Abstract
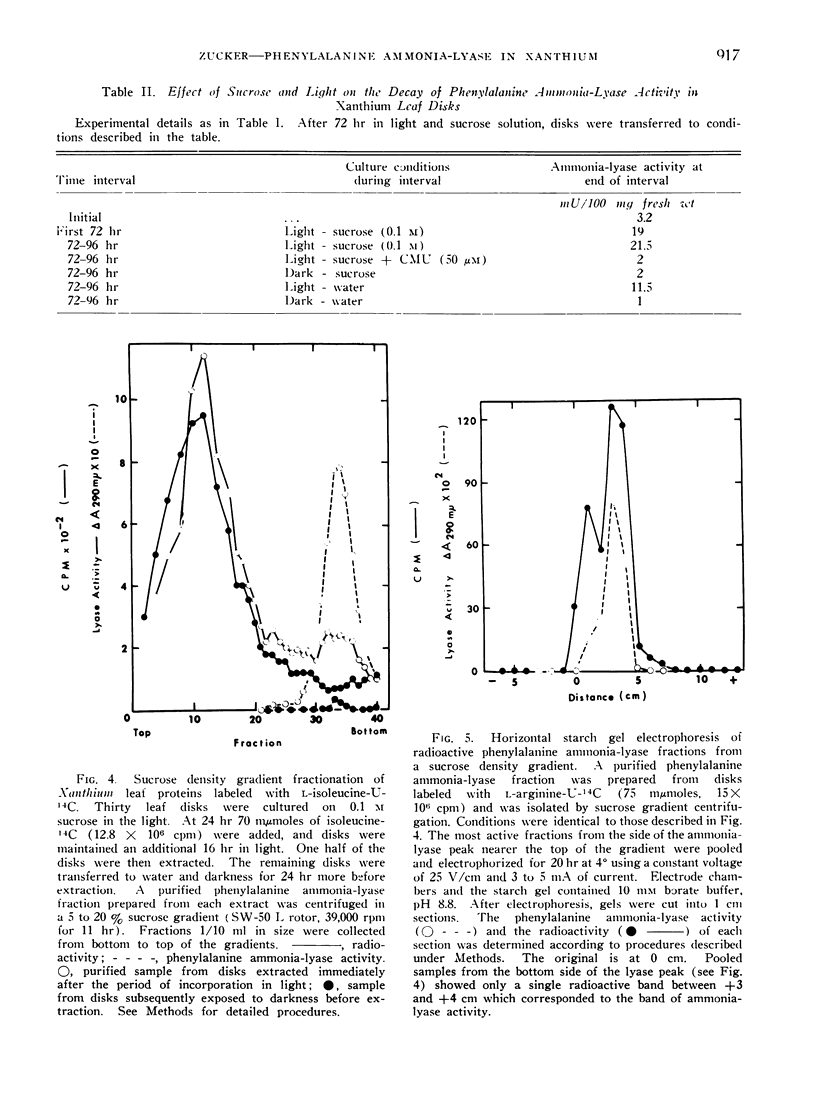
A cycloheximide-sensitive increase in the activity of phenylalanine ammonia-lyase (EC 4.3.1.5) occurs in Xanthium leaf disks exposed to light. Radioactive ammonia-lyase has been isolated by means of sucrose density gradient centrifugation and starch gel electrophoresis from disks fed l-isoleucine-U-14C or l-arginine-U-14C. The incorporation of radioactive amino acids into phenylalanine ammonia-lyase together with the inhibitory effects of cycloheximide indicate that the observed increase in enzyme activity involves the induction of lyase synthesis.
