Abstract
Adult female prairie (Microtus ochrogaster) and meadow (M. pennsylvanicus) voles were compared to examine neural cell proliferation and the effects of estrogen manipulation on cell proliferation in the amygdala, ventromedial hypothalamus (VMH), and dentate gyrus of the hippocampus (DG). Unlike prior studies, our study focused on the amygdala and VMH, because they are involved in social behaviors and may underlie behavioral differences between the species. Meadow voles had a higher density of cells labeled with the cell proliferation marker 5-bromo-2′-deoxyuridine (BrdU) in the amygdala and DG than did prairie voles. Treatment with estradiol benzoate (EB) for 3 days increased the density of BrdU-labeled cells in the amygdala, particularly in the posterior cortical (pCorA) and medial (pMeA) nuclei, in meadow, but not prairie, voles. Furthermore, the majority of the BrdU-labeled cells in the pCorA and pMeA displayed either a neuronal or a glial progenitor phenotype, but no species or treatment differences were found in the percentage of neuronal or glial progenitor cells. To understand better estrogen’s effects on adult neurogenesis, we also examined estrogen receptor-α (ERα) distribution. Meadow voles had more ERα-labeled cells in the pCorA and VMH, but not in the pMeA or DG, than did prairie voles. In addition, more than one-half of the BrdU-labeled cells in the amygdala of both species coexpressed ERα labeling. Together, these data indicate that estrogen alters cell proliferation in a species- and region-specific manner, and some of these effects may lie in the specific localization of estrogen receptors in the adult vole brain.
Indexing terms: neurogenesis, ERα, amygdala, hypothalamus, progenitor
In adult mammals, newly proliferated cells have been found in the dentate gyrus of the hippocampus (DG) and subventricular zone (SVZ) of almost all species examined, including rats (Kaplan and Hinds, 1977; Peretto et al., 1999; Tanapat et al., 1999), mice (Kempermann et al., 1997; Shingo et al., 2003), hamsters (Huang et al., 1998), voles (Fowler et al., 2002; Galea and McEwen, 1999; Smith et al., 2001), nonhuman primates (Bedard et al., 2002; Gould et al., 1999a), and humans (Curtis et al., 2003; Eriksson et al., 1998). In some of these species, newly proliferated cells have also been identified in other forebrain regions, such as the striatum, septum, amygdala, neocortex, thalamus, and hypothalamus (Fowler et al., 2002; Gould et al., 1999b; Huang et al., 1998; Magavi et al., 2000; Nunes et al., 2003; Pencea et al., 2001). This widespread presence of new cells in varying regions of the adult brain has led researchers to investigate the mechanisms regulating new cell proliferation and survival (Fowler and Wang, 2003; Goldman, 1998). Because hormones play an important role in brain development and functioning (Dohler et al., 1983; Gould et al., 1991; McEwen et al., 1997; Vom Saal, 1983), several lines of research have begun to focus on the role of hormones. For example, in rats, corticosterone decreases the density of new cells in the DG (Cameron and Gould, 1994), whereas, in mice, prolactin enhances the number of new cells in the SVZ (Shingo et al., 2003). The gonadal steroid hormone estradiol also appears to be important. In the female rat DG, an increase in the proliferation of new cells occurs during proestrus, when estrogen reaches its highest level, and ovariectomy diminishes the number of new cells, whereas estradiol replacement transiently reverses this effect (Ormerod et al., 2003; Tanapat et al., 1999).
In most female mammals, serum estrogen levels fluctuate in accordance with an estrus cycle. In contrast, in voles—a group of microtine (Microtus) rodents—females are induced ovulators and exposure to a conspecific male results in increases in serum estrogen levels and estrogen receptor (ER) binding in the brain (Dluzen and Carter, 1979; Seabloom, 1985; Hnatczuk et al., 1994). It is interesting to note that some vole species display remarkable differences in life strategy and social behaviors. For example, prairie voles (Microtus ochrogaster) have been characterized as monogamous and highly social; a male and female share a nest and form long-lasting partnerships, or pair bonds (Carter et al., 1986; Getz et al., 1987). On the other hand, meadow voles (M. pennsylvanicus) are promiscuous and asocial; a male and female usually do not share a nest, nor do they form pair bonds (Lim et al., 2004; Madison, 1980). [However, meadow voles can show plasticity in their social behaviors; nest sharing and selective partner preferences have been observed under some laboratory conditions (Parker et al., 2001; Storey et al., 1994)]. These animals have provided an excellent comparative model for the study of brain organization, development, social behaviors, and underlying mechanisms of varying life strategies. For example, in comparison with monogamous voles, promiscuous voles develop more rapidly in their overall brain growth (Gutierrez et al., 1989) and in the development of specific central systems, such as brain-derived neurotrophic factor (Liu et al., 2001b). Female meadow voles are induced to undergo behavioral estrus more rapidly after exposure to a conspecific male (Taylor et al., 1992) and appear to perform better in navigational mazes (Gaulin et al., 1990) compared with female prairie voles. Monogamous and promiscuous voles also differ in their neuropeptide systems, such as vasopressin and oxytocin (Insel and Shapiro, 1992; Wang et al., 1997b), and such differences appear to underlie their ability to form and express pair bonding behavior (Insel and Hulihan, 1995; Liu et al., 2001a).
Insofar as social environment may alter serum estrogen levels in voles, the effects of estrogen on cell proliferation could differ in species displaying alternate reproductive strategies. Indeed, attempts have been made to investigate the role of steroid hormones in adult neurogenesis in the vole brain. In the DG of meadow voles, enhanced cell survival, but not proliferation, occurs during periods of reproductive activity in males (Ormerod and Galea, 2003), whereas a decrease in cell proliferation is found during the breeding season in females, coincident with higher levels of corticosterone and estrogen (Galea and McEwen, 1999). Testosterone or estradiol, but not dihydrotestosterone, increases cell proliferation in the amygdala, but not DG, of male meadow voles (Fowler et al., 2003), and estradiol treatment transiently enhances cell proliferation in the DG of female meadow voles (Ormerod and Galea, 2001). In female prairie voles, male exposure and mating enhance cell proliferation and survival in the amygdala and hypothalamus (Fowler et al., 2002), and estradiol treatment increases cell proliferation in the SVZ (Smith et al., 2001). Although these data establish a hormonal influence on adult neurogenesis and indicate that steroid hormones may have differential effects on neurogenesis in vole species with differing life strategies and social behaviors, variations in the experimental paradigms, procedures, and methods of cell labeling as well as in the aspects of the natural vs. laboratory environments have made direct comparisons between these studies/species impossible.
Therefore, the primary goal of the present study was to compare systematically the effects of estradiol on cell proliferation in female prairie and meadow voles. Unlike prior studies, our study focused on the amygdala and ventromedial hypothalamus (VMH), because these brain areas have been implicated in species-specific social behaviors (Cushing et al., 2004; Demas et al., 1997; Insel and Shapiro, 1992; Kirkpatrick et al., 1994; Wang et al., 1997a), and cell proliferation in these brain areas can be influenced by manipulation of social environment, possibly resulting from altered circulating levels of estrogen, in female prairie voles (Fowler et al., 2002). We also included the DG in our analysis so that our data may be compared with data from prior studies. Our secondary goal was to determine the localization of ERα in the brain of both species. In prairie voles, ERs have been identified in both the amygdala and the VMH, indicating that these brain regions may respond to estradiol actions (Cushing et al., 2004; Hnatczuk et al., 1994). However, to date, the localization of ERs in other species of voles, including meadow voles, has not been examined. Therefore, to understand better estrogen’s regulation of adult neurogenesis, we compared the distribution of ERα and examined newly proliferated cells in the amygdala and VMH for the presence of ERα in both species.
MATERIALS AND METHODS
Subjects
Sexually naive adult female prairie (Microtus ochrogaster) and meadow (M. pennsylvanicus) voles that were offspring of the F4 generation of laboratory breeding colonies were used as subjects when they reached 4–5 months of age. The prairie voles were derived from wild-caught animals from Illinois, whereas the meadow voles were derived from wild-caught animals from northwestern Pennsylvania and southwestern New York State. The voles were weaned at 21 days of age and housed in same-sex sibling pairs in plastic cages (29 × 18 × 13 cm) containing cedar chip bedding. All cages were maintained under 14L:10D photoperiod, with lights on at 0700. Temperature was maintained at 21°C ± 1°C, and subjects were provided ad libitum food (rabbit chow and sunflower seeds) and water.
