Abstract
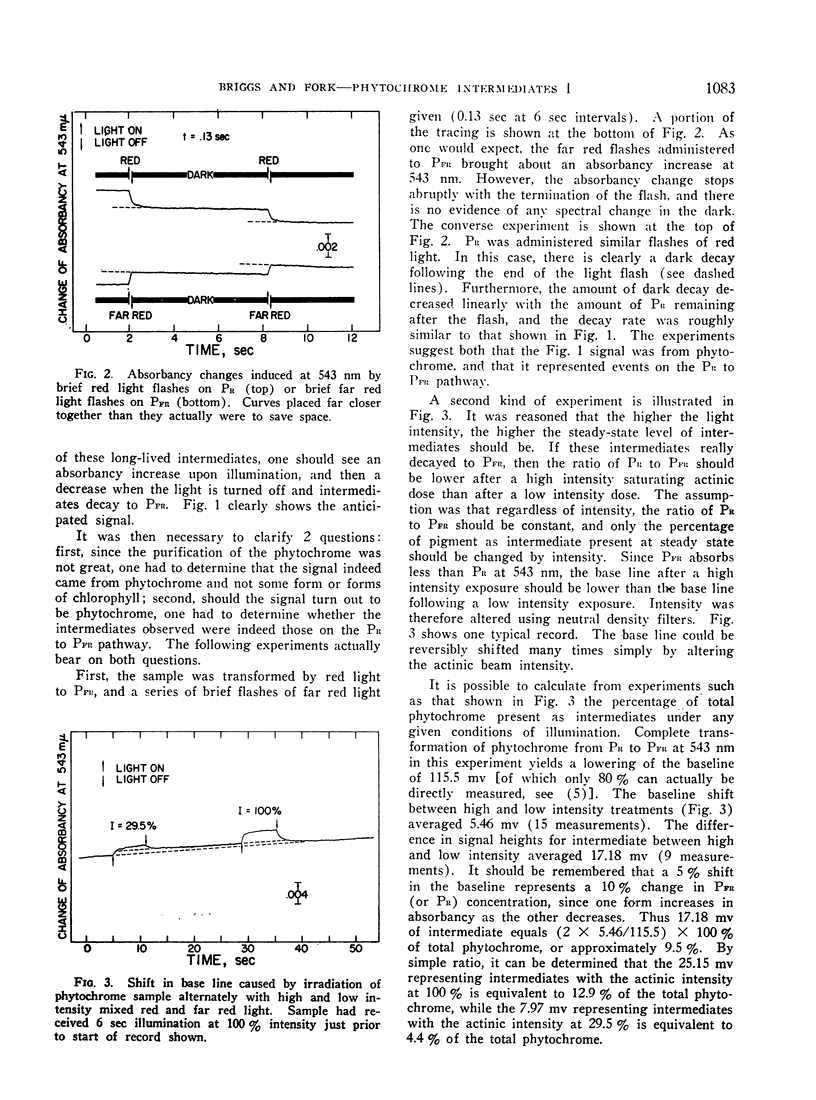
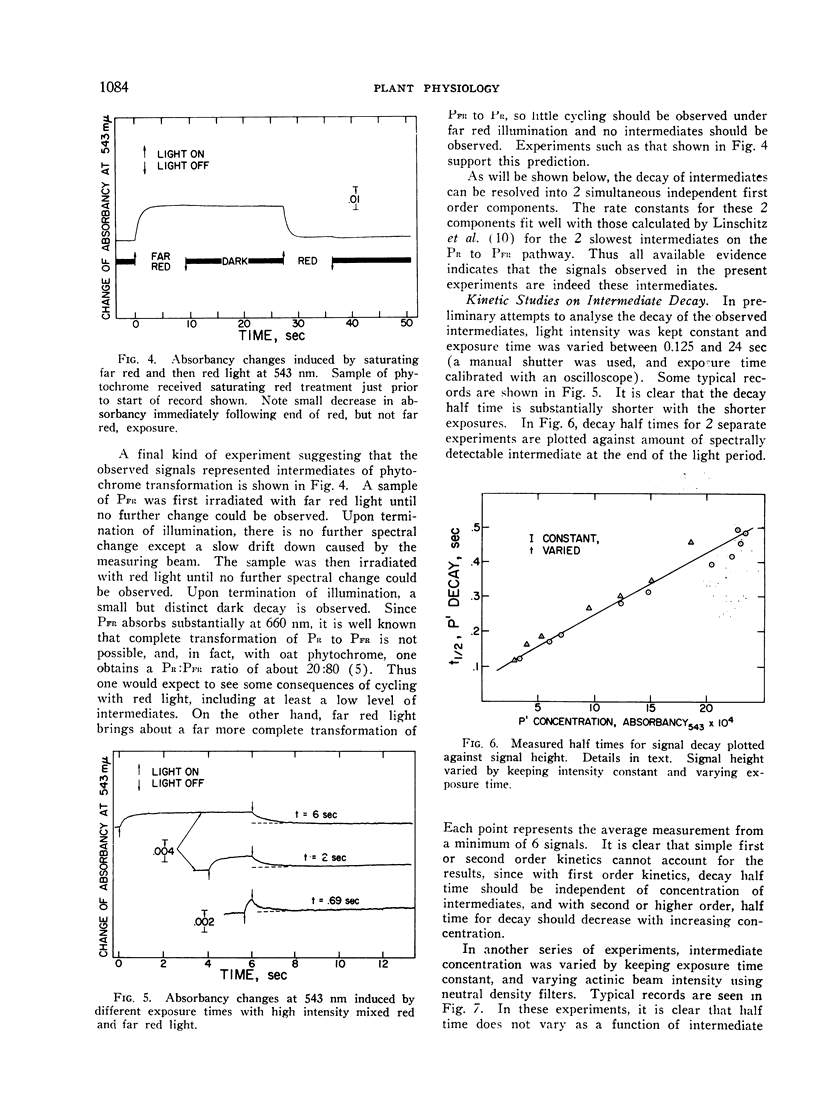
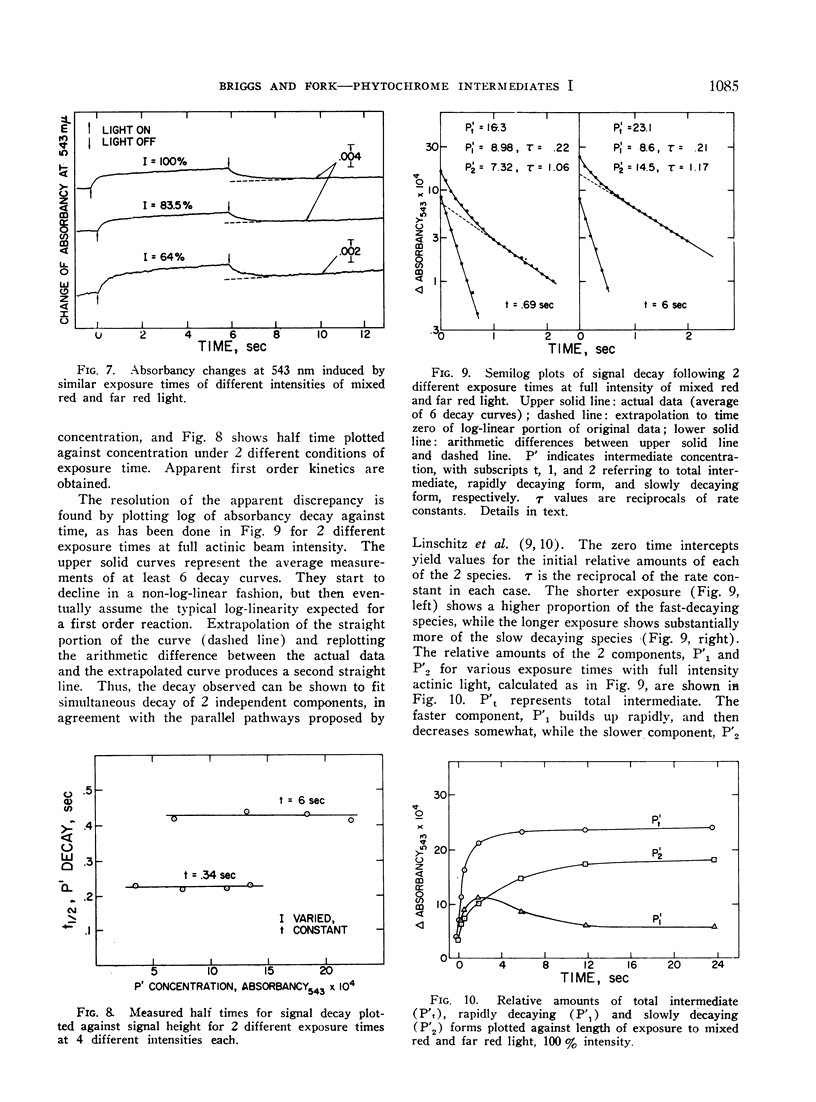
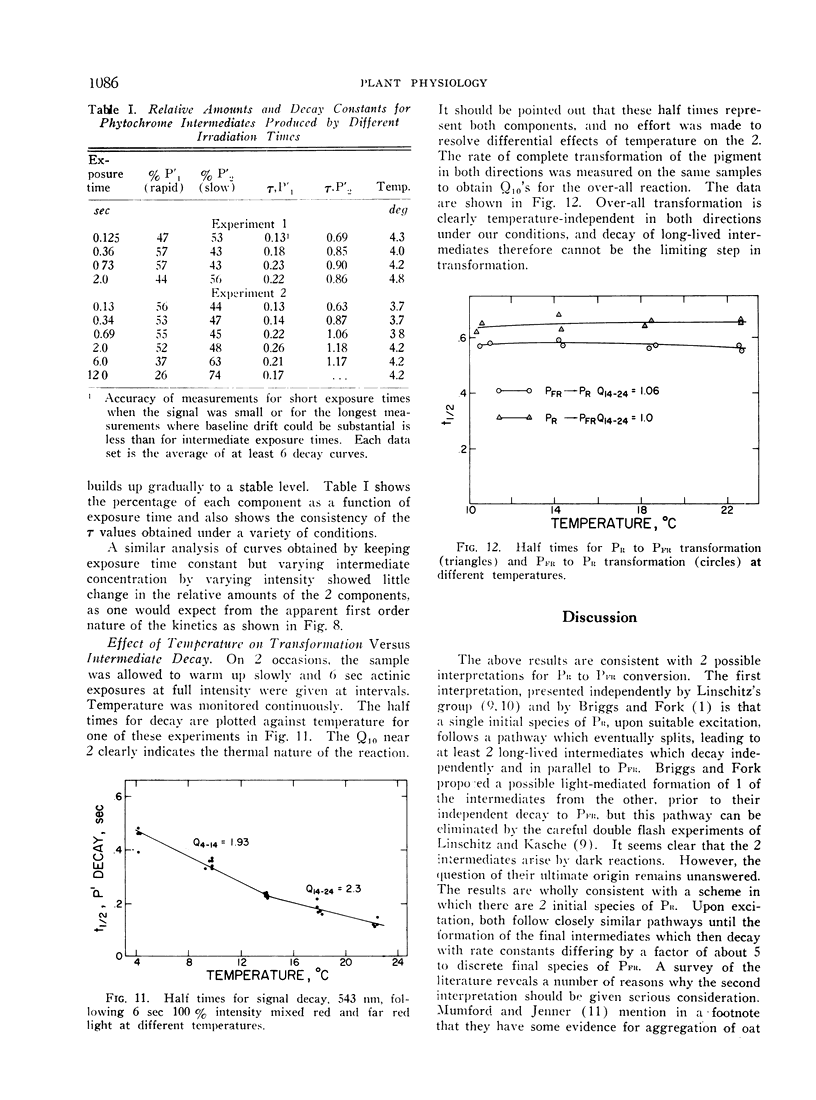
Irradiation of phytochrome solutions with a high-intensity mixed red and far red light source causes measurable absorbancy increases at 543 nm. Evidence is presented that these absorbancy increases are caused by accumulation of intermediates on the PR to PFR pathway with relatively slow thermal decay constants. Kinetic analysis of the decay signals is consistent with the interpretation that the signals represent simultaneous independent and parallel decay of 2 species by first order kinetics to PFR. If actinic light intensity is kept constant and exposure time changed, the relative amounts of the 2 components change, with proportionately more of the rapidly decaying species present following short exposure times. If the amount of the intermediates is decreased by decreasing actinic light intensity at constant exposure time, however, the relative amounts of the 2 remain constant. The Q10 for intermediate decay following illumination