Abstract
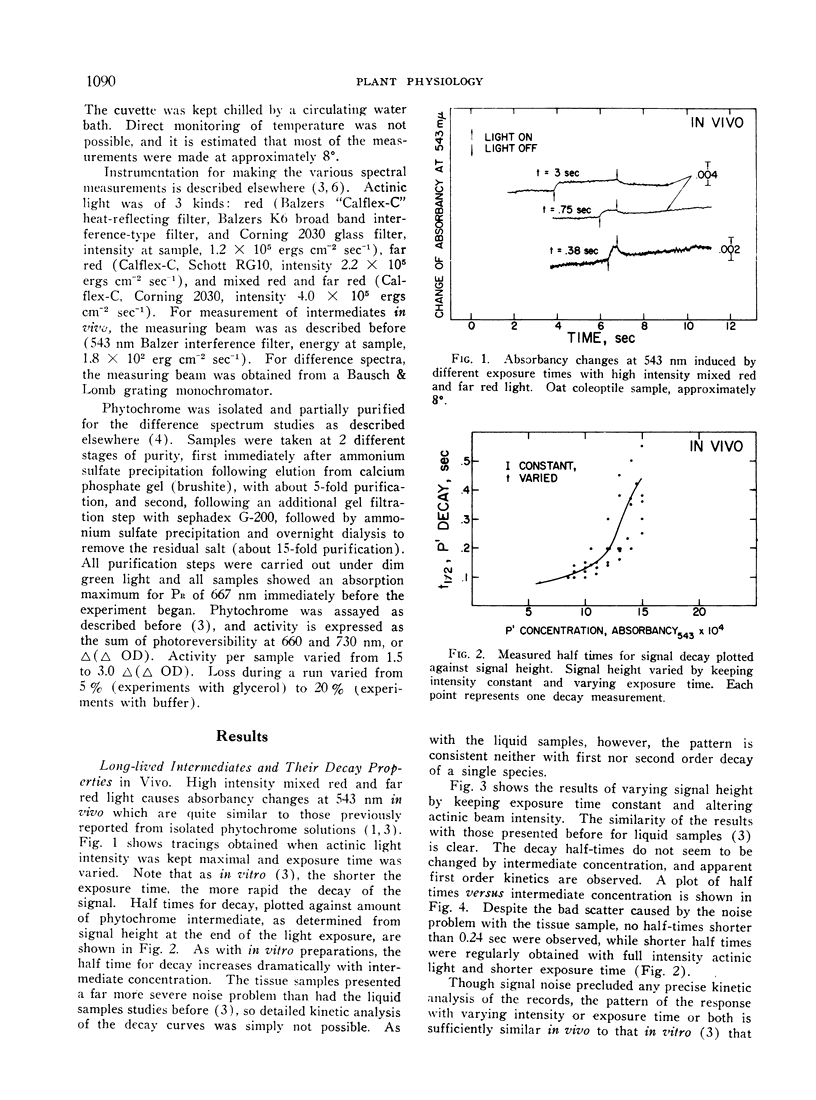
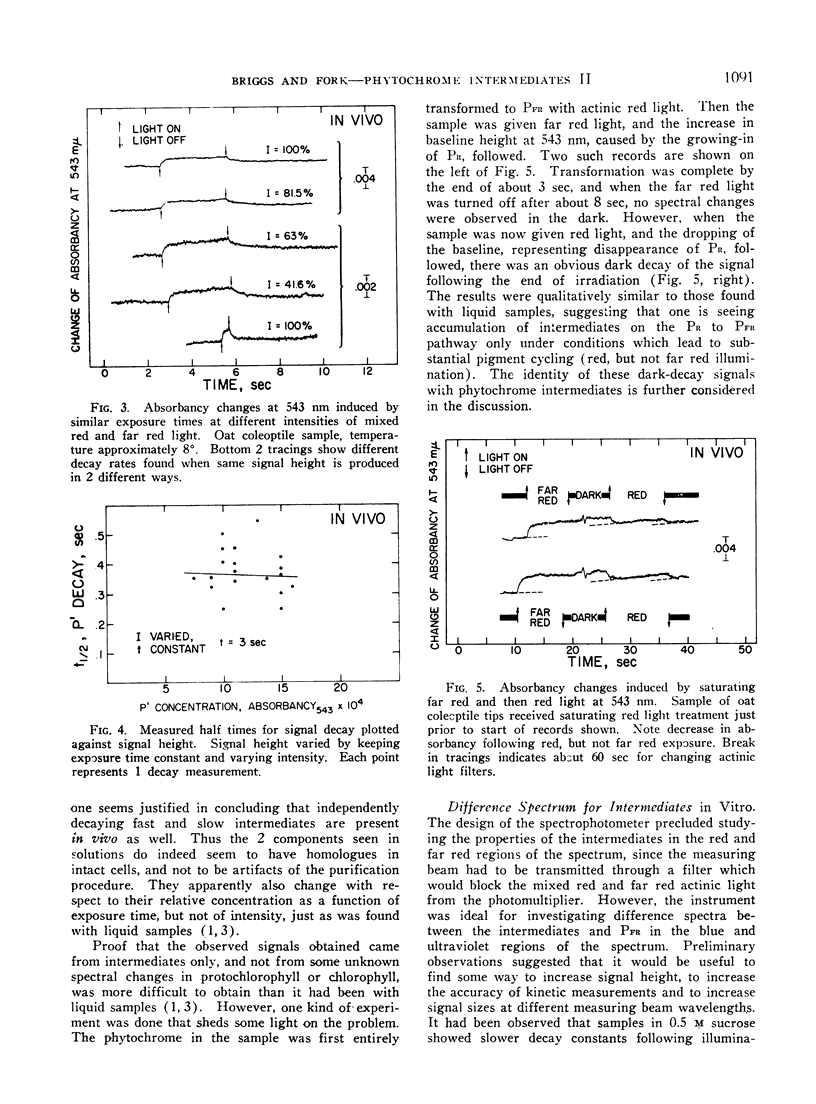
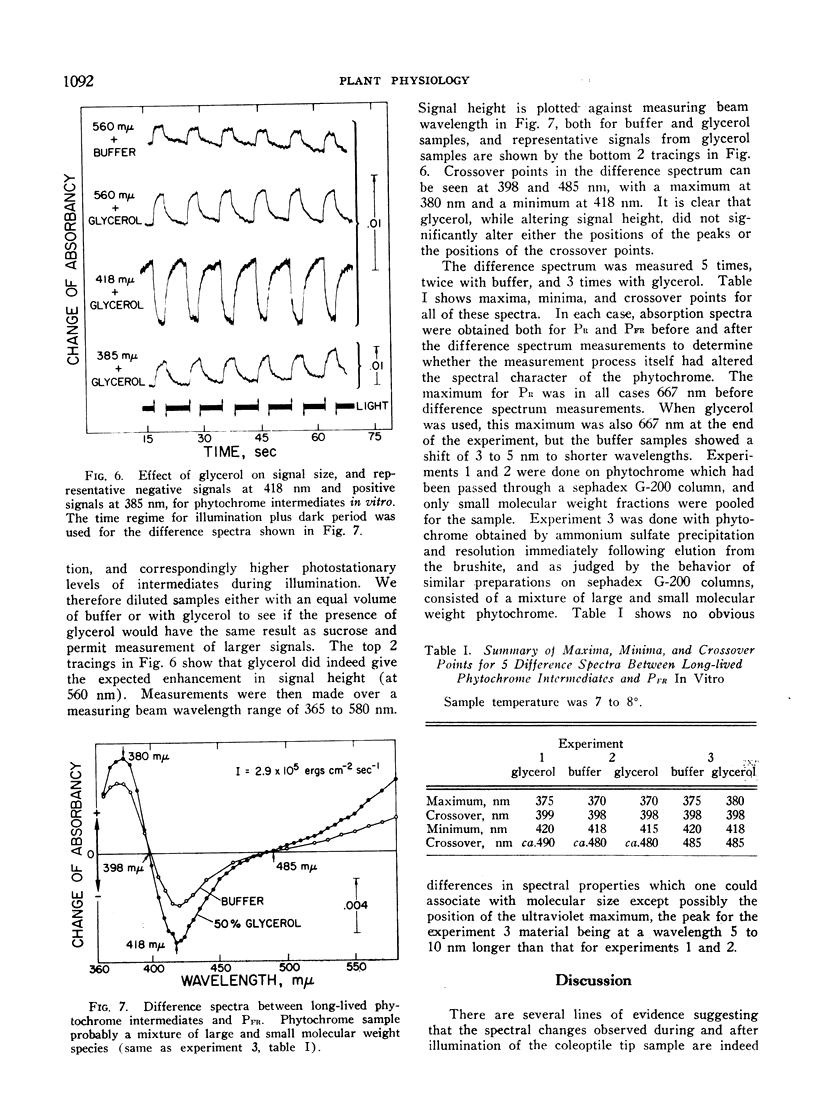
Conditions of illumination which cause phytochrome to cycle rapidly from PR to PFR and back lead to the accumulation in vivo of detectable amounts of long-lived intermediates on the PR to PFR pathway in oat coleoptile tissue. They appear to decay independently and in parallel to PFR. Their behavior under different intensities of illumination and exposure time suggests that they are homologous with 2 similar intermediates previously observed in vitro. Available evidence favoring this suggestion is discussed. Equivalent illumination apparently causes far higher steady state levels of absorption by intermediates in vivo than in vitro, suggestion that native phytochrome is in a different physical state in the cell than it is in solution. A difference spectrum for the intermediates in vitro between 365 and 580 nm is presented. It has a maximum at 380 nm, a minimum at 418 nm, and crossover points at 398 and 485 nm. Glycerol in the phytochrome sample enhances the signal without otherwise changing the spectrum in any way. The difference spectrum represents the difference in absorption between the combined intermediates and PFR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation I: In Vitro Studies. Plant Physiol. 1969 Aug;44(8):1081–1088. doi: 10.1104/pp.44.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D. R., Linschitz H., Kasche V., Tenenbaum J. Low-temperature studies on phytochrome: light and dark reactions in the red to far-red transformation and new intermediate forms of phytochrome. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1095–1101. doi: 10.1073/pnas.61.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Stabilization of phytochrome intermediates by low temperature. Photochem Photobiol. 1968 Nov;8(5):477–485. doi: 10.1111/j.1751-1097.1968.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruit C. J. Low-temperature action spectra for transformations of photoperiodic pigments. Biochim Biophys Acta. 1966 Jul 13;120(3):454–456. doi: 10.1016/0926-6585(66)90312-8. [DOI] [PubMed] [Google Scholar]
- Spruit C. J. Photoreversible pigment transformations in etiolated plants. Biochim Biophys Acta. 1966 Jan 4;112(1):186–188. doi: 10.1016/s0926-6585(96)90025-4. [DOI] [PubMed] [Google Scholar]