Experimental design and manipulations
Study 1
Exposure to a male for 72 hours altered cell proliferation in the amygdala and hypothalamus of female prairie voles (Fowler et al., 2002). Male exposure also induces an increase in circulating levels of estrogen (Dluzen and Carter, 1979), so this hormone may play a role in influencing cell proliferation in female prairie voles. Therefore, the first study was designed to test this hypothesis. In addition, because prairie and meadow voles differ in life strategy and social behaviors, these species were both examined to determine whether estrogen’s effects are species specific. Adult females were ovariectomized, followed by a recovery period of 2–3 weeks. Thereafter, they were randomly assigned to one of two treatment groups: implantation with empty Silastic tubing (10 mm long, 1.98 mm i.d., 3.18 mm o.d.; control, n = 6 for each species) or tubing filled with estradiol benzoate (EB; Sigma, St. Louis, MO; n = 6 for each species). After tubing implantation, subjects received a series of BrdU injections at 48 hours (see below) and were perfused at 72 hours. A radioimmunoassay was performed to confirm the efficacy of the EB implants by using the Coat-a-Count estradiol kit (Diagnostic Products Corp., Los Angeles, CA); this kit has been previously verified for use in voles (Fowler et al., 2003). The sensitivity of the estradiol kit was 8 pg/ml, and the intra- and interassay coefficients of variation were both <5%. The mean plasma concentration of estradiol was higher for EB-treated voles (408.21 ± 31.27 pg/ml) than for control voles with empty tubing (10.03 ± 2.88 pg/ml). After perfusion of the subjects, brains were removed and examined for BrdU labeling and for BrdU, TuJ1, and NG2 fluorescence triple labeling.
Study 2
Estrogen may influence cell proliferation by acting on specific receptors in the brain. Although the presence of ERs has been reported for the prairie vole brain (Cushing et al., 2004; Hnatczuk et al., 1994), no study to date has been conducted in meadow voles. It is also still unknown whether the two species differ in their distribution pattern and regional density of ERs, potentially indicating a differential brain responsiveness to estrogen. Therefore, the second study was designed to compare the distribution of ERα in the brains of prairie and meadow voles. In addition, we also investigated whether ERα was present on the newly proliferated cells in the amygdala and VMH of both species. Intact, untreated female prairie and meadow voles (n = 6 for each species) received the series of 5-bromo-2′-deoxyuridine (BrdU) injections (see below) and were perfused, and their brains were examined for the presence of ERα immunostaining and for ERα with BrdU double labeling. All of this research was approved by Florida State University’s Animal Care and Use Committee and conformed to NIH guidelines.
BrdU injections
To label proliferating cells, subjects were injected with the cell proliferation marker BrdU (Sigma) intraperitoneally (ip; 50 μg/g body weight) in 0.9% NaCl and 0.007 N NaOH. All subjects received four injections of BrdU at 6-hour intervals and were sacrificed 6 hours after the last BrdU injection (Fowler et al., 2002). For the subjects that received Silastic tubing implantation, BrdU injections began 48 hours following the tubing implantation.
Brain perfusion/fixation
All subjects were anesthetized with sodium pentobarbital (0.1 mg/10 g body weight). For study 1, subjects were perfused through the ascending aorta with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer solution (PBS; pH 7.4). Brains were harvested, postfixed for 2 hours in 4% paraformaldehyde, and then stored in 30% sucrose in PBS. For study 2, a more immediate and extensive tissue fixation was necessary for ERα immunoreactive staining (Cushing et al., 2004). Therefore, subjects were perfused with 0.9% saline, followed by 3% acrolein and 4% paraformaldehyde in PBS. Brains were postfixed in 4% paraformaldehyde overnight and then stored in 30% sucrose in PBS. All brains then were cut into 40-μm coronal sections on a microtome, and the floating sections were stored in 0.1 M PBS with 1% sodium azide at 4°C until processing for immunocytochemistry.
BrdU immunocytochemistry
Floating brain sections at 120-μm intervals were processed for BrdU immunostaining as described previously (Fowler et al., 2002). Briefly, sections were treated with 2 N HCl for 30 minutes at 60°C and then with 0.1 M borate buffer at room temperature for 25 minutes. After being rinsed in 0.1 M PBS, sections were incubated in 0.3% hydrogen peroxide and 10% methanol in 0.1 M PBS for 15 minutes, 0.5% Triton X-100 in 0.1 M PBS (0.5% PBT) with 10% normal goat serum for 60 minutes, and rat anti-BrdU monoclonal antibody (1:1,000; Accurate Chemical, Westbury, NY) in 0.5% PBT with 10% normal goat serum at 4°C overnight. This antibody detects BrdU incorporated into the DNA while cells are in S-phase of the cell cycle. Sections then were rinsed and incubated in biotinylated goat anti-rat IgG (1:200; Jackson Immunoresearch, West Grove, PA) in 0.5% PBT for 2 hours at room temperature. Thereafter, sections were incubated in ABC Elite (Vector, Burlingame, CA) in 0.1 M PBS for 90 minutes, and immunoreactivity was revealed by using 3′-diaminobenzidine (DAB; Sigma). To reduce variability in the background and to standardize the staining, sections from all subjects were processed concurrently. Controls included processing brain sections without the primary antibody and processing brain sections from animals that did not receive BrdU injections; in either case, BrdU immunoreactive staining was not detected.
ERα immunocytochemistry
To identify ERα immunoreactivity, an antibody directed against the last 14 amino acids of the rat ERα (C1355; Upstate, Lake Placid, NY) was used. The specificity of the C1355 ERα antibody has previously been verified by pre-absorbtion with the peptide against which it was produced (Moffatt et al., 1998) and by immunoblot with rat ERα-transfected Cos-1 cells (Schreihofer et al., 1999). Furthermore, this antibody has been previously used to identify ERα in the vole brain (Cushing et al., 2004). Floating brain sections at 120-μm intervals were rinsed in PBS, incubated in sodium borohydride (0.1 g/10 ml of 0.1 M PBS), rinsed in PBS, blocked in 10% normal goat serum in 0.5% PBT, and then incubated in rabbit anti-ERα primary IgG (1:25,000) in 0.5% PBT for 1 hour at room temperature, 48 hours at 4°C, and overnight at room temperature. The sections then were rinsed in 0.1 M PBS and incubated in goat anti-rabbit secondary IgG (1:200; Jackson Immunoresearch) in 0.5% PBT for 2 hours and ABC complex in 0.5% PBT for 90 minutes, and staining was revealed with nickel-DAB. Sections were mounted on slides and cover-slipped with Permount. As a control, additional brain sections were processed without the primary antibody, and specific labeling was not present. To reduce variability in the background and to standardize the staining, sections from all subjects were processed concurrently.
Double- or triple-fluorescence immunocytochemistry
For study 1, to determine the phenotype of the BrdU-labeled cells, we examined the amygdala regions in which estradiol treatment influenced BrdU labeling and the VMH as a control area. Floating sections at 120-μm intervals were processed for BrdU, TuJ1, and NG2 fluorescence triple labeling. TuJ1, a mouse monoclonal IgG, recognizes a neuron-specific class III β-tubulin which is considered to be the earliest marker for cells that have begun to differentiate into neurons (Alexander et al., 1991; Kameda et al., 1993). NG2, a polyclonal IgG, recognizes NG2, which is an integral membrane proteoglycan expressed on glial progenitor cells (Nishiyama et al., 1995); this proteoglycan has been identified in cells that mature into astrocytes (Fidler et al., 1999), oligodendrocytes (Butt and Berry, 2000), or microglia (Jones et al., 2002). Both TuJ1 and NG2 have been used to identify the phenotypes of newly proliferated cells in the adult brain (Fowler et al., 2002; Shihabuddin et al., 2000).
