Abstract
Many bacteriophages produce small proteins that specifically interfere with the bacterial host transcription machinery and thus contribute to the acquisition of the bacterial cell by the bacteriophage. We recently described how a small protein, called P7, produced by the Xp10 bacteriophage inhibits bacterial transcription initiation by causing the dissociation of the promoter specificity sigma factor subunit from the host RNA polymerase holoenzyme. In this addendum to the original publication, we present the highlights of that research.
Keywords: bacterial RNA polymerase, bacteriophage, inhibitor, sigma factor, transcription
Central to the regulation of bacterial gene expression is the RNA polymerase (RNAp), the enzyme which catalyzes transcription, the stage during which RNA is synthesized from a DNA template. The catalytic core of the bacterial RNAp consists of five polypeptide subunits, α2, β, β’, and ω. The β and β’ subunits contain the catalytic site. Promoter specificity is conferred upon the catalytic core by dissociable RNAp σ subunits. Thus, the bacterial transcription cycle begins with the association of a σ subunit with the catalytic core, and subsequent σ-directed binding of RNAp holoenzyme to promoter DNA. Not surprisingly, bacteriophages (phages) have evolved strategies to alter the activity of bacterial (host) RNAp during infection and shift host resources toward the production of viral progeny.1,2 This modulation can occur in two ways, either through covalent modifications, such as phosphorylation or ADP ribosylation of target sites in the RNAp, or through small phage-encoded proteins that bind to RNAp. Many phages, which depend on both phage-encoded single-subunit RNAp and the bacterial RNAp, synthesize small proteins that are potent bacterial RNAp inhibitors. For example, the T7 phage relies on Escherichia coli host RNAp to catalyze the entry of its DNA into the cell and to transcribe a handful of early T7 phage genes. After which, the host RNAp is shut down by a middle T7 phage gene product, called Gp2, and the T7 phage single-subunit RNAp takes over and transcribes the rest of viral genes.3 In previous work, we studied the molecular and structural basis of the mechanism by which T7 Gp2 inhibits the bacterial RNAp and our analyses revealed that Gp2 uses a multipronged strategy to inactivate the bacterial RNAp.4,5 Such studies have broad implications not only in elucidating novel paradigms of bacterial transcription regulation, but also for uncovering strategies for development of novel antibacterial compounds targeting bacterial RNAp.
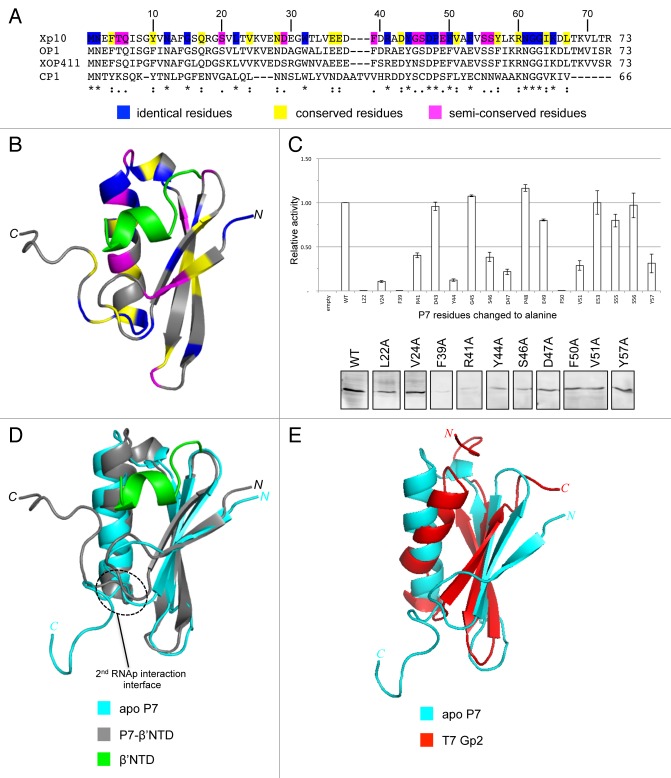
Our recent work examined the functional mechanism of a phage-encoded bacterial RNAp binding protein, called P7, which interferes with the bacterial transcription cycle at the initiation and termination stages.6 The 8 kDa P7 protein is produced by the lytic phage Xp10, which infects the bacterial phytopathogen Xanthomonas oryzae, the causal agent of rice blight. Xp10 is an unusual phage, combining elements of T7-like and λ-like phages.7,8 The genome of Xp10 consists of two groups of genes, called L and R genes, which are divergently transcribed. The L and R genes are separated by a regulatory intergenic region with multiple promoters, which are recognized either by the Xp10-encoded single-subunit T7-like RNAp or the X. oryzae host RNAp. It is believed that the ability of P7 to inhibit transcription initiation and effectively terminate transcription by the bacterial RNAp enables a switch between host to phage RNAp for the efficient transcription of Xp10 genes. An earlier study by Nechaev et al. revealed that the binding of P7 to the bacterial RNAp prevented conformational changes in σ70 (the σ subunit that directs the