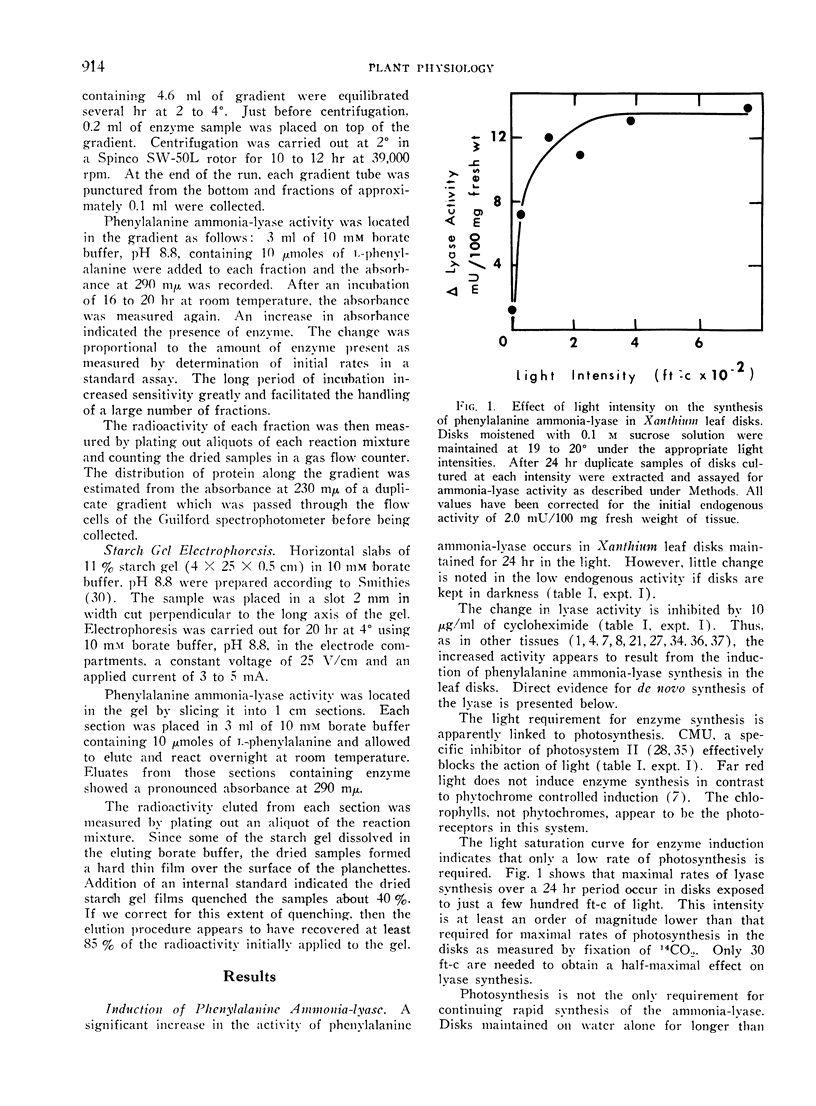
The light-dependent synthesis of the ammonia-lyase is completely inhibited by 50 μm 3-(4-chlorophenyl)-1,1-dimethylurea (CMU) indicating that photosynthesis is involved. Only a trace quantity of some photosynthetic product must be needed because half light saturation occurs at very low intensity (ca. 30 ft-c). Exogenous carbohydrate is also required for continuing enzyme synthesis over a 72 hr period. But carbohydrate does not replace the photosynthetic requirement in darkness.