Sections were blocked with 10% normal donkey serum in 0.1% PBT and incubated in rabbit anti-NG2 (1:150; Chemicon, Temecula, CA) in 0.3% PBT at 4°C overnight. On day 2, the sections were rinsed and incubated in Cy5-conjugated donkey anti-rabbit IgG (1:100; Jackson Immunoresearch) in 0.3% PBT for 2 hours. Next, the sections were rinsed, blocked in 10% normal goat serum in 0.1% PBT, and incubated in mouse anti-TuJ1 (1:500; Covance, Berkeley, CA) at 4°C overnight. Thereafter, the sections were rinsed, incubated in Alexa-488-conjugated goat anti-mouse IgG (1:400; Molecular Probes, Eugene, OR) in 0.3% PBT for 2 hours, and then processed for BrdU immunocytochemistry by blocking in 10% normal donkey serum and incubating in rat anti-BrdU (1:200; Accurate Chemical) in 0.1% PBT at 4°C 36 hours and then Texas red-conjugated donkey anti-rat IgG (1:200; Jackson Immunoresearch) in 0.3% PBT for 2 hours at room temperature. Sections were rinsed, mounted on slides with SlowFade component A (Molecular Probes), and coverslipped. Controls included processing the secondary antibodies alone to verify background staining, processing each primary with the secondary antibodies to verify laser-specific excitation, and using sequential scans to avoid cross-talk among channels.
For study 2, BrdU and ERα double-fluorescence immunolabeling was performed. Floating brain sections were rinsed in 0.1% PBT, incubated in sodium borohydride (0.1 g/10 ml of 0.1 M PBS), and stained for BrdU fluorescence using rat anti-BrdU (1:200; Accurate Chemical) as the primary antibody and Texas red-conjugated donkey anti-rat IgG (1:200; Jackson Immunoresearch) as the second antibody, as described above. Thereafter, sections were rinsed, blocked in 10% normal donkey serum in 0.3% PBT, and then incubated in rabbit anti-ERα primary IgG (1: 250; Upstate) in 0.3% PBT overnight at room temperature. The sections were rinsed again and incubated in fluorescein isothiocyanate (FITC) donkey anti-rabbit secondary IgG (1:200; Jackson Immunoresearch) in 0.3% PBT for 2 hours. Finally, sections were rinsed, mounted on slides with SlowFade component A, and coverslipped. Controls included processing the secondary antibodies alone to verify background staining, processing each primary with the secondary antibodies to verify laser-specific excitation, and using sequential scans to avoid cross-talk between channels.
Data quantification and analysis
All slides were coded to disguise group identity. BrdU-labeled cells were examined and quantified bilaterally throughout the entire rostrocaudal extent of the DG (granule and hilus combined); central (CeA), medial (MeA), and cortical (CorA) subnuclei of the amygdala; and VMH on coronal sections. For the DG and amygdala, cell numbers and region volumes were quantified by using unbiased stereological methods and the optical fractionator probe with Stereo Investigator software (MicroBrightField, Inc., Williston, VT) on a Leica DMRB microscope. This method of assessing total volume and cell number has been validated and employed in many prior studies (see, e.g., Benner et al., 2004; Bothwell et al., 2001; Brown et al., 2003; Glaser and Glaser, 2000). The coefficient of error for both of these areas was less than 0.1, which is within the acceptable range for stereological analysis. Total cell counts and area measurements were determined for each brain area, and cell density (number of cells per cubic millimeter) was calculated for each subject. Because stereological methods were employed in which a structure must be examined in its entirety, we did not differentiate between the dorsal and the ventral arms of the DG. For the VMH, stereological measurements for BrdU labeling yielded inappropriately high coefficients of error because of small numbers of BrdU-labeled cells. Therefore, profile methods of cell counting were employed in this area, and area measurements (square millimeters) were taken on each section analyzed to determine cell densities. Group differences for each region were analyzed by a two-way analysis of variance (ANOVA), using species and treatment as independent variables, followed by a Student-Newman-Keul’s (SNK) posthoc test. The criterion for significance was set at P < 0.05.
ERα-labeled cells were examined and quantified bilaterally throughout the entire rostrocaudal extent of the DG, VMH, and posterior cortical (pCorA) and posterior medial (pMeA) nuclei of the amygdala on coronal sections. Cell number and region volumes were quantified by using stereological methods. Large numbers of ERα-labeled cells were present in the VMH, in contrast to the small number of BrdU-labeled cells in this area, allowing us to use stereological methods with an acceptable coefficient of error for the ERα quantification. The coefficient of error was 0.1 for all brain areas measured, except for the DG (coefficient of error mean 0.23) because of smaller cell numbers. Total cell counts and area measurements were determined for each brain area, and cell density (number of cells per cubic millimeter) was calculated for each subject. Species differences in the density of ERα-labeled cells for each brain region were analyzed by t-test. The criterion for significance was set at P < 0.05.
For BrdU, TuJ1, and NG2 fluorescence triple immunostaining, labeled cells were examined and quantified in the pCorA and pMeA of the amygdala and in the VMH. Cells were visualized under ×63 magnification with a Zeiss 510NLO confocal microscope. At least 92 cells were counted in the pCorA, 119 cells in the pMeA, and 70 cells in the VMH for each species from two sections per animal. Individual cells stained for BrdU/ TuJ1, BrdU/NG2, or BrdU only were quantified. Percentages of BrdU-labeled cells containing a neuronal (TuJ1) or glial progenitor (NG2) marker were calculated, and group differences were analyzed by a two-way ANOVA. The criterion for significance was set at P < 0.05.
For BrdU and ERα double labeling, cells were quantified in the DG, pCorA, pMeA, and VMH by using a Zeiss 510NLO confocal microscope. At least 85 BrdU-labeled cells for the pCorA, 64 for the pMeA, 60 for the VMH, and 195 for the DG were counted for each species from two sections/animal. Percentages of BrdU-labeled cells containing ERα labeling were calculated. Species differences in the percentage of BrdU/ERα colocalized cells were analyzed by t-test for each brain region, and differences across the brain regions were analyzed by one-way ANOVA, followed by a SNK post hoc test. The criterion for significance was set at P < 0.05.
Photomicrographic images were obtained on a Leica DMRB microscope with Stereo Investigator software (Figs. 1, 3, 6) or a Zeiss 510NLO confocal with Zeiss software (Figs. 5, 8). For Figures 5 and 8, z series were merged and y-z and x-z views were obtained with MetaMorph software. Final photomicrograph images were stored and minimally processed (only contrast and/or brightness modifications) with Adobe Photo-shop.
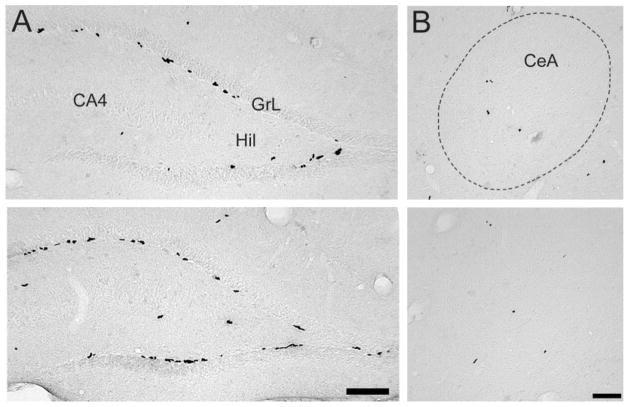
Fig. 1.
Photomicrographs displaying BrdU-labeled cells in prairie and meadow voles. For the dentate gyrus of the hippocampus (A), prairie voles (top) had fewer BrdU-labeled cells than did meadow voles (bottom). CA4, CA4 layer of the hippocampus; GrL, granule cell layer; Hil, hilus. For the central nucleus of amygdala (CeA; B), BrdU-labeled cells did not appear to differ between prairie (top) and meadow (bottom) voles. Scale bars = 200 μm.
