Abstract
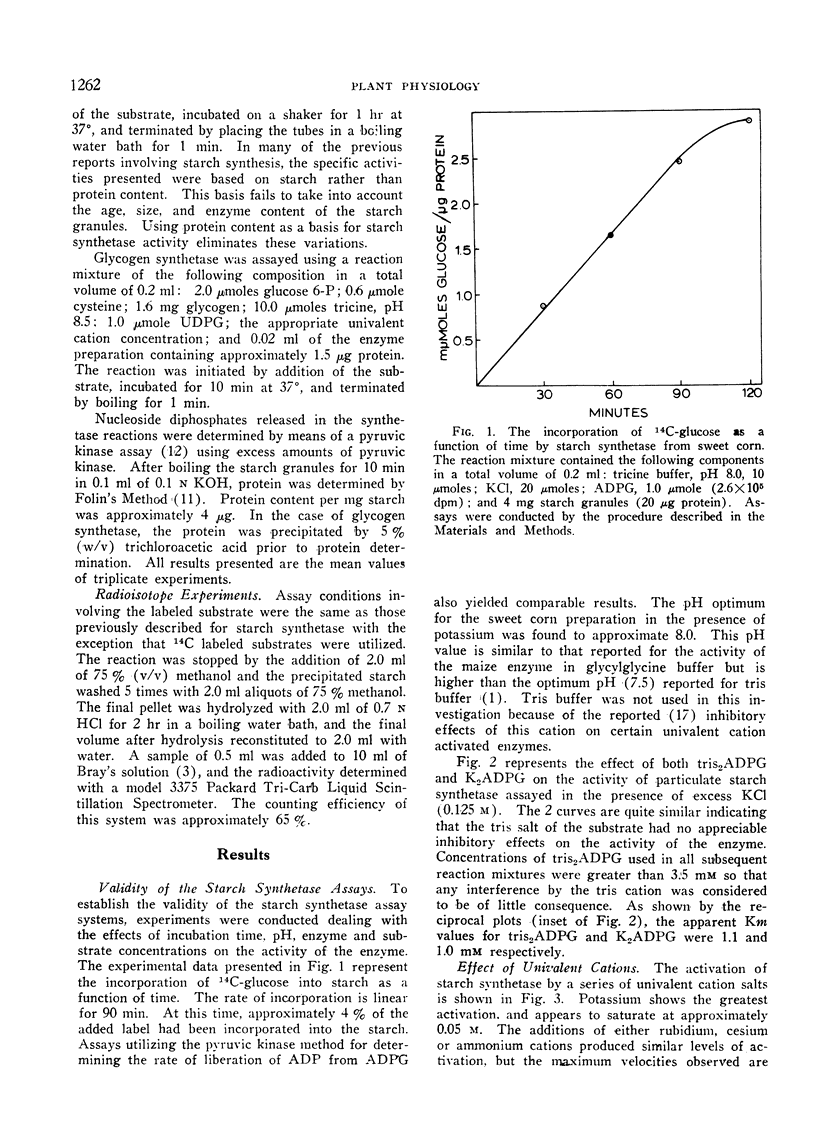
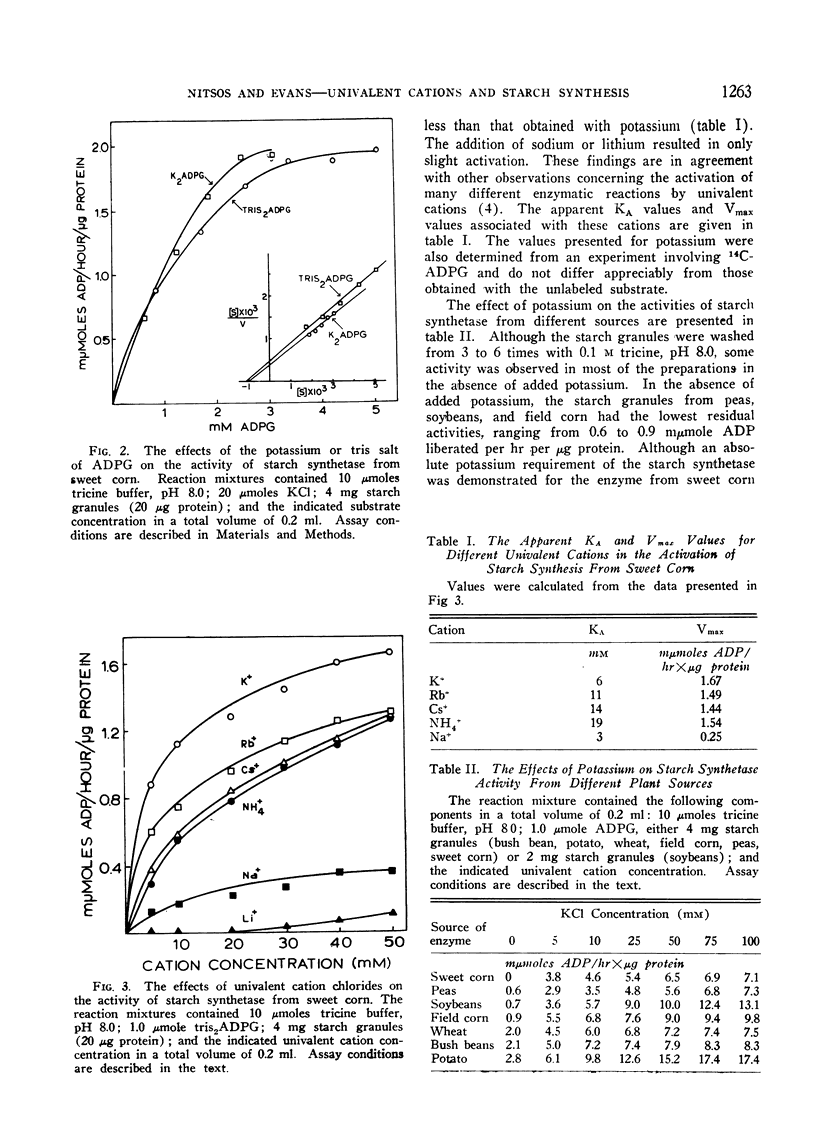
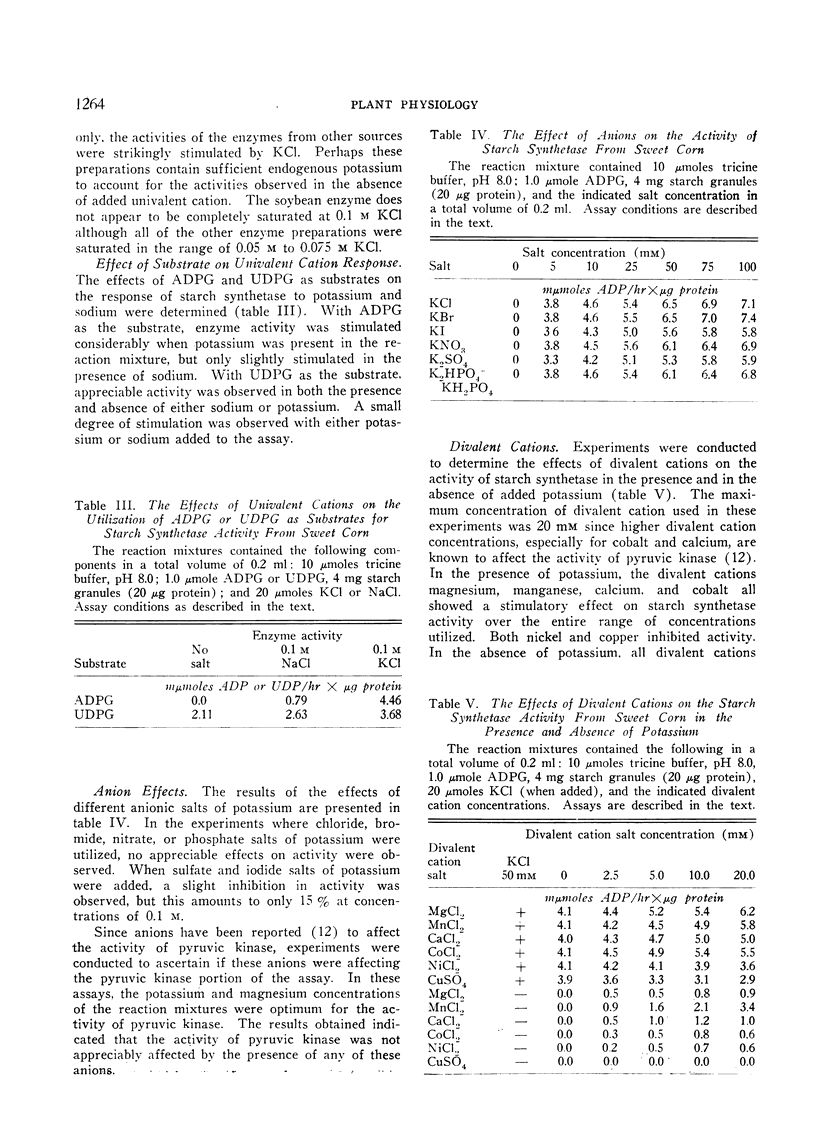
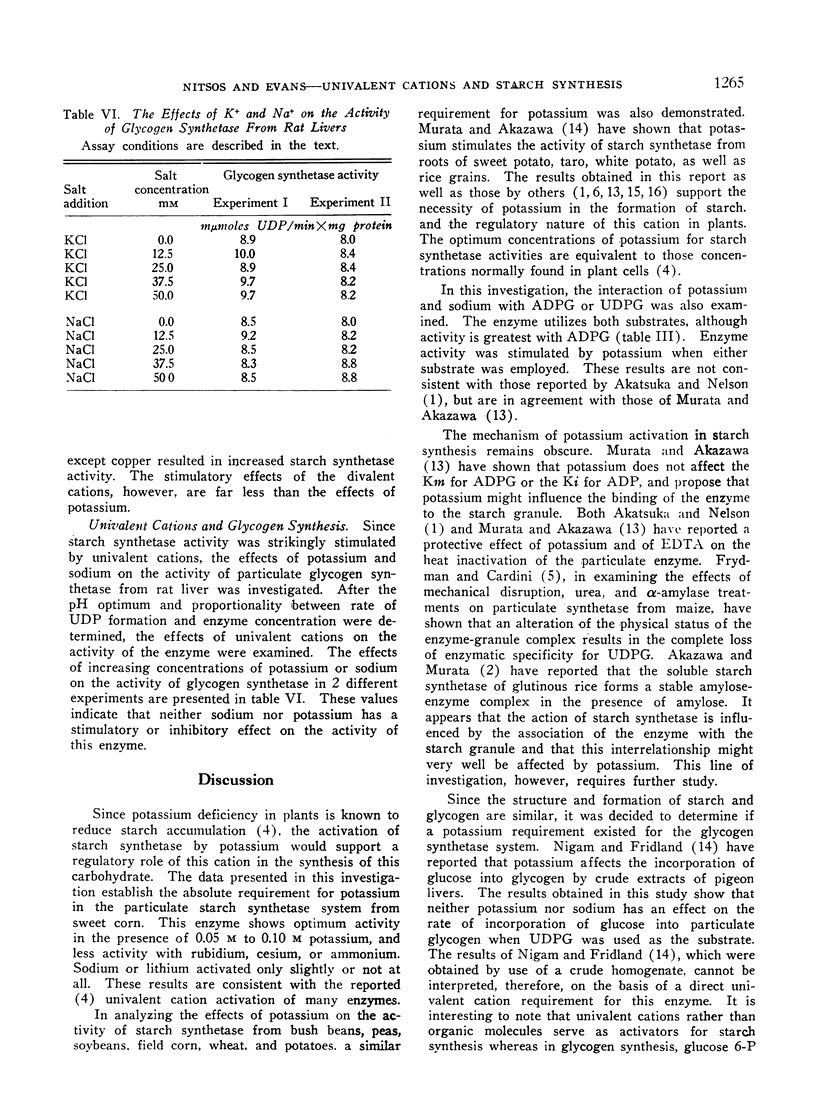
An investigation was made to determine the univalent cation requirements of starch synthetase from a variety of plant species of economic importance. The particulate enzyme from sweet corn was shown to have an absolute requirement for potassium, with the optimum activation occurring at 0.05 M KCl. Rubidium, cesium, and ammonium were 80% as effective as potassium while sodium and lithium were respectively 21% and 8% as effective as potassium. The KA for potassium was determined to be 6 mM. In the case of the particulate starch synthetase from wheat, bush beans, field corn, soybeans, peas, or potatoes, considerable stimulation of enzyme activity was obtained by the addition of potassium to the reaction mixture. In these studies, low enzyme activity was observed in the absence of added potassium, but the content of endogenous univalent