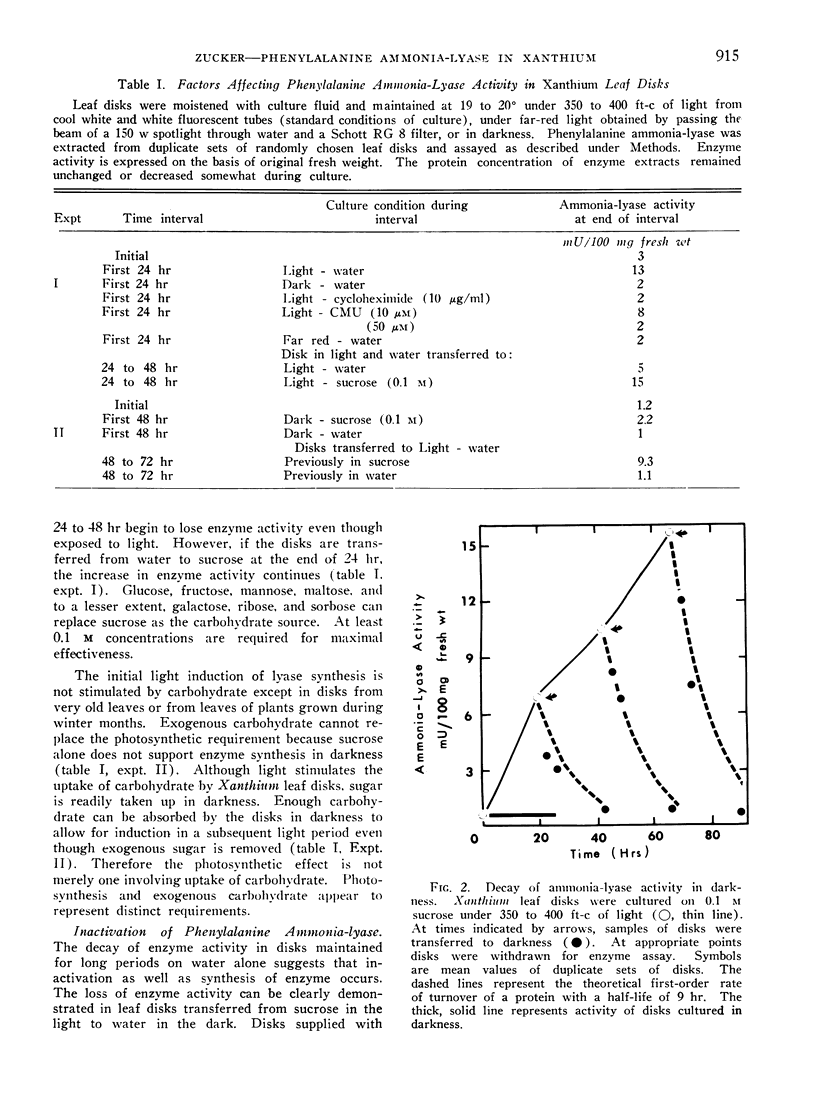
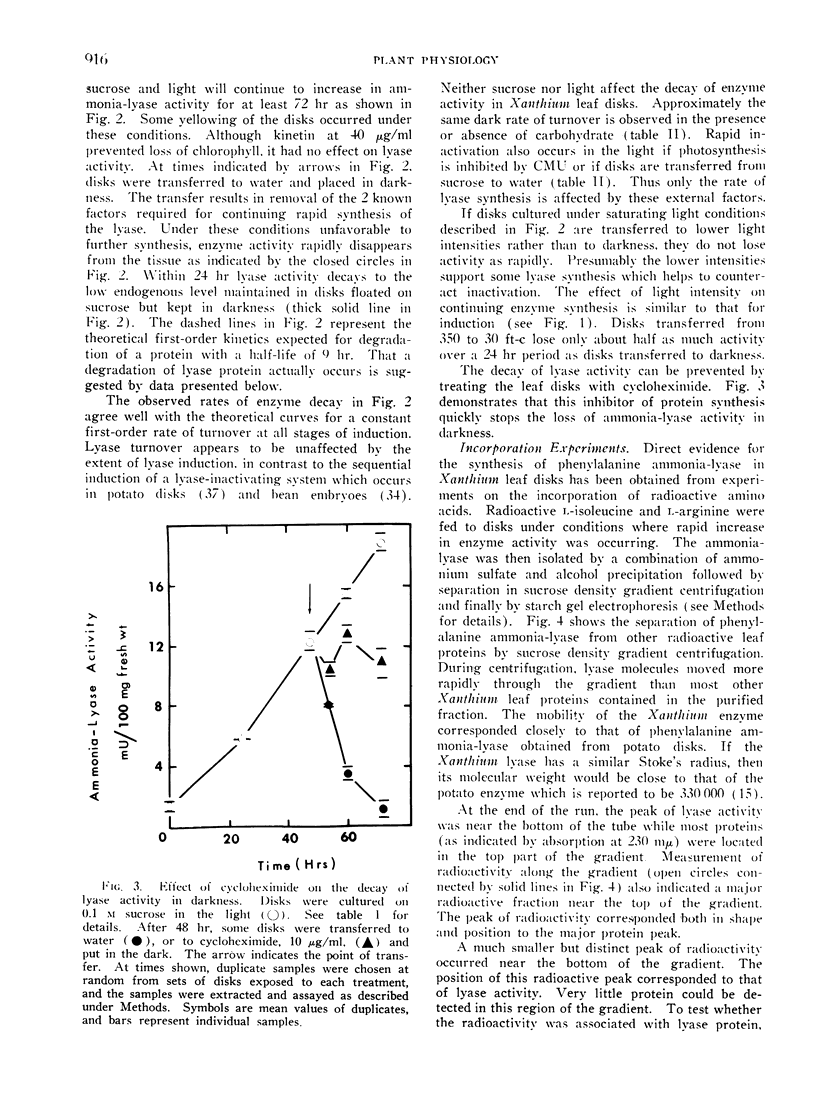
Enzyme formed in light disappears rapidly from disks placed in the dark. The decay of ammonia-lyase activity follows first order kinetics. The half-life of the lyase ranged from 6 to 15 hr in leaf material used. Cyoloheximide inhibits the decay of lyase activity. Thus the maintenance of turnover in Xanthium leaf disks requires de novo synthesis of protein. That turnover, i.e., degradation as well as synthesis of lyase protein occurs is suggested by the apparent loss of radioactive ammonia-lyase from leaf disks placed in darkness. Light-induced synthesis coupled with rapid turnover can produce a diurnal fluctuation of ammonia-lyase activity in Xanthium leaf disks. Alternating periods of enzyme synthesis and degradation were observed in disks exposed to 24 hr cycles of light and dark. The average level of enzyme activity maintained in the tissue was directly related to the length of the light period. Induction of lyase synthesis was also observed in excised leaves and to a lesser extent in leaves of whole plants.
Full text
PDF

