M2-type macrophage polarization by doxycycline is not limited to an attenuation of MRC1 expression or limited to the THP-1 myeloid cell line, we tested whether M2-type macrophage marker expression is inhibited by doxycycline in primary murine BMDMs as well. Prototypical M2-type macrophage markers in mouse macrophages include arginase 1 (Arg1) and Fizz-1. As observed for MRC1 expression in human THP-1 cell-derived macrophages, doxycycline inhibited, dose-dependently, M2-type macrophage marker expression (Arg1, Fizz-1, and CCL17) in murine BMDMs without having this effect on general macrophage differentiation markers (CD68 and F4/80) or the M1 type marker Cox-2 (Fig. 3A). Western blotting experiments confirmed this observation and showed a dose-dependent inhibition of Arg1 protein levels in IL-4-induced BMDMs that were treated with doxycycline, whereas the general macrophage differentiation marker F4/80 was unchanged (Fig. 3B). Thus, our findings in human THP-1 cell-derived macrophages and mouse BMDMs demonstrate that doxycycline dose-dependently inhibits IL-4-induced M2-type macrophage polarization in vitro.
FIGURE 3.
Doxycycline inhibits expression of M2-type markers in murine BMDMs. A, BMDMs were isolated from 9-month-old female C57Bl/6j mice. After cell culture for 6 days and subsequent splitting, cells were pretreated with Dox overnight (in DMEM + 1% FBS + M-CSF at 10 ng/ml), followed by treatment with Dox or Dox + IL-4 at the indicated concentrations (micromolar) for an additional 3 days. The graphs indicate semiquantitative RT-PCR data (n = 3/group). Doxycycline inhibits expression of the M2-type macrophage markers (Arg1, Fizz1, and CCL17) but not of the M1-type marker COX-2 or the macrophage differentiation markers CD68 and F4/80. Values are normalized to the housekeeping gene 36B4. B, Western blotting for Arg1 and F4/80 shows that doxycycline inhibits Arg1 protein levels in BMDMs treated with IL-4 without affecting F4/80 levels. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Doxycycline Inhibits M2-type Macrophage Polarization Induced by Either IL-4, IL-13, PGE2, or PMA
Next, we tested whether the inhibitory effect of doxycycline on M2-type macrophage polarization is specific to induction by IL-4. We observed that macrophages can be polarized toward the M2-type by either IL-4 or IL-13 treatment but also, to a lesser extent, by PGE2 or prolonged treatment with PMA alone (Fig. 4A). Doxycycline dose-dependently inhibited M2-type macrophage polarization induced by IL-4, IL-13, PGE2, and PMA in the MRC1 promoter-driven luciferase assay (Fig. 4A). Thus, the effect of doxycycline as an inhibitor of M2-type macrophage polarization is not limited to IL-4-induction.
FIGURE 4.
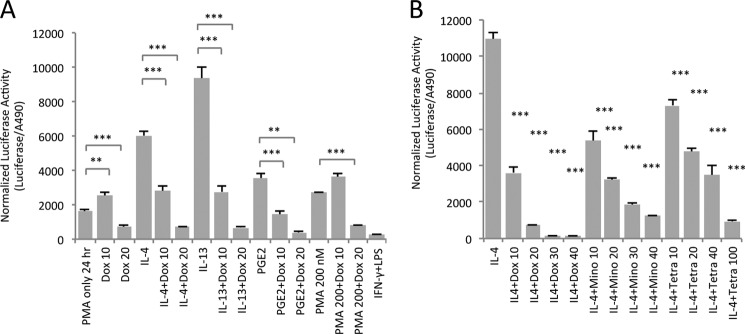
Doxycycline inhibits M2-type macrophage polarization induced by either IL-4, IL-13, PGE2, or PMA, and this inhibitory activity can also be observed with minocycline and tetracycline. A, MRC1 promoter-driven luciferase assay using IL-4, IL-13, PGE2, or PMA as inducers. Cells were treated with 100 nm PMA for 24 h, pretreated for 24 h with doxycycline at the indicated concentrations, and then induced to polarize with either IL-4, IL-13, PGE2, or PMA for 3 days with or without doxycycline treatment. B, dose-dependent inhibition of M2-type macrophage polarization can be observed with doxycycline as well as with minocycline and tetracycline. The concentrations used are indicated. p values are relative to IL-4 treatment alone. A and B, the indicated values are luciferase activity normalized to cell viability (A490 in the cell viability assay). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Inhibition of M2-type Macrophage Polarization by Doxycycline, Minocycline, and Tetracycline
Doxycycline belongs to the group of tetracycline antibiotics. Thus, we tested whether the observed inhibition of M2-type macrophage polarization is specific for doxycycline or whether this effect could be observed with other tetracycline antibiotics as well. We found that doxycycline, minocycline, and tetracycline all dose-dependently inhibited MRC1 promoter-driven luciferase expression without affecting cell viability (Fig. 4B). Notably, doxycycline was the most potent inhibitor of M2-type macrophage polarization (Fig. 4B).