is approximately 2.0, while that for complete phototransformation of the pigment in either direction is very close to 1.0. Incomplete transformation of PR to PFR, caused by overlapping absorption of the 2 forms, is shown by the presence of intermediates (indicating cycling of the pigment) in continuous red light. Such intermediates do not appear in continuous far red, indicating a rate of pigment cycling below detection by the available instrumentation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation II: In Vitro and In Vivo Studies. Plant Physiol. 1969 Aug;44(8):1089–1094. doi: 10.1104/pp.44.8.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Cross D. R., Linschitz H., Kasche V., Tenenbaum J. Low-temperature studies on phytochrome: light and dark reactions in the red to far-red transformation and new intermediate forms of phytochrome. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1095–1101. doi: 10.1073/pnas.61.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linschitz H., Kasche V. KINETICS OF PHYTOCHROME CONVERSION: MULTIPLE PATHWAYS IN THE P(r) TO P(fr) REACTION, AS STUDIED BY DOUBLE-FLASH TECHNIQUE. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1059–1064. doi: 10.1073/pnas.58.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linschitz H., Kasche V. The kinetics of phytochrome conversion. J Biol Chem. 1966 Jul 25;241(14):3395–3403. [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Purification and characterization of phytochrome from oat seedlings. Biochemistry. 1966 Nov;5(11):3657–3662. doi: 10.1021/bi00875a039. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Stabilization of phytochrome intermediates by low temperature. Photochem Photobiol. 1968 Nov;8(5):477–485. doi: 10.1111/j.1751-1097.1968.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruit C. J. Low-temperature action spectra for transformations of photoperiodic pigments. Biochim Biophys Acta. 1966 Jul 13;120(3):454–456. doi: 10.1016/0926-6585(66)90312-8. [DOI] [PubMed] [Google Scholar]
- Spruit C. J. Photoreversible pigment transformations in etiolated plants. Biochim Biophys Acta. 1966 Jan 4;112(1):186–188. doi: 10.1016/s0926-6585(96)90025-4. [DOI] [PubMed] [Google Scholar]