RNAp core to promoters of housekeeping bacterial genes) that are necessary for productive engagement with the promoter DNA.6 A subsequent study by Yuzenkova et al. identified the N-terminus of the β’ subunit on the X. oryzae RNAp,9 specifically a region comprising amino acid (aa) residues 1–10 (hereafter referred to as the β’ NTD), as the P7 binding site. Since the β’ NTD is absent from the electron density and likely disordered in the available structures of bacterial RNAp from Thermus thermophillus, Thermus aquaticus, and Escherichia coli,10-13 we initially sought to study the molecular and structural basis by which P7 inhibits transcription initiation by solving the solution structures of apo P7 and the P7-β’NTD complex by nuclear magnetic resonance (NMR) spectroscopy and conducting a mutational analysis of P7. The β’NTD sits in a largely hydrophobic cavity of P7.14 A BLAST search conducted using standard search parameters and Xp10 P7 as a query sequence found three P7 homologs encoded by three different Xanthomonas phages (Fig. 1A). The multiple protein sequence alignment of the P7 homologs conducted using COBALT16 showed that aa residues that surround the β’NTD in the cavity of P7 display high degree of conservation (Fig. 1A). Notably, the side chains of conserved aa residues L22, V24, F39, R41, D43, Y44, G45, S46, D47, P48, E49, F50, V51, E53, S55, S56, and Y57 are proximal to β’NTD and therefore likely contribute directly to the binding of P7 to the RNAp (Fig. 1B). As expected, alanine substitutions at 6 of 17 of these aa residues adversely affect the interaction between P7 and the β’NTD in a bacterial two-hybrid interaction assay, without having significant effects on the stability of the mutant protein under the assay conditions (Fig. 1C).
Figure 1. (A) Sequence alignment analysis of Xp10 phage P7-like proteins. The “*,” “:”, and “.” symbols indicate identical, conserved and semi-conserved aa residues, which are also color-coded (in blue, yellow, and magenta, respectively) in the Xp10 phage P7 sequence. (B) Ribbon representation of P7-β’NTD complex.14 The β’NTD is shown in green and the aa residues in P7 that are identical, conserved, and semi-conserved among P7-like proteins are color coded as in (A). (C) Top. Graph showing the ability of P7 mutants harboring alanine substitution at selected aa residues (see text) to interact with the β’NTD relative to the interaction between wild-type P7 and β’NTD. Bottom. Western blot analysis to assess intracellular levels of P7 mutants using polyclonal anti-P7 antibodies. The results rule out the possibility that the failure of the P7 mutants L22A, V24A, D47A, F50A, V51A, and Y57A to interact with the β’NTD is attributable to protein instability under the bacteria two hybrid interaction assay conditions. (D) Overlay of the ribbon representations of apo P7 and P7-β’NTD complex.14 The region on P7 that interacts with the β flap domain is circled. (E) Overlay of the ribbon representations of apo P7 and T7 Gp2.14,15
The comparison of the structures P7-β’NTD complex and apo P7 reveals that the flexible carboxyl terminal tail of P7 (aa residues K65-R73), which is disordered in the apo P7 structure folds back and contacts the β’NTD region in the complex (Fig. 1D). The model of P7-RNAp based on our P7-β’NTD complex and the crystal structure of the Escherichia coli RNAp10 indicates that the rearrangement of the carboxyl terminal tail of P7 that occurs upon binding the β’NTD unveils a second RNAp interaction interface, which enables P7 to interact with a flexible and conserved domain of the bacterial RNAp, known as the β flap domain.14 The β flap domain plays a major role in the association of σ factors with the RNAp core by interacting with a DNA binding region of σ termed