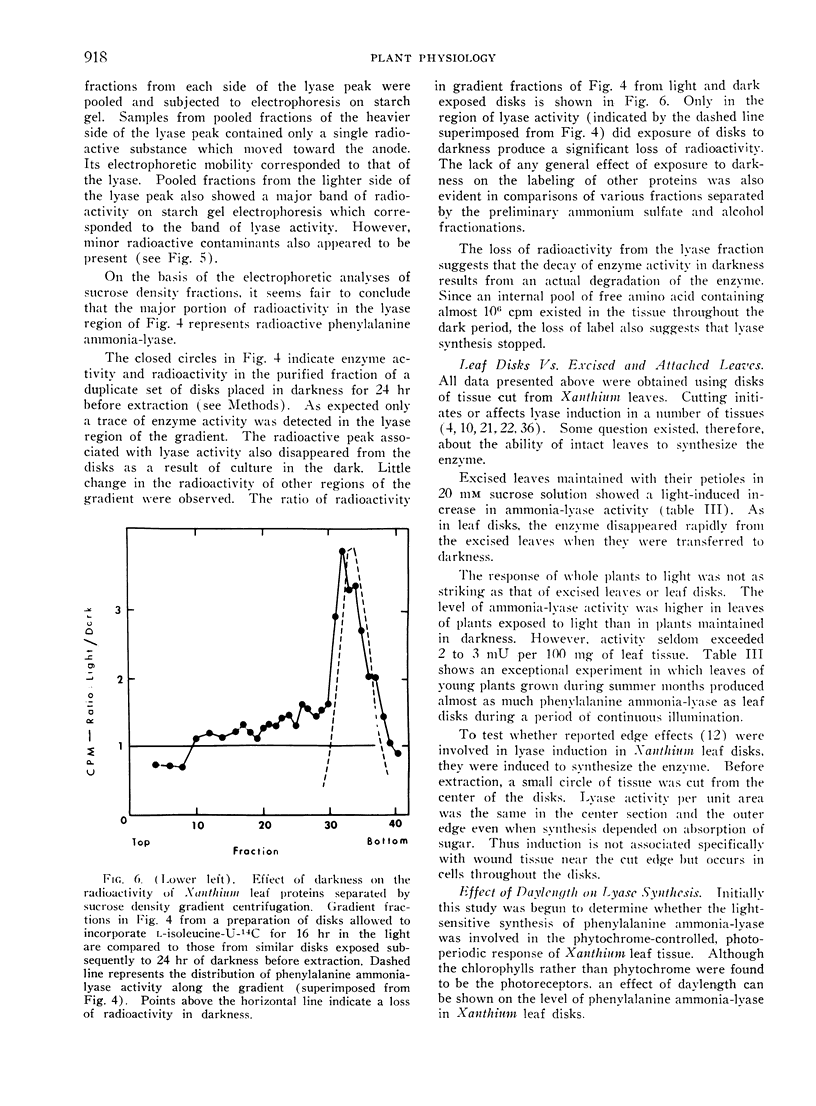



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARK M. F., MATTHEWS R. E., RALPH R. K. RIBOSOMES AND POLYRIBOSOMES IN BRASSICA PEKINENSIS. Biochim Biophys Acta. 1964 Oct 16;91:289–304. doi: 10.1016/0926-6550(64)90253-1. [DOI] [PubMed] [Google Scholar]
- Cheng C. K., Marsh H. V. Gibberellic Acid-Promoted Lignification and Phenylalanine Ammonia-lyase Activity in a Dwarf Pea (Pisum sativum). Plant Physiol. 1968 Nov;43(11):1755–1759. doi: 10.1104/pp.43.11.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F., Mohr H. Phytochrome-mediated induction of enzyme synthesis in mustard seedlings(Sinapis alba L.). Naturwissenschaften. 1966 Oct;53(20):531–532. doi: 10.1007/BF00600655. [DOI] [PubMed] [Google Scholar]
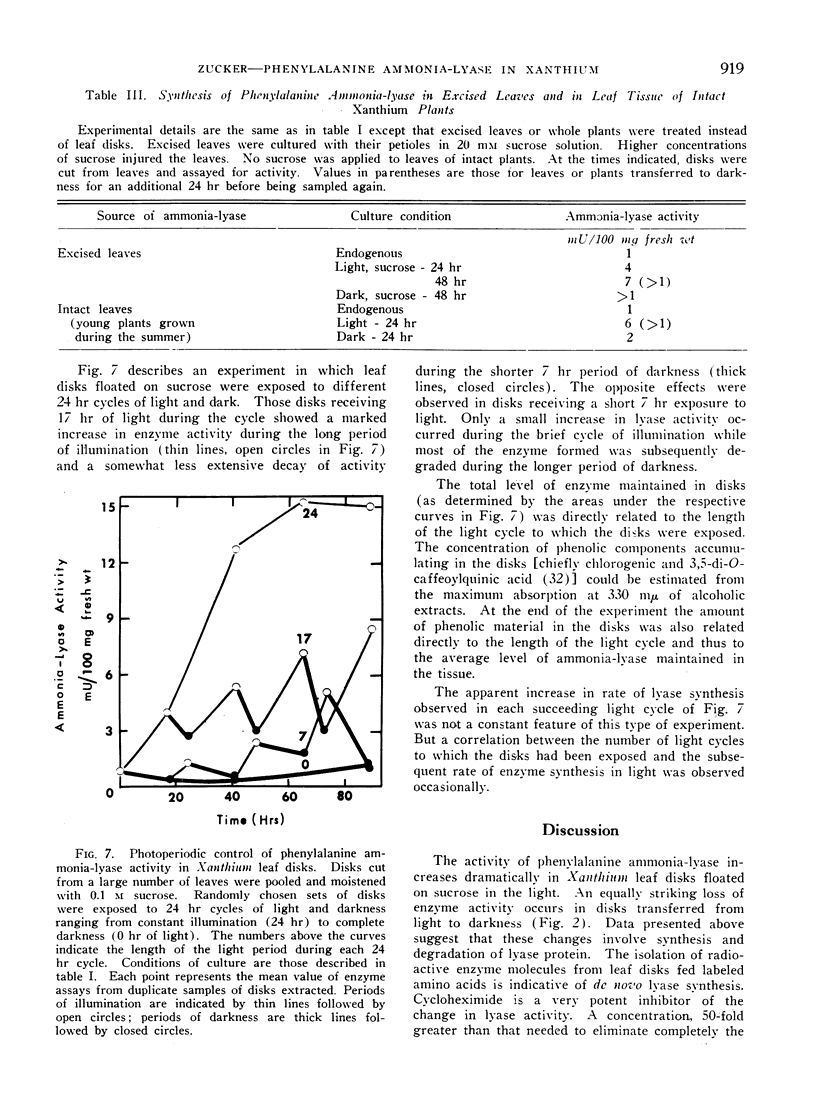
- Engelsma G. Effect of cycloheximide on the inactivation of phenylalanine deaminase in gherkin seedlings. Naturwissenschaften. 1967 Jun;54(12):319–320. doi: 10.1007/BF00640619. [DOI] [PubMed] [Google Scholar]