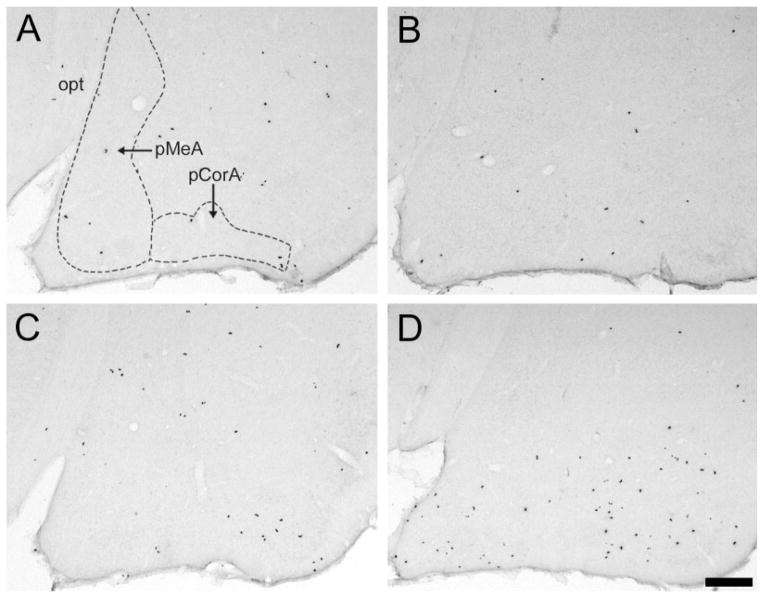
Fig. 3.
Photomicrographs displaying the species–treatment interaction on BrdU-labeled cells in the posterior cortical (pCorA) and posterior medial (pMeA) nuclei of the amygdala. In both areas, BrdU-labeled cells did not seem to differ between control (A) and estradiol benzoate (EB)-treated (B) prairie voles. However, meadow voles treated with EB (D) appeared to have significantly more BrdU-labeled cells in both the pCorA and the pMeA than controls (C). opt, Optic tract. Scale bar – 200 μm in D (applies to A–D).
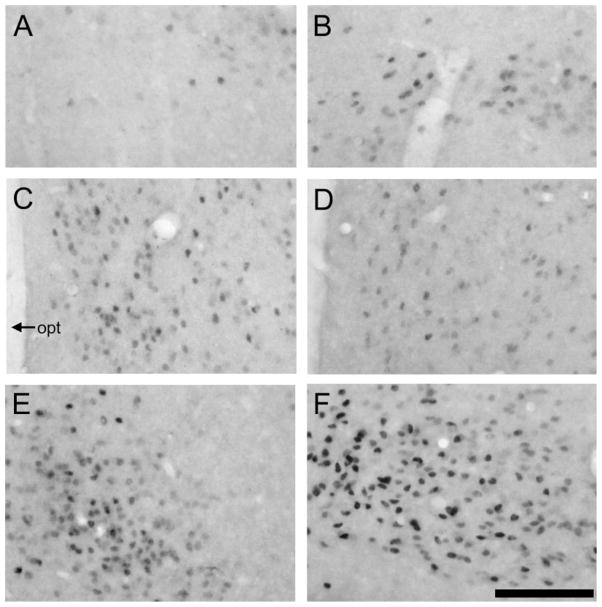
Fig. 6.
Photomicrographs displaying ERα labeling in the amyg-dala and ventromedial hypothalamus (VMH) in prairie (left) and meadow (right) voles. In the posterior cortical nucleus of the amyg-dala (A,B) and VMH (E, F), meadow voles (B, F) appeared to have more ERα-labeled cells than did the prairie voles (A, E). However, no species differences were found in the posterior medial nucleus of the amyg-dala (C, D). opt, optic tract. Scale bar – 200 μm in F (applies to A–F).
Fig. 5.
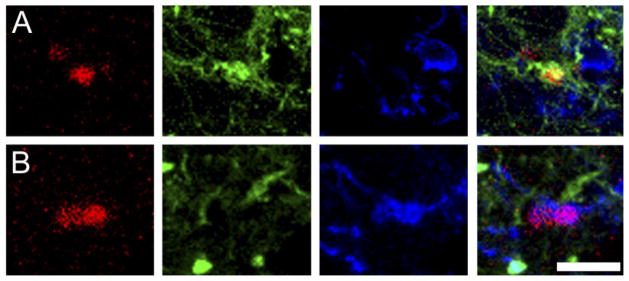
Phenotype of BrdU-labeled cells in the amygdala. Confocal laser microscope images display labeling for BrdU (red; left), TuJ1 (green; center left), NG2 (blue; center right), and all three markers (right) in the posterior cortical nucleus (pCorA) of the amygdala in the vole brain. Some BrdU-labeled cells coexpressed the neuronal (TuJ1; A) or glial progenitor (NG2; B) marker. Scale bar – 5 μm in B (applies to A–B, all panels).
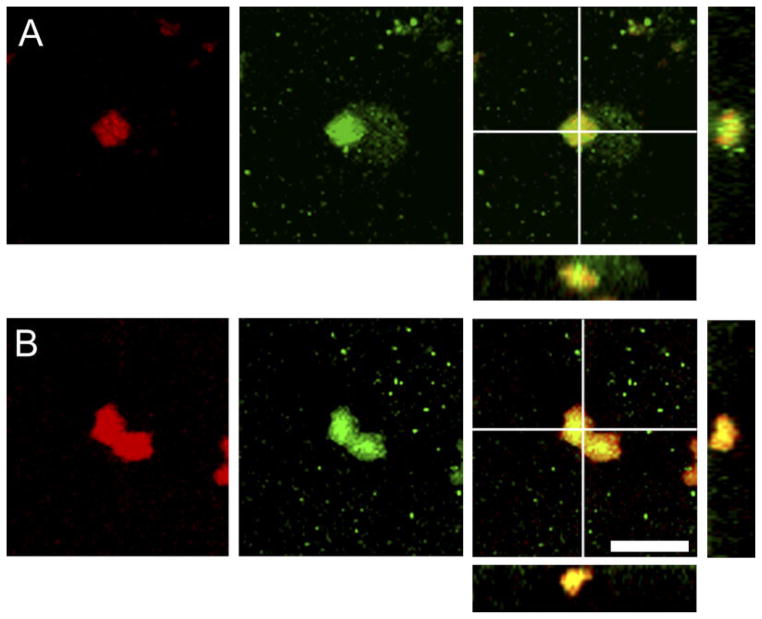
Fig. 8.
Nuclei coexpressing BrdU and ERα labeling in the posterior cortical nucleus of the amygdala in prairie (A) and meadow (B) voles. Confocal laser microscope images display labeling for BrdU (red; left), ERα (green; center), and both markers (right). In the right panels, the BrdU and ERα colocalized cells display a yellow image, and cross marks on the larger image indicate the location of views along the y-z axis (right) and x-z axis (below) to demonstrate 3D colocalization of BrdU and ERα. Scale bar – 5 μm in B (applies to A, B).
RESULTS
Study 1
Species differences in BrdU-labeled cells
Prairie and meadow voles differed in the densities of BrdU-labeled cells in certain brain regions. For the DG, meadow voles displayed a higher density of BrdU-labeled cells than did prairie voles [F(1,20) = 10.17, P < 0.01; Figs. 1A, 2A]. For the amygdala, meadow voles also had a higher density of BrdU-labeled cells than did prairie voles [F(1,20) = 5.42, P < 0.05; Fig. 2B]. This difference was due specifically to the results in the pCorA, in which meadow voles had a higher density of BrdU-labeled cells compared with prairie voles [F(1,20) = 46.36, P < 0.001]. No species differences were found in the densities of BrdU-labeled cells in the aCorA, aMeA, pMeA, or CeA or in the VMH (Figs. 1B, 2B).
Fig. 2.
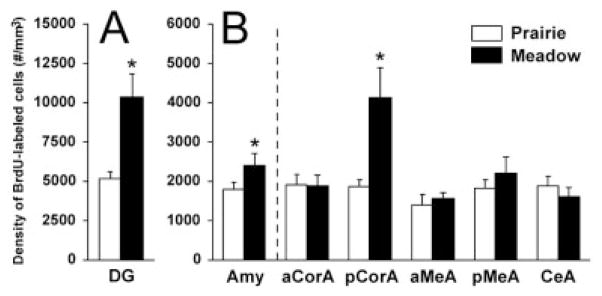
Species differences in the densities of BrdU-labeled cells in the dentate gyrus of the hippocampus (DG) and amygdala. For the DG (A), meadow voles displayed a higher density of BrdU-labeled cells than did prairie voles. For the amygdala (AMY; B), meadow voles also had a higher density of BrdU-labeled cells than did prairie voles. This difference was due largely to meadow voles having more BrdU-labeled cells in the posterior cortical subnucleus of the amygdala (pCorA). No species differences were found in other subnuclei of the amygdala, including the anterior cortical (aCorA), anterior medial (aMeA), posterior medial (pMeA), and central (CeA) subnuclei. *P < 0.05; error bars indicate SEM.
