Abstract
Interstitial cells of Cajal (ICC) generate slow waves in gastrointestinal (GI) muscles. Previous studies have suggested that slow wave generation and propagation depends on a voltage-dependent Ca2+ entry mechanism with the signature of a T-type Ca2+ conductance. We studied voltage-dependent inward currents in isolated ICC. ICC displayed two phases of inward current upon depolarization: a low voltage-activated inward current and a high voltage-activated current. The latter was of smaller current density and blocked by nicardipine. Ni2+ (30 μM) or mibefradil (1 μM) blocked the low voltage-activated current. Replacement of extracellular Ca2+ with Ba2+ did not affect the current, suggesting that either charge carrier was equally permeable. Half-activation and half-inactivation occurred at −36 and −59 mV, respectively. Temperature sensitivity of the Ca2+ current was also characterized. Increasing temperature (20–30°C) augmented peak current from −7 to −19 pA and decreased the activation time from 20.6 to 7.5 ms [temperature coefficient (Q10) = 3.0]. Molecular studies showed expression of Cacna1g (Cav3.1) and Cacna1h (Cav3.2) in ICC. The temperature dependence of slow waves in intact jejunal muscles of wild-type and Cacna1h−/− mice was tested. Reducing temperature decreased the upstroke velocity significantly. Upstroke velocity was also reduced in muscles of Cacna1h−/− mice, and Ni2+ or reduced temperature had little effect on these muscles. Our data show that a T-type conductance is expressed and functional in ICC. With previous studies our data suggest that T-type current is required for entrainment of pacemaker activity within ICC and for active propagation of slow waves in ICC networks.
Keywords: gastrointestinal smooth muscle, pacemaker, slow wave, gastrointestinal motility
coordination of contractions, in some cases over many centimeters of bowel wall, requires regulatory mechanisms beyond the spontaneous excitability of smooth muscle cells (SMCs). For example, rhythmic electrical depolarizations, known as slow waves, time the phasic contractions of segmentation and peristalsis in the small intestine (21, 32). Interstitial cells of Cajal (ICC) are the cells that generate pacemaker activity and propagate slow waves actively in gastrointestinal muscles (4, 11, 19, 25, 28, 33, 36). Slow waves conduct to SMCs via gap junctions, but SMCs lack the ionic mechanisms necessary for active propagation of slow waves (30). Depolarization of SMCs caused by slow waves leads to enhanced open probability of voltage-dependent, dihydropyridine-sensitive Ca2+ channels, and the resulting influx of Ca2+ induces excitation-contraction coupling (26).
Slow waves result from activation of a large inward current (aka slow wave current) carried by Cl− ions (9, 39). The basic pacemaker event is release of Ca2+ from intracellular stores and localized activation of Cl− channels, encoded by Ano1 (12, 39). These events are stochastic in nature and referred to as spontaneous transient inward currents (STICs; see Refs. 10, 40). Cells in situ, in the absence of voltage control, depolarize in response to STICs producing spontaneous transient depolarization events [STDs; also termed “unitary potentials” by Hirst and Edwards (10)]. A voltage-dependent mechanism appears to entrain STICs into whole cell slow wave currents, and this is why step depolarization can activate (or pace) slow waves (7, 39). A similar mechanism is likely to be responsible for cell-to-cell active propagation of slow waves.
Most investigators agree that propagation of slow waves through networks of ICC depends on a voltage-dependent mechanism (5, 15, 34), but the precise mechanism has been controversial. It is clear that propagation depends on gap junction coupling between cells because β-glycyrrhetinic acid, which does not block pacemaker activity in individual cells, blocks the ability of slow waves to spread cell-to-cell coherently (27). It also appears that the voltage-dependent step includes, or is reinforced by, release of Ca2+ from inositol triphosphate (IP3) receptor-operated stores because 2-aminoethoxydiphenyl borate and xestospongin C block slow waves and propagation (8, 21, 27, 35). Some investigators have suggested that depolarization activates phospholipase C and causes generation of IP3, release of Ca2+, and activation of Ca2+-activated Cl− current (24). However, there is also evidence suggesting that depolarization activates a Ca2+ conductance, and Ca2+ entry could couple to release from stores via Ca2+-induced Ca2+ release (1, 17, 18). Pharmacological evidence provided in these studies suggests that a T-type conductance may be responsible for voltage-dependent Ca2+ entry because T channel blockers inhibited slow wave propagation. A study of cultured cells reported the presence of a T-type current in ICC (15); however, concerns about this finding have been raised since ion channel expression changes in culture (39). In the present study we have examined the expression of voltage-dependent inward currents in freshly dispersed ICC from the murine small intestine that were identified unequivocally by constitutive expression of a fluorescent reporter.
MATERIALS AND METHODS
Tissue preparation.
C57BL/6 (Jackson Laboratory, Bar Harbor, ME), smMHC/Cre/eGFP (donated by Michael Kotlikoff, Cornell University), Cacna1h−/− mice (donated by Dr. Kevin P. Campbell, University of Iowa), and KitcopGFP/+ mice were used for these experiments. Animals were anaesthetized with isoflurane (Aerrane; Baxter, Deerfield, IL) before decapitation and then small intestines were removed. The Institutional Animal Use and Care Committee at the University of Nevada approved all procedures used in the breeding and killing of animals.
Isolation of cells.
ICC and SMCs were isolated from Kit+/copGFP mice described previously (39) and smMHC/Cre/eGFP mice, respectively. Small strips of jejunal muscle were dissected and equilibrated in Ca2+-free Hanks' solution for 20 min. Cells were dispersed from these strips, with an enzyme solution containing the following (per ml): collagenase (1.3 mg. Worthington Type II), bovine serum albumin (2 mg; Sigma, St. Louis, MO), trypsin inhibitor (2 mg; Sigma) and ATP (0.27 mg). Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 mg/ml; BD Falcon, Franklin Lakes, NJ) in 35-mm culture dishes. Freshly dispersed copGFP+ cells were allowed to stabilize for 3 to 6 h at 37°C in an incubator (atmosphere 95% O2-5% CO2) in smooth muscle growth medium (Clonetics, San Diego, CA) supplemented with 2% antibiotic-antimycotic (GIBCO, Grand Island, NY) and stem cell factor (5 ng/ml; Sigma).