cations in the reactions may be sufficient to account for the activities observed. Anions of various types had no effect on starch synthetase activity. Divalent cations produced slight activation in the presence or absence of potassium. All efforts to show a potassium requirement for glycogen synthetase from rat liver have been negative.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akatsuka T., Nelson O. E. Starch granule-bound adenosine diphosphate glucose-starch glucosyltransferases of maize seeds. J Biol Chem. 1966 May 25;241(10):2280–2285. [PubMed] [Google Scholar]
- Frydman R. B., Cardini C. E. Studies on the biosynthesis of starch. II. Some properties of the adenosine diphosphate glucose:starch glucosyltransferase bound to the starch granule. J Biol Chem. 1967 Jan 25;242(2):312–317. [PubMed] [Google Scholar]
- GRAZI E., RIPPA M., PONTREMOLI S. THE NATURE OF THE AMINO ACID RESIDUES INVOLVED IN THE INACTIVATION OF GLUCONATE 6-PHOSPHATE DEHYDROGENASE BY IODOACETATE. J Biol Chem. 1965 Jan;240:234–237. [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- LELOIR L. F., OLAVARRIA J. M., GOLDEMBERG S. H., CARMINATTI H. Biosynthesis of glycogen from uridine diphosphate glucose. Arch Biochem Biophys. 1959 Apr;81(2):508–520. doi: 10.1016/0003-9861(59)90232-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Akazawa T. Enzymic mechanism of starch synthesis in sweet potato roots. I. Requirement of potassium lons for potassium ions for starch synthetase. Arch Biochem Biophys. 1968 Sep 10;126(3):873–879. doi: 10.1016/0003-9861(68)90481-5. [DOI] [PubMed] [Google Scholar]
- Nigam V. N., Fridland A. Studies on glycogen synthesis in pigeon liver homogenates. Incorporation of hexose into glycogen. Biochem J. 1967 Nov;105(2):505–513. doi: 10.1042/bj1050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H., Evans H. J., Becker R. R. The effect of univalent cation salts on the stability and on certain physical properties of pyruvate kinase. J Biol Chem. 1967 Sep 10;242(17):3825–3832. [PubMed] [Google Scholar]