Estrogen regulation of BrdU-labeled cells
In the amygdala, EB treatment elicited a higher density of BrdU-labeled cells than did control treatment [F(1,20) = 6.35, P < 0.05], and a significant treatment-by-species interaction was also found [F(1,20) = 13.23, P < 0.01]. The post hoc test indicated that EB treatment significantly increased the density of BrdU-labeled cells in meadow, but not prairie, voles (Figs. 3, 4B). For the specific subnuclei of the amygdala, significant treatment-by-species interactions were also found in the pMeA [F(1,20) = 10.56, P < 0.01] and pCorA [F(1,20) = 37.76, P < 0.001]; the post hoc test indicated that EB treatment significantly increased the density of BrdU-labeled cells for meadow but not prairie voles in both subnuclei (Figs. 3, 4B). No significant effects of EB treatment or treatment-by-species interaction were found on the density of BrdU-labeled cells in the aCorA, aMeA, or CeA; DG (Fig. 4A); or VMH.
Fig. 4.
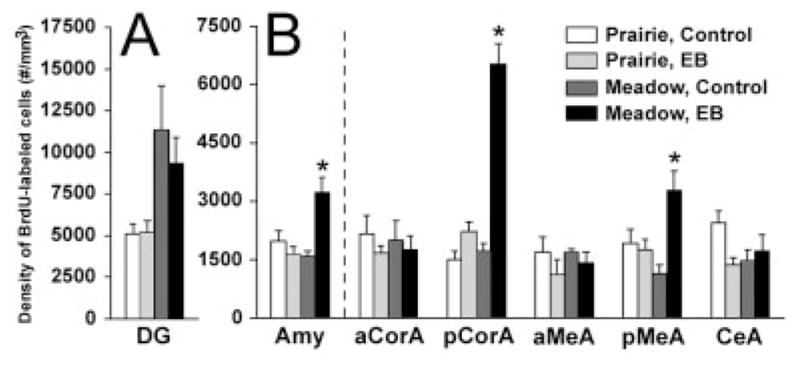
Species–treatment interactions in the density of BrdU-labeled cells in the vole brain. In the dentate gyrus of the hippocampus (DG; A), treatment with estradiol benzoate (EB) did not alter BrdU labeling in either species. In the amygdala (AMY; B), particularly in the posterior cortical (pCorA) and posterior medial (pMeA) subnuclei, EB treatment elicited a significant increase in the density of BrdU-labeled cells only in meadow voles. No species or treatment differences were found in the anterior cortical (aCorA), anterior medial (aMeA), or central (CeA) subnuclei of the amygdala. *P < 0.05; error bars indicate SEM.
Phenotype of the BrdU-labeled cells
BrdU-labeled cells in the pCorA, pMeA, and VMH were examined to determine phenotype. Triple-immunoreactive staining resulted in cells coexpressing BrdU labeling with either the neuronal (TuJ1) or the glial progenitor (NG2) markers (Fig. 5) and in cells expressing BrdU alone (BrdU only). In the pCorA and pMeA, approximately 40.5% of BrdU-labeled cells coexpressed the neuronal marker, 45.5% co-expressed the glial progenitor marker, and 14.0% were undifferentiated or of undetermined phenotype (BrdU only; see Table 1). In the VMH, approximately 26.8% of the BrdU-labeled cells coexpressed the neuronal marker, 48.6% coexpressed the glial progenitor marker, and 24.6% were undifferentiated or of undetermined phenotype. No statistically significant species or treatment effects were found in the relative percentage of BrdU-labeled cells colocalized with either TuJ1 or NG2 in any of the measured brain regions. It was noted, however, that some of the pCorA groups displayed high variability, so group differences could be revealed if a larger number of subjects is examined.
TABLE 1.
Percentage (Mean ± SEM) of Cells Labeled for BrdU or BrdU with a Neuronal (TuJ1) or Glial Progenitor (NG2) Marker in the Posterior Cortical (pCorA) and Posterior Medial (pMeA) Nuclei of the Amygdala in Ovariectomized Control or Estradiol Benzoate (EB)-Treated Prairie and Meadow Voles1
| Prairie vole
|
Meadow vole
|
F(1,19) | P | |||
|---|---|---|---|---|---|---|
| Control | EB | Control | EB | |||
| pCorA | ||||||
| BrdU/TuJ1 | 5.56 ± 3.0 | 34.13 ± 14.1 | 45.14 ± 18.0 | 32.75 ± 14.9 | 2.21 | 0.15 |
| BrdU/NG2 | 80.56 ± 5.0 | 52.15 ± 17.1 | 48.61 ± 16.7 | 47.12 ± 15.0 | 0.91 | 0.35 |
| BrdU only | 13.89 ± 6.3 | 13.73 ± 7.3 | 6.25 ± 6.3 | 20.14 ± 6.8 | 1.11 | 0.31 |
| pMeA | ||||||
| BrdU/TuJ1 | 51.66 ± 7.5 | 57.66 ± 19.1 | 57.75 ± 12.3 | 40.25 ± 13.8 | 0.78 | 0.39 |
| BrdU/NG2 | 36.75 ± 8.1 | 31.87 ± 16.6 | 30.74 ± 14.4 | 36.28 ± 14.4 | 0.15 | 0.70 |
| BrdU only | 11.59 ± 5.9 | 10.47 ± 5.9 | 11.52 ± 7.9 | 23.48 ± 15.6 | 0.43 | 0.52 |
F and P values represent the two-way ANOVA interaction results.
Study 2
Species differences in the density of ERα-labeled cells
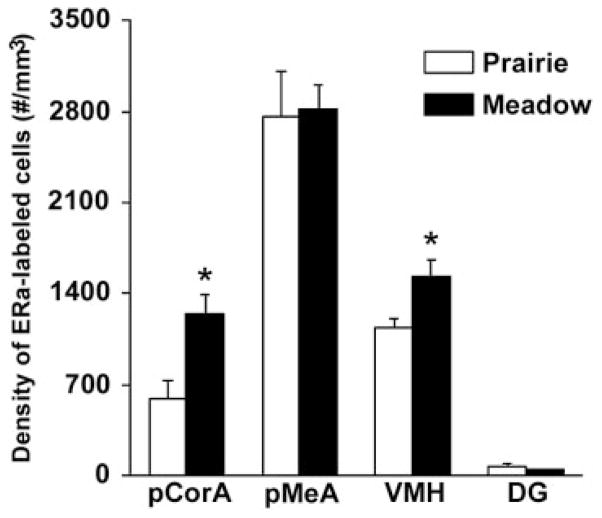
ERα immunocytochemistry resulted in the specific staining of cells in the brains of intact, untreated females of both species. In prairie voles, dense clusters of ERα-labeled cells were found in the amygdala and hypothalamus, similar to those reported previously (Cushing et al., 2004; Hnatczuk et al., 1994), and some scattered ERα-labeled cells were found in the DG. In meadow voles, ERα-labeled cells were also found in these brain areas. Interesting species differences were detected, in that meadow voles displayed higher densities of ERα-labeled cells in the pCorA (t = 2.63, P < 0.05) and VMH (t = 2.63, P < 0.05) than did prairie voles (Figs. 6, 7). Significant species differences were not found in the pMeA or DG (Fig. 7).
Fig. 7.
Species differences in the densities of ERα-labeled cells. Meadow voles displayed higher densities of ERα-labeled cells in the posterior cortical amygdala (pCorA) and ventromedial hypothalamus (VMH) than did prairie voles. Significant species differences were not found in the posterior medial amygdala (pMeA) or dentate gyrus of the hippocampus (DG). *P < 0.05; error bars indicate SEM.