region 4.2. This interaction induces conformational changes in σ, allowing it to productively engage the promoter.17 The interaction between P7 and β flap domain was confirmed by bacterial two-hybrid interaction assays and mutagenesis of P7.14 Thus, P7 adopts a two-step strategy to simultaneously interact with the RNAp β and β’ subunits to inhibit transcription initiation. We propose that the initial “docking” of P7 to the β’-NTD facilitates the interaction with the β flap domain first by bringing P7 in the close vicinity of the β flap domain and, second, by inducing conformational rearrangements in the carboxyl terminal tail of P7 to establish interaction with the β flap domain. A conserved positively charged aa residue (R60 in Xp10 P7; Fig. 1A) seems to play an important role in the interaction with the β flap domain.14 The interaction between P7 with the β flap domain is functionally important: The results show that P7 inhibits transcription initiation by sabotaging the integrity of RNAp, causing the displacement of σ70 from the RNAp during engagement of promoter DNA.14 A charge-reversal substitution at R60 in Xp10 P7 does not affect the ability of P7 to bind the β’NTD but abolishes P7-mediated displacement of σ70 from the RNAp.14 Intriguingly, the P7-like protein encoded by the CP1 phage differs from Xp10 P7 in that the former is missing residues that contribute to the carboxyl terminal tail (Fig. 1A). However, CP1 P7 contains two extra cysteine residues at the carboxyl terminal end, which leads us to speculate that CP1 P7 may interface with the RNAp in a different manner compared with P7 homologs encoded by other phages.
At present, the precise sequence of events that leads to the P7 mediated displacement of σ70 from the RNAp remains unknown. The structural model of the E. coli RNAp-P7 complex places P7 in close proximity to σ70 region 4.2 and the ω subunit of the RNAp. Although bacterial two-hybrid interaction analyses rule out an interaction between P7 and σ70 region 4.2, an interaction between P7 and the ω subunit of the RNAp remains a possibility. An altered interaction between ω and the β’ subunit has been recently implied to affect the stability of RNAp and thereby adversely affect transcription initiation.18 The involvement of the P7-ω interaction in the transcription anti-termination function of P7 is also possible. Thus, investigating the structural and molecular details of the interaction between the ω subunit and P7 is of great interest and is currently the subject of investigation in our laboratory.
In conclusion, the P7 protein is an unusual inhibitor of bacterial RNAp. Although a search for proteins with structural similarities to P7 using bioinformatics tools such as DALI19 and PDBeFold20 was unsuccessful, intriguingly, the topology of P7 is reminiscent of T7 phage Gp2, which interacts with a different site on the RNAp and inhibits transcription initiation using an entirely different mechanism (Fig. 1E). The elucidation of the structural and mechanistic basis for the action of P7 not only underscores the diversity of strategies employed by phages to sabotage the bacterial transcription machinery and thereby acquire their bacterial prey, but, perhaps more importantly, serves as a much-needed opportunity and inspiration to uncover strategies for the development of novel antibacterial compounds.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Project grants from the Wellcome Trust (to SW) and Biological and Biotechnological Research Council (to SM and SW) supported this work. SM and SW are recipients of Wellcome Trust Investigator Awards. Work in KS laboratory was supported by an NIH GM59295 grant and grant from the Ministry of Education and Science of Russian Federation, project 14.B25.31.0004.
