Abstract
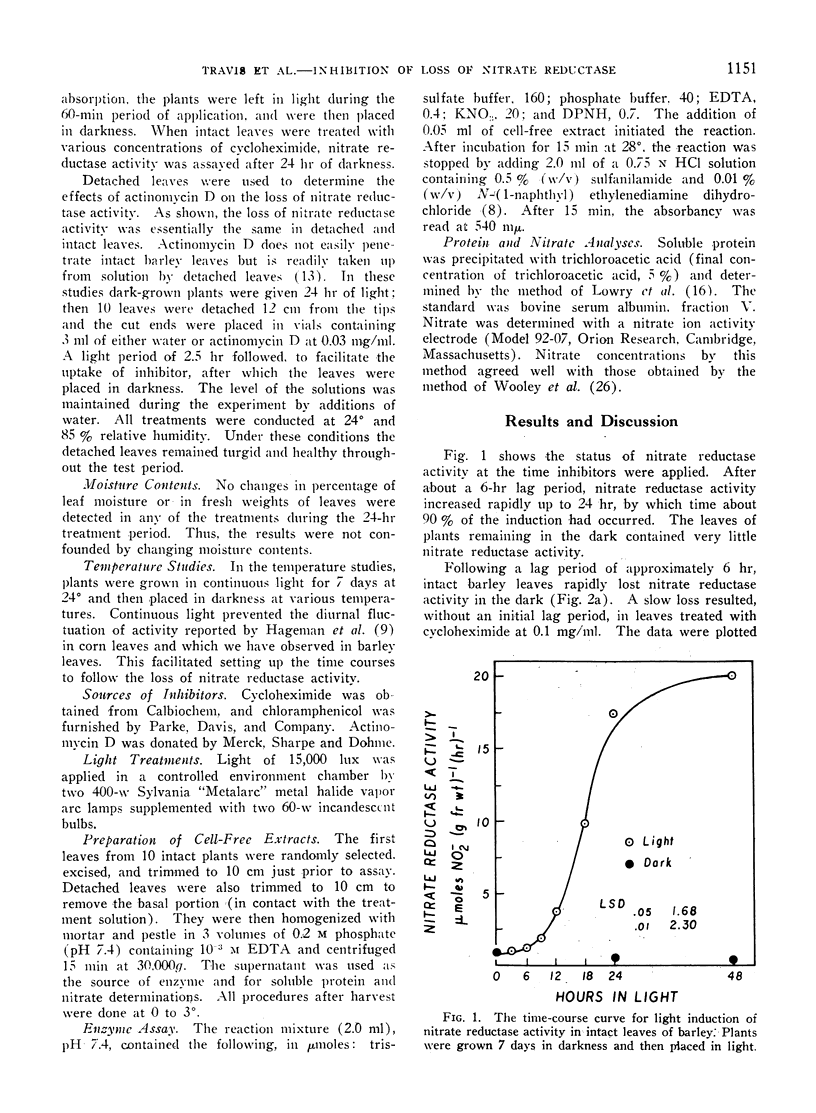
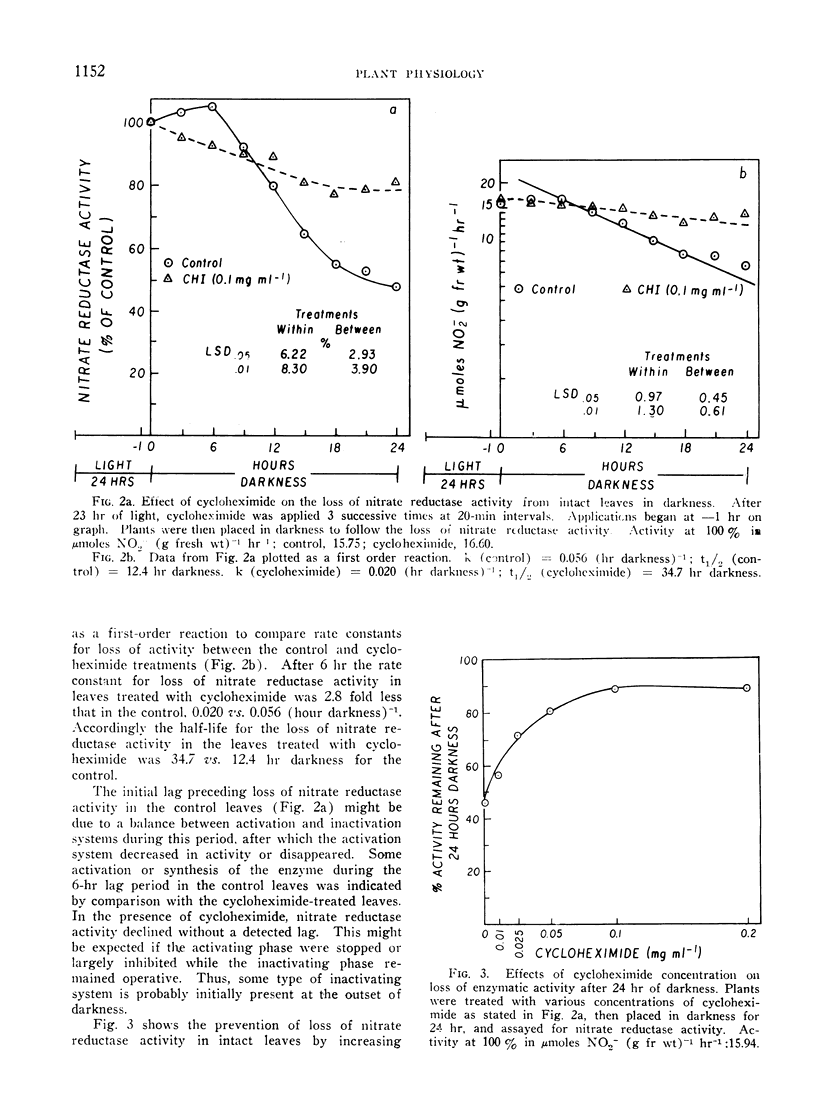
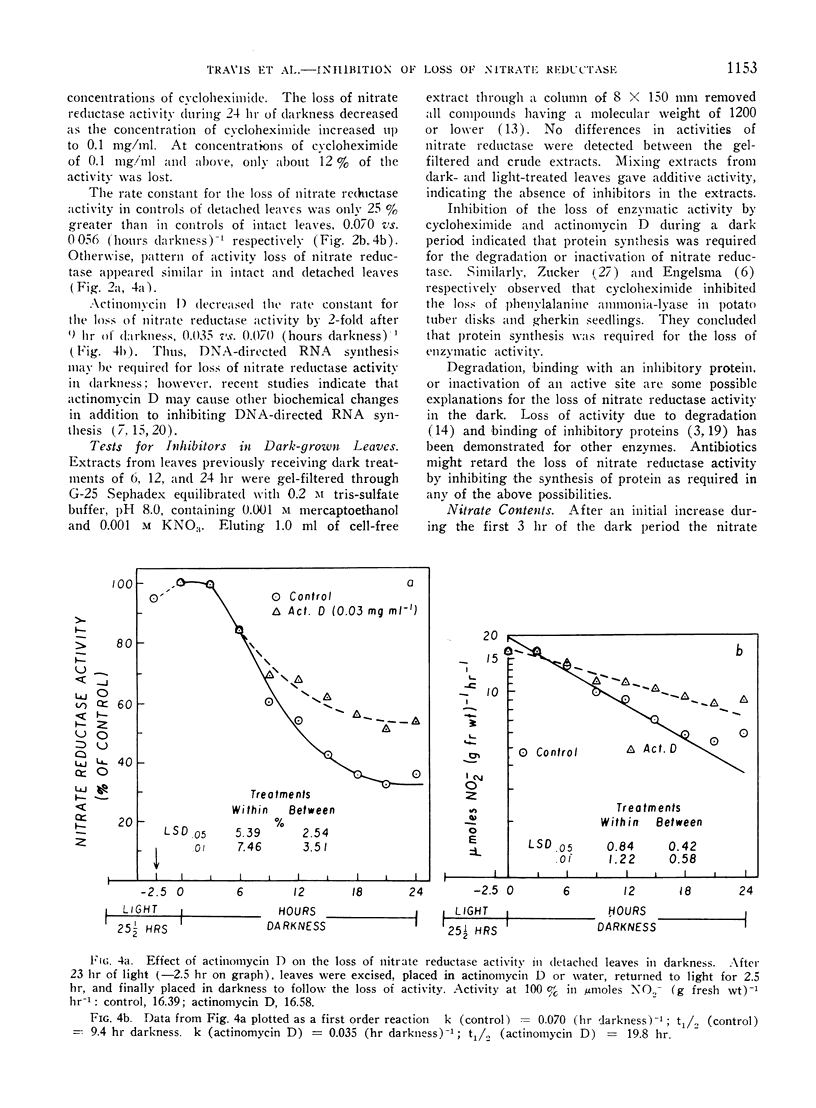
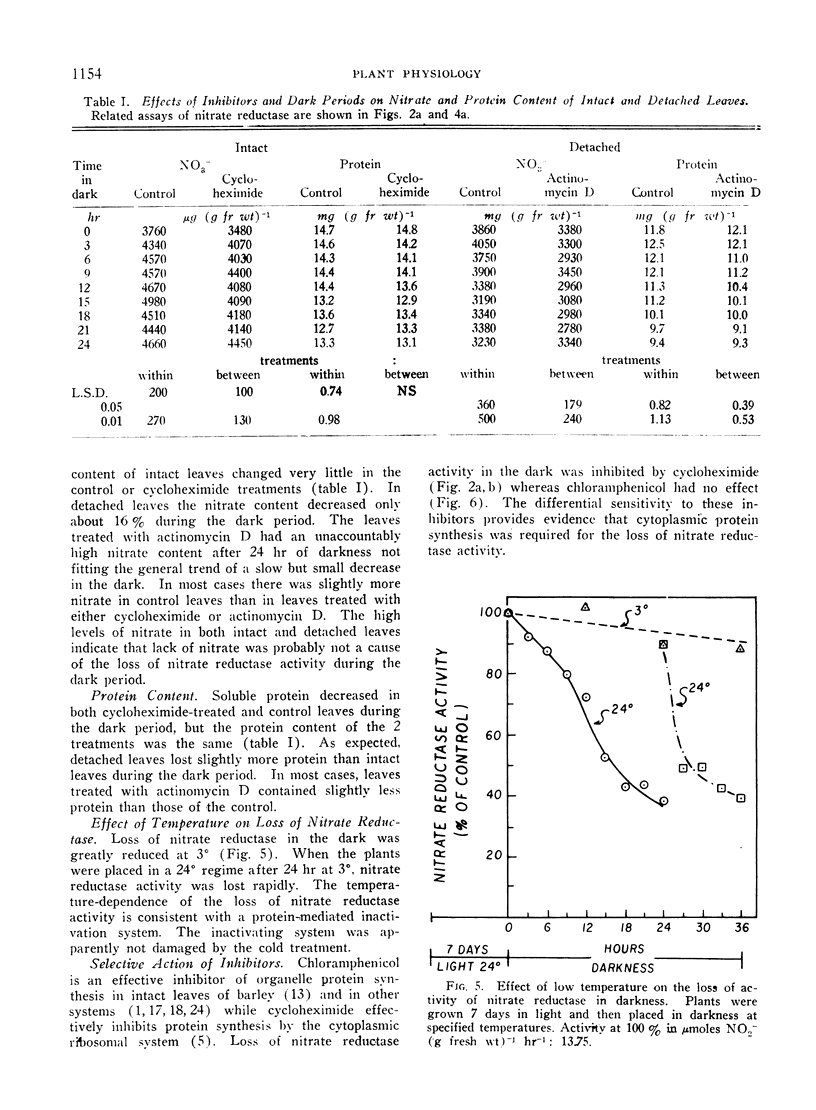
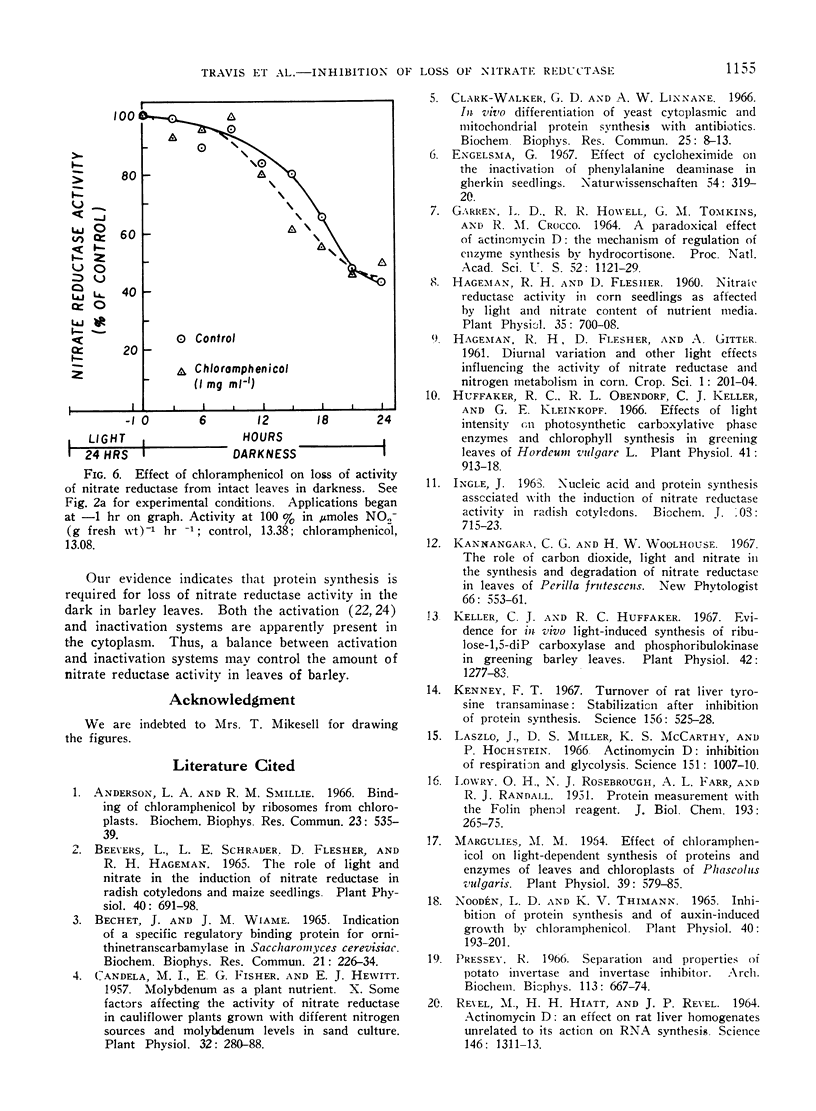
The disappearance of nitrate reductase activity in leaves of Hordeum vulgare L. during darkness was inhibited by cycloheximide, actinomycin D, and low temperature. Thus, protein synthesis was probably required for the disappearance of nitrate reductase in the dark. Since chloramphenicol did not affect the rate of loss of activity, the degradation or inactivation apparently required protein synthesis by the cytoplasmic ribosomal system. Consistent with this observation, nitrate reductase is also reportedly located in the cytoplasm. Thus, the amount of nitrate reductase activity present in leaves of barley may be controlled by a balance between activating and inactivating systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. A., Smillie R. M. Binding of chloramphenicol by ribosomes from chloroplasts. Biochem Biophys Res Commun. 1966 May 25;23(4):535–539. doi: 10.1016/0006-291x(66)90762-5. [DOI] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Beevers L., Schrader L. E., Flesher D., Hageman R. H. The Role of Light and Nitrate in the Induction of Nitrate Reductase in Radish Cotyledons and Maize Seedlings. Plant Physiol. 1965 Jul;40(4):691–698. doi: 10.1104/pp.40.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela M. I., Fisher E. G., Hewitt E. J. Molybdenum as a Plant Nutrient. X. Some Factors Affecting the Activity of Nitrate Reductase in Cauliflower Plants Grown with Different Nitrogen Sources and Molybdenum Levels in Sand Culture. Plant Physiol. 1957 Jul;32(4):280–288. doi: 10.1104/pp.32.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. In vivo differentiation of yeast cytoplasmic and mitochondrial protein synthesis with antibiotics. Biochem Biophys Res Commun. 1966 Oct 5;25(1):8–13. doi: 10.1016/0006-291x(66)90631-0. [DOI] [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker R. C., Obendorf R. L., Keller C. J., Kleinkopf G. E. Effects of Light Intensity on Photosynthetic Carboxylative Phase Enzymes and Chlorophyll Synthesis in Greening Leaves of Hordeum vulgare L. Plant Physiol. 1966 Jun;41(6):913–918. doi: 10.1104/pp.41.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C. J., Huffaker R. C. Evidence for in vivo Light-induced Synthesis of Ribulose-1,5-diP Carboxylase and Phosphoribulokinase in Greening Barley Leaves. Plant Physiol. 1967 Sep;42(9):1277–1283. doi: 10.1104/pp.42.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laszlo J., Miller D. S., McCarty K. S., Hochstein P. Actinomycin D: inhibition of respiration and glycolysis. Science. 1966 Feb 25;151(3713):1007–1010. doi: 10.1126/science.151.3713.1007. [DOI] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. Inhibition of protein synthesis and of auxin-induced growth by chloramphenicol. Plant Physiol. 1965 Jan;40(1):193–201. doi: 10.1104/pp.40.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- REVEL M., HIATT H. H., REVEL J. P. ACTINOMYCIN D: AN EFFECT ON RAT LIVER HOMOGENATES UNRELATED TO ITS ACTION ON RNA SYNTHESIS. Science. 1964 Dec 4;146(3649):1311–1313. doi: 10.1126/science.146.3649.1311. [DOI] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Beevers L., Hageman R. H. Differential effects of chloramphenicol on the induction of nitrate and nitrite reductase in green leaf tissue. Biochem Biophys Res Commun. 1967 Jan 10;26(1):14–17. doi: 10.1016/0006-291x(67)90244-6. [DOI] [PubMed] [Google Scholar]


