Abstract
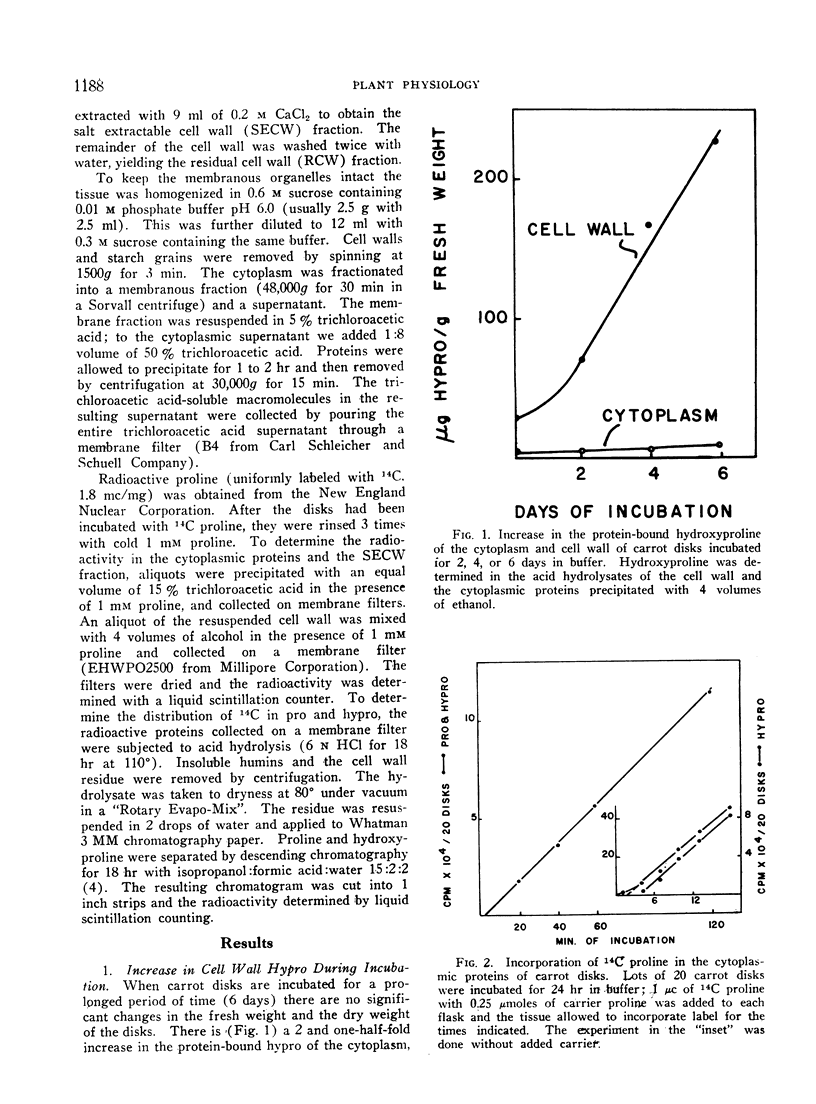
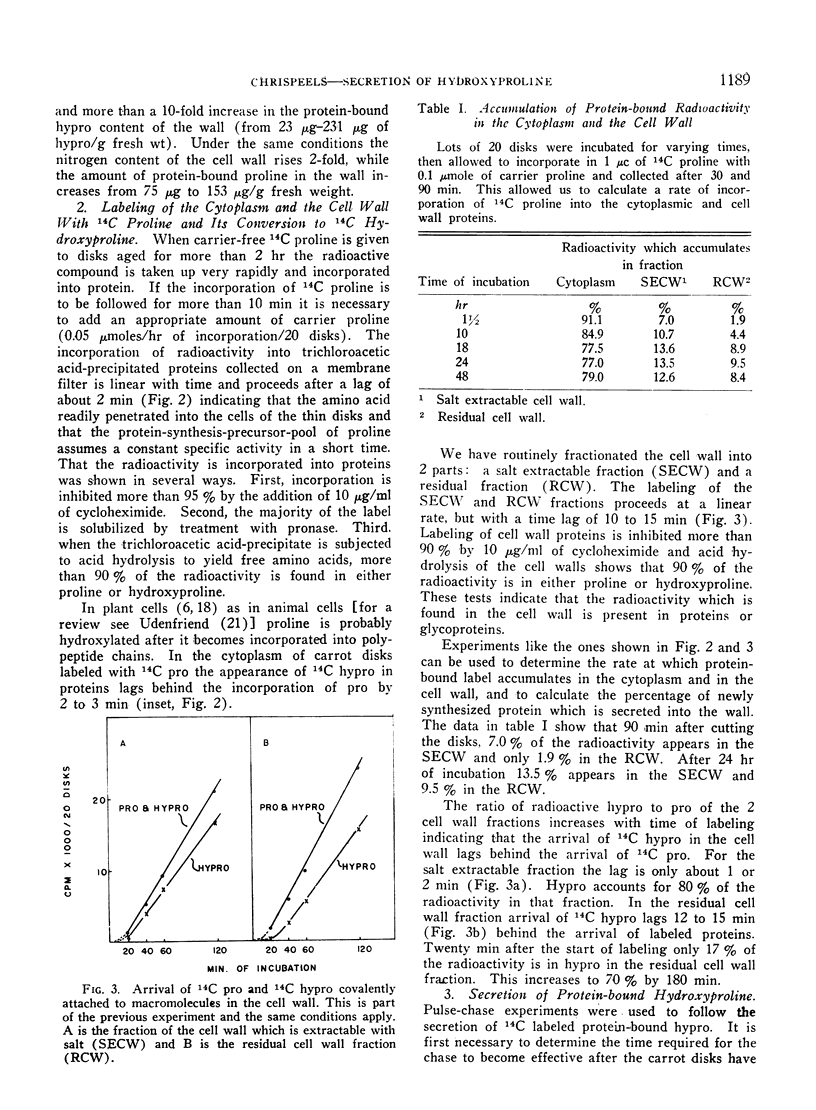
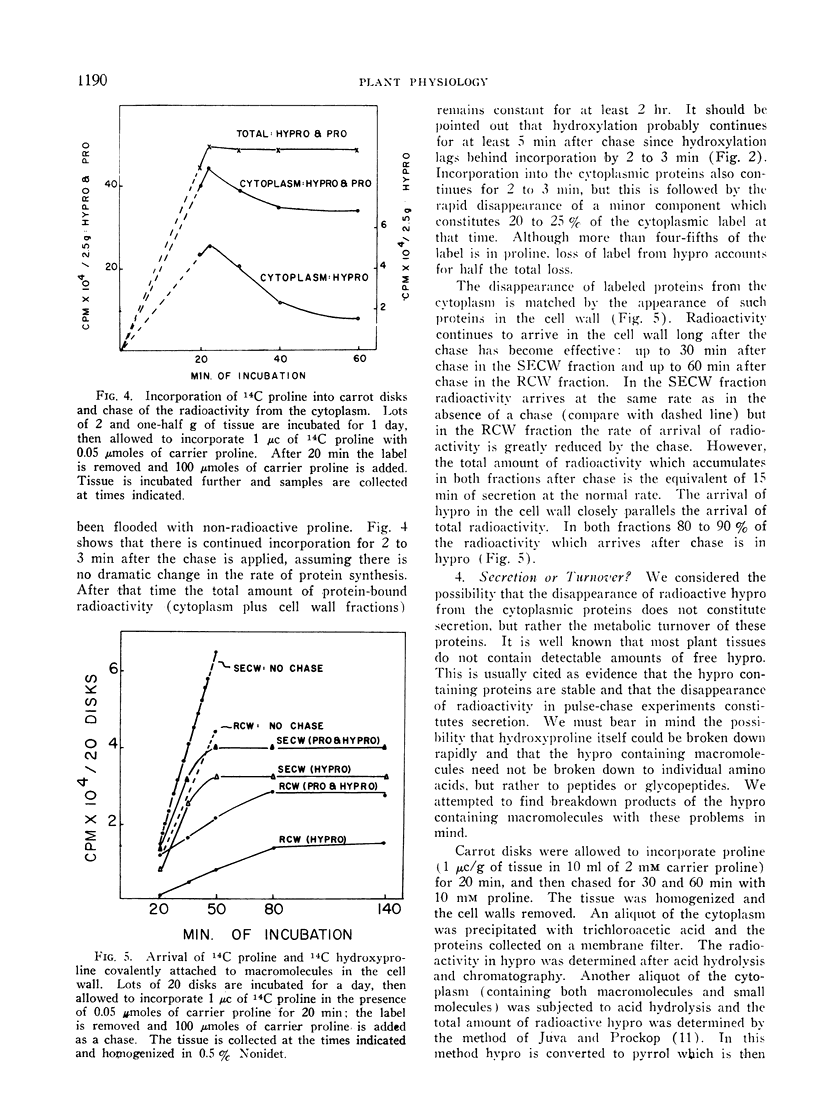
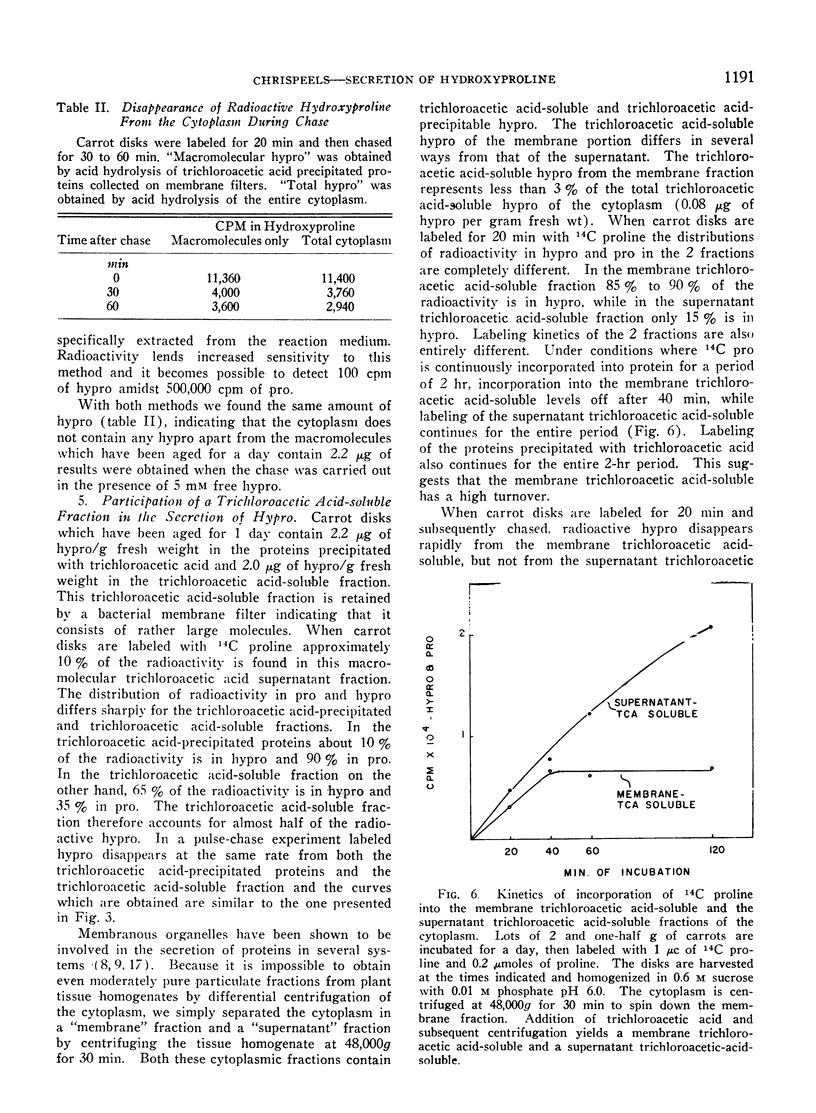
When disks of carrot (Daucus carota) phloem parenchyma are incubated for 6 days there is a 10-fold increase in cell wall hydroxyproline due to the synthesis and secretion of hydroxyproline-containing macromolecules. The synthesis of these molecules and their secretion are demonstrated by measuring the kinetics of incorporation and of chase of 14C-proline and hydroxyproline in different fractions of the cytoplasm and the cell wall. The hydroxyproline-containing molecules which are secreted are associated with the membranous organelles of the cytoplasm. They can be fractionated into trichloroacetic acid-soluble