Inhibition of M2-type Macrophage Polarization in Vivo, Expression of the Proangiogenic Cytokine IL-1β, and Laser-induced CNV by Doxycycline
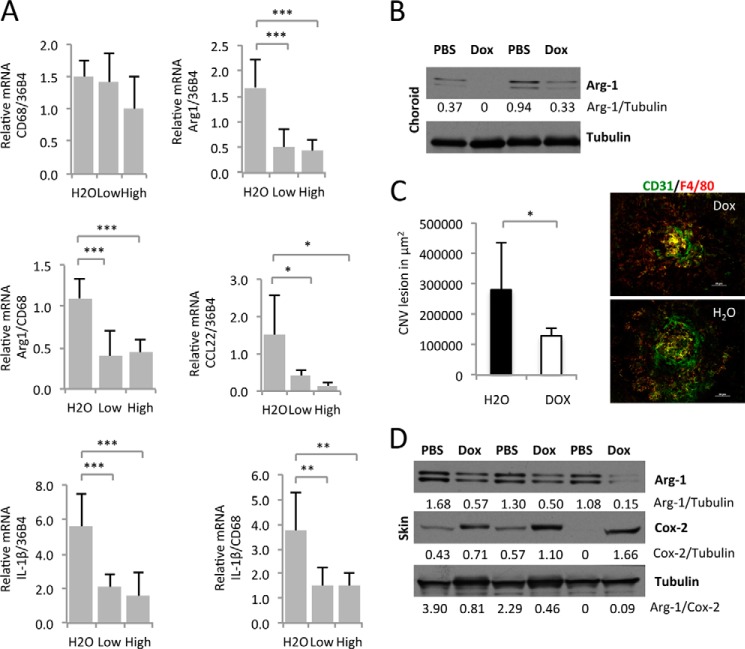
Because M2-type macrophages are proangiogenic (5), we speculated that inhibition of M2-type polarization inhibits the proangiogenic potential of activated macrophages. M2-type macrophages are the predominant macrophage population in several proangiogenic conditions, including in laser-induced choroidal neovascularization, a model for neovascular AMD (2). In this model, laser injury results in the infiltration of F4/80+Arg1+YM1+ macrophages into areas of injury, which leads to an increased expression of the proangiogenic cytokine IL-1β in adjacent retinal glia cells and subsequent neovascularization at that site. Immunolabeling identified high Arg1 expression only in infiltrating macrophages and in no other cell types in these lasered RPE/choroidal flat mounts (2). Consistent with this observation, ablation of macrophages results in loss of Arg1 protein levels in lasered RPE/choroidal tissue lysates and inhibition of neovascularization (2, 3).