Colocalization of BrdU with ERα
Many of the BrdU-labeled cells coexpressed ERα in both species (Fig. 8). Species differences were detected in the DG, with prairie voles displaying a greater percentage of BrdU/ERα colocalized cells than meadow voles (prairie: 79.3% ± 10.9%, meadow: 43.1% ± 8.6%; t = 2.66, P < 0.05), but such differences were not found in the other brain regions examined (Table 2). Across brain regions, a higher percentage of the BrdU-labeled cells contained ERα labeling in the pCorA (87.6% ± 4.7%) than in the DG (59.6% ± 8.6%) or VMH (56.2% ± 7.6%), whereas the percentage in the pMeA (76.1% ± 8.5%) did not differ from the percent-age in any other area [F(3,37) = 3.78, P < 0.05].
TABLE 2.
Percentage (Mean ± SEM) of BrdU/ERα Double-Labeled Cells in the Posterior Cortical (pCorA) and Posterior Medial (pMeA) Amygdala, Ventromedial Hypothalamus (VMH), and Dentate Gyrus of the Hippocampus (DG) of Prairie and Meadow Voles
| Prairie vole | Meadow vole | P | |
|---|---|---|---|
| pCorA | 88.7 ± 6.4 | 86.4 ± 7.6 | ns |
| pMeA | 80.7 ± 10.6 | 71.5 ± 14.0 | ns |
| VMH | 56.2 ± 9.3 | 56.3 ± 11.8 | ns |
| DG | 79.3 ± 10.9 | 43.1 ± 8.6 | <0.05 |
DISCUSSION
Vole species with differing life strategies and social behaviors provide an excellent opportunity for comparative studies. We found in the present experiments that female meadow voles had higher densities of BrdU-labeled cells in the pCorA and DG compared with female prairie voles. EB treatment significantly increased the density of BrdU-labeled cells in the pCorA and pMeA of meadow, but not prairie, voles, indicating species- and region-specific effects on adult neurogenesis. In the pCorA and pMeA, most of the BrdU-labeled cells displayed neuronal or glial progenitor phenotypes; although variability existed among groups, no statistical differences were found in the percentage of BrdU-labeled cells displaying either phenotype, indicating that EB increased the proliferation of both new neurons and glial cells. Species-specific patterns of ERα labeling were present; meadow voles had higher densities of ERα-labeled cells in the pCorA and VMH than did prairie voles, and a large population of the BrdU-labeled cells in the amygdala coexpressed ERα labeling in both species. Together, these data indicate that estrogen differentially influences cell proliferation in a species- and region-specific manner, and some of these effects may lie in the specific localization of ERs in the adult vole brain.
Species-specific effects of estrogen on cell proliferation in the amygdala
In our previous study, male exposure and mating significantly enhanced the number of BrdU-labeled cells in the amygdala and hypothalamus of female prairie voles (Fowler et al., 2002). Because exposure to a male or to male sensory cues results in an increase in serum estrogen and ER binding in the brain (Cohen-Parsons and Carter, 1987; Dluzen and Carter, 1979) and EB treatment enhances cell proliferation in the SVZ of female prairie voles (Smith et al., 2001), we hypothesized that the increased cell proliferation in the amygdala and hypothalamus seen in our previous study (Fowler et al., 2002) was due to an increase in serum estrogen associated with male experience. In the present study, however, EB treatment did not increase the density of BrdU-labeled cells in the amygdala or VMH of female prairie voles, indicating that the enhanced cell proliferation found in the previous study most likely was not regulated solely by estrogen. However, it still appears that estrogen has site-specific effects on cell proliferation in the prairie vole brain: it enhances cell proliferation in the SVZ (Smith et al., 2001) but not in the amygdala and hypothalamus (present study). What factors could be responsible for the increased cell proliferation in the amygdala and hypothalamus of female prairie voles in the previous study (Fowler et al., 2002)?
One possibility is that male-associated cues or interactions with a male (e.g., mating) were essential for the up-regulation of cell proliferation in the brain of prairie voles. The amygdala receives direct inputs from both the main and the accessory olfactory systems and projects into several forebrain areas, including the medial preoptic area, bed nucleus of the stria terminalis, and VMH (Dominguez et al., 2001; Kevetter and Winans, 1981; Lehman and Winans, 1982; Luiten et al., 1983; Meredith, 1991), and this circuit plays an important role in mediating the effects of chemosensory inputs on behavior. In female voles, male chemosensory cues can influence estrus induction, social behavior, and ability to form a socially relevant memory (Curtis et al., 2001; Lepri and Wysocki, 1987; Williams et al., 1992b), and mating significantly facilitates the formation of a social memory (Williams et al., 1992a). Furthermore, it has been hypothesized that male experience is capable of reorganizing the female’s brain for future experience (Carter et al., 1988; Cushing and Hite, 1996). Therefore, exposure to male-associated cues or interactions with a male may lead to downstream effects that could have increased cell proliferation in the amygdala and hypothalamus of the adult female prairie vole (Fowler et al., 2002).
Another possibility is that other hormones involved during the male experience could have contributed to cell proliferation. For example, male exposure and mating increase progestin binding sites in the brain of female prairie voles (Cohen-Parsons and Carter, 1988), allowing for a putative effect of progestin on cell proliferation. However, progestin increases 72 hours after the mating episode in female prairie voles (Carter et al., 1989), which would have been after BrdU’s incorporation into dividing cells in our previous study (Fowler et al., 2002), and progesterone treatment did not have any significant effect on cell proliferation in the SVZ of female mice (Shingo et al., 2003). Prolactin is also involved in reproduction, and it increases following mating for at least 48 hours in female voles (Meek and Lee, 1994). In vitro, prolactin stimulates neural stem cell proliferation under specific culture conditions, and, in vivo, prolactin enhances new cell number in the SVZ of female and male rats, an effect that could not be induced in females with EB or progesterone treatment (Shingo et al., 2003). Prolactin is also increased during pregnancy, so it could have accounted for the enhanced cell proliferation previously found in the amygdala and hypothalamus of female prairie voles 3 weeks after mating and impregnation (Fowler et al., 2002). If the increased cell proliferation in the amygdala is due to prolactin, it may also indicate a feedback loop, insofar as the posterodorsal medial amygdala appears to regulate prolactin secretions following mating behaviors in female rats (Polston and Erskine, 2001).
One drawback of the present study is that we cannot completely rule out the possibility that estrogen was involved in the cell proliferation in the amygdala of female prairie voles. The temporal pattern of naturally occurring estrogen release might have been important for enhanced cell proliferation in the amygdala. In female prairie voles, serum estrogen levels increase following male exposure, reach a peak during lordosis, and then decrease dramatically (Carter et al., 1989). In the present study, estrogen was administrated via constant diffusion from implanted Silastic tubing. Such EB treatment does not result in a temporal pattern mimicking the naturally occurring changes of estrogen and thus might have potentially led to the lack of effect on cell proliferation in prairie voles. It should also be noted that the implanted EB pellets in the present study resulted in a level of circulating estradiol about 5–10 times higher than that following male-induced estrus found in prior studies (Cohen-Parson and Carter, 1987; Cushing et al., 1995; Prentice and Shepard, 1978). Therefore, this pharmacological level of estradiol might not have the same effect on cell proliferation as the physiological level of estradiol associated with male-induced estrus. Furthermore, the time point we chose was based on the observation that male experience altered cell proliferation after 72 hours in female prairie voles (Fowler et al., 2002). An earlier time point may be needed to detect optimally any immediate effects of estradiol on cell proliferation, in that a temporal effect of estradiol has been demonstrated in the DG of meadow voles (Ormerod et al., 2003). Finally, estrogen could also have acted synergistically with any of the above-mentioned or other factors to contribute to the enhanced cell proliferation seen in our prior study (Fowler et al., 2002).
Although EB treatment did not alter BrdU labeling in the prairie vole brain, it significantly enhanced the density of BrdU-labeled cells in the amygdala, specifically in the pCorA and pMeA, of female meadow voles, indicating that estrogen does have species-specific effects on cell proliferation. The finding that estrogen enhances cell proliferation in the amygdala of female meadow voles is consistent with our recent finding in males of the same species (Fowler et al., 2003). The enhanced cell proliferation was found specifically in the cortical and medial subnuclei of the amygdala in both studies, indicating that these brain regions have a similar susceptibility to estrogen in both male and female meadow voles. Another study in female meadow voles has reported that EB treatment initially enhances cell proliferation in the DG within 4 hours and then suppresses cell proliferation by 48 hours (Ormerod and Galea, 2001). In the present study, we examined BrdU labeling 72 hours after beginning EB treatment, so our results are consistent with the notion that estrogen’s effects on cell proliferation in the adult DG may be short-lived in female meadow voles.