and trichloroacetic acid-precipitated fractions. The properties of the trichloroacetic acid-soluble fraction associated with the membranous organelles are consistent with its role as a cell wall precursor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. Distribution and metabolism of protein-bound hydroxyproline in an elongating tissue, the Avena coleoptile. Plant Physiol. 1968 Jun;43(6):865–870. doi: 10.1104/pp.43.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall D. K., Shimbayashi K. Factors Affecting Growth of Tobacco Callus Tissue and Its Incorporation of Tyrosine. Plant Physiol. 1960 May;35(3):396–404. doi: 10.1104/pp.35.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman J. Direct incorporation of hydroxyproline into protein of sycamore cells incubated at growth-inhibitory levels of hydroxyproline. Proc Natl Acad Sci U S A. 1967 Jan;57(1):50–54. doi: 10.1073/pnas.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. Synthesis of the carbohydrate of mucus in the golgi complex as shown by electron microscope radioautography of goblet cells from rats injected with glucose-H3. J Cell Biol. 1966 Jul;30(1):119–136. doi: 10.1083/jcb.30.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. C. Proteins and Plant Cell Walls. Proline to Hydroxyproline in Tobacco Suspension Cultures. Plant Physiol. 1964 Jul;39(4):543–550. doi: 10.1104/pp.39.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S. Formation of hydroxyproline in collagen. Science. 1966 Jun 3;152(3727):1335–1340. doi: 10.1126/science.152.3727.1335. [DOI] [PubMed] [Google Scholar]