Electrophysiological recording.
ICC were identified as cells with green fluorescent protein using an inverted fluorescence microscope. The standard whole cell patch clamp configuration was employed to record membrane currents (voltage clamp). Currents were amplified with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Union City, CA) and digitized with a 16-bit analog to digital converter (Digidata 1440A; Axon Instruments) and stored directly online using pCLAMP software (version 10.2; Axon Instruments). Data were sampled at 4 kHz and filtered at 2 kHz using an eight-pole Bessel filter for whole cell experiments. All data were analyzed using clampfit (pCLAMP version, 10.2; Axon Instruments) and Graphpad Prism (version 3.0; Graphpad Software, San Diego, CA) software. Average capacitance of freshly dispersed ICC was 5.2 ± 0.6 pF. External solution for whole cell recordings was a Ca2+-containing physiological salt solution (CaPSS) containing the following (in mM): 5 KCl, 135 NaCl, 2 CaCl2, 10 glucose, 1.2 MgCl2, and 10 HEPES adjusted to pH 7.4 with Tris. The pipette solutions contained the following (in mM): 120 CsCl, 20 TEACl, 10 BAPTA, 10 HEPES, 2 MgATP, and 0.1 NaGTP, pH adjusted to 7.2 by Tris.
For the measurement of intracellular membrane potentials, jejunal muscles from C57BL/6 and Cacna1h−/− mice were prepared by removing the mucosa. Muscle strips (10 × 5 mm) were pinned to Sygard (Dow Corning, Midland, MI) floor of a recording chamber with circular muscle facing upward. The bath chamber was perfused with Krebs-Ringer buffer (KRB) containing the following (in mM): 120 NaCl, 5.9 KCl, 1.2 MgCl2, 15.5 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, and 2.5 CaCl2 pH 7.4 adjusted by bubbling with 95% O2−5% CO2 at 37°C. Circular muscle cells were impaled with glass microelectrodes (50–80 MΩ) filled with 3 M KCl. Membrane potentials were measured with a high impedance electrometor (Duo-773; WPI, Sarasota, FL) and digitized using an analog to digital converter (Digidata 1322A; Axon Instruments). All data were analyzed using pCLAMP 10.2 (Clampfit; Axon Instruments) and Graphpad Prism 3.0 (Graphpad Software, San Diego, CA).
Cell collecting and PCR.
copGFP+ ICCs and eGFP-SMCs were purified by fluorescence-activated cell sorting (FACS; Becton-Dickinson FACSAria) using 488-nm excitation and a 530/30-nm bandpass filter for green fluorescent protein. Total RNA was isolated from copGFP+ ICC, eGFP-SMCs, and unsorted cells using illustra RNAspin Mini RNA isolation kit (GE Healthcare, Little Chalfont, UK), and first-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. PCR was performed with specific primers using AmpliTaq Gold PCR Master Mix (Applied Biosystems, Foster City, CA). Primer sequences are shown in Table 1. PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers used for PCR using Syber green chemistry on the 7300 Real Time PCR System (Applied Biosystems). Regression analysis of the mean values of eight multiplex qPCRs for the log10 diluted cDNA was used to generate standard curves. Unknown amounts of messenger RNA (mRNA) were plotted relative to the standard curve for each set of primers and graphically plotted using Microsoft Excel. This gave transcriptional quantification of each gene relative to the endogenous hypoxanthine guanine phosphoribosyltransferase (Hprt) standard after log transformation of the corresponding raw data.
Table 1.
Primer sequences
| Gene Name/Primer Sequence | Product Length, bp | Accession Number |
|---|---|---|
| Hprt | ||
| F-GACTTGCTCGAGATGTCATGAAGGAGAT | 198 | NM_013556 |
| R-TGTCCCCCGTTGACTGATCATTACAGTA | (Exons 3–4) | |
| Cacna1g | ||
| F-ACA ACG GCA TGG CCT CCA CGT | 137 | NM_009783 |
| R-CCG TTT GCC GAT TTC CTC TGC CTG | (Exons 13–14) | |
| Cacna1h | ||
| F-TGG AGA CCT ACA CAG GCC CGG T | 149 | NM_021415 |
| R-CAG AGA GCG GGG CGT ATC CC | (Exons 40–41) | |
| Cacna1i | ||
| F-ACC AAC CCT GAC GTC CCG CA | 120 | NM_001044308 |
| R-CAC ACA CTC GAA CCA CGG GTT ACA | (Exons 3–4) | |
| Myh11 | ||
| F-CAGCTGGAAGAGGCAGAGGAGG | 198 | NM_013607 |
| R-AACAAATGAAGCCTCGTTTCCTCTC | (Exons 40–41) | |
| cKit | ||
| F-CGCCTGCCGAAATGTATGACG | 162 | NM_021099 |
| R-GGTTCTCTGGGTTGGGGTTGC | (Exons 19–20) | |
| Pdgfra | ||
| F-ATGACAGCAGGCAGGGCTTCAACG | 195 | NM_011058 |
| R-CGGCACAGGTCACCACGATCGTTT | (Exons 5–6) |
Statistical analyses.
Data are expressed as means ± SE of n cells. All statistical analyses were performed using Graphpad Prism. We used Student's t-test to compare single values under control and experimental conditions or ANOVA with Dunnett's post-hoc analysis to compare groups of data. In all statistical analyses, P < 0.05 was considered statistically significant.
RESULTS
Characterization of two types of inward currents in ICC.