- Hartt C. E., Kortschak H. P. Sugar Gradients and Translocation of Sucrose in Detached Blades of Sugarcane. Plant Physiol. 1964 May;39(3):460–474. doi: 10.1104/pp.39.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartt C. E. Light and Translocation of C in Detached Blades of Sugarcane. Plant Physiol. 1965 Jul;40(4):718–724. doi: 10.1104/pp.40.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Ross C. Influence of cycloheximide (Actidione) upon pyrimidine nucleotide metabolism and rna synthesis in cocklebur leaf discs. Biochim Biophys Acta. 1968 Aug 23;166(1):40–47. doi: 10.1016/0005-2787(68)90488-7. [DOI] [PubMed] [Google Scholar]
- Rubery P. H., Northcote D. H. Site of phenylalanine ammonia--lyase activity and synthesis of lignin during xylem differentiation. Nature. 1968 Sep 21;219(5160):1230–1234. doi: 10.1038/2191230a0. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. An inducible protein in potato and tomato leaflets. Plant Physiol. 1968 Nov;43(11):1880–1881. doi: 10.1104/pp.43.11.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Synthesis of chymotrypsin inhibitor I protein in potato leaflets induced by detachment. Plant Physiol. 1968 Nov;43(11):1859–1865. doi: 10.1104/pp.43.11.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Taylor A. O. Some Effects of Photoperiod on the Biosynthesis of Phenylpropane Derivatives in Xanthium. Plant Physiol. 1965 Mar;40(2):273–280. doi: 10.1104/pp.40.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Zucker M. Turnover and metabolism of chlorogenic Acid in xanthium leaves and potato tubers. Plant Physiol. 1966 Oct;41(8):1350–1359. doi: 10.1104/pp.41.8.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSELS J. S., VAN DER VEEN R. The action of some derivatives of phenylurethan and of 3-phenyl-1, 1-dimethylurea on the Hill reaction. Biochim Biophys Acta. 1956 Mar;19(3):548–549. doi: 10.1016/0006-3002(56)90481-4. [DOI] [PubMed] [Google Scholar]
- Walton D. C., Sondheimer E. Effects of Abscisin II on Phenylalanine Ammonia-Lyase Activity in Excised Bean Axes. Plant Physiol. 1968 Mar;43(3):467–469. doi: 10.1104/pp.43.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Sequential Induction of Phenylalanine Ammonia-lyase and a Lyase-inactivating System in Potato Tuber Disks. Plant Physiol. 1968 Mar;43(3):365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