Importantly, several studies have shown that doxycycline dose-dependently inhibits CNV lesion formation in this model, demonstrating a potent antiangiogenic activity of doxycycline (9, 11, 12). Thus, we hypothesized that inhibition of M2-type macrophage polarization by doxycycline results in reduced proangiogenic cytokine expression and subsequent neovascularization in this model. We tested, in the laser-induced CNV model of neovascular AMD, whether doxycycline treatment inhibits M2-type macrophage marker expression and IL-1β transcript levels. We found that doxycycline treatment of mice significantly reduced expression of the M2-type macrophage marker Arg1 in choroidal tissue lysates 3 days after laser injury, a time point when Arg1 and IL-1β expression is maximal after laser injury-induced infiltration of M2-type macrophages (Fig. 5A). Arg1 levels and the ratio of Arg1/CD68 levels were reduced significantly, whereas CD68 levels (normalized to 36b4) were not affected (Fig. 5A). These findings are consistent with the inhibition of M2-type polarization of macrophages in laser-induced CNV when treated with doxycycline. Similarly, the M2-type macrophage marker CCL22 was reduced by doxycycline treatment (Fig. 5A). Furthermore, IL-1β transcript levels in lasered choroidal tissues were reduced in doxycycline-treated mice, concomitant with the suppression of M2-type macrophage polarization (Fig. 5A).
FIGURE 5.
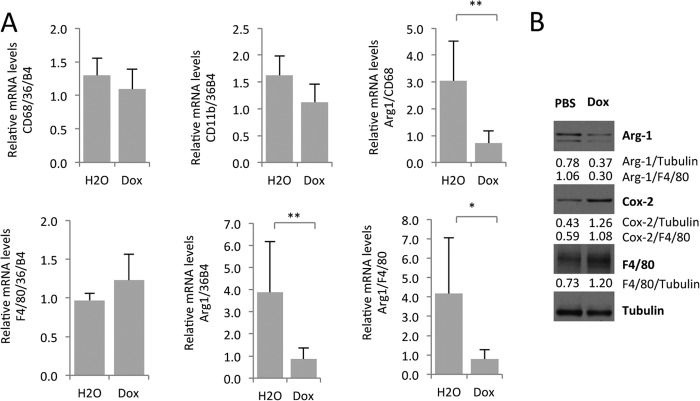
Doxycycline inhibits M2-type macrophage polarization in vivo, IL-1β expression, and subsequent neovascularization in a laser injury CNV model. A, 12-month-old female C57Bl/6j mice (6 mice in the control group, 8 mice in the low-dose Dox-treated group (daily intraperitoneal injection of doxycycline at 0.5 mg/30 g of BW), and 7 mice in high-dose Dox-treated group (daily intraperitoneal injection of doxycycline at 1.8 mg/30 g of BW)) were pretreated with doxycycline 1 day before laser injury to the choroid and, thereafter, daily. Three days after laser injury, mRNA was extracted from RPE/choroid tissues and used for semiquantitative RT-PCR experiments. Doxycycline inhibits expression of the M2-type macrophage markers Arg1 and CCL22 without affecting CD68 levels, suggesting an inhibition of M2-type macrophage polarization without reducing overall macrophage infiltration into lasered choroids. Reduction in M2-type marker expression correlated with inhibition of IL-1β transcript levels. B, Western blotting of choroidal/RPE tissue lysates 3 days after laser injury confirms reduced Arg1 protein levels, consistent with an inhibition of M2-type macrophage polarization in lasered eyes. C, doxycycline treatment (1.8 mg/30 g of BW) inhibited CNV lesion size significantly at day 5 after laser injury (5 control mice and 7 mice treated with doxycycline). Values indicate average CNV lesion size per mouse in square micrometers. Representative CNV lesions are shown in H20- or Dox-treated mice. CD31 stains neovessels and macrophages in green, and F4/80 stains macrophages in red. Scale bars = 50 μm. D, similar as observed in lasered choroidal/RPE tissues, doxycycline inhibits Arg1 protein levels in skin wounds, whereas the M1-type marker Cox-2 is not decreased. Densitometric band intensity ratios are indicated. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Western blotting of choroidal tissue lysates 3 days after laser injury confirmed that doxycycline reduced Arg1 protein levels (Fig. 5B). Importantly, the inhibition of M2-type macrophage polarization by doxycycline treatment correlated with its antiangiogenic effect on CNV lesion formation (Fig. 5C).