For characterization of voltage-dependent inward currents, a Cs+-rich pipette solution with a high concentration of BAPTA (10 mM, see materials and methods) was used to minimize contamination from the large amplitude of Ca2+-activated Cl− currents expressed by these cells (39). We tested the effects of varying external Ca2+ ([Ca2+]o) from 0 to 10 mM. The cells were held at −80 mV and stepped from −80 to +60 mV in 10-mV increments. Figure 1, A–D, shows representative current responses to step depolarizations in the presence of different [Ca2+]o. After each solution change, a voltage ramp protocol (from −80 to +80 mV for 400 ms) was applied to show the effects of each solution on the current-voltage (I–V) relationship (Fig. 1E). When cells were exposed to 2 mM [Ca2+]o, two types of voltage gated inward currents were recorded (Fig. 1, A and E). One conductance (low voltage-activated inward current) activated and reached a peak at between −30 and −20 mV (−6.0 ± 0.2 pA/pF; n = 5) and the second conductance (high-voltage activated inward current) activated and reached peak at 0 mV (−5.5 ± 0.7 pA/pF; n = 5; Fig. 1E). Equimolar replacement of extracellular Na+ with TEA to test the contribution of a Na+ conductance did not affect the inward current responses (Fig. 1, B and E). When [Ca2+]o was reduced to a nominally Ca2+-free solution (0 mM), both phases of the inward current response was abolished (Fig. 1, C and E). The reversal potential for the whole cell current shifted dramatically to negative potentials under these circumstances (Fig. 1E). Increasing [Ca2+]o from 2 to 10 mM increased both types of inward currents (e.g., from −33.0 ± 5.1 to −87.8 ± 5.0 pA at −20 mV and from 30.3 ± 5.5 to −77.4 ± 8.0 pA at 0 mV; n = 5) and shifted the reversal potential from +41.9 ± 0.6 to +57.5 ± 0.4 mV (n = 5; Fig. 1, D and F). These data show that the voltage-dependent inward currents in ICC are both due to Ca2+ conductances. Current-voltage relationships obtained from step protocols performed on 5 cells are summarized in Fig. 1F. These data indicate that both the inward currents are Ca2+-permeable and low voltage-activated inward currents are highly expressed in ICC.
Fig. 1.
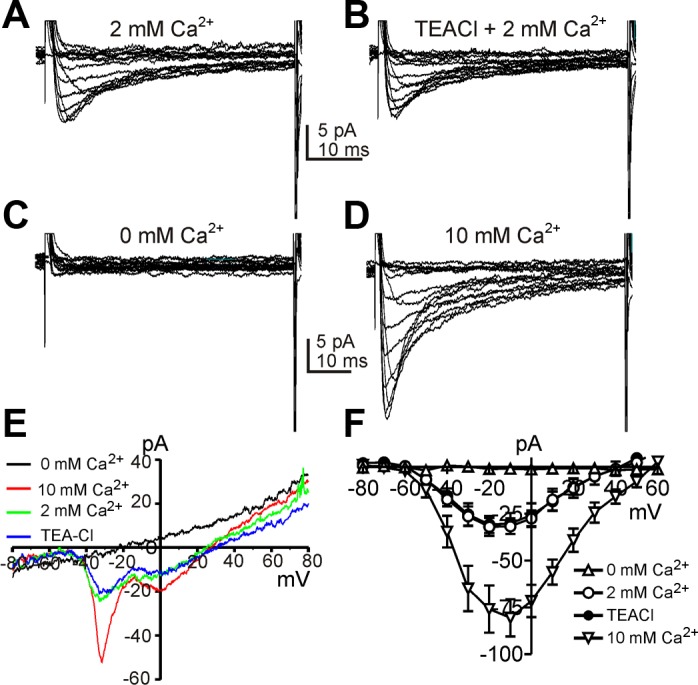
Two types of voltage-dependent Ca2+ currents in interstitial cells of Cajal (ICC). A–D: representative traces recorded in 2 mM Ca2+, 0 mM Na+ (TEA), 0 mM Ca2+, and 10 mM Ca2+, respectively. Data were obtained by steps from −80 to 60 mV every 10 s with 10-mV increments. E: cells were also depolarized from −80 to 80 mV by ramp protocols. The inward current was sensitive to extracellular Ca2+. F: current-voltage (I–V) relationships for the inward current (means ± SE) from 5 cells using step protocols to more accurately determine reversal potentials in 0 mM Ca2+ (△), 2 mM Ca2+ (○), 0 mM Na+ (TEA; ●), and 10 mM Ca2+ (▽). The pattern of the I–V relationships is different using ramp protocols (E) and step protocols (F) due to the inactivation properties of the Ca2+ conductances.
L-type Ca2+ channels have been shown to be more permeable to Ba2+ than to Ca2+, and T-type Ca2+ channels are equally permeable to Ca2+ and Ba2+ (3). We examined inward current responses after extracellular Ba2+ ([Ba2+]o) replacement of [Ca2+]o. In these experiments we first tested the effects of increasing [Ca2+]o from 2 to 10 mM (Fig. 2, B and C). Elevated [Ca2+]o increased both components of inward current. Replacement of [Ca2+]o (10 mM) with [Ba2+]o (10 mM) increased the high voltage-activated inward current by 76 ± 16% at 0 mV but did not enhance the low voltage-activated inward current (Fig. 2, A–C; n = 5). These data suggest that ICC express both L-type and T-type Ca2+ conductances.
Fig. 2.
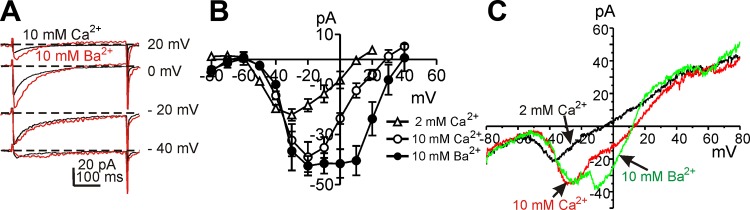
Ba2+ permeability of two types of inward currents in ICC. A. Representative current traces recorded with 10 mM Ca2+ or 10 mM Ba2+, respectively. Steps were applied to −40,−20, 0, and 20 mV from a holding of −80 mV in 10 mM Ca2+ (black lines) or 10 mM Ba2+ (red lines). B. current-voltage relationships for peak current (means ± SE) in 2 mM Ca2+ (△), 10 mM Ca2+ (○), and 10 mM Ba2+ (●). C: currents were obtained by ramp depolarization from −80 to +80 mV in 2 mM Ca2+, 10 mM Ca2+, and 10 mM Ba2+.