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/28520
References
- 1.Nechaev S, Severinov K. Bacteriophage-induced modifications of host RNA polymerase. Annu Rev Microbiol. 2003;57:301–22. doi: 10.1146/annurev.micro.57.030502.090942. [DOI] [PubMed] [Google Scholar]
- 2.Nechaev S, Severinov K. The elusive object of desire--interactions of bacteriophages and their hosts. Curr Opin Microbiol. 2008;11:186–93. doi: 10.1016/j.mib.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savalia D, Robins W, Nechaev S, Molineux I, Severinov K. The role of the T7 Gp2 inhibitor of host RNA polymerase in phage development. J Mol Biol. 2010;402:118–26. doi: 10.1016/j.jmb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekler V, Minakhin L, Sheppard C, Wigneshweraraj S, Severinov K. Molecular mechanism of transcription inhibition by phage T7 gp2 protein. J Mol Biol. 2011;413:1016–27. doi: 10.1016/j.jmb.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James E, Liu M, Sheppard C, Mekler V, Cámara B, Liu B, Simpson P, Cota E, Severinov K, Matthews S, et al. Structural and mechanistic basis for the inhibition of Escherichia coli RNA polymerase by T7 Gp2. Mol Cell. 2012;47:755–66. doi: 10.1016/j.molcel.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nechaev S, Yuzenkova Y, Niedziela-Majka A, Heyduk T, Severinov K. A novel bacteriophage-encoded RNA polymerase binding protein inhibits transcription initiation and abolishes transcription termination by host RNA polymerase. J Mol Biol. 2002;320:11–22. doi: 10.1016/S0022-2836(02)00420-5. [DOI] [PubMed] [Google Scholar]
- 7.Semenova E, Djordjevic M, Shraiman B, Severinov K. The tale of two RNA polymerases: transcription profiling and gene expression strategy of bacteriophage Xp10. Mol Microbiol. 2005;55:764–77. doi: 10.1111/j.1365-2958.2004.04442.x. [DOI] [PubMed] [Google Scholar]
- 8.Yuzenkova J, Nechaev S, Berlin J, Rogulja D, Kuznedelov K, Inman R, Mushegian A, Severinov K. Genome of Xanthomonas oryzae bacteriophage Xp10: an odd T-odd phage. J Mol Biol. 2003;330:735–48. doi: 10.1016/S0022-2836(03)00634-X. [DOI] [PubMed] [Google Scholar]
- 9.Yuzenkova Y, Zenkin N, Severinov K. Mapping of RNA polymerase residues that interact with bacteriophage Xp10 transcription antitermination factor p7. J Mol Biol. 2008;375:29–35. doi: 10.1016/j.jmb.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami KS. X-ray crystal structure of Escherichia coli RNA polymerase σ70 holoenzyme. J Biol Chem. 2013;288:9126–34. doi: 10.1074/jbc.M112.430900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami KS, Masuda S, Campbell EA, Muzzin O, Darst SA. Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science. 2002;296:1285–90. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- 12.Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science. 2002;296:1280–4. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 13.Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417:712–9. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Shadrin A, Sheppard C, Mekler V, Xu Y, Severinov K, Matthews S, Wigneshweraraj S. A bacteriophage transcription regulator inhibits bacterial transcription initiation by sigma-factor displacement. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku080. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cámara B, Liu M, Reynolds J, Shadrin A, Liu B, Kwok K, Simpson P, Weinzierl R, Severinov K, Cota E, et al. T7 phage protein Gp2 inhibits the Escherichia coli RNA polymerase by antagonizing stable DNA strand separation near the transcription start site. Proc Natl Acad Sci U S A. 2010;107:2247–52. doi: 10.1073/pnas.0907908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulos JS, Agarwala R. COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007;23:1073–9. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- 17.Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science. 2002;295:855–7. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar P, Sardesai AA, Murakami KS, Chatterji D. Inactivation of the bacterial RNA polymerase due to acquisition of secondary structure by the ω subunit. J Biol Chem. 2013;288:25076–87. doi: 10.1074/jbc.M113.468520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm L, Sander C. Dali: a network tool for protein structure comparison. Trends Biochem Sci. 1995;20:478–80. doi: 10.1016/S0968-0004(00)89105-7. [DOI] [PubMed] [Google Scholar]
- 20.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–68. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]