To show that the inhibitory effect of doxycycline on M2-type macrophage polarization is not limited to laser injury-induced CNV in choroidal tissue, we also tested the effect of doxycycline on M2-type macrophage polarization in a skin wound healing model. In this model, a full-thickness skin punch biopsy also induced infiltration of M2-type (F4/80+Arg1+YM1+) macrophages (13). Similarly as observed in laser-induced CNV lesions, skin wounds showed reduced Arg1 protein levels after doxycycline treatment, whereas this inhibition was not observed for the M1-type macrophage marker Cox-2 (Fig. 5D).
Next, we tested whether the inhibition of M2-type macrophage polarization is limited to the early phase of the laser-injury response in vivo (day 3) or whether this effect continues right through the time point when a neovascular lesion has formed fully (day 5). Similarly, as in RPE/choroid tissues 3 days after laser injury, Arg1 expression was reduced significantly 5 days after laser injury without affecting the expression of general macrophage differentiation markers (CD11b, CD68, and F4/80) (Fig. 6A). A reduction of Arg1 protein levels was further confirmed by Western blotting of choroidal tissue lysates 5 days after laser treatment, whereas F4/80 or Cox-2 levels were not reduced (Fig. 6B).
FIGURE 6.
Inhibition of M2-type polarization of macrophages in fully formed CNV lesions in vivo. A, low-dose doxycycline treatment (0.5 mg/30 g of BW) is sufficient to inhibit expression of the M2-type macrophage marker Arg1 in choroids at day 5 after laser injury, whereas the macrophage differentiation markers CD68, CD11b, or F4/80 were not inhibited. 17-month-old female mice were used (3 mice in the control group and 7 mice in the Dox-treated group). Shown are semiquantitative RT-PCR experiments for Arg1, CD11b, CD68, and F4/80. B, Western blotting of choroidal tissue lysates confirms an inhibition of Arg1 protein levels by doxycycline treatment. *, p < 0.05; **, p < 0.01.
Thus, doxycycline treatment inhibits M2-type macrophage polarization and reduces IL-1β transcript levels in laser-induced CNV, and this inhibition correlates with the antiangiogenic activity of doxycycline in this CNV model.
DISCUSSION
Doxycycline has been shown to have potent antiangiogenic activity in various experimental in vivo models (10). However, through which cellular or molecular effects doxycycline exerts this antiangiogenic activity is not known. Although doxycycline has been shown to inhibit several matrix metalloproteinases (MMPs) (14), it has been suggested that the antiangiogenic activity of doxycycline is not mediated by MMP inhibition (15).
In mouse tumor models, doxycycline inhibited tumor vascular permeability and tumor growth (10). Furthermore, several studies have reported that doxycycline inhibits ocular angiogenesis, for example in the corneal alkali burn model of angiogenesis, in which it has been observed that doxycycline reduced IL-1β levels and increased the potency of anti-VEGF-A treatment in inhibiting corneal angiogenesis (16, 17). It has been suggested that, although the inhibitory activity of doxycycline on MMPs may contribute to this antiangiogenic activity in this assay, MMP-independent antiangiogenic mechanisms contribute to the reduction of the angiogenic response with doxycycline treatment (18). For example, doxycycline inhibited VEGF-A-induced endothelial cell proliferation in a dose-dependent manner and reduced PI3K activity, AKT phosphorylation, and NO production in human umbilical vein endothelial cells (18). Thus, it is possible that doxycycline inhibits angiogenesis to some extent by attenuating VEGF-A-induced endothelial cell proliferation.