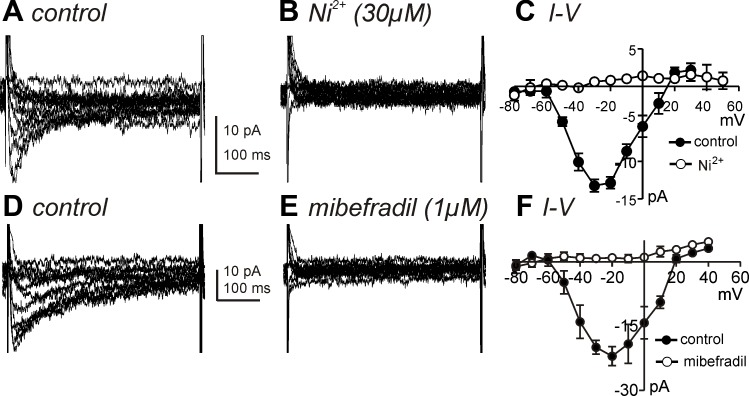
We also examined the pharmacological profiles of the Ca2+ conductances in ICC by testing drugs and ions traditionally described as T-type and L-type Ca2+ channel blockers. The same step depolarization and ramp (400 ms) protocols described in Fig. 1 were utilized in these experiments. Nicardipine (1 μM, L-type Ca2+ channel blocker) completely blocked the high voltage-activated currents, but the low voltage-activated current was not blocked, and in fact, the magnitude of the low voltage-activated current increased significantly in the presence of nicardipine (Fig. 3, A and B). Similar results were observed during ramp depolarization (Fig. 3C). Threshold potentials for resolvable low voltage-activated currents were around −60 mV (Fig. 3B). Current density at −20 mV was 6.6 ± 1.3 pA/pF in the presence of nicardipine, and nicardipine-sensitive current densities at 0 mV was 5.0 ± 1.3 pA/pF. Thus the T-type and L-type current density were not different in ICC (P = 0.4; n = 5). Summarized data are shown in Fig. 3B (n = 5). We further tested the effects of T-type Ca2+ channel blockers on the nicardipine-resistant currents. Ni2+ (30 μM, T-type channel blocker) completely abolished the low-voltage activated current (Fig. 4, A and B). Summarized data are shown in Fig. 4C (n = 5). Mibefradil (1 μM), another T-type channel blocker, had the same effect as Ni2+ on the low voltage-activated current (Fig. 4, D and F; n = 5). Taken together, these data suggest expression of L-type and T-type Ca2+ conductances in ICC. Because of the importance of T-type conductances in slow waves in intact muscles (37), we concentrated the remainder of the study on this component of inward current.
Fig. 3.
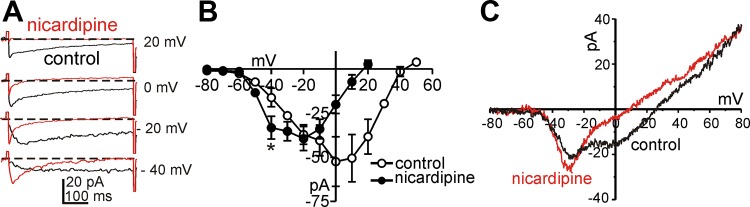
Effects of nicardipine on Ca2+ currents in ICC. A: Ca2+ currents were recorded by voltage steps at −40,−20, 0, and 20 mV in control (black line) and nicardipine (1 μM, red line) presence. B: current-voltage relationships for peak current in control (○) and nicardipine (●). Nicardipine blocked high voltage-activated Ca2+ currents. C: currents traces recorded by ramp pulses from −80 to +80 mV in control (black line) and the presence of nicardipine (1 μM, red line).
Fig. 4.
Effects of T-type Ca2+ channel blockers on low voltage-activated Ca2+ currents of ICC in the presence of nicardipine. A and B: T-type Ca2+ currents were recorded by voltage steps from −80 to +50 mV in control and Ni2+ (30 μM) in the presence of nicardipine (1 μM). C: I–V relationships for peak current in control (●) and Ni2+ (○). Ni2+ completely blocked low voltage-activated Ca2+ currents. D and E: T-type Ca2+ currents were recorded by voltage steps from −80 to +60 mV in control and mibefradil (1 μM) in the presence of nicardipine (1 μM). F: I–V relationships for peak current in control (●) and mibefradil (○). Mibefradil completely blocked low voltage-activated Ca2+ currents.
Voltage and temperature dependence of T-type Ca2+ channel in ICC.
Further experiments to examine the voltage dependence of the T-type Ca2+ conductance in ICC were performed in the presence of nicardipine (1 μM; Fig. 5A). A double-pulse protocol was used to characterize the steady-state inactivation of the T-type conductance. Prepulse potentials (150 ms) ranging from −80 to +60 mV in 5-mV increments were applied. Following a 1-ms interpulse step to −80 mV, membrane potential was stepped to a test potential of −30 mV for 90 ms. The currents resulting from the test depolarization were normalized to the current obtained at −80 mV (I/Imax) and plotted against the prepulse potentials. Plotted data were fitted by a Boltzmann equation. The half-inactivation voltage was −59.2 ± 0.4 mV with a mean slope of 4.9 ± 0.4 mV (Fig. 5B; n = 5). To evaluate steady-state activation relationships, the peak conductance at each test potential was calculated using the equation: ICa = gCa × (V − Erev), where gCa, V, and Erev are peak conductance, test potential, and reversal potential, respectively. Steady-state activation curves were constructed by normalization of the maximum conductance (G/Gmax). The voltage of half-activation was −35.7 ± 3.2 mV with a mean slope of 12.1 ± 3.7 mV (Fig. 5B; n = 5).
Fig. 5.
