Abstract
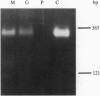
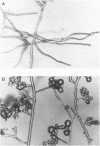
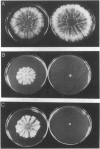
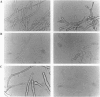
Two-component signal transduction systems are most often found in prokaryotic organisms where they are responsible for mediating the cellular responses to many environmental stimuli. These systems are composed of an autophosphorylating histidine kinase and a response regulator. We have found evidence for the existence of two-component histidine kinases in the eukaryotic filamentous fungus Neurospora crassa based on screening with degenerate primers to conserved regions of these signaling proteins. Subsequent cloning and sequencing of one member of this newly discovered group, nik-1+, shows that the predicted protein sequence shares homology with both the kinase and response regulator modules of two-component signaling proteins. In addition, the N-terminal region of the protein has a novel repeating 90-amino acid motif. Deletion of the nik-1+ gene in N. crassa results in an organism that displays aberrant hyphal structure, which is enhanced under conditions of high osmostress. Increased osmotic pressure during growth on solid medium leads to restricted colonial growth, loss of aerial hyphae formation, and no subsequent conidiophore development. This finding may have implications for mechanisms of fungal colonization and pathogenicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aatsinki J. T., Lakkakorpi J. T., Pietilä E. M., Rajaniemi H. J. A coupled one-step reverse transcription PCR procedure for generation of full-length open reading frames. Biotechniques. 1994 Feb;16(2):282-4, 286-8. [PubMed] [Google Scholar]
- Alex L. A., Simon M. I. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994 Apr;10(4):133–138. doi: 10.1016/0168-9525(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Brown J. L., North S., Bussey H. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall beta-glucan assembly, encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J Bacteriol. 1993 Nov;175(21):6908–6915. doi: 10.1128/jb.175.21.6908-6915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Kwok S. F., Bleecker A. B., Meyerowitz E. M. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993 Oct 22;262(5133):539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Hua J., Chang C., Sun Q., Meyerowitz E. M. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science. 1995 Sep 22;269(5231):1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Maeda H., Ishida N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem. 1967 Aug;62(2):276–278. doi: 10.1093/oxfordjournals.jbchem.a128660. [DOI] [PubMed] [Google Scholar]
- Maeda T., Wurgler-Murphy S. M., Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994 May 19;369(6477):242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Mishra N. C. Genetics and biochemistry of morphogenesis in Neurospora. Adv Genet. 1977;19:341–405. doi: 10.1016/s0065-2660(08)60248-5. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Tokishita S., Aiba H., Mizuno T. A novel sensor-regulator protein that belongs to the homologous family of signal-transduction proteins involved in adaptive responses in Escherichia coli. Mol Microbiol. 1992 Mar;6(6):799–807. doi: 10.1111/j.1365-2958.1992.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Okamoto P. M., Fu Y. H., Marzluf G. A. Nit-3, the structural gene of nitrate reductase in Neurospora crassa: nucleotide sequence and regulation of mRNA synthesis and turnover. Mol Gen Genet. 1991 Jun;227(2):213–223. doi: 10.1007/BF00259673. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Sachs M. S., Yanofsky C. The Neurospora crassa arg-2 locus. Structure and expression of the gene encoding the small subunit of arginine-specific carbamoyl phosphate synthetase. J Biol Chem. 1990 Jul 5;265(19):10981–10987. [PubMed] [Google Scholar]
- Ota I. M., Varshavsky A. A yeast protein similar to bacterial two-component regulators. Science. 1993 Oct 22;262(5133):566–569. doi: 10.1126/science.8211183. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Kofoid E. C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Barry E. G. The cytogenetics of Neurospora. Adv Genet. 1977;19:133–285. doi: 10.1016/s0065-2660(08)60246-1. [DOI] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert W. R., Patel V. B., Giles N. H. Genetic regulation of the qa gene cluster of Neurospora crassa: induction of qa messenger ribonucleic acid and dependency on qa-1 function. Mol Cell Biol. 1981 Sep;1(9):829–835. doi: 10.1128/mcb.1.9.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Springer M. L. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays. 1993 Jun;15(6):365–374. doi: 10.1002/bies.950150602. [DOI] [PubMed] [Google Scholar]
- Swanson R. V., Alex L. A., Simon M. I. Histidine and aspartate phosphorylation: two-component systems and the limits of homology. Trends Biochem Sci. 1994 Nov;19(11):485–490. doi: 10.1016/0968-0004(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Yao V. J., Spudich J. L. Primary structure of an archaebacterial transducer, a methyl-accepting protein associated with sensory rhodopsin I. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11915–11919. doi: 10.1073/pnas.89.24.11915. [DOI] [PMC free article] [PubMed] [Google Scholar]