Doxycycline has also been reported to dose-dependently reduce laser-induced CNV lesions in mice, as shown in this study as well (9, 11, 12), and preliminary observations suggest a beneficial effect of doxycycline in treating neovascular AMD. One study suggests that this effect may be mediated by increased FasL expression by RPE cells after doxycycline treatment (11). Taken together, doxycycline inhibits pathologic angiogenesis and is likely to do so through diverse mechanisms.
Here we demonstrate a novel effect of doxycycline as an inhibitor of M2-type macrophage polarization and suggest that this effect may contribute to the antiangiogenic activity of doxycycline. Our data demonstrate that doxycycline dose-dependently inhibits polarization of macrophages toward the proangiogenic M2-type, which correlates with reduced expression of the proangiogenic factor IL-1β in laser-induced CNV and the antiangiogenic activity of doxycycline. Because doxycycline is a well tolerated and clinically widely used antibiotic, the data suggest that it could be used therapeutically to inhibit neovascularization in various conditions with pathological angiogenesis that is driven by M2-type macrophages. Notably, we also observed an inhibitory activity of other tetracycline group antibiotics on M2-type macrophage polarization, such as minocycline and tetracycline. However, doxycycline showed the highest potency as an inhibitor of M2-type macrophage polarization.
Several signaling pathways have been implicated to be important for M2-type macrophage polarization (5). For example, the IL-10 or the IL-4/STAT6 signaling pathways have been suggested to promote M2-type macrophage polarization (5). However, genetic inactivation of these pathways does not prevent M2-type polarization of macrophages in vivo, for example in the laser-induced CNV assay or in skin wound healing experiments (in STAT6 or IL-10 null mice) (2, 5, 13). Thus, in the in vivo setting, M2-type macrophage polarization seems to involve several pathways, and targeting IL-4/STAT6 or IL-10 alone is not sufficient to inhibit this polarization. This makes it challenging to inhibit M2-type polarization of macrophages by pharmacological targeting of a specific signaling pathway. It also suggests that doxycycline inhibits M2-type macrophage polarization in vivo not only by inhibiting IL-4 signaling in macrophages but also by inhibiting pathways that are activated by various other cytokines and factors that promote M2-type polarization. This hypothesis is consistent with our observation that doxycycline could inhibit M2-type macrophage polarization not only when induced by IL-4 or IL-13 but also when induced by PGE2 or PMA.
Therefore, our finding that a commonly used antibiotic that is well tolerated, doxycycline, can potently inhibit M2-type polarization of macrophages at concentrations that do not cause cell toxicity provides an important therapeutic opportunity to inhibit this proangiogenic macrophage population in several pathologic conditions. The data suggest that combining anti-VEGF-A therapies with doxycycline treatment may improve overall outcome in proangiogenic pathologic conditions, such as in neovascular AMD or glioblastoma multiforme.
In addition, doxycycline is commonly used to regulate expression of transgenes in various genetic mouse models, and our findings suggest that it is important to distinguish the effects of doxycycline on macrophages from the effects of the particular transgene studied.
Supplementary Material
Acknowledgment
We thank Dr. Julia Fox for technical assistance.
This study was supported, in whole or in part, by NEI, National Institutes of Health Grant NEI R01-EY019297 (to A. G. M.). This work was also supported by a Shiseido research grant (to A. G. M.) and by a Dermatology Foundation research grant (to L. H.).

This article contains supplemental Figs. S1 and S2 and Tables S1 and S2.
- AMD
- age-related macular degeneration
- CNV
- choroidal neovascularization
- BMDM
- bone marrow-derived macrophage
- PG
- prostaglandin
- Dox
- doxycycline
- PMA
- phorbol 12-myristate 13-acetate
- BW
- body weight
- RPE
- retinal pigment epithelium
- MMP
- matrix metalloproteinase.