Species differences in ERα distribution
Differences between prairie and meadow voles in the estrogen regulation of cell proliferation may be due to differential abilities of their brains to respond to estrogen. One way to examine this possibility would be to compare ERs between the two species. In the current study, the presence of ERα-labeled cells in the amygdala and hypothalamus in female prairie voles is consistent with findings from previous studies (Cushing et al., 2004; Hnatczuk et al., 1994). In addition, ERα-labeled cells were also present in these brain areas in female meadow voles. Meadow voles had a higher density of ERα-labeled cells in the pCorA and VMH than did prairie voles. This may indicate an enhanced brain responsiveness to estrogen in meadow voles, which could account for the species differences in estrogen’s regulation of cell proliferation. However, such a difference in ERα was not found in the pMeA, where EB also enhanced BrdU labeling in meadow voles. Therefore, estrogen’s effects may be dependent on the specificity of ERs on certain cells; although one species may have a higher density of ERs, the receptors might not be on the appropriate cells to stimulate cell proliferation.
Potential mechanism of estrogen action
In the current study, ERαs were present on many BrdU-labeled cells within the adult amygdala, indicating that newly proliferated cells could be estrogen responsive. To our knowledge, this is the first documentation in vivo of ERαs on newly proliferated cells in the adult brain. In vitro, ERαs and ERαs have been localized on neural stem cells in embryonic and adult rats (Brannvall et al., 2002), and estrogen has been shown to regulate the proliferation and/or survival of new neurons in the fetal rat amygdala and hypothalamus (Arimatsu and Hatanaka, 1986; Chowen et al., 1992). Therefore, estrogen may be able to stimulate the progenitor cells into a proliferative cell cycle in the adult brain, a contention that should be investigated further. In our study, a higher percentage of BrdU and ERα colocalized cells was found in the pCorA compared with the other brain regions, consistent with the estrogen-induced increase in BrdU-labeled cells in this brain region for meadow voles. However, no differences in ERα labeling were revealed in the pMeA—an area in which EB treatment increased BrdU labeling in meadow, but not prairie, voles—and differences in BrdU/ERα double labeling were not found between the species in either of these brain regions. Therefore, although estrogen may differently affect the prairie and meadow vole brains, our data could not completely explain estrogen’s effects on cell proliferation in specific brain regions or the species differences in BrdU labeling. Of course, it is possible that limitations lie in the specificity of our methods. For example, we could not determine the percentage of BrdU cells that, if possible, could be induced into additional proliferative cell cycles by estrogen, because some of the colabeled cells might have undergone their final cell cycle and begun to mature. It will be essential to examine, in further studies, whether estrogen is able to stimulate directly the proliferation of adult progenitors in vivo.
Alternatively, estrogen may be acting via alternate receptors. We chose to examine ERαs because of their apparent importance in behavioral aspects of reproduction and in female receptivity and lordosis (Kudwa and Rissman, 2003; Lonstein et al., 2000; McEwen and Alves, 1999; Rissman et al., 1997), their presence in neurogeneic regions of the adult brain (Brannvall et al., 2002; Shughrue et al., 1997; Weiland et al., 1997), and their involvement in the induction of proliferation in epithelial cells from the mammary glands of mice (Cheng et al., 2004). Another nuclear estrogen receptor, ERβ, is also located in similar areas of the adult brain (Shughrue and Merchenthaler, 2001). ERβ activation appears to influence cell migration and/or survival (Wang et al., 2003), neurite elongation (Patrone et al., 2000), and cortical development (Wang et al., 2003) and thus may play a role in the regulation of neurogenesis in adult vole brains. The currently available ERβ antibodies display inconsistent labeling (McEwen and Alves, 1999), and we could not achieve reliable staining with the vole tissue, a finding substantiated by another laboratory (B. Cushing, unpublished observations). Finally, estrogen could also bind to a recently identified G-protein-coupled transmembrane-bound receptor (Kelly and Wagner, 1999; Qiu et al., 2003; Singh et al., 2000; Toran-Allerand et al., 2002), leading to effects on cell proliferation and survival in the adult brain.
It is also possible that estrogen acted through other neurotransmitter systems to influence adult cell proliferation. For example, in the adult rat, serotonin and insulin-like growth factor-1 (IGF-1) can regulate cell proliferation in the DG (Aberg et al., 2000; Banasr et al., 2001), and estrogen increases serotonin receptor mRNA and IGF-1 mRNA expression in the brain (Shingo and Kito, 2003; Zhou et al., 2002). Estrogen may also interact with the IGF-1 receptor to affect its phosphorylation (Mendez et al., 2003). Furthermore, astrocytes contain steroid hormone receptors (Finley and Kritzer, 1999), secrete neurotrophic factors such as brain-derived neurotrophic factor (BDNF; Ikeda et al., 2001), and can induce cell proliferation in vitro (Lim and Alvarez-Buylla, 1999; Song et al., 2002). Given that EB treatment affected cell proliferation in the amygdala differentially between prairie and meadow voles but that no species differences were found in the colocalization of BrdU and ERα labeling, EB may act through a neurotransmitter-mediated mechanism to regulate cell proliferation. For instance, BDNF enhances cell proliferation and survival (Pencea et al., 2001; Zigova et al., 1998) and differs in distribution throughout the brain between monogamous and promiscuous vole species (Liu et al., 2001b), so it may play an important role in mediating the species-specific effects of estrogen on adult neurogenesis. This speculation should be tested in further studies.
Acknowledgments
Grant sponsor: National Institutes of Health (NIH)/National Research Service Award; Grant number: MH-64352 (to C.D.F.); Grant sponsor: NIH; Grant number: MH-58616 (to Z.W.); Grant number: MH-66734 (to Z.W.).
We are grateful to Dr. Thomas Curtis, Dr. Yan Liu, Dr. Brandon Aragona, Mike Smeltzer, and Kyle Gobrogge for critical reading of the article.