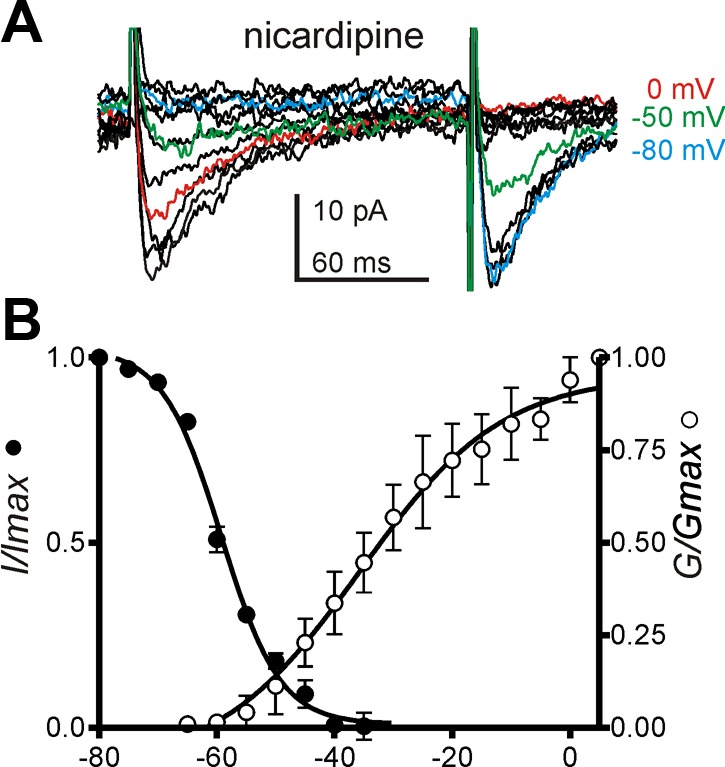
Voltage dependence of activation and inactivation of T-type Ca2+ channel in ICC. A: representative traces of current evoked by applying a prepulse from −80 to 60 mV with 5-mV increments following by currents evoked at −30 mV from a brief holding potential of −80 mV in the presence of nicardipine. Blue, green, and red lines denote the traces elicited using prepotentials at −80, −50, and 0 mV, respectively. B: voltage dependence of steady-state activation (G/Gmax) and inactivation (I/Imax) was fit with Boltzmann equation.
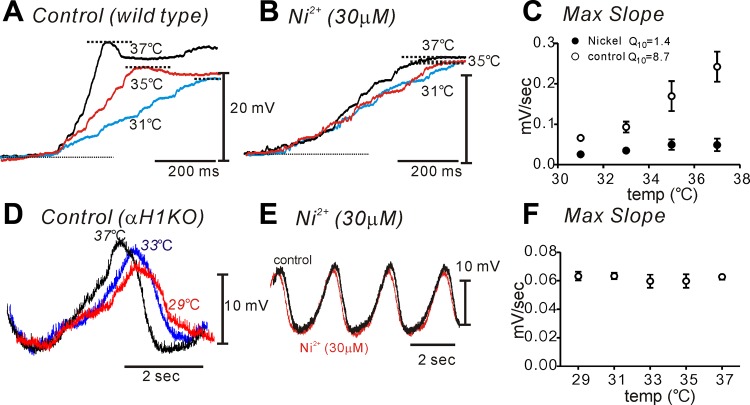
Next, we tested the temperature sensitivity of the T-type Ca2+ conductance, because this is an important property of T-type Ca2+ channels (13). Step depolarizations were applied repetitively from a holding potential of −80 to −30 mV (Fig. 6A). Increasing temperature from 20°C to 30°C increased the amplitude of the T-type Ca2+ currents from −7.0 ± 1.9 to −19.5 ± 3.8 pA (Fig. 6B; n = 5; P = 0.004; F = 17.84 by ANOVA) and decreased the activation time constant from 19.0 ± 1.9 to 7.5 ± 0.1 ms (Fig. 6C; n = 5; P = 0.001; F = 15.09 by ANOVA). The temperature coefficient (Q10) for amplitude was 2.8 and for the rate of activation time constant was 2.5.
Fig. 6.
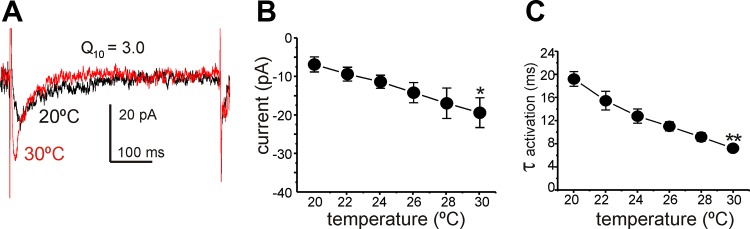
Temperature sensitivity of T-type Ca2+ currents in ICC. A: representative traces of current recorded by applying a pulse from −80 to −30 mV in 20°C (black trace) and 30°C (red trace). B: summary of peak current at various temperature (from 20 to 30°C; n = 5). C: summary of activation time constant at various temperatures (n = 5) *P < 0.05, **P < 0.01 vs. 20°C.
Molecular candidate for T-type Ca2+ channels in ICC and its functional role on slow waves.
Transcriptional expression of Cacna1g (CaV3.1), Cacna1h (CaV3.2), and Cacna1i (CaV3.3) subunits of T-type Ca2+ channel were tested by RT-PCR on extracts of ICC and SMC purified by FACS (see Methods). Primers are provided in Table 1. Cacna1h (Cav3.2) was expressed in SMC and ICC; however, Cacna1g (Cav3.1) was resolved only in extracts of ICC (Fig. 7A). Kit mRNA was amplified in sorted ICC, and Myh11 was amplified in sorted SMCs vs. unsorted cells, confirming the relative purity of the sorted cells. Quantitative PCR showed Cacna1h is highly expressed in ICC and SMC. Cacna1g is also expressed in ICC but at a lower level compared with Cacna1h. There was negligible expression of Cacna1i in either ICC or SMC (Fig. 7B).
Fig. 7.