REFERENCES
- 1. Sica A., Schioppa T., Mantovani A., Allavena P. (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression. Potential targets of anti-cancer therapy. Eur. J. Cancer 42, 717–727 [DOI] [PubMed] [Google Scholar]
- 2. He L., Marneros A. G. (2013) Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am. J. Pathol. 182, 2407–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marneros A. G. (2013) NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 4, 945–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavalette S., Raoul W., Houssier M., Camelo S., Levy O., Calippe B., Jonet L., Behar-Cohen F., Chemtob S., Guillonneau X., Combadière C., Sennlaub F. (2011) Interleukin-1β inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am. J. Pathol. 178, 2416–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sica A., Mantovani A. (2012) Macrophage plasticity and polarization. In vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pyonteck S. M., Akkari L., Schuhmacher A. J., Bowman R. L., Sevenich L., Quail D. F., Olson O. C., Quick M. L., Huse J. T., Teijeiro V., Setty M., Leslie C. S., Oei Y., Pedraza A., Zhang J., Brennan C. W., Sutton J. C., Holland E. C., Daniel D., Joyce J. A. (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CATT Research Group, Martin D. F., Maguire M. G., Ying G. S., Grunwald J. E., Fine S. L., Jaffe G. J. (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364, 1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rofagha S., Bhisitkul R. B., Boyer D. S., Sadda S. R., Zhang K. (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON. A multicenter cohort study (SEVEN-UP). Ophthalmology 120, 2292–2299 [DOI] [PubMed] [Google Scholar]
- 9. Samtani S., Amaral J., Campos M. M., Fariss R. N., Becerra S. P. (2009) Doxycycline-mediated inhibition of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 50, 5098–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fainaru O., Adini I., Benny O., Bazinet L., Pravda E., D'Amato R., Folkman J. (2008) Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 22, 3728–3735 [DOI] [PubMed] [Google Scholar]
- 11. Roychoudhury J., Herndon J. M., Yin J., Apte R. S., Ferguson T. A. (2010) Targeting immune privilege to prevent pathogenic neovascularization. Invest. Ophthalmol. Vis. Sci. 51, 3560–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox C. A., Amaral J., Salloum R., Guedez L., Reid T. W., Jaworski C., John-Aryankalayil M., Freedman K. A., Campos M. M., Martinez A., Becerra S. P., Carper D. A. (2010) Doxycycline's effect on ocular angiogenesis. An in vivo analysis. Ophthalmology 117, 1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daley J. M., Brancato S. K., Thomay A. A., Reichner J. S., Albina J. E. (2010) The phenotype of murine wound macrophages. J. Leukocyte Biol. 87, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suomalainen K., Sorsa T., Golub L. M., Ramamurthy N., Lee H. M., Uitto V. J., Saari H., Konttinen Y. T. (1992) Specificity of the anticollagenase action of tetracyclines. Relevance to their anti-inflammatory potential. Antimicrob. Agents Chemother. 36, 227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbertson-Beadling S., Powers E. A., Stamp-Cole M., Scott P. S., Wallace T. L., Copeland J., Petzold G., Mitchell M., Ledbetter S., Poorman R. (1995) The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother. Pharmacol. 36, 418–424 [DOI] [PubMed] [Google Scholar]
- 16. Dan L., Shi-long Y., Miao-li L., Yong-ping L., Hong-jie M., Ying Z., Xiang-gui W. (2008) Inhibitory effect of oral doxycycline on neovascularization in a rat corneal alkali burn model of angiogenesis. Curr. Eye Res. 33, 653–660 [DOI] [PubMed] [Google Scholar]
- 17. Su W., Li Z., Li Y., Lin M., Yao L., Liu Y., He Z., Wu C., Liang D. (2011) Doxycycline enhances the inhibitory effects of bevacizumab on corneal neovascularization and prevents its side effects. Invest. Ophthalmol. Vis. Sci. 52, 9108–9115 [DOI] [PubMed] [Google Scholar]
- 18. Su W., Li Z., Li F., Chen X., Wan Q., Liang D. (2013) Doxycycline-mediated inhibition of corneal angiogenesis. An MMP-independent mechanism. Invest. Ophthalmol. Vis. Sci. 54, 783–788 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.