Abstract
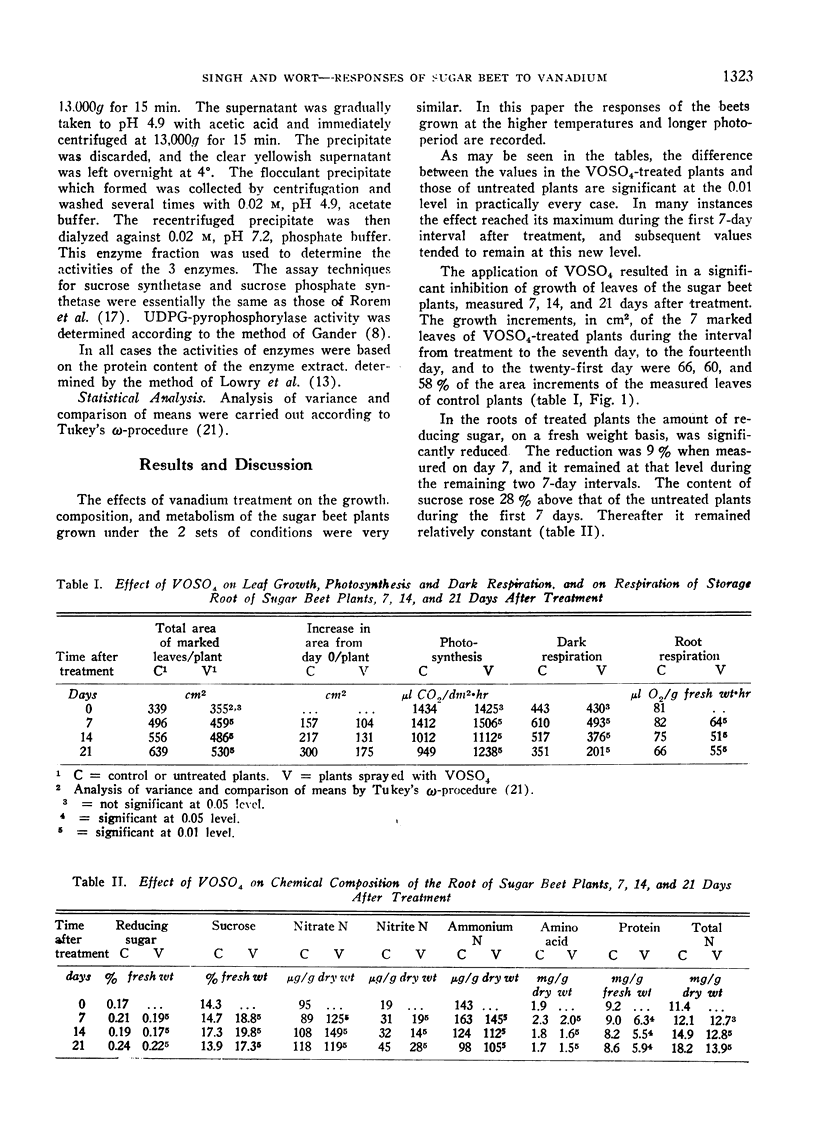
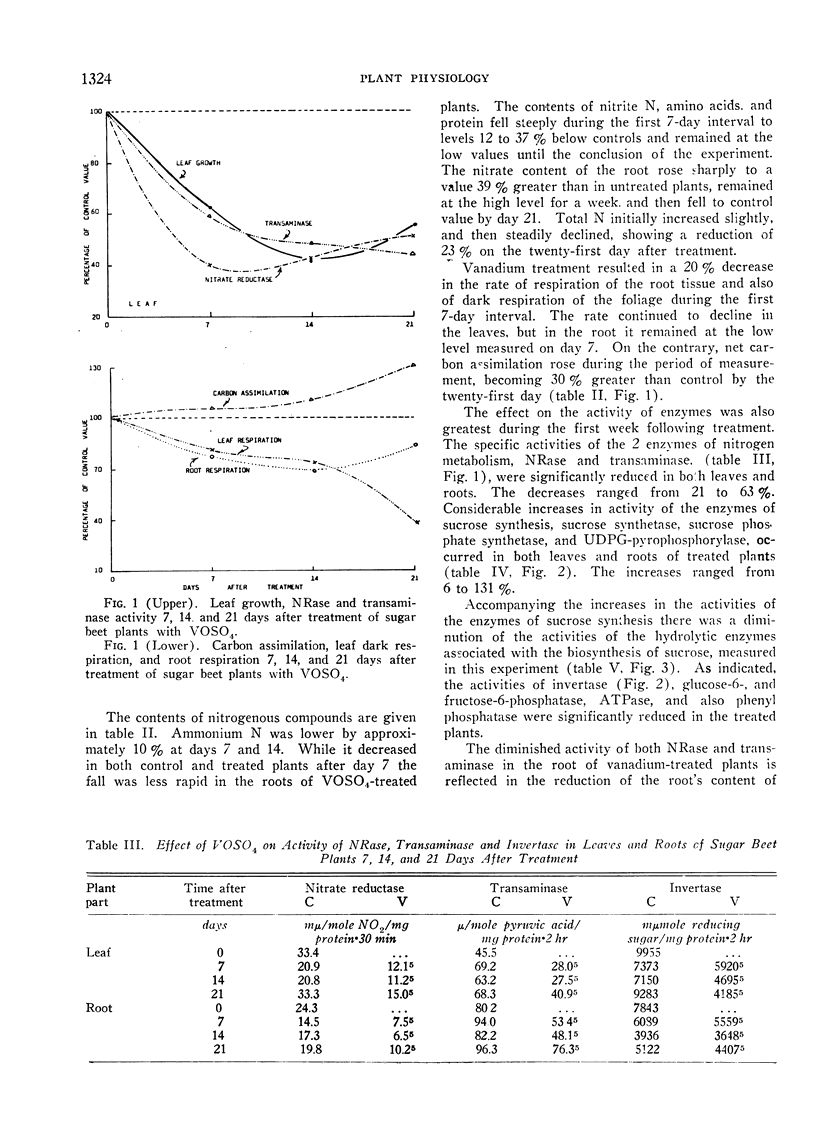
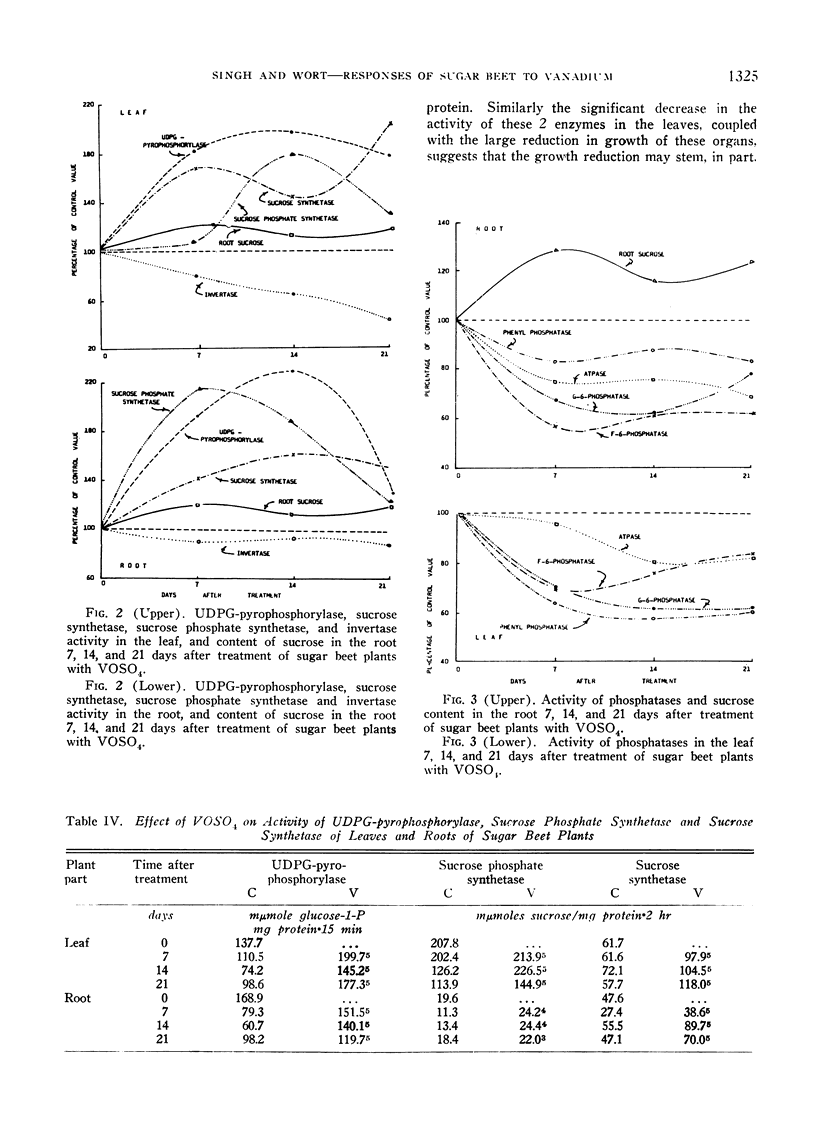
As measured 7, 14, and 21 days after the application of 10−2 M vanadyl sulfate solution to the foliage of 4.5-month-old sugar beet plants, significantly less growth of the leaves and an increase in the sucrose content of the storage root resulted. Accompanying these alterations were a higher rate of carbon dioxide fixation, a lower rate of respiration, and a decreased rate of nitrate reductase, glutamic-pyruvic transaminase, phosphatase, and invertase activity. The enzymes of sucrose synthesis, sucrose synthetase, sucrose phosphate synthetase and uridine diphosphate glucose-pyrophosphorylase were stimulated. The content of reducing sugar, nitrite N, amino acids and protein was less, and that of nitrate N was greater in the vanadium-treated plants. In the majority of cases the greatest magnitude of change occurred during the first 7 days following treatment. The changes in growth and chemical composition are believed to be closely related to the stimulation or inhibition of the various enzymes by vanadyl sulfate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COSTELLO R. L., HEDGECOCK L. W. Effect of metavanadate ion on the growth in vitro of Mycobacterium tuberculosis. J Bacteriol. 1959 Jun;77(6):794–799. doi: 10.1128/jb.77.6.794-799.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN G. L., COSTELLO R. L. Reduction of excess cholesterol in the rabbit aorta by inhibition of endogenous cholesterol synthesis. J Exp Med. 1956 Jan 1;103(1):49–56. doi: 10.1084/jem.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock J. N., Hill C. H., Tove S. B. Uncoupling of oxidative phosphorylation by vanadate. Can J Biochem. 1966 Jul;44(7):983–988. doi: 10.1139/o66-115. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- REITMAN S., FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957 Jul;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Rorem E. S., Walker H. G., McCready R. M. Biosynthesis of Sucrose and Sucrose-Phosphate by Sugar Beet Leaf Extracts. Plant Physiol. 1960 Mar;35(2):269–272. doi: 10.1104/pp.35.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. H. Protein, Nucleotide, & Ribonucleid Acid Metabolism in Corn During Germination Under Water Stress. Plant Physiol. 1962 Sep;37(5):565–571. doi: 10.1104/pp.37.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]