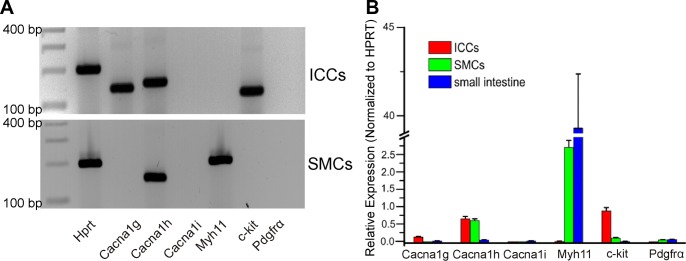
Qualitative and quantitative PCR of T type calcium channels in sorted ICC, smooth muscle cells (SMCs), and unsorted (whole small intestine smooth muscle). A: sorted ICC displayed no contamination of SMC or PDGFRα+ cells. Top: Cacna1g and Cacna1h transcripts were detected in sorted ICC. Bottom: Cacna1h detection in sorted SMC. B: quantitative analysis of PCR products demonstrated that Kit was highly enriched in ICC. Cacna1h was dominantly expressed in ICC and SMCs compared with whole small intestine. Relative expressions of all transcripts were normalized to Hprt.
Role of T-type Ca2+ channel in slow waves in situ.
Ni2+ and mibefradil were shown to reduce the slope of the upstroke phase of slow waves from small intestinal ICC of wild-type mice previously (18). In this study we tested the role of T-type Ca2+ channels in slow waves by testing the effects of temperature on slow waves in jejunal smooth muscles in wild-type and Cacna1h−/− mouse. The frequency of slow waves in Cacna1h−/− mice decreased significantly compared with wild-type mice as previously reported (6). Reducing temperature decreased the upstroke velocity of slow waves significantly from 0.24 ± 0.05 at 37 to 0.07 ± 0.01 at 31°C in wild type (Q10 = 8.7; n = 6; Fig. 8A). Q10 was determined by the changes in the maximum slope. Ni2+ (30 μM) also decreased the maximum slope of the upstroke phase of slow waves. In the presence of Ni2+ (30 μM), changing temperature had little effect on the slow wave upstroke slope (Fig. 8B). Figure 8C shows the average change in maximum slope before and after Ni2+ following temperature changes applied to muscles of five animals. Ni2+ significantly decreased the temperature sensitivity on the rate of rise of slow waves (Q10 = 1.4). Slow waves recorded from jejunal muscles of Cacna1h−/− mice were not decreased significantly by reduced temperature (Fig. 8, D and F) or Ni2+ (Fig. 8E; n = 4). These data suggested that Cav3.2 is a major conductance generating the slow wave upstroke in the small intestine.
Fig. 8.
Role of T-type Ca2+ currents on initial upstroke of slow waves in murine jejunal smooth muscle from wild-type and Cacna1h−/− mice. A: raising temperature from 31 to 37°C increased the initial upstroke slope of slow wave in wild-type jejunum. B: pretreatment of Ni2+ (30 μM) abolished temperature sensitivity of the initial slope in wild-type jejunum. C.: summarized data of maximum slope of initial upstroke before (○) and after Ni2+ (●) at various temperatures in wild-type jejunum. D: Cacna1h−/− mouse did not show the temperature sensitivity. E: Ni2+ treatment also did not show significant changes of initial upstroke slope and frequency of slow waves in Cacna1h−/−. F: summarized data of maximum slope of initial upstroke in Cacna1h−/− jejunum.
DISCUSSION
It has been proposed that voltage-dependent Ca2+ entry, possibly via a T-type conductance, is responsible for entrainment and propagation of pacemaker currents in ICC (1, 30). In this study we showed that ICC, freshly isolated from the murine small intestine, expresses voltage-dependent Ca2+ currents. Two components of voltage-dependent Ca2+ conductance, a low voltage-activated Ca2+ current and a high voltage-activated Ca2+ current, were distinguished in whole cell patch-clamp recordings. Since slow waves in intact muscles are not typically blocked by dihydropyridines, it is unlikely that the high voltage-activated conductance is of prime importance. Therefore, we concentrated our experiments on the properties and pharmacology of the low voltage-activated conductance and concluded that it results from T-type Ca2+ channels, most probably encoded by Cacna1h and Cacna1g. Electrophysiological evidence supported the idea that the low-voltage activated Ca2+ current was due to T-type conductance, because the currents were as follows: 1) activated and inactivated at potentials (i.e., half activation = −36 mV and half inactivation = −59 mV) consistent with the properties of expressed Cacna1h channels (13); 2) blocked by Ni2+ and mibefradil; 3) not blocked by nicardipine; 4) the same magnitude when charge was carried by Ba2+ or Ca2+ (implying equal permeable); and 5) temperature sensitive and consistent with properties of expressed Cacna1h channels (13). Many of the properties and pharmacology that would be consistent with the involvement of a T-like conductance have been demonstrated, as discussed below, in experiments on muscles from several regions of the gastrointestinal tract and from several species previously. The temperature dependence of slow waves in wild-type muscles and the absence of this property in muscles lacking Cacna1h were demonstrated in the current study. Our molecular data also showed a low level of expression of Cacna1g; however, both Ni2+ and temperature sensitivity were absent in Cacna1h−/− mice suggesting that Cacna1h is the dominant species of T-type Ca2+ channels in murine jejunum. Thus our data document a conductance that is likely to be involved in organizing localized STICs in ICC into whole cell slow wave currents and providing a voltage-dependent mechanism for cell-to-cell propagation of slow waves in ICC networks.
Slow waves recorded directly from ICC in the murine small intestine were greatly reduced in frequency, amplitude, and upstroke velocity by Ni2+ and mibefradil, as shown previously (17, 18). Slow waves were also reduced in frequency, amplitude, and upstroke velocity by reducing [Ca2+]o to nominally Ca2+ free conditions or by elevated (20 mM) [K+]o, which depolarized ICC to −40 mV (17). The effects of high [K+]o might be explained by the inactivation of the low voltage-activated conductance we observed in ICC, because at −40 mV the conductance was <10% available for activation. Propagation of slow waves in intact muscles is also strongly dependent on a Ca2+ entry mechanism that appears to be consistent with a T-type Ca2+ conductance. Nifedipine had no effect on slow wave propagation rate or upstroke velocity, but Ni2+ progressively decreased these parameters and blocked propagation at 100 μM (1). The effects of mibefradil and reduced [Ca2+]o were similar, with total block of slow wave propagation at 25 and 0.5 μM, respectively. Depolarization with 15 mM [K+]o also blocked propagation. The current study demonstrates a conductance in ICC that can explain previous observations and suggests that a T-type conductance is a fundamental component of the apparatus in ICC required for pacemaker activity.