LITERATURE CITED
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci U S A. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimatsu Y, Hatanaka H. Estrogen treatment enhances survival of cultured fetal rat amygdala neurons in a defined medium. Brain Res. 1986;391:151–159. doi: 10.1016/0165-3806(86)90017-9. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Bedard A, Levesque M, Bernier PJ, Parent A. The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur J Neurosci. 2002;16:1917–1924. doi: 10.1046/j.1460-9568.2002.02263.x. [DOI] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE. Therapeutic immunizatioon protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell S, Meredith GE, Phillips J, Staunton H, Doherty C, Grigorenko E, Glazier S, Deadwyler SA, O’Donovan CA, Farrell M. Neuronal hypertrophy in the neocortex of patients with temporal lobe epilepsy. J Neurosci. 2001;21:4789–4800. doi: 10.1523/JNEUROSCI.21-13-04789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, VanPraag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Butt AM, Berry M. Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum. J Neurosci Res. 2000;59:477–488. doi: 10.1002/(SICI)1097-4547(20000215)59:4<477::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL, Cohen-Parsons M. Relationships between social organization and behavioral endocrinology in a monogamous mammal. Adv Study Behav. 1986;16:109–145. [Google Scholar]
- Carter CS, Witt DM, Thompson EG, Carlstead K. Effects of hormonal, sexual, and social history on mating and pair bonding in prairie voles. Physiol Behav. 1988;44:691–697. doi: 10.1016/0031-9384(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Manock SR, Adams KA, Bahr JM, Carlstead K. Hormonal correlates of sexual behavior and ovulation in male-induced and postpartum estrus in female prairie voles. Physiol Behav. 1989;46:941–948. doi: 10.1016/0031-9384(89)90195-9. [DOI] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Warner M, Gustafsson JA. Estrogen receptors ER alpha and ER beta in proliferation in the rodent mammary gland. Proc Natl Acad Sci U S A. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen JA, Torres-Aleman I, Garcia-Segura LM. Trophic effects of estradiol on fetal rat hypothalamic neurons. Neuroendocrinology. 1992;56:895–901. doi: 10.1159/000126321. [DOI] [PubMed] [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiol Behav. 1987;39:309–314. doi: 10.1016/0031-9384(87)90227-7. [DOI] [PubMed] [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase progestin receptor binding in brain of female voles. Physiol Behav. 1988;42:191–197. doi: 10.1016/0031-9384(88)90297-1. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Wang ZX. Lesions of the vomeronasal organ disrupt pair bonding in female prairie voles (Microtus ochrogaster) Brain Res. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, Van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Hite R. Effects of estradiol on sexual receptivity, wheel-running behavior, and vaginal estrus in virgin prairie voles. Physiol Behav. 1996;60:829–832. doi: 10.1016/0031-9384(96)00084-4. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Marhenke S, McClure PA. Estradiol concentration and the regulation of locomotor activity. Physiol Behav. 1995;58:953–957. doi: 10.1016/0031-9384(95)00158-f. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le WW, Hoffman GE. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016:247–254. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Demas GE, Williams JM, Nelson RJ. Amygdala but not hippocampal lesions impair olfactory membory for mate in prairie voles (Microtus ochrogaster) Am J Physiol. 1997;273:R1683–R1689. doi: 10.1152/ajpregu.1997.273.5.R1683. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Carter CS. Ovarian hormones regulating sexual and social behaviors in female prairie voles, Microtus ochrogaster. Physiol Behav. 1979;23:597–600. doi: 10.1016/0031-9384(79)90063-5. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Hines M, Davis F, Shryne JE, Gorski RA. Hormonal influence on sexual differentiation of rat brain anatomy. In: Balthaazart J, Prove E, Gilles R, editors. Hormones and behavior in higher vertebrates. Berlin: Springer-Verlag; 1983. pp. 194–203. [Google Scholar]
- Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J Neurosci. 2001;21:349–355. doi: 10.1523/JNEUROSCI.21-01-00349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir E, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci. 1999;19:8778–8788. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40:446–457. [PubMed] [Google Scholar]
- Fowler CD, Wang ZX. Adult neurogenesis in the mammalian brain: Exogenous and endogenous influences. Acta Zool Sin. 2003;49:151–162. [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang ZX. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang ZX. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neurosci. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Gaulin SJ, FitzGerald RW, Wartell MS. Sex differences in spatial ability and activity in two vole species (Microtus ochrogaster and M. pennsylvanicus) J Comp Psychol. 1990;104:88–93. doi: 10.1037/0735-7036.104.1.88. [DOI] [PubMed] [Google Scholar]
- Getz LL, Hofmann JE, Carter CS. Mating system and population fluctuations of the prairie vole, Microtus ochrogaster. Am Zool. 1987;27:909–920. [Google Scholar]
- Glaser JR, Glaser EM. Stereology, morphometry, and mapping: the whole is greater than the sum of its parts. J Chem Neuroanat. 2000;20:115–126. doi: 10.1016/s0891-0618(00)00073-9. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36:267–286. [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate post-natal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999a;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999b;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gutierrez PJ, Meyer JS, Novak MA. Comparison of postnatal brain development in meadow voles (Microtus pennsylvanicus) and pine voles (Microtus pinetorum) J Mammal. 1989;70:292–299. [Google Scholar]
- Hnatczuk OC, Lisciotto CA, DonCarlos LL, Carter CS, Morrell JI. Estrogen receptor immunoreactivity in specific brain areas of the prairie vole (Microtus ochrogaster) is altered by sexual receptivity and genetic sex. J Neuroendocrinol. 1994;6:89–100. doi: 10.1111/j.1365-2826.1994.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ikeda O, Murakami M, Ino H, Yamazaki M, Nemoto T, Koda M, Nakayama C, Moriya H. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol. 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda Y, Kameya T, Frankfurter A. Immunohistochemical localization of a neuron-specific β-tubulin isotype in the developing chicken ultimobranchial glands. Brain Res. 1993;628:121–127. doi: 10.1016/0006-8993(93)90946-k. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ. Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kirpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240:27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Lepri JJ, Wysocki CJ. Removal of the vomeronasal organ disrupts the activation of reproduction in female voles. Physiol Behav. 1987;40:349–355. doi: 10.1016/0031-9384(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang ZX, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang ZX. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001a;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Wang ZX. Ontogeny of brain-derived neurotrophic factor gene expression in the forebrain of prairie and montane voles. Brain Res Dev Brain Res. 2001b;127:51–61. doi: 10.1016/s0165-3806(01)00111-0. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Greco B, De Vries GJ, Stern JM, Blaustein JD. Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology. 2000;72:91–101. doi: 10.1159/000054576. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Ono T, Nishijo H, Fukuda M. Differential input from the amygdaloid body to the ventromedial hypothalamic nucleus in the rat. Neurosci Lett. 1983;35:253–258. doi: 10.1016/0304-3940(83)90326-9. [DOI] [PubMed] [Google Scholar]
- Madison DM. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol. 1980;7:65–71. [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48:S8–S15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- Meek LR, Lee TM. Luteinizing hormone and prolactin in mated female meadow voles housed in long and short day lengths. Biol Reprod. 1994;51:725–730. doi: 10.1095/biolreprod51.4.725. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Meredith M. Sensory processing in the main and accessory olfactory systems: comparisons and contrasts. J Steroid Biochem Mol Biol. 1991;39:601–614. doi: 10.1016/0960-0760(91)90258-7. [DOI] [PubMed] [Google Scholar]
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-α gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Stallcup WB. Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell. 1995;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett. 2003;346:25–28. doi: 10.1016/s0304-3940(03)00546-9. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Lee TM. Development of selective partner preferences in captive male and female meadow voles, Microtus pennsylvanicus. Anim Behav. 2001;61:1217–1226. [Google Scholar]
- Patrone C, Pollio G, Vegeto E, Enmark E, de Curtis I, Gustafsson JA, Maggi A. Estradiol induces differential neuronal phenotypes by activating estrogen receptor alpha or beta. Endocrinology. 2000;141:1839–1845. doi: 10.1210/endo.141.5.7443. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res Bull. 1999;49:221–243. doi: 10.1016/s0361-9230(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Polston EK, Erskine MS. Excitotoxic lesions of the medial amygdala differentially disrupt prolactin secretory responses in cycling and mated female rats. J Neuroendocrinol. 2001;13:13–21. doi: 10.1046/j.1365-2826.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- Prentice RC, Shepard BA. The relationship between ovarian vesicular follicle population and serum estradiol-17β concentration in Microtus ochrogaster. Trans IL State Acad Sci. 1978;71:286–290. [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- Seabloom RW. Endocrinology. In: Tamarin RH, editor. Biology of new world Microtus. Shippensburg, PA: American Society of Mammalogists; 1985. pp. 685–724. [Google Scholar]
- Schreihofer DA, Resnick EM, Soh AY, Shupnik MA. Transcriptional regulation by a naturally occurring truncated rat estrogen receptor (ER), truncated ER product-1 (TERP-1) Mol Endocrinol. 1999;13:320–329. doi: 10.1210/mend.13.2.0236. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estrogen induces insulin-like growth factor-1 mRNA expression in the immortalized hippocampal cell: determination by quantitative real-time polymerase chain reaction. Neurochem Res. 2003;28:1379–1383. doi: 10.1023/a:1024900616704. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang ZX, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens C, Gage F. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Storey AE, Bradbury CG, Joyce TL. Nest attendance in male meadow voles: the role of the female in regulating male interactions with pups. Anim Behav. 1994;47:1037–1046. [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SA, Salo AL, Dewsbury DA. Estrus induction in four species of voles (Microtus) J Comp Psychol. 1992;106:366–373. doi: 10.1037/0735-7036.106.4.366. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS. The interaction of circulating oestrogens and androgens in regulating mammalian sexual differentiation. In: Balthaazart J, Prove E, Gilles R, editors. Hormones and behaviour in higher vertebrates. Berlin: Springer-Verlag; 1983. pp. 159–177. [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci U S A. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Hulihan T, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997a;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: comparative anatomic studies in prairie and montane voles. J Comp Neurol. 1997b;378:535–546. [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992a;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Slotnick BM, Kirkpatrick BW, Carter CS. Olfactory bulb removal affects partner preference development and estrus induction in female prairie voles. Physiol Behav. 1992b;52:635–639. doi: 10.1016/0031-9384(92)90390-n. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]