Some investigators have suggested that the voltage sensor in ICC responsible for propagation is voltage-dependent enhancement of phospholipase C activity and/or voltage-dependent “sensitization” of IP3 receptors (5, 14, 24). Increasing IP3 synthesis or increasing the sensitivity of IP3 receptors might facilitate synchronization of Ca2+ release in ICC and serve to entrain STICs into slow wave currents. In fact based on the properties of IP3 receptors, increasing either IP3 or [Ca2+]i would tend to enhance the probability of Ca2+ release, so either mechanism might be considered an effective means of “sensitizing” IP3 receptors (20). Although voltage-dependent regulation of IP3 receptor-operated Ca2+ release has been demonstrated in megakaryocytes (22), such a mechanism has not been observed directly in ICC. Testing this hypothesis will require monitoring subcellular Ca2+ responses to agonists or IP3 under voltage-clamp conditions, and these experiments have not yet been accomplished in isolated ICC. Therefore, at present we cannot exclude the possibility that a voltage sensor linked to IP3 production and/or receptor affinity is involved in slow wave propagation, but results to date strongly support the importance of voltage-dependent Ca2+ entry through a T-type conductance in both entrainment of localized pacemaker events (STICs) into whole cell slow wave currents and in cell-to-cell slow wave propagation.
The slopes of the upstroke depolarizations of slow waves of guinea-pig antrum and murine small intestine are sensitive to changes in temperature (16, 23). Higher temperatures increase upstroke velocity and overall frequency without changing resting membrane potentials. The temperature dependence of the upstroke depolarization may be another indication of the role of T-type currents in initiation of slow waves. In the present study lowering temperature decreased the slope of the upstroke depolarization significantly. The temperature sensitivity of the upstroke was abolished by pretreatment with Ni2+. Thus, based on the significant temperature sensitivity of T-type Ca2+ currents in ICC, it is possible that these channels contribute to slow wave upstrokes. Our molecular data suggest that Cacna1h is the dominant T-type Ca2+ channel expressed in ICC. Therefore, we also examined slow wave activity and slopes of upstroke potentials in jejunal muscles from Cacna1h−/− mice. The upstroke depolarizations of slow waves in these muscles lacked significant temperature dependence and responses to Ni2+. These results are consistent with a role for channels encoded by Cacna1h in the initial depolarization phase of slow waves.
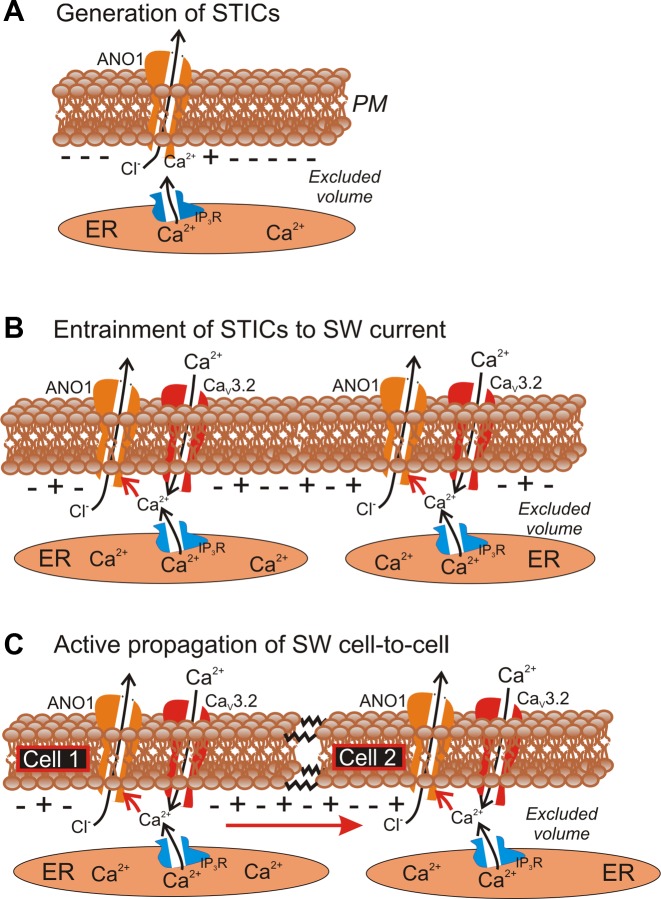
This study provides direct measurement of another critical element of the pacemaker mechanism driving slow wave activity in ICC. Previous studies showed voltage-dependent activation of inward currents in freshly isolated ICC from murine small intestine (7, 39), and this conductance was later shown to be due to Ca2+-activated Cl− channels encoded by Ano1 (aka Tmem16a). Although the Ca2+ dependence of Ano1 is well documented (2, 31, 38) and the requirement for Ca2+ entry for depolarization-dependent generation of slow wave currents was demonstrated (39), the mechanism for Ca2+ entry in native, freshly isolated ICC had not been described previously. ICC display a continuous discharge of STICs when held at negative potentials, and depolarization to about −65 mV elicits large amplitude slow wave currents. The voltage-dependent generation of slow wave currents is blocked by reduced [Ca2+]o, by replacing [Ca2+]o with equimolar [Ba2+]o and by Ni2+ (30 μM). These data suggest that entrainment of STICs into slow wave currents requires Ca2+ entry through the T-type conductance identified in the present study. We also envision a role for these channels in cell-to-cell propagation because depolarization caused by Ano1 slow wave currents will depolarize and activate T-type channels in coupled ICC. In the present study 30 μM Ni2+ decreased the frequency of slow waves and slowed upstroke velocities; however, this concentration of Ni2+ did not block pacemaker activity. As discussed above, propagation experiments are consistent with the involvement of a T-type conductance in propagation. Together with the data from the present study, pharmacological studies suggest that the following sequence of events in the generation and propagation of slow waves (Fig. 9): 1) stochastic localized Ca2+ release events (puffs) are linked to local activation of clusters of Ca2+-activated Cl− channels, STICs; 2) STICs cause variable amplitude transient depolarizations (STDs); 3) STDs reach a threshold for regenerative activation of T-type Ca2+ channels; 4) ensuing depolarization can also recruit L-type channels; 5) Ca2+ entry through channels distributed through the cell membrane can elicit Ca2+-induced Ca2+ release nearly simultaneously from Ca2+ stores (i.e., entraining Ca2+ release); 6) simultaneous Ca2+ release, summing with Ca2+ entry, elicits whole cell slow wave currents; and 7) these currents result in slow wave depolarization that conducts to coupled cells and evokes whole cell slow wave currents progressively cell-to-cell through the ICC network. Missing from the above scheme, at present, are direct measurements of Ca2+ puffs or entrainment of localized Ca2+ transients into multisite or whole cell Ca2+ transients in ICC in response to depolarization and Ca2+ entry via T-type Ca2+. The small size of ICC and the need to locate these cells in mixed cell dispersions with a fluorescent reporter have made such measurements difficult to obtain.
Fig. 9.
Schematic showing role of T-current in pacemaker activity and propagation. A: localized Ca2+ release events in ICC activate nearby Ca2+-activated Cl− channels (Ano1) to create spontaneous transient inward currents (STICs). STICs produce spontaneous transient depolarizations of cell membrane. B: when a threshold potential is reached for regenerative activation of T-type channels (CaV3.2) Ca2+ entry reinforces activation of Ano1 and further depolarizes cell. This leads to generation of whole cell slow wave current. C: slow wave currents conduct (red arrow) to coupled cells (resistor symbols) and depolarize adjacent cells. Depolarization activates T-type currents in adjacent cells and Ca2+ entry and release in these cells activates Ano1 channels. This sequence explains cell-to-cell propagation of slow waves in ICC networks. IP3, inositol triphosphate; ER, endoplasmic reticulum; SW, slow wave; PM, plasma membrane.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01-DK-41315.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.Z., K.S.P., S.D.K., and K.M.S. conception and design of research; H.Z. and K.S.P. performed experiments; H.Z., K.S.P., and S.D.K. analyzed data; H.Z., S.D.K., and K.M.S. interpreted results of experiments; H.Z. and K.S.P. prepared figures; H.Z., S.D.K., and K.M.S. drafted manuscript; H.Z., S.D.K., and K.M.S. edited and revised manuscript; H.Z., K.S.P., S.D.K., and K.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Lauren E. Peri for performing the PCR studies of Ca2+ channel gene expression, Jared B. Townsend for sorting cells by FACS, Nancy Horowitz for technical assistance, and Huili Zheng for help with breeding and maintenance of the transgenic animals. smMHC/Cre/eGFP mice were donated by Michael Kotlikoff, Cornell University, and Cacna1h−/− mice were provided by Kevin P Campbell, University of Iowa.
REFERENCES
- 1.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol 293: C1645–C1659, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol 514: 515–531, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards FR, Hirst GD. An electrical analysis of slow wave propagation in the guinea-pig gastric antrum. J Physiol 571: 179–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons SJ, Strege PR, Lei S, Roeder JL, Mazzone A, Ou Y, Rich A, Farrugia G. The alpha1H Ca2+ channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J Cell Mol Med 13: 4422–4431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol 559: 411–422, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol 556: 585–599, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol 540: 907–919, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach–a stochastic process. J Physiol 535: 165–180, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iftinca M, McKay BE, Snutch TP, McRory JE, Turner RW, Zamponi GW. Temperature dependence of T-type calcium channel gating. Neuroscience 142: 1031–1042, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Imtiaz MS, Katnik CP, Smith DW, van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+ oscillations. Biophys J 90: 1–23, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol 541: 797–810, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kito Y, Suzuki H. Effects of temperature on pacemaker potentials in the mouse small intestine. Pflügers Arch 454: 263–275, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol 553: 803–818, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol 288: C710–C720, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA 86: 7280–7284, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci USA 95: 15821–15825, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP3-sensitive calcium release in the murine small intestine. Am J Physiol Gastrointest Liver Physiol 280: G439–G448, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Mason MJ, Mahaut-Smith MP. Voltage-dependent Ca2+ release in rat megakaryocytes requires functional IP3 receptors. J Physiol 533: 175–183, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura E, Kito Y, Hashitani H, Suzuki H. Metabolic component of the temperature-sensitivity of slow waves recorded from gastric muscle of the guinea-pig. J Smooth Muscle Res 42: 33–48, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Nose K, Suzuki H, Kannan H. Voltage dependency of the frequency of slow waves in antrum smooth muscle of the guinea-pig stomach. Jpn J Physiol 50: 625–633, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Ordog T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol 518: 257–269, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol Cell Physiol 260: C917–C925, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol 290: C1411–C1427, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111: 492–515, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol 54: 439–453, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol 68: 307–343, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscle. In: Smooth Muscle, An Assessment of Current Knowledge. Austin, TX: Univ. of Texas Press, 1981, p. 127–156 [Google Scholar]
- 33.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 280: 97–111, 1995 [DOI] [PubMed] [Google Scholar]
- 34.van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol 548: 271–296, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward SM, Baker SA, de Faoite A, Sanders KM. Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J Physiol 549: 207–218, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480: 91–97, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward SM, Vogalis F, Blondfield DP, Ozaki H, Fusetani N, Uemura D, Publicover NG, Sanders KM. Inhibition of electrical slow waves and Ca2+ currents of gastric and colonic smooth muscle by phosphatase inhibitors. Am J Physiol Cell Physiol 261: C64–C70, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O'Driscoll K, Koh SD, Sanders KM. Muscarinic activation of Ca2+-activated Cl− current in interstitial cells of Cajal. J Physiol 589: 